Plant-Inspired Layer-by-Layer Self-Assembly of Super-Hydrophobic Coating for Oil Spill Cleanup
Abstract
1. Introduction
2. Experimental
2.1. Materials
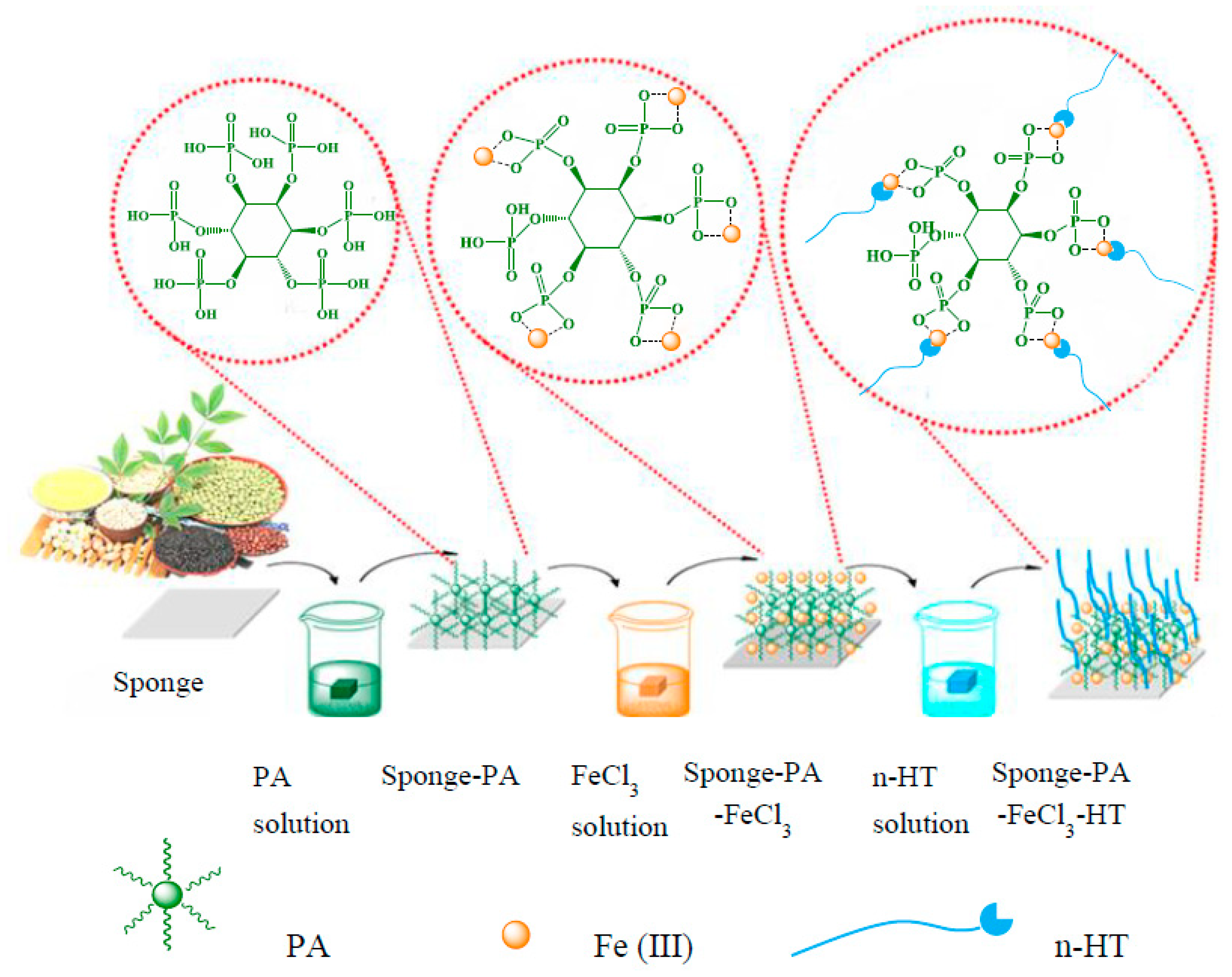
2.2. Layer by Layer Self-Assembly Super-Hydrophobic Modification of Various Highly Hydrophilic Polymer Materials
2.3. Characterizations and Measurements
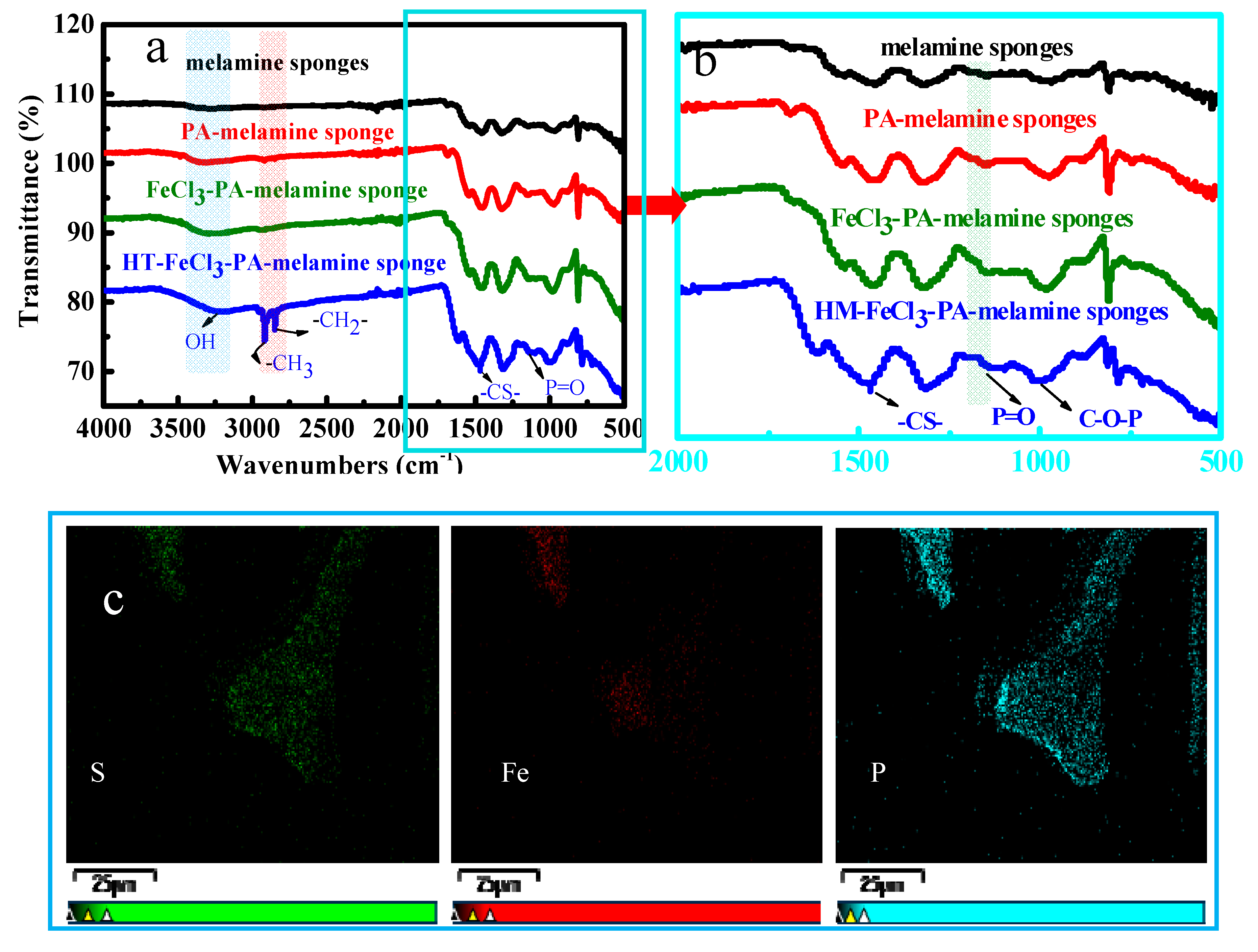
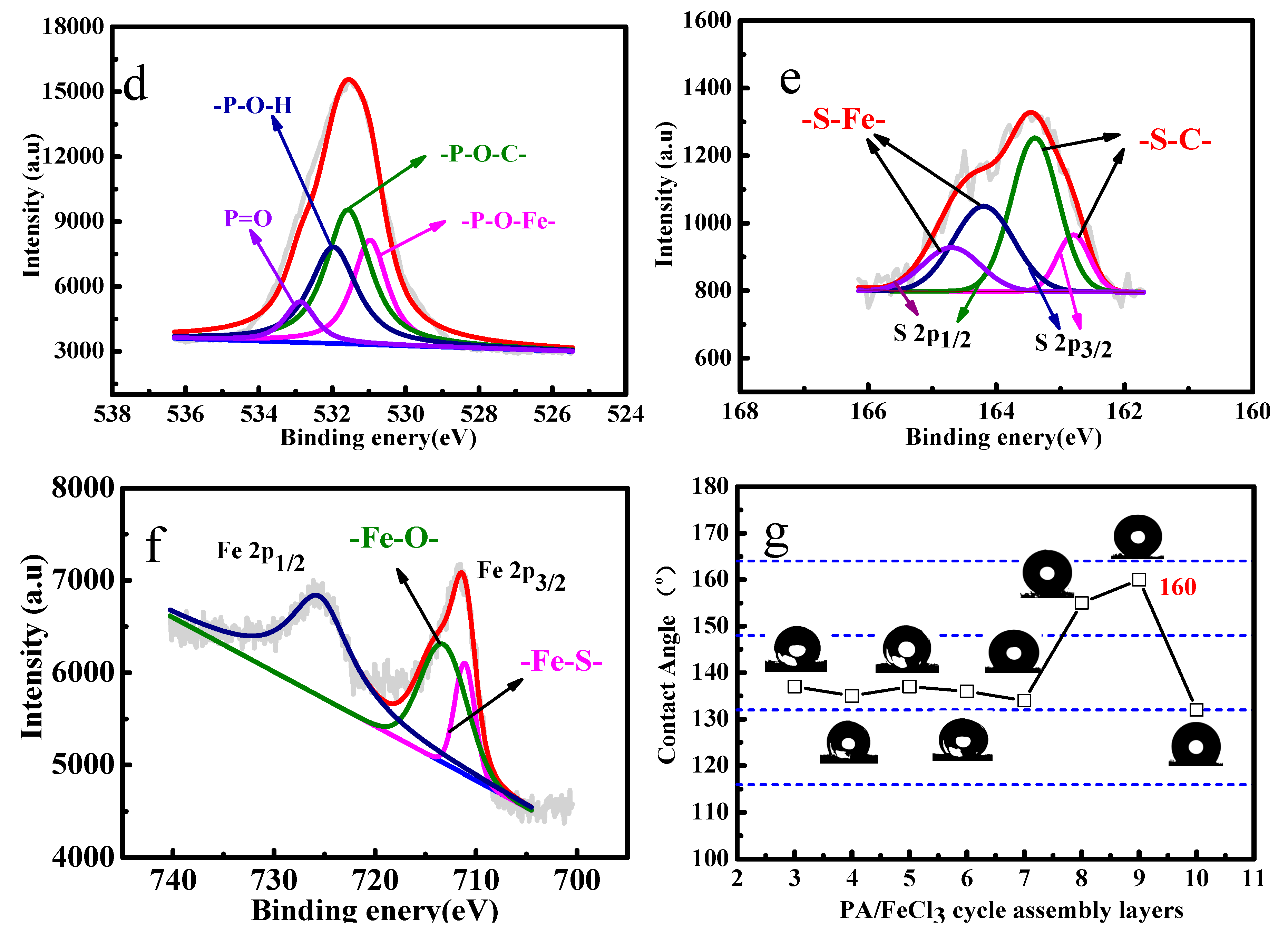
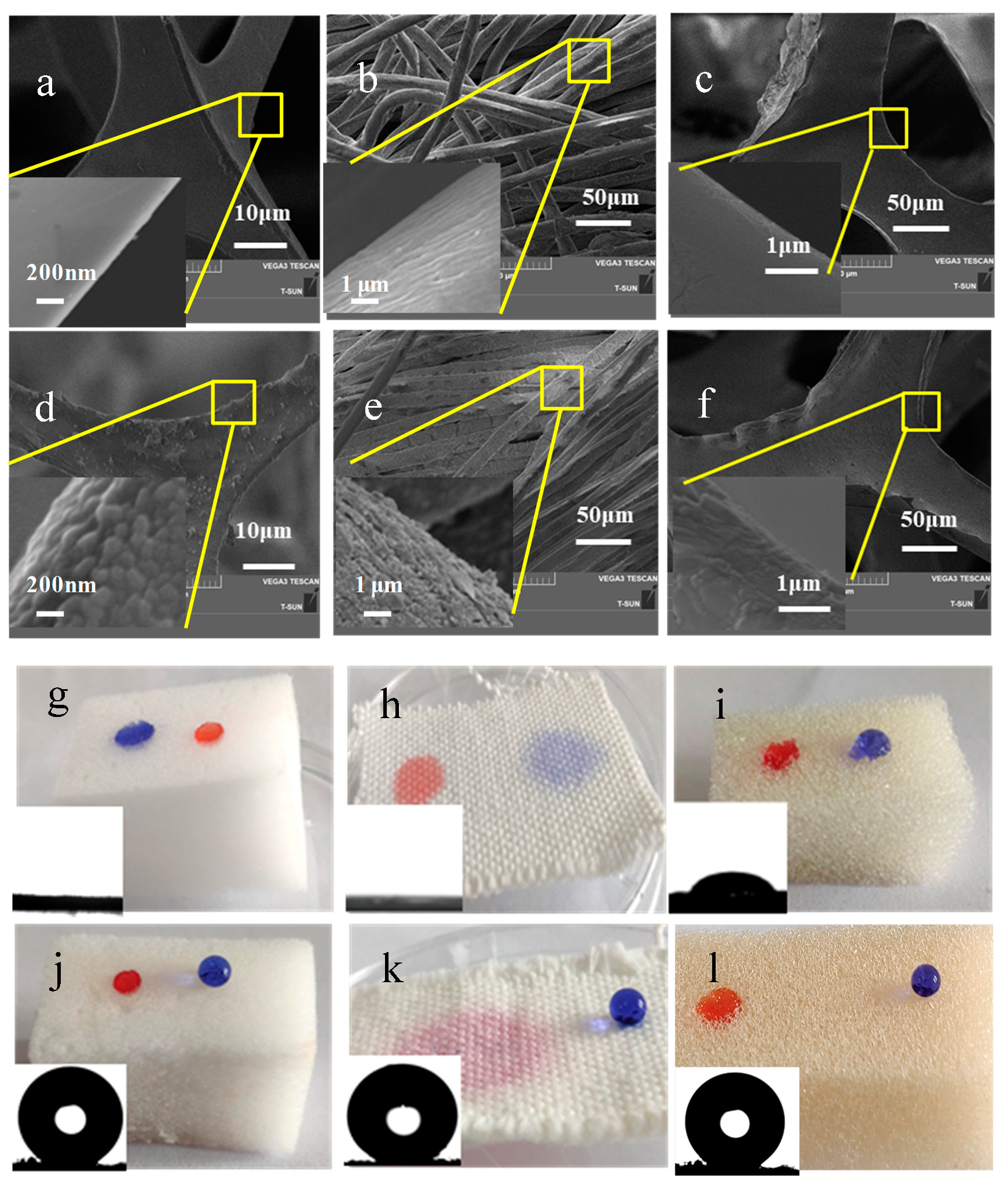
2.3.1. Structure and Morphology Characterizations
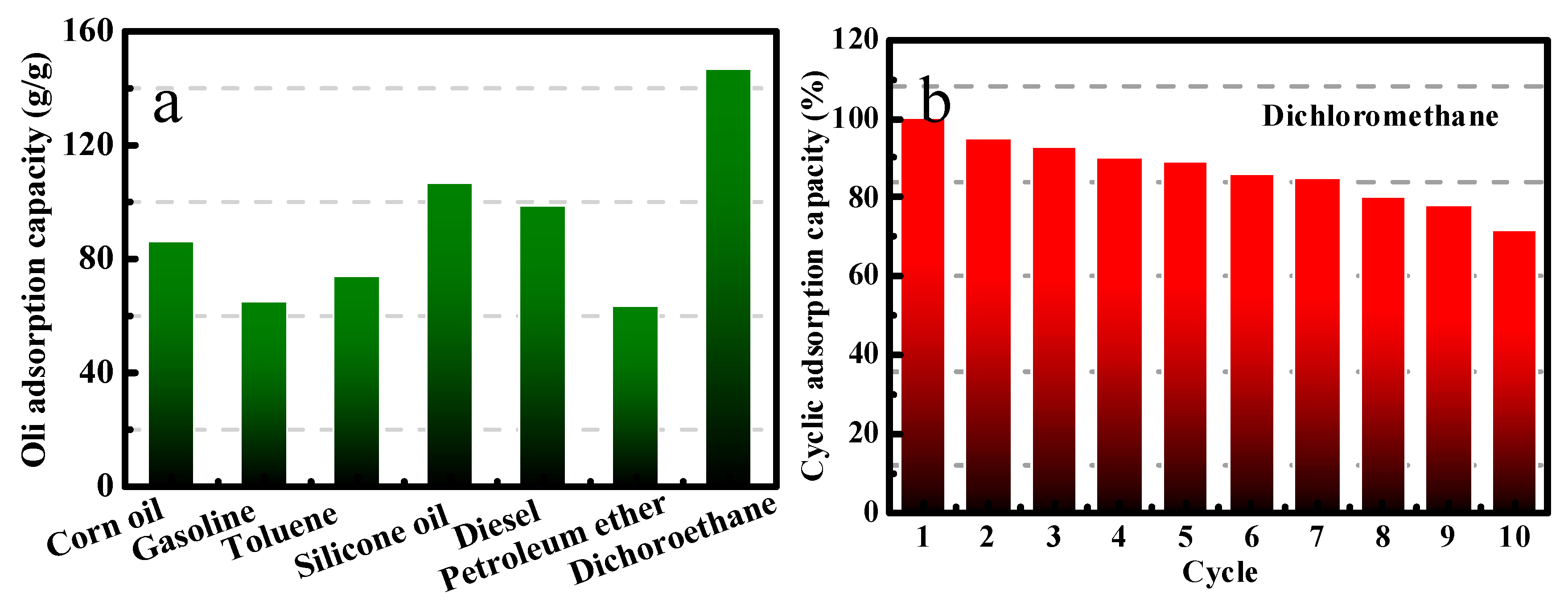
2.3.2. Oil/Water Separation Experiments
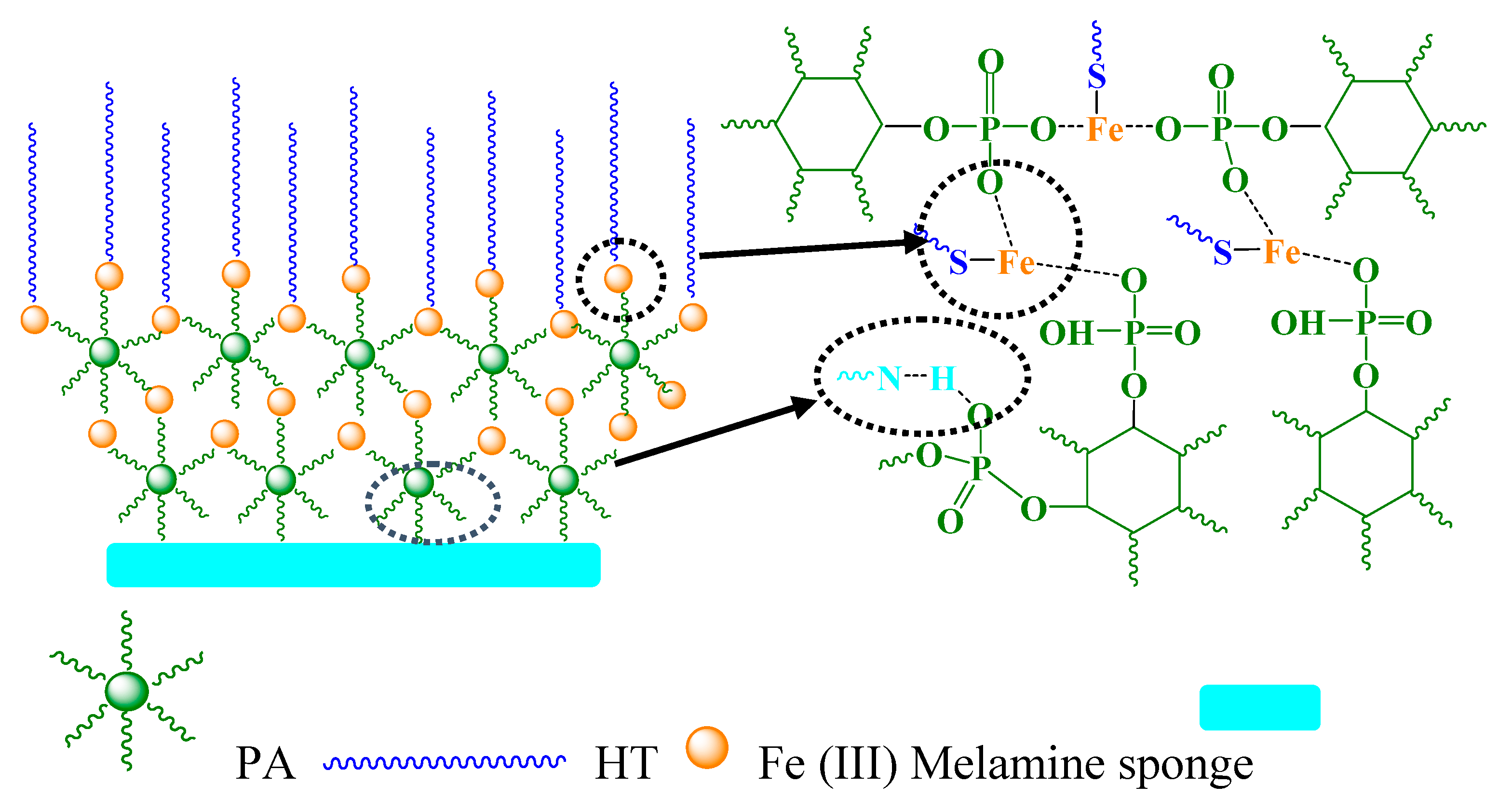
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ge, J.; Shi, L.-A.; Wang, Y.-C.; Zhao, H.-Y.; Yao, H.-B.; Zhu, Y.-B.; Zhang, Y.; Zhu, H.-W.; Wu, H.-A.; Yu, S.-H. Joule-heated graphene-wrapped sponge enables fast clean-up of viscous crude-oil spill. Nat. Nanotechnol. 2017, 12, 434. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Zhao, H.-Y.; Zhu, H.-W.; Huang, J.; Shi, L.-A.; Yu, S.-H. Advanced Sorbents for Oil-Spill Cleanup: Recent Advances and Future Perspectives. Adv. Mater. 2016, 28, 10459–10490. [Google Scholar] [CrossRef] [PubMed]
- Doshi, B.; Sillanpää, M.; Kalliola, S. A review of bio-based materials for oil spill treatment. Water Res. 2018, 135, 262–277. [Google Scholar] [CrossRef] [PubMed]
- Dombrowski, N.; Donaho, J.A.; Gutierrez, T.; Seitz, K.W.; Teske, A.P.; Baker, B.J. Reconstructing metabolic pathways of hydrocarbon-degrading bacteria from the Deepwater Horizon oil spill. Nat. Microbiol. 2016, 1, 16057. [Google Scholar] [CrossRef]
- Kwok Richard, K.; Engel Lawrence, S.; Miller Aubrey, K.; Blair, A.; Curry Matthew, D.; Jackson, W.B.; Stewart Patricia, A.; Stenzel Mark, R.; Birnbaum Linda, S.; Sandler Dale, P.; et al. The GuLF STUDY: A Prospective Study of Persons Involved in the Deepwater Horizon Oil Spill Response and Clean-Up. Environ. Health Perspect. 2017, 125, 570–578. [Google Scholar] [CrossRef]
- Sergy, G.A.; Guénette, C.C.; Owens, E.H.; Prince, R.C.; Lee, K. In-situ Treatment of Oiled Sediment Shorelines. Spill Sci. Technol. Bull. 2003, 8, 237–244. [Google Scholar] [CrossRef]
- Fragouli, D.; Athanassiou, A. Graphene heaters absorb faster. Nat. Nanotechnol. 2017, 12, 406. [Google Scholar] [CrossRef]
- Chu, Z.; Feng, Y.; Seeger, S. Oil/Water Separation with Selective Superantiwetting/Superwetting Surface Materials. Angew. Chem. Int. Ed. 2015, 54, 2328–2338. [Google Scholar] [CrossRef]
- Gao, X.; Zhou, J.; Du, R.; Xie, Z.; Deng, S.; Liu, R.; Liu, Z.; Zhang, J. Robust Superhydrophobic Foam: A Graphdiyne-Based Hierarchical Architecture for Oil/Water Separation. Adv. Mater. 2016, 28, 168–173. [Google Scholar] [CrossRef]
- Zhou, J.; Sun, Z.; Chen, M.; Wang, J.; Qiao, W.; Long, D.; Ling, L. Macroscopic and Mechanically Robust Hollow Carbon Spheres with Superior Oil Adsorption and Light-to-Heat Evaporation Properties. Adv. Funct. Mater. 2016, 26, 5368–5375. [Google Scholar] [CrossRef]
- Wang, J.; Shang, L.; Cheng, Y.; Ding, H.; Zhao, Y.; Gu, Z. Microfluidic Generation of Porous Particles Encapsulating Spongy Graphene for Oil Absorption. Small 2015, 11, 3890–3895. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Cheng, H.; Fane, A.G.; Wang, R.; Zhang, H. Recent Development of Advanced Materials with Special Wettability for Selective Oil/Water Separation. Small 2016, 12, 2186–2202. [Google Scholar] [CrossRef] [PubMed]
- Padaki, M.; Surya Murali, R.; Abdullah, M.S.; Misdan, N.; Moslehyani, A.; Kassim, M.A.; Hilal, N.; Ismail, A.F. Membrane technology enhancement in oil–water separation. A review. Desalination 2015, 357, 197–207. [Google Scholar] [CrossRef]
- Liu, Y.; Su, Y.; Guan, J.; Cao, J.; Zhang, R.; He, M.; Gao, K.; Zhou, L.; Jiang, Z. 2D Heterostructure Membranes with Sunlight-Driven Self-Cleaning Ability for Highly Efficient Oil–Water Separation. Adv. Funct. Mater. 2018, 28, 1706545. [Google Scholar] [CrossRef]
- Ding, L.; Gao, J.; Chung, T.-S. Schiff base reaction assisted one-step self-assembly method for efficient gravity-driven oil-water emulsion separation. Sep. Purif. Technol. 2019, 213, 437–446. [Google Scholar] [CrossRef]
- Ding, L.; Wang, Y.; Zhu, P.; Bai, Y. One-step plant-inspired reaction that transform membrane hydrophobicity into high hydrophilicity and underwater super oleophobicity for oil-in-water emulsion separation. Appl. Surf. Sci. 2019, 479, 423–429. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, Y.; Yang, C.; Liu, J.; Jiang, W.; Liang, B. Facile Two-Step Strategy for the Construction of a Mechanically Stable Three-Dimensional Superhydrophobic Structure for Continuous Oil–Water Separation. ACS Appl. Mater. Interfaces 2018, 10, 24149–24156. [Google Scholar] [CrossRef]
- Qiang, F.; Hu, L.-L.; Gong, L.-X.; Zhao, L.; Li, S.-N.; Tang, L.-C. Facile synthesis of super-hydrophobic, electrically conductive and mechanically flexible functionalized graphene nanoribbon/polyurethane sponge for efficient oil/water separation at static and dynamic states. Chem. Eng. J. 2018, 334, 2154–2166. [Google Scholar] [CrossRef]
- Mi, H.-Y.; Jing, X.; Xie, H.; Huang, H.-X.; Turng, L.-S. Magnetically driven superhydrophobic silica sponge decorated with hierarchical cobalt nanoparticles for selective oil absorption and oil/water separation. Chem. Eng. J. 2018, 337, 541–551. [Google Scholar] [CrossRef]
- Yang, Y.; Li, X.; Zheng, X.; Chen, Z.; Zhou, Q.; Chen, Y. 3D-Printed Biomimetic Super-Hydrophobic Structure for Microdroplet Manipulation and Oil/Water Separation. Adv. Mater. 2018, 30, 1704912. [Google Scholar] [CrossRef]
- Liu, H.; Kang, Y. Superhydrophobic and superoleophilic modified EPDM foam rubber fabricated by a facile approach for oil/water separation. Appl. Surf. Sci. 2018, 451, 223–231. [Google Scholar] [CrossRef]
- Saththasivam, J.; Yiming, W.; Wang, K.; Jin, J.; Liu, Z. A Novel Architecture for Carbon Nanotube Membranes towards Fast and Efficient Oil/water Separation. Sci. Rep. 2018, 8, 7418. [Google Scholar] [CrossRef] [PubMed]
- Dashairya, L.; Rout, M.; Saha, P. Reduced graphene oxide-coated cotton as an efficient absorbent in oil-water separation. Adv. Compos. Hybrid Mater. 2018, 1, 135–148. [Google Scholar] [CrossRef]
- Lei, Z.; Zhang, G.; Deng, Y.; Wang, C. Thermoresponsive Melamine Sponges with Switchable Wettability by Interface-Initiated Atom Transfer Radical Polymerization for Oil/Water Separation. ACS Appl. Mater. Interfaces 2017, 9, 8967–8974. [Google Scholar] [CrossRef]
- Guan, Y.; Cheng, F.; Pan, Z. Superwetting Polymeric Three Dimensional (3D) Porous Materials for Oil/Water Separation: A Review. Polymers 2019, 11, 806. [Google Scholar] [CrossRef]
- Pinto, J.; Athanassiou, A.; Fragouli, D. Surface modification of polymeric foams for oil spills remediation. J. Environ. Manag. 2018, 206, 872–889. [Google Scholar] [CrossRef]
- Lei, Z.; Deng, Y.; Wang, C. Multiphase surface growth of hydrophobic ZIF-8 on melamine sponge for excellent oil/water separation and effective catalysis in a Knoevenagel reaction. J. Mater. Chem. A 2018, 6, 3258–3263. [Google Scholar] [CrossRef]
- Chen, X.; Weibel, J.A.; Garimella, S.V. Continuous Oil–Water Separation Using Polydimethylsiloxane-Functionalized Melamine Sponge. Ind. Eng. Chem. Res. 2016, 55, 3596–3602. [Google Scholar] [CrossRef]
- Qiu, S.; Jiang, B.; Zheng, X.; Zheng, J.; Zhu, C.; Wu, M. Hydrophobic and fire-resistant carbon monolith from melamine sponge: A recyclable sorbent for oil–water separation. Carbon 2015, 84, 551–559. [Google Scholar] [CrossRef]
- Wang, C.-F.; Huang, H.-C.; Chen, L.-T. Protonated Melamine Sponge for Effective Oil/Water Separation. Sci. Rep. 2015, 5, 14294. [Google Scholar] [CrossRef]
- Yilgör, E.; Söz, C.K.; Yilgör, I. Wetting behavior of superhydrophobic poly(methyl methacrylate). Prog. Org. Coat. 2018, 125, 530–536. [Google Scholar] [CrossRef]
- Jiaqiang, E.; Jin, Y.; Deng, Y.; Wei, Z.; Zhao, X.; Han, D.; Peng, Q.; Zhang, Z. Wetting Models and Working Mechanisms of Typical Surfaces Existing in Nature and Their Application on Superhydrophobic Surfaces: A Review. Adv. Mater. Interfaces 2018, 5, 1701052–1701091. [Google Scholar] [CrossRef]
- Zulfiqar, U.; Hussain, S.Z.; Subhani, T.; Hussain, I. Mechanically robust superhydrophobic coating from sawdust particles and carbon soot for oil/water separation. Colloids Surf. A 2018, 539, 391–398. [Google Scholar] [CrossRef]
- Li, H.; Liang, T.; Lai, X.; Su, X.; Zhang, L.; Zeng, X. Vapor-liquid interfacial reaction to fabricate superhydrophilic and underwater superoleophobic thiol-ene/silica hybrid decorated fabric for oil/water separation. Appl. Surf. Sci. 2018, 427, 92–101. [Google Scholar] [CrossRef]
- Li, F.; Wang, Z.; Huang, S.; Pan, Y.; Zhao, X. Flexible, Durable, and Unconditioned Superoleophobic/Superhydrophilic Surfaces for Controllable Transport and Oil–Water Separation. Adv. Funct. Mater. 2018, 28, 1706867. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, H.-C.; He, F.; Peng, S.; Li, Y.; Shao, L.; Darling, S.B. Mussel-Inspired Surface Engineering for Water-Remediation Materials. Matter 2019, 1, 115–155. [Google Scholar] [CrossRef]
- Fang, Y.; Gonuguntla, S.; Soh, S. Universal Nature-Inspired Coatings for Preparing Noncharging Surfaces. ACS Appl. Mater. Interfaces 2017, 9, 32220–32226. [Google Scholar] [CrossRef]
- Yang, Z.; Qiu, H.; Li, X.; Gao, P.; Huang, N. Plant-inspired gallolamine catalytic surface chemistry for engineering an efficient nitric oxide generating coating. Acta Biomater. 2018, 76, 89–98. [Google Scholar] [CrossRef]
- Gan, D.; Xing, W.; Jiang, L.; Fang, J.; Zhao, C.; Ren, F.; Fang, L.; Wang, K.; Lu, X. Plant-inspired adhesive and tough hydrogel based on Ag-Lignin nanoparticles-triggered dynamic redox catechol chemistry. Nat. Commun. 2019, 10, 1487. [Google Scholar] [CrossRef]
- Dong, L.; Gao, Z.A.; Lin, N. Self-assembly of metal–organic coordination structures on surfaces. Prog. Surf. Sci. 2016, 91, 101–135. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, Z.; Shi, J.; Liang, Y.; Zhang, C.; Wu, H. Metal–Organic Coordination-Enabled Layer-by-Layer Self-Assembly to Prepare Hybrid Microcapsules for Efficient Enzyme Immobilization. ACS Appl. Mater. Interfaces 2012, 4, 3476–3483. [Google Scholar] [CrossRef] [PubMed]
- Altman, M.; Shukla, A.D.; Zubkov, T.; Evmenenko, G.; Dutta, P.; van der Boom, M.E. Controlling Structure from the Bottom-Up: Structural and Optical Properties of Layer-by-Layer Assembled Palladium Coordination-Based Multilayers. J. Am. Chem. Soc. 2006, 128, 7374–7382. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, K.; Son, Y.; Zhang, W.; Spindler, L.M.; Han, Z.; Ren, L. A smart switchable bioinspired copper foam responding to different pH droplets for reversible oil–water separation. J. Mater. Chem. A 2017, 5, 2603–2612. [Google Scholar] [CrossRef]
- Li, L.; Zhang, G.; Su, Z. One-Step Assembly of Phytic Acid Metal Complexes for Superhydrophilic Coatings. Angew. Chem. Int. Ed. 2016, 55, 9093–9096. [Google Scholar] [CrossRef] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, L.; Wang, Y.; Xiong, J.; Lu, H.; Zeng, M.; Zhu, P.; Ma, H. Plant-Inspired Layer-by-Layer Self-Assembly of Super-Hydrophobic Coating for Oil Spill Cleanup. Polymers 2019, 11, 2047. https://doi.org/10.3390/polym11122047
Ding L, Wang Y, Xiong J, Lu H, Zeng M, Zhu P, Ma H. Plant-Inspired Layer-by-Layer Self-Assembly of Super-Hydrophobic Coating for Oil Spill Cleanup. Polymers. 2019; 11(12):2047. https://doi.org/10.3390/polym11122047
Chicago/Turabian StyleDing, Liping, Yanqing Wang, Jinxin Xiong, Huiying Lu, Mingjian Zeng, Peng Zhu, and Haiyan Ma. 2019. "Plant-Inspired Layer-by-Layer Self-Assembly of Super-Hydrophobic Coating for Oil Spill Cleanup" Polymers 11, no. 12: 2047. https://doi.org/10.3390/polym11122047
APA StyleDing, L., Wang, Y., Xiong, J., Lu, H., Zeng, M., Zhu, P., & Ma, H. (2019). Plant-Inspired Layer-by-Layer Self-Assembly of Super-Hydrophobic Coating for Oil Spill Cleanup. Polymers, 11(12), 2047. https://doi.org/10.3390/polym11122047