SEBS-Grafted Itaconic Acid as Compatibilizer for Elastomer Nanocomposites Based on BaTiO3 Particles
Abstract
1. Introduction
2. Experimental
2.1. Materials
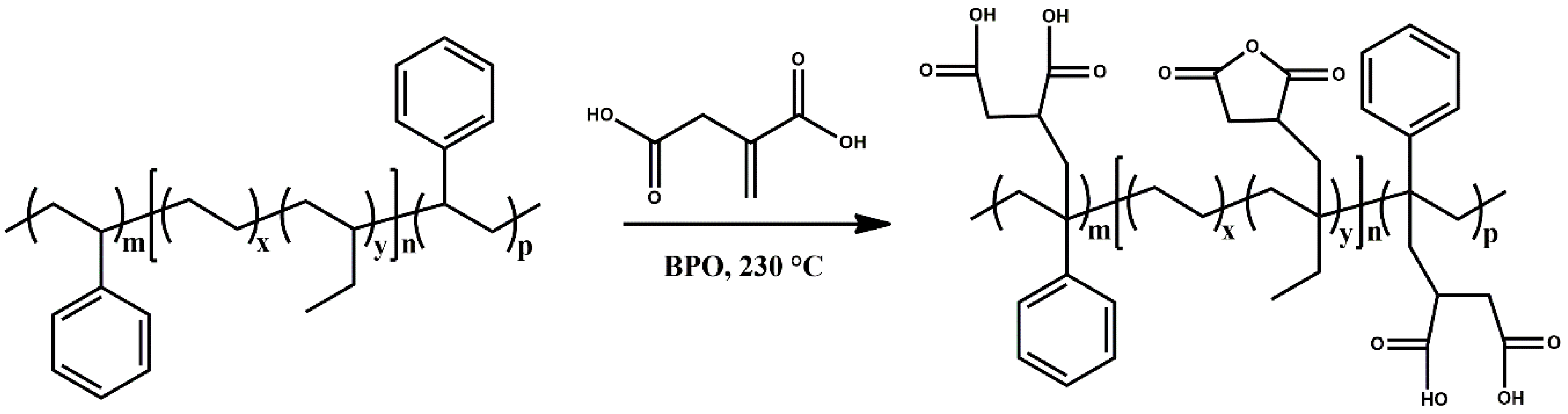
2.2. Grafting of Itaconic Acid onto SEBSF
2.3. Preparation of SEBS/BaTiO3 Composites
2.4. Characterization
3. Results and Discussion
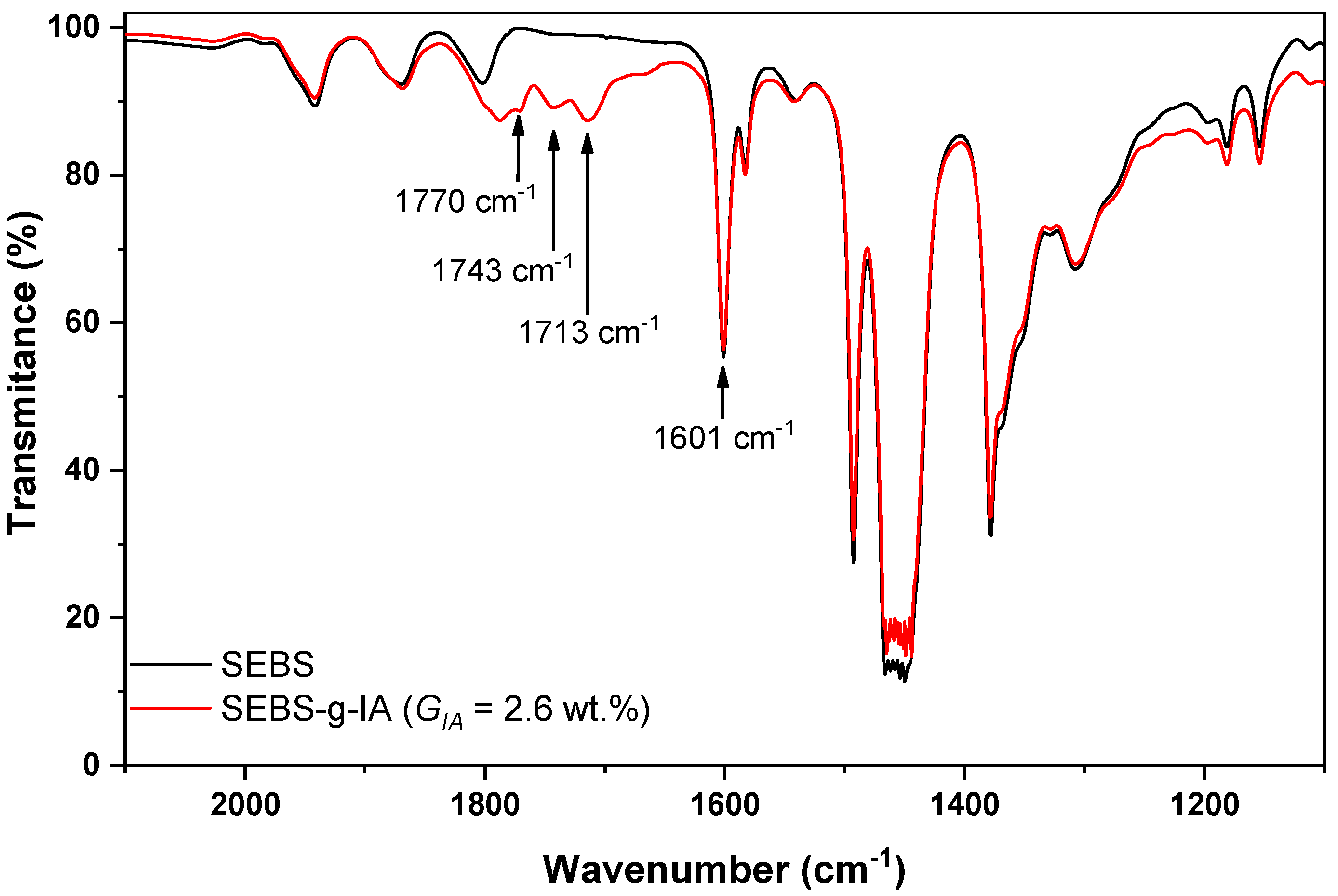
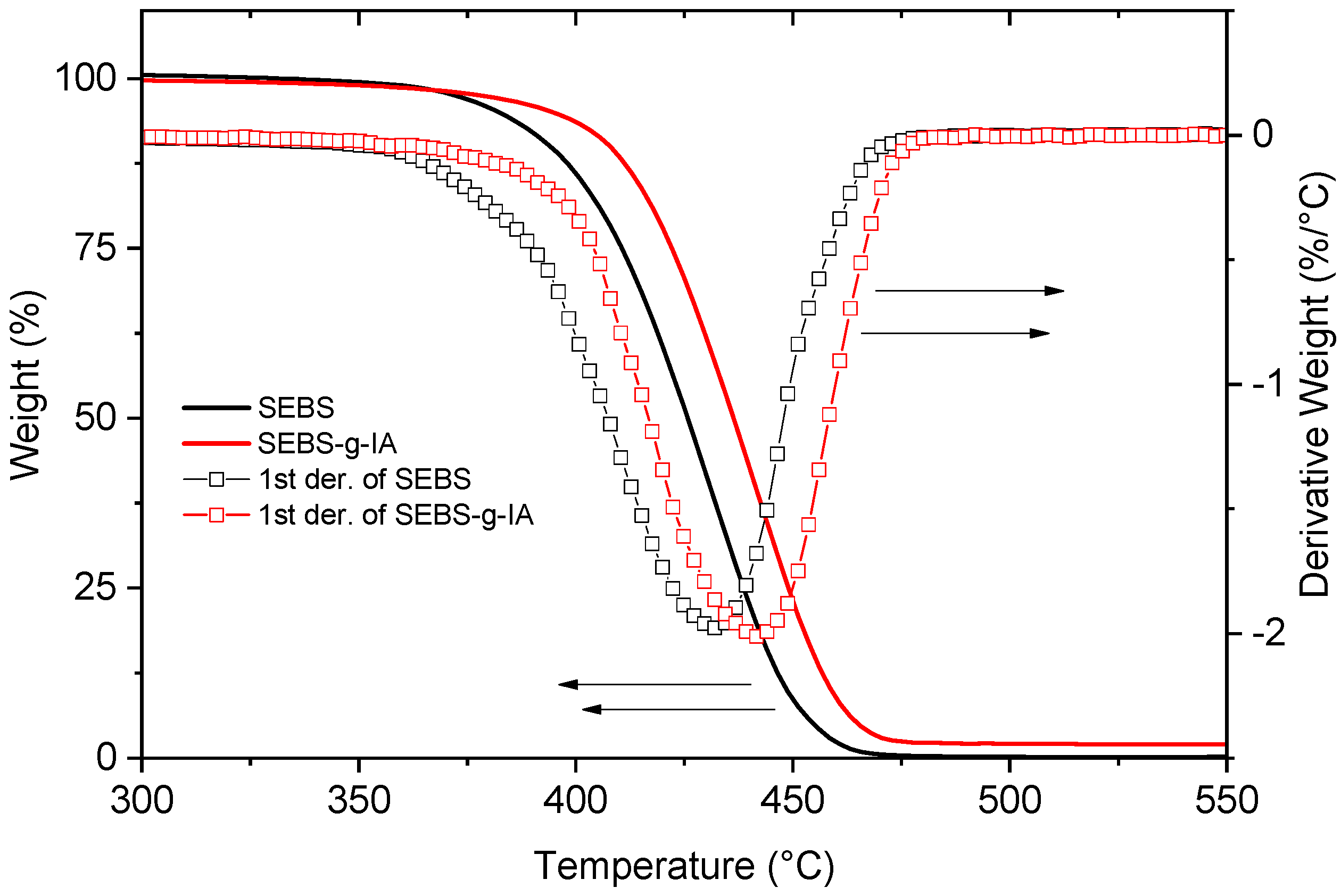
3.1. Grafting of Itaconic Acid onto SEBS
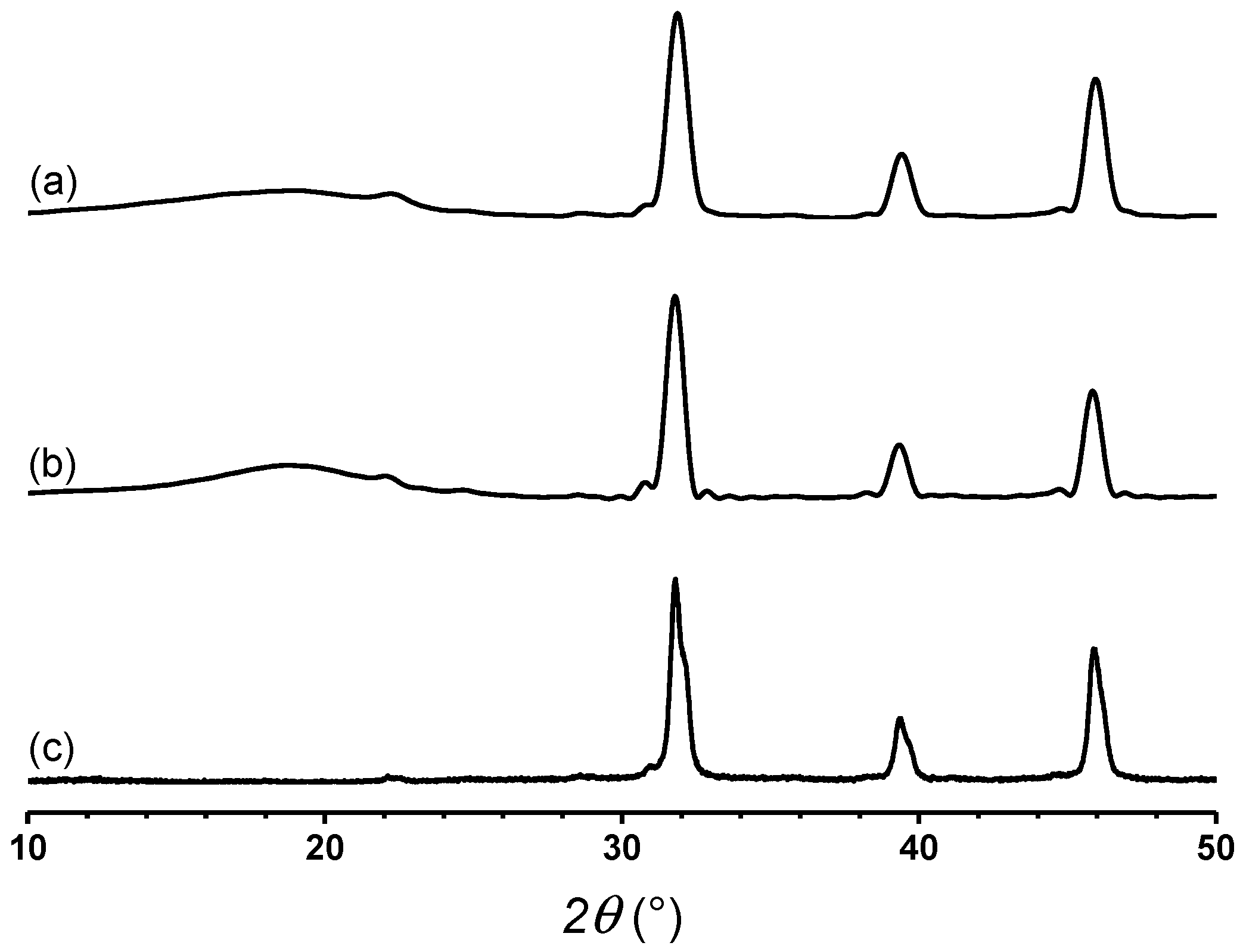
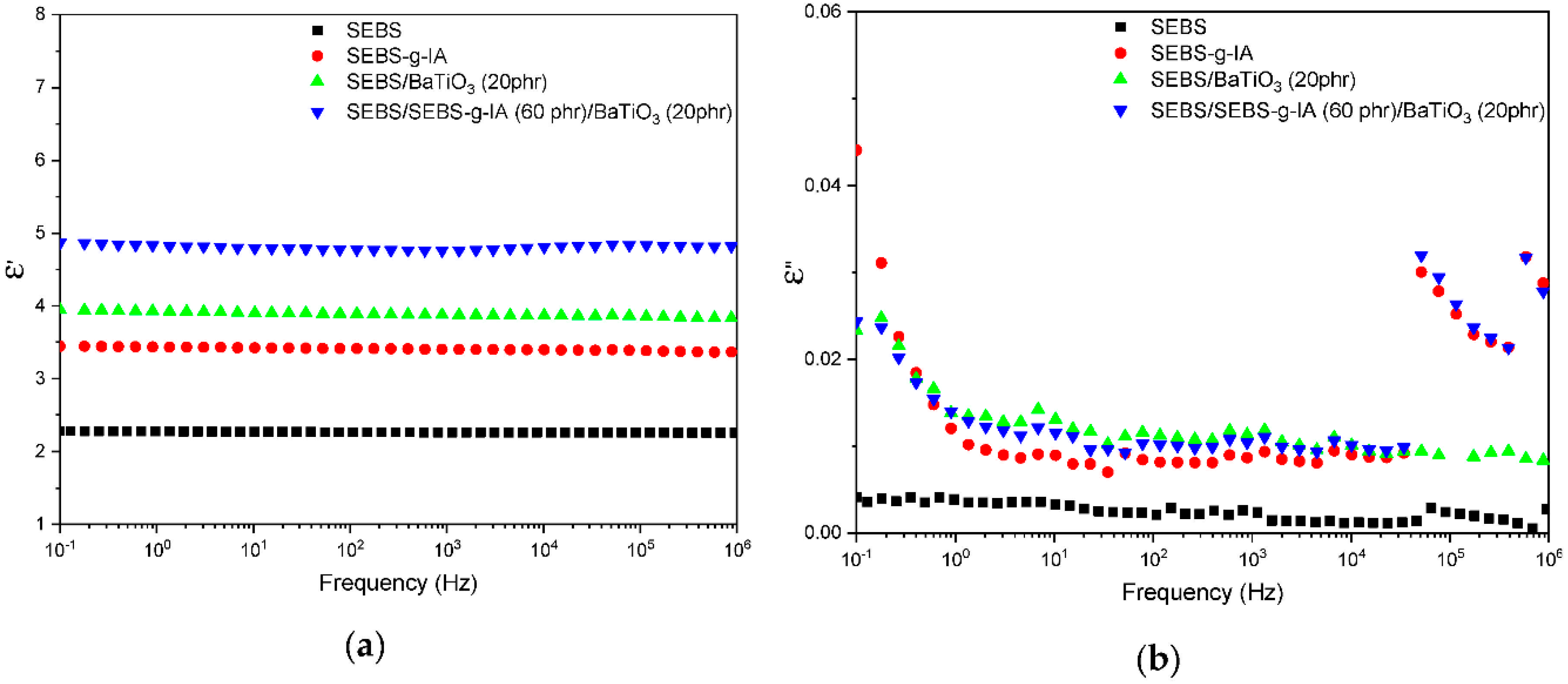
3.2. Properties of SEBS/BaTiO3 Composites Using Itaconic Acid as Compatibilizer
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Drobny, J.G. 1—Introduction. In Handbook of Thermoplastic Elastomers; Drobny, J.G., Ed.; William Andrew Publishing: Norwich, NY, USA, 2007; pp. 1–8. [Google Scholar]
- Spontak, R.J.; Patel, N.P. Thermoplastic elastomers: Fundamentals and applications. Curr. Opin. Colloid Interface Sci. 2000, 5, 333–340. [Google Scholar] [CrossRef]
- Handlin, D.L. Thermoplastic Elastomers. In Encyclopedia of Materials: Science and Technology; Buschow, K.H.J., Cahn, R.W., Flemings, M.C., Ilschner, B., Kramer, E.J., Mahajan, S., Veyssière, P., Eds.; Elsevier: Oxford, UK, 2001; pp. 9197–9204. [Google Scholar]
- Chuang, P.-L.; Nien, Y.-H. Synthesis and characterization of maleic anhydride grafted SEBS modified with ethanolamine, 2-amino-2-methyl-1-propanol or glycerine. J. Polymer Res. 2019, 26, 66. [Google Scholar] [CrossRef]
- Braga, F.N.; Zaggo, M.H.; Montanheiro, L.A.T.; Passador, R.F. Preparation of Maleic Anhydride Grafted Poly(trimethylene terephthalate) (PTT-g-MA) by Reactive Extrusion Processing. J. Manuf. Mater. Process. 2019, 3, 37. [Google Scholar] [CrossRef]
- Fink, J.K. 15—Reactive Extrusion. In Reactive Polymers: Fundamentals and Applications, 3rd ed.; Fink, J.K., Ed.; William Andrew Publishing: Amsterdam, The Netherlands, 2018; pp. 449–496. [Google Scholar]
- Moncada, E.; Quijada, R.; Lieberwirth, I.; Yazdani-Pedram, M. Use of PP grafted with itaconic acid as a new compatibilizer for PP/clay nanocomposites. Macromol. Chem. Phys. 2006, 207, 1376–1386. [Google Scholar] [CrossRef]
- Doble, M.; Kruthiventi, A. Industrial Examples. In Green Chemistry and Engineering; Doble, M., Kruthiventy, A., Eds.; Acedemic Press: Cambridge, MA, USA, 2007; pp. 245–296. [Google Scholar]
- Tostar, S.; Stenvall, E.; Foreman, R.S.J.M.; Boldizar, A. The Influence of Compatibilizer Addition and Gamma Irradiation on Mechanical and Rheological Properties of a Recycled WEEE Plastics Blend. Recycling 2016, 1, 101–110. [Google Scholar] [CrossRef]
- Mengual, A.; Juárez, D.; Balart, R.; Ferrándiz, S. PE-g-MA, PP-g-MA and SEBS-g-MA compatibilizers used in material blends. Procedia Manuf. 2017, 13, 321–326. [Google Scholar] [CrossRef]
- Guo, C.G.; Wang, Q.W. Compatibilizing Effect of Maleic Anhydride Grafted Styrene-Ethylene-Butylene-Styrene (MAH-g-SEBS) on the Polypropylene and Wood Fiber Composites. J. Reinf. Plast. Comp. 2007, 26, 1743–1752. [Google Scholar] [CrossRef]
- Zhan, Z.; He, H.; Zhu, Z.; Xue, B.; Wang, G.; Chen, M.; Xiong, C. Blends of rABS and SEBS: Influence of In-Situ Compatibilization on the Mechanical Properties. Materials 2019, 12, 2352. [Google Scholar] [CrossRef]
- Sheldon, R.A. Green and sustainable manufacture of chemicals from biomass: State of the art. Green Chem. 2014, 16, 950–963. [Google Scholar] [CrossRef]
- Hepp, A.; Kulis, M.; De La Ree, A.; Zubrin, R.; Beggren, M.; Hensel, J.; Kimble, M. Green Aerospace Fuels from Non-Petroleum Sources. In 49th AIAA Aerospace Sciences Meeting including the New Horizons Forum and Aerospace Exposition, Orlando, FL, USA, 4–7 January 2011; American Institute of Aeronautics and Astronautics: Reston, VA, USA, 2011. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, C.; Fan, Z.; Shi, S.; Zhang, Z.; Oeser, M. Feasibility analysis of bio-binder as non-petroleum alternative for bituminous materials. Mater. Res. Express 2020, 6, 125115. [Google Scholar] [CrossRef]
- Saha, B.C.; Kennedy, G.J.; Bowman, M.J.; Qureshi, N.; Dunn, R.O. Factors Affecting Production of Itaconic Acid from Mixed Sugars by Aspergillus terreus. App. Biochem. Biotech. 2019, 187, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Yazdani-Pedram, M.; Abarca, R.Q.; Acevedo, E.A.M. Compatibilizers for Producing Nanocomposites, Microcomposites and Polymer Blends and Process for Obtaining Them. U.S. Patent 20100160509A1, 4 March 2014. [Google Scholar]
- Equiza, N.; Yave, W.; Quijada, R.; Yazdani-Pedram, M. Use of SEBS/EPR and SBR/EPR as binary compatibilizers for PE/PP/PS/HIPS blends: A work oriented to the recycling of thermoplastic wastes. Macromol. Mater. Eng. 2007, 292, 1001–1011. [Google Scholar] [CrossRef]
- Yazdani-Pedram, M.; Menzel, C.; Toro, P.; Quijada, R.; May-Pat, A.; Aviles, F. Mechanical and thermal properties of multiwalled carbon nanotube/polypropylene composites using itaconic acid as compatibilizer and coupling agent. Macromol. Res. 2013, 21, 153–160. [Google Scholar] [CrossRef]
- Bruna, J. Estudio de Funcionalización de Polipropilenos y Elastomeros y su Uso Como Compatibilizantes en Mezclas. Ph.D. Thesis, Universidad de Chile, Sntiago, Chile, 2007. Available online: http://repositorio.uchile.cl/handle/2250/102954 (accessed on 10 March 2020).
- Teleky, B.-E.; Vodnar, C.D. Biomass-Derived Production of Itaconic Acid as a Building Block in Specialty Polymers. Polymers 2019, 11, 1035. [Google Scholar] [CrossRef] [PubMed]
- Klement, T.; Büchs, J. Itaconic acid—A biotechnological process in change. Bioresour. Technol. 2013, 135, 422–431. [Google Scholar] [CrossRef]
- Werpy, T.; Holladay, J.; White, J. Top Value Added Chemicals From Biomass: I. Results of Screening for Potential Candidates from Sugars and Synthesis Gas; U.S. Department of Energy Office of Scientific and Technical Information: Oak Ridge, TN, USA, 2004. [CrossRef]
- Galia, A.; De Gregorio, R.; Spadaro, G.; Scialdone, O.; Filardo, G. Grafting of Maleic Anhydride onto Isotactic Polypropylene in the Presence of Supercritical Carbon Dioxide as a Solvent and Swelling Fluid. Macromolecules 2004, 37, 4580–4589. [Google Scholar] [CrossRef]
- Bruna, J.; Yazdani-Pedram, M.; Quijada, R.; Valentín, J.L.; López-Manchado, M.A. Melt grafting of itaconic acid and its derivatives onto an ethylene-propylene copolymer. React. Funct. Polym. 2005, 64, 169–178. [Google Scholar] [CrossRef]
- Yazdani-Pedram, M.; Vega, H.; Quijada, R. Melt functionalization of polypropylene with methyl esters of itaconic acid. Polymer 2001, 42, 4751–4758. [Google Scholar] [CrossRef]
- López-Manchado, M.A.; Kenny, J.M.; Quijada, R.; Yazdani-Pedram, M. Effect of Grafted PP on the Properties of Thermoplastic Elastomers Based on PP-EPDM Blends. Macromol. Chem. Phys. 2001, 202, 1909–1916. [Google Scholar] [CrossRef]
- Chércoles Asensio, R.; San Andrés Moya, M.; de la Roja, J.M.; Gómez, M. Analytical characterization of polymers used in conservation and restoration by ATR-FTIR spectroscopy. Anal. Bioanal. Chem. 2009, 395, 2081–2096. [Google Scholar] [CrossRef]
- Aguilar Bolados, H.; Hernández-Santana, M.; Romasanta, L.J.; Yazdani-Pedram, M.; Quijada, R.; López-Manchado, M.A.; Verdejo, R. Electro-mechanical actuation performance of SEBS/PU blends. Polymer 2019, 171, 25–33. [Google Scholar] [CrossRef]
- Guerra, G.; De Rosa, C.; Vitagliano, V.M.; Petraccone, V.; Corradini, P. Effects of blending on the polymorphic behavior of melt-crystallized syndiotactic polystyrene. J. Polym. Sci. Polym. Phys. 1991, 29, 265–271. [Google Scholar] [CrossRef]
- Gowd, E.B.; Tashiro, K.; Ramesh, C. Structural phase transitions of syndiotactic polystyrene. Prog. Polym. Sci. 2009, 34, 280–315. [Google Scholar] [CrossRef]
- Daniel, C.; Galdi, N.; Montefusco, T.; Guerra, G. Syndiotactic Polystyrene Clathrates with Polar Guest Molecules. Chem. Mater. 2007, 19, 3302–3308. [Google Scholar] [CrossRef]
- Tan, W.T.; Radhi, M.M.; Ab Rahman, M.Z.; Kassim, A.B. Synthesis and characterization of grafted polystyrene with acrylonitrile using gamma-irradiation. J. Appl. Sci. 2010, 10, 139–144. [Google Scholar] [CrossRef]
- Thanki, A.A.; Goyal, R.K. Study on effect of cubic- and tetragonal phased BaTiO3 on the electrical and thermal properties of polymeric nanocomposites. Mater. Chem. Phys. 2016, 183, 447–456. [Google Scholar] [CrossRef]
- Dalle Vacche, S.; Damjanovic, D.; Michaud, V.; Leterrier, Y. Interface-Dominated Time-Dependent Behavior of Poled Poly(Vinylidene Fluoride–Trifluoroethylene)/Barium Titanate Composites. Materials 2020, 13, 225. [Google Scholar] [CrossRef]
- Poudel, A.; Coffey, A.; Kennedy, J.; Lyons, S.; Thomas, K.; Walsh, P. Dielectric Polarization Enhancement of Thermoplastic Elastomers for Sensing and Energy Harvesting Applications. Int. J. Mater. Mech. Manuf. 2016, 4, 237–242. [Google Scholar] [CrossRef]
- Jiang, L.; Betts, A.; Kennedy, D.; Jerrams, S. Improving the electromechanical performance of dielectric elastomers using silicone rubber and dopamine coated barium titanate. Mater. Design 2015, 85, 733–742. [Google Scholar] [CrossRef]
- Yang, D.; Ge, F.; Tian, M.; Ning, N.; Zhang, L.; Zhao, C.; Ito, K.; Nishi, T.; Wang, H.; Luan, Y. Dielectric elastomer actuator with excellent electromechanical performance using slide-ring materials/barium titanate composites. J. Mater. Chem. A 2015, 3, 9468–9479. [Google Scholar] [CrossRef]



| Sample | SEBS (phr) | SEBS-g-IA (phr) | BaTiO3 (phr) |
|---|---|---|---|
| SEBS | 100 | 0 | 0 |
| SEBS/BaTiO3 | 100 | 0 | 20 |
| SEBS/SEBS-g-IA 60 | 100 | 60 | 0 |
| SEBS/SEBS-g-IA 60/BaTiO3 | 100 | 60 | 20 |
| SEBS | SEBS-g-IA | ||||
|---|---|---|---|---|---|
| 2ϴ (°) | d (Å) | D (Å) | 2ϴ (°) | d (Å) | D (Å) |
| 9.802 | 9.016 | 19.85 | 10.06 | 8.788 | 17.09 |
| 15.78 | 5.613 | 12.33 | 15.89 | 5.572 | 11.11 |
| 19.37 | 4.578 | 10.73 | 19.38 | 4.572 | 10.96 |
| 22.56 | 3.938 | 45.85 | 22.55 | 3.940 | 48.94 |
| Sample | Young Modulus | E50 | E100 | E300 | Tensile Strength | Elongation at Break |
|---|---|---|---|---|---|---|
| (MPa) | (MPa) | (MPa) | (MPa) | (MPa) | (%) | |
| SEBS | 13.7 ± 1.1 | 1.88 ± 0.11 | 1.98 ± 0.11 | 2.65 ± 0.19 | 2.53 ± 0.32 | 609 ± 30 |
| SEBS/BaTiO3 | 17.0 ± 1.4 | 1.98 ± 0.07 | 2.05 ± 0.07 | 2.74 ± 0.04 | 3.05 ± 0.04 | 555 ± 22 |
| SEBS/SEBS-g-IA 60 | 20.5 ± 0.2 | 2.18 ± 0.02 | 2.33 ± 0.04 | 3.23 ± 0.28 | 3.61 ± 0.40 | 353 ± 49 |
| SEBS/SEBS-g-IA 60/BaTiO3 | 30.2 ± 1.2 | 2.23 ± 0.04 | 2.37 ± 0.05 | 3.36 ± 0.17 | 3.82 ± 0.31 | 445 ± 31 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguilar-Bolados, H.; Quijada, R.; Yazdani-Pedram, M.; Maldonado-Magnere, S.; Verdejo, R.; Lopez-Manchado, M.A. SEBS-Grafted Itaconic Acid as Compatibilizer for Elastomer Nanocomposites Based on BaTiO3 Particles. Polymers 2020, 12, 643. https://doi.org/10.3390/polym12030643
Aguilar-Bolados H, Quijada R, Yazdani-Pedram M, Maldonado-Magnere S, Verdejo R, Lopez-Manchado MA. SEBS-Grafted Itaconic Acid as Compatibilizer for Elastomer Nanocomposites Based on BaTiO3 Particles. Polymers. 2020; 12(3):643. https://doi.org/10.3390/polym12030643
Chicago/Turabian StyleAguilar-Bolados, Héctor, Raúl Quijada, Mehrdad Yazdani-Pedram, Santiago Maldonado-Magnere, Raquel Verdejo, and Miguel A. Lopez-Manchado. 2020. "SEBS-Grafted Itaconic Acid as Compatibilizer for Elastomer Nanocomposites Based on BaTiO3 Particles" Polymers 12, no. 3: 643. https://doi.org/10.3390/polym12030643
APA StyleAguilar-Bolados, H., Quijada, R., Yazdani-Pedram, M., Maldonado-Magnere, S., Verdejo, R., & Lopez-Manchado, M. A. (2020). SEBS-Grafted Itaconic Acid as Compatibilizer for Elastomer Nanocomposites Based on BaTiO3 Particles. Polymers, 12(3), 643. https://doi.org/10.3390/polym12030643







