Enhanced Surface Properties of Carbon Fiber Reinforced Plastic by Epoxy Modified Primer with Plasma for Automotive Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Epoxy Modified Primer
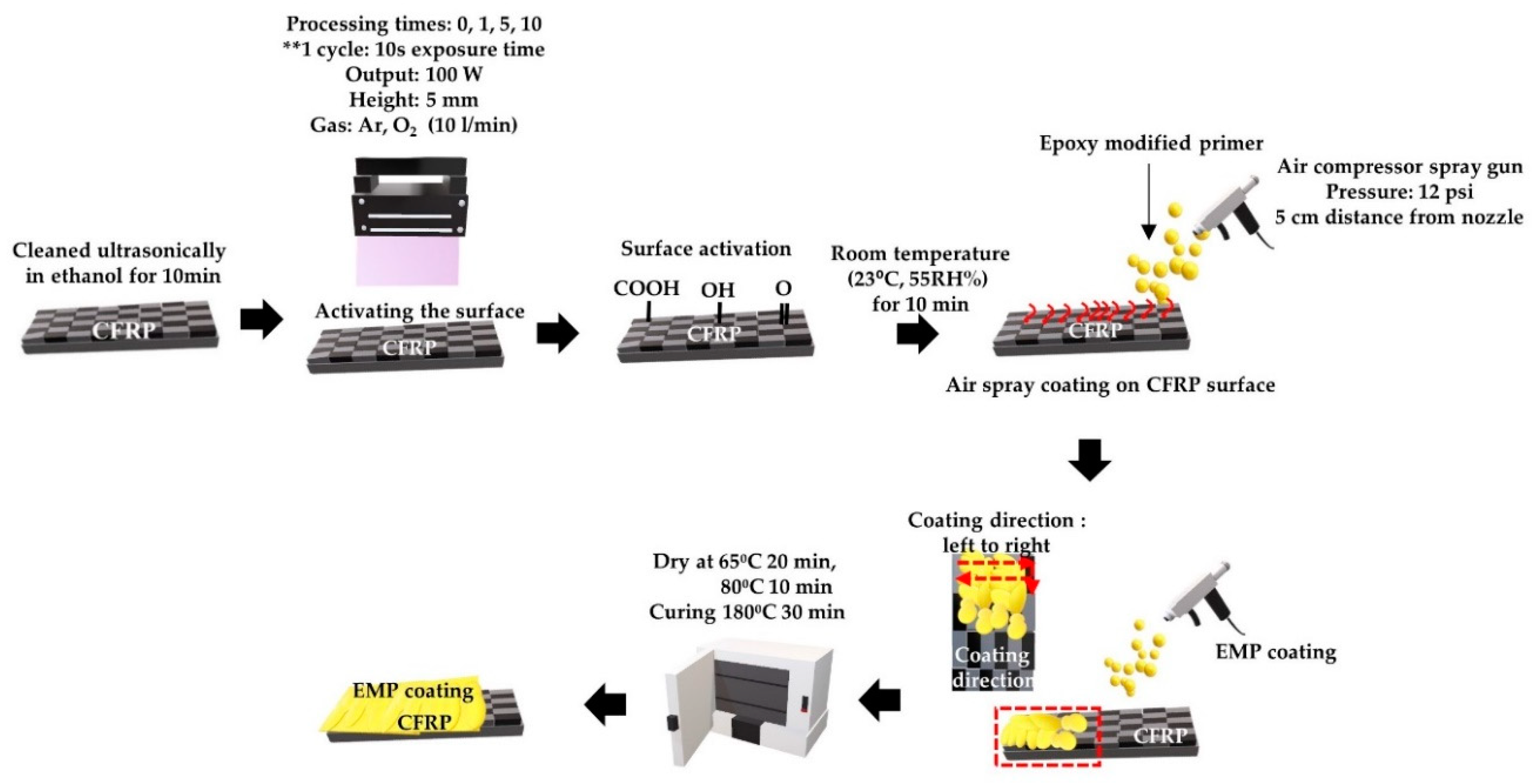
2.1.2. Plasma Treatment
2.2. Epoxy-Modified Primer Coating
2.3. Contact Angle
2.4. X-Ray Photoelectron Spectroscopy
2.5. Surface Analysis
2.6. Surface and Interfacial Cutting Analysis System
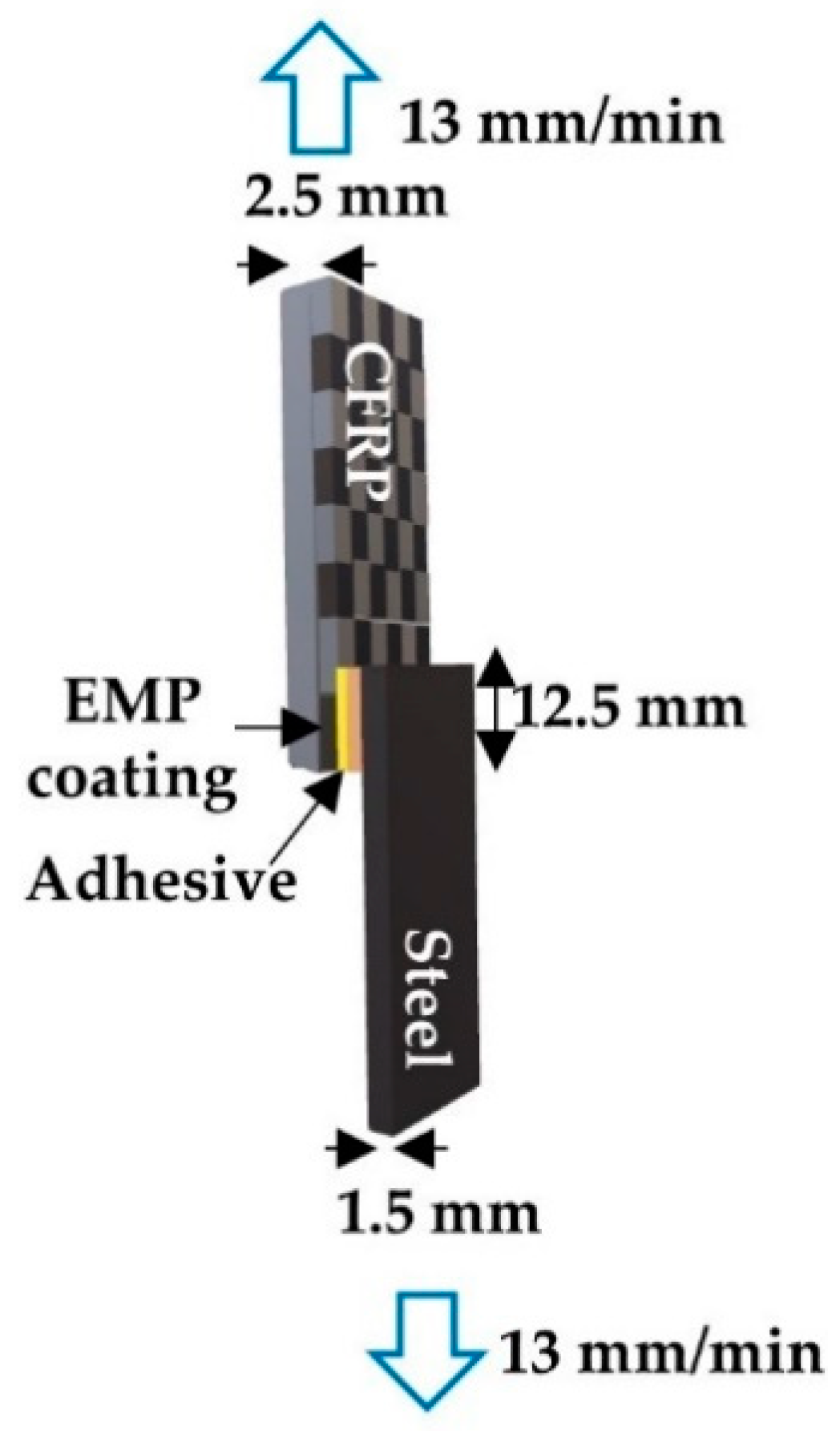
2.7. Lap Shear Test
3. Results and Discussion
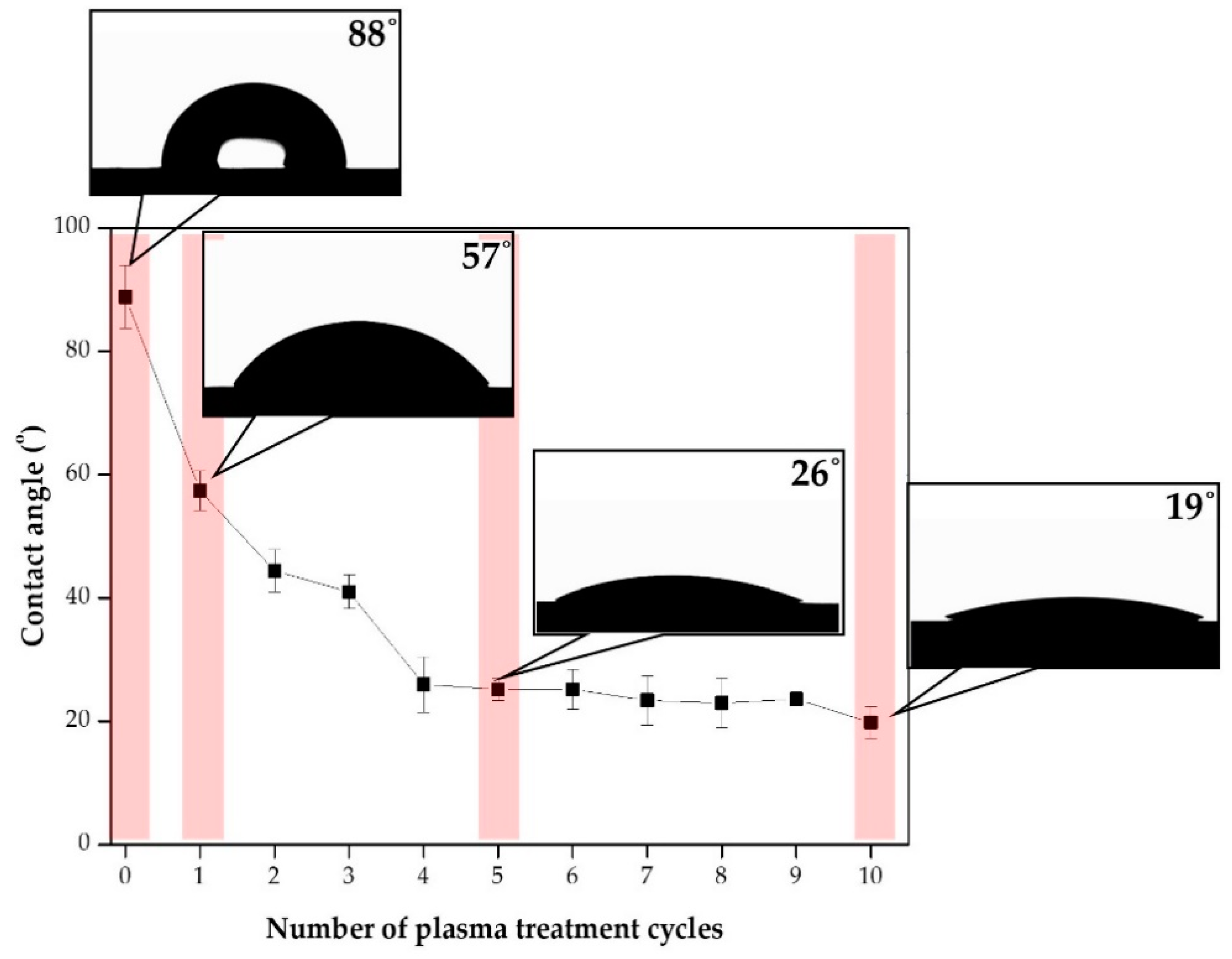
3.1. Surface Modification through Plasma Treatment
3.2. EMP Coatings
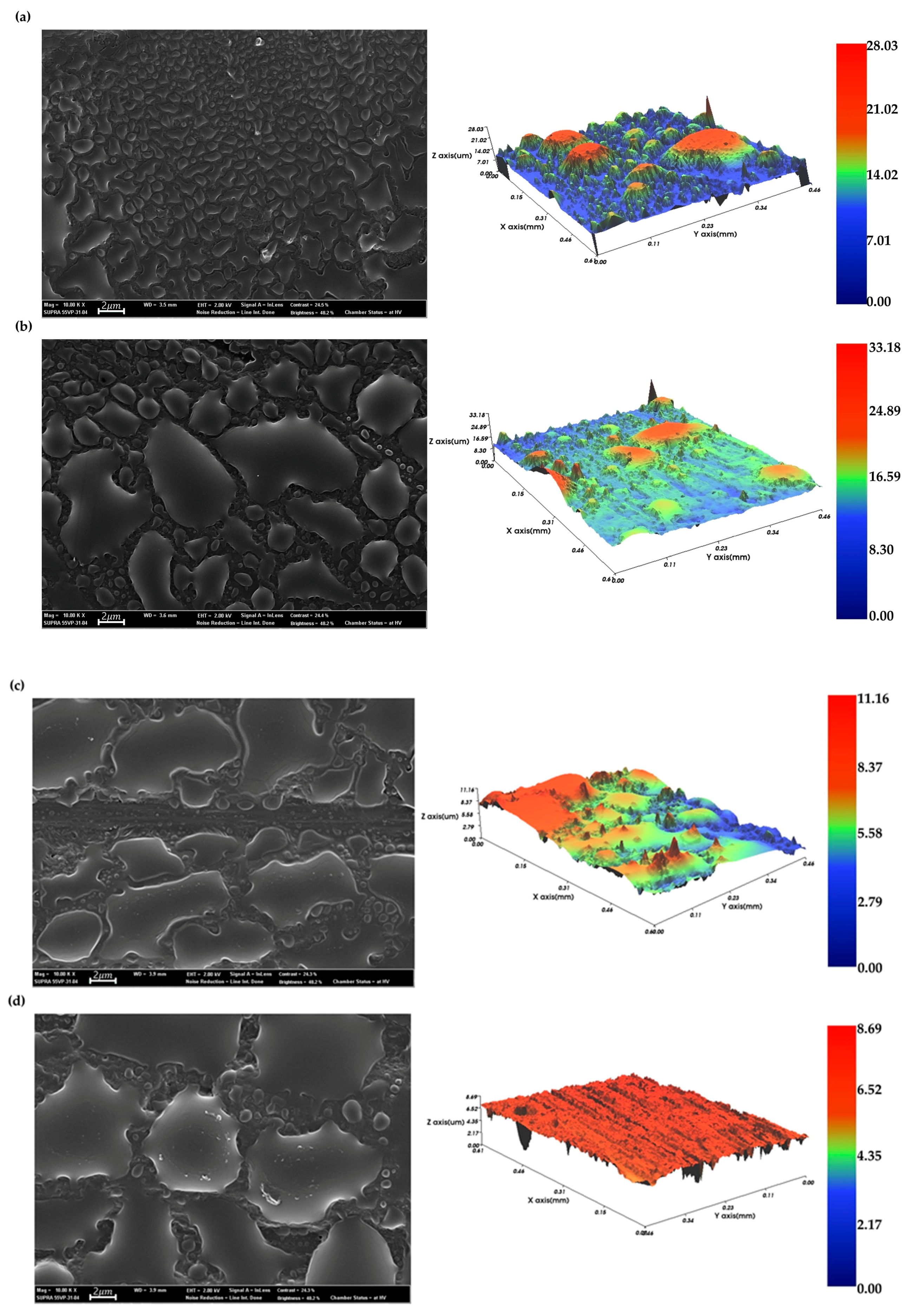
3.2.1. Surface Morphology
3.2.2. EMP Coating Adhesion Properties
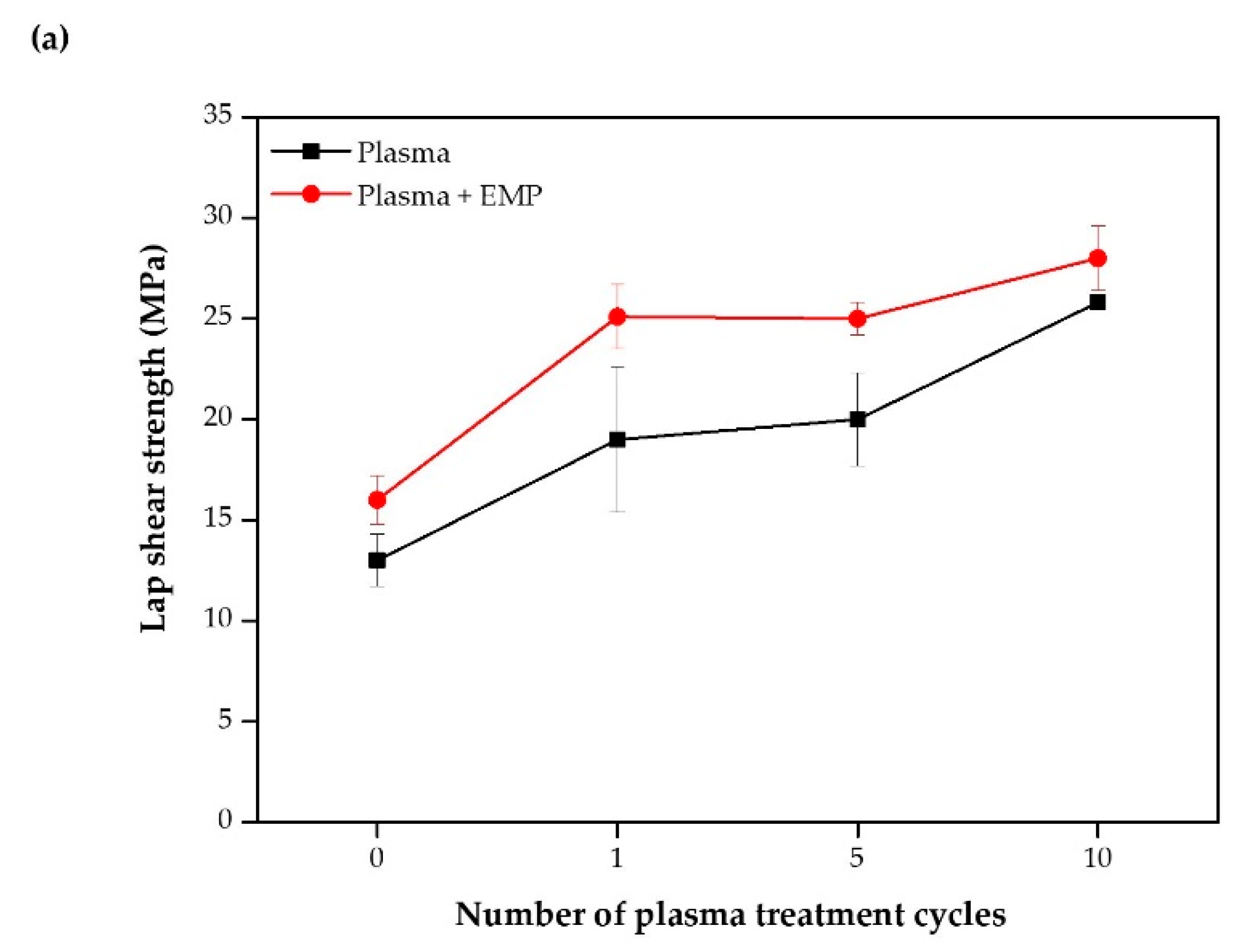
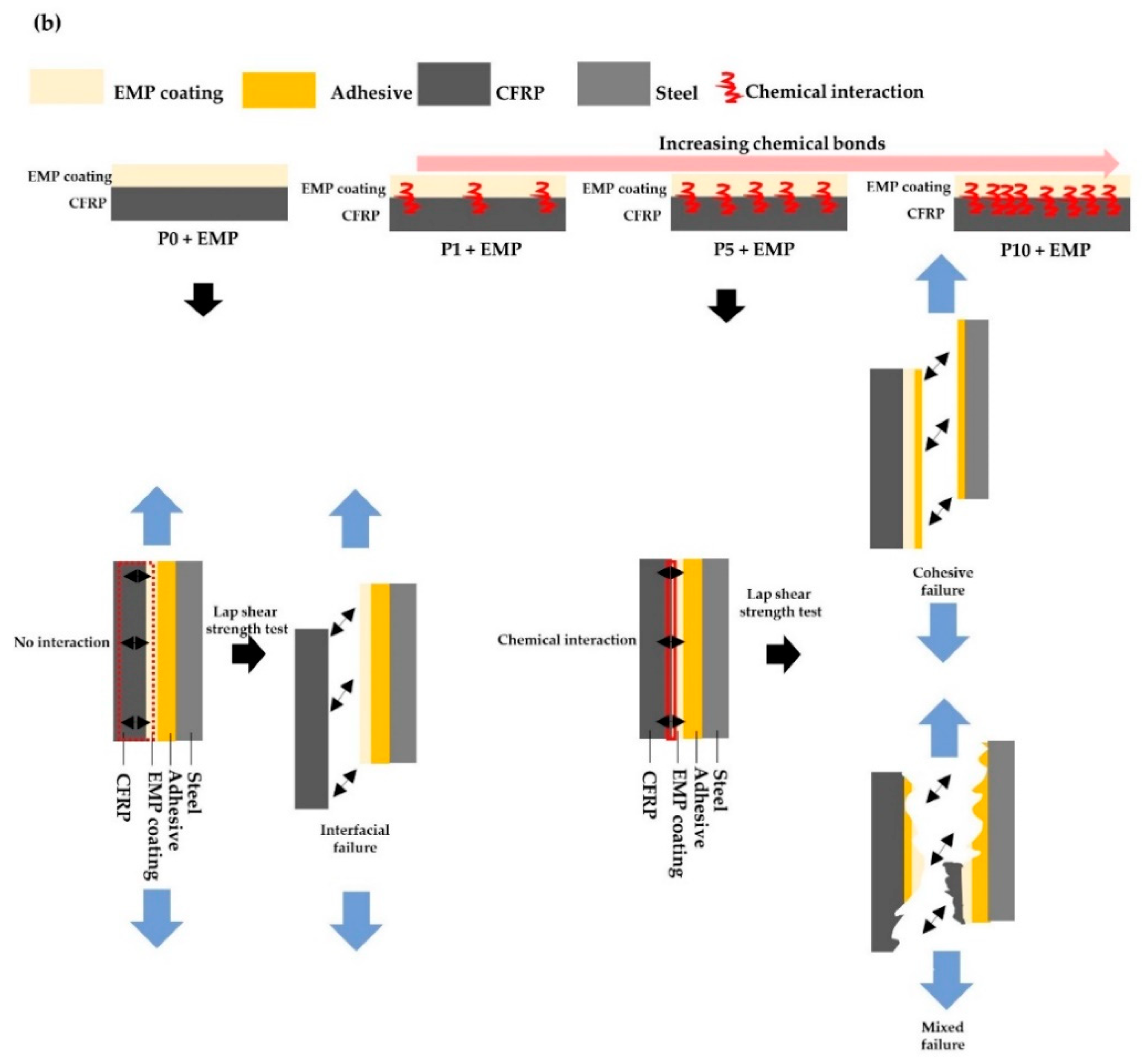
3.3. Mechanical Test and Fracture Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, S.; Sun, T.; Liu, C.; Yang, W.; Tang, Q. A study of laser surface treatment in bonded repair of composite aircraft structures. R. Soc. Open Sci. 2018, 5, 171272. [Google Scholar] [CrossRef]
- Zhang, X.; Yamauchi, M.; Takahashi, J. Life Cycle Assessment of CFRP in Application of Automobile. In Proceedings of the ICCM International Conferences on Composite Materials, Jeju Island, Korea, 21–26 August 2011. [Google Scholar]
- Russo, S. First investigation on mixed cracks and failure modes in multi-bolted FRP plates. Compos. Struct. 2016, 154, 17–30. [Google Scholar] [CrossRef]
- Camanho, P.P.; Bowron, S.; Matthews, F.L. Failure mechanisms in bolted CFRP. J. Reinf. Plast. Compos. 1998, 17, 205–233. [Google Scholar] [CrossRef]
- Feo, L.; Marra, G.; Mosallam, A.S. Stress analysis of multi-bolted joints for FRP pultruded composite structures. Compos. Struct. 2012, 94, 3769–3780. [Google Scholar] [CrossRef]
- Cooper, C.; Turvey, G.J. Effects of joint geometry and bolt torque on the structural performance of single bolt tension joints in pultruded GRP sheet material. Compos. Struct. 1995, 32, 217–226. [Google Scholar] [CrossRef]
- Starikov, R.; Schön, J. Local fatigue behaviour of CFRP bolted joints. Compos. Sci. Technol. 2002, 62, 243–253. [Google Scholar] [CrossRef]
- Goncalves, J.P.; De Moura, M.F.; De Castro, P.M. A three-dimensional finite element model for stress analysis of adhesive joints. Int. J. Adhes. Adhes. 2002, 22, 357–365. [Google Scholar] [CrossRef]
- Sawa, T.; Liu, J.; Nakano, K.; Tanaka, J. Two-dimensional stress analysis of single-lap adhesive joints of dissimilar adherends subjected to tensile loads. J. Adhes. Sci. Technol. 2000, 14, 43–66. [Google Scholar] [CrossRef]
- Davis, G.D. Contamination of surfaces: Origin, detection and effect on adhesion. Surf. Interface Anal. 1993, 20, 368–372. [Google Scholar] [CrossRef]
- Rauh, B.; Kreling, S.; Kolb, M.; Geistbeck, M.; Boujenfa, S.; Suess, M.; Dilger, K. UV-laser cleaning and surface characterization of an aerospace carbon fibre reinforced polymer. Int. J. Adhes. Adhes. 2018, 82, 50–59. [Google Scholar] [CrossRef]
- Blass, D.; Dilger, K. CFRP-Part Quality as the Result of Release Agent Application—Demoldability, Contamination Level, Bondability. Procedia CIRP 2017, 66, 33–38. [Google Scholar] [CrossRef]
- Wingfield, J.R. Treatment of composite surfaces for adhesive bonding. Int. J. Adhes. Adhes. 1993, 13, 151–156. [Google Scholar] [CrossRef]
- Cui, N.Y.; Brown, N.M. Modification of the surface properties of a polypropylene (PP) film using an air dielectric barrier discharge plasma. Appl. Surf. Sci. 2002, 189, 31–38. [Google Scholar] [CrossRef]
- Lai, J.; Sunderland, B.; Xue, J.; Yan, S.; Zhao, W.; Folkard, M.; Michael, B.D.; Wang, Y. Study on hydrophilicity of polymer surfaces improved by plasma treatment. Appl. Surf. Sci. 2006, 252, 3375–3379. [Google Scholar] [CrossRef]
- Shenton, M.J.; Lovell-Hoare, M.C.; Stevens, G.C. Adhesion enhancement of polymer surfaces by atmospheric plasma treatment. J. Phys. D Appl. Phys. 2001, 34, 2754. [Google Scholar] [CrossRef]
- Mund, M.; Lippky, K.; Blass, D.; Dilger, K. Influence of production based surface topography and release agent amount on bonding properties of CFRP. Compos. Struct. 2019, 216, 104–111. [Google Scholar] [CrossRef]
- Walther, F.; Davydovskaya, P.; Zürcher, S.; Kaiser, M.; Herberg, H.; Gigler, A.M.; Stark, R.W. Stability of the hydrophilic behavior of oxygen plasma activated SU-8. J. Micromech. Microeng. 2007, 17, 524. [Google Scholar] [CrossRef]
- Liu, D.; Chen, P.; Mu, J.; Yu, Q.; Lu, C. Improvement and mechanism of interfacial adhesion in PBO fiber/bismaleimide composite by oxygen plasma treatment. Appl. Surf. Sci. 2011, 257, 6935–6940. [Google Scholar] [CrossRef]
- Blank, W.J.; He, Z.A.; Picci, M. Catalysis of the epoxy-carboxyl reaction. J. Coat. Technol. 2002, 74, 33–41. [Google Scholar] [CrossRef]
- Vesel, A.; Mozetic, M.; Zalar, A. XPS study of oxygen plasma activated PET. Vacuum 2007, 82, 248–251. [Google Scholar] [CrossRef]
- Park, J.M.; Kim, D.S.; Kim, S.R. Improvement of interfacial adhesion and nondestructive damage evaluation for plasma-treated PBO and Kevlar fibers/epoxy composites using micromechanical techniques and surface wettability. J. Colloid Interface Sci. 2003, 264, 431–445. [Google Scholar] [CrossRef]
- Kim, K.; Byun, S.; Cho, I.; Ryou, M.H.; Lee, Y.M. Three-Dimensional Adhesion Map Based on Surface and Interfacial Cutting Analysis System for Predicting Adhesion Properties of Composite Electrodes. ACS Appl. Mater. Interfaces 2016, 8, 23688–23695. [Google Scholar] [CrossRef] [PubMed]
- Alam, P.; Mamalis, D.; Robert, C.; Floreani, C.; Ó Brádaigh, C.M. The fatigue of carbon fibre reinforced plastics—A review. Compos. Part B Eng. 2019, 166, 555–579. [Google Scholar] [CrossRef]






| Epoxy Modified Primer | |||
|---|---|---|---|
| Material | Composition | Equivalent Weight (g/eq) | Form |
| YD-128 (Kukdo Chem.) | Bisphenol-A epoxy | 187 | Liquid |
| Dyhard 100 s (Alzchem.) | Dicyandiamide | 21 | Powder |
| Dyhard UR500 (Alzchem.) | Substituted urea | 3 | Powder |
| Ethyl Acetate | Solvent | Liquid | |
| Adhesive | |||
|---|---|---|---|
| Materials | Composition | Equivalent Weight (g/eq) | Form |
| KSR-177 (Kukdo Chem.) | Bisphenol-A Type | 205 | Liquid |
| G-5022 (Kukdo Chem.) | Polyamide | 175 | Liquid |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sim, K.-B.; Baek, D.; Shin, J.-H.; Shim, G.-S.; Jang, S.-W.; Kim, H.-J.; Hwang, J.-W.; Roh, J.U. Enhanced Surface Properties of Carbon Fiber Reinforced Plastic by Epoxy Modified Primer with Plasma for Automotive Applications. Polymers 2020, 12, 556. https://doi.org/10.3390/polym12030556
Sim K-B, Baek D, Shin J-H, Shim G-S, Jang S-W, Kim H-J, Hwang J-W, Roh JU. Enhanced Surface Properties of Carbon Fiber Reinforced Plastic by Epoxy Modified Primer with Plasma for Automotive Applications. Polymers. 2020; 12(3):556. https://doi.org/10.3390/polym12030556
Chicago/Turabian StyleSim, Kyeng-Bo, Dooyoung Baek, Jae-Ho Shin, Gyu-Seong Shim, Seong-Wook Jang, Hyun-Joong Kim, Jong-Won Hwang, and Jeong U. Roh. 2020. "Enhanced Surface Properties of Carbon Fiber Reinforced Plastic by Epoxy Modified Primer with Plasma for Automotive Applications" Polymers 12, no. 3: 556. https://doi.org/10.3390/polym12030556
APA StyleSim, K.-B., Baek, D., Shin, J.-H., Shim, G.-S., Jang, S.-W., Kim, H.-J., Hwang, J.-W., & Roh, J. U. (2020). Enhanced Surface Properties of Carbon Fiber Reinforced Plastic by Epoxy Modified Primer with Plasma for Automotive Applications. Polymers, 12(3), 556. https://doi.org/10.3390/polym12030556








