Roles of Salicylate Donors in Enhancement of Productivity and Isotacticity of Ziegler–Natta Catalyzed Propylene Polymerization
Abstract
1. Introduction
2. Computational Details
2.1. Adsorption Modes
2.2. Activation Energies and Stereoselectivities
3. Results and Discussion
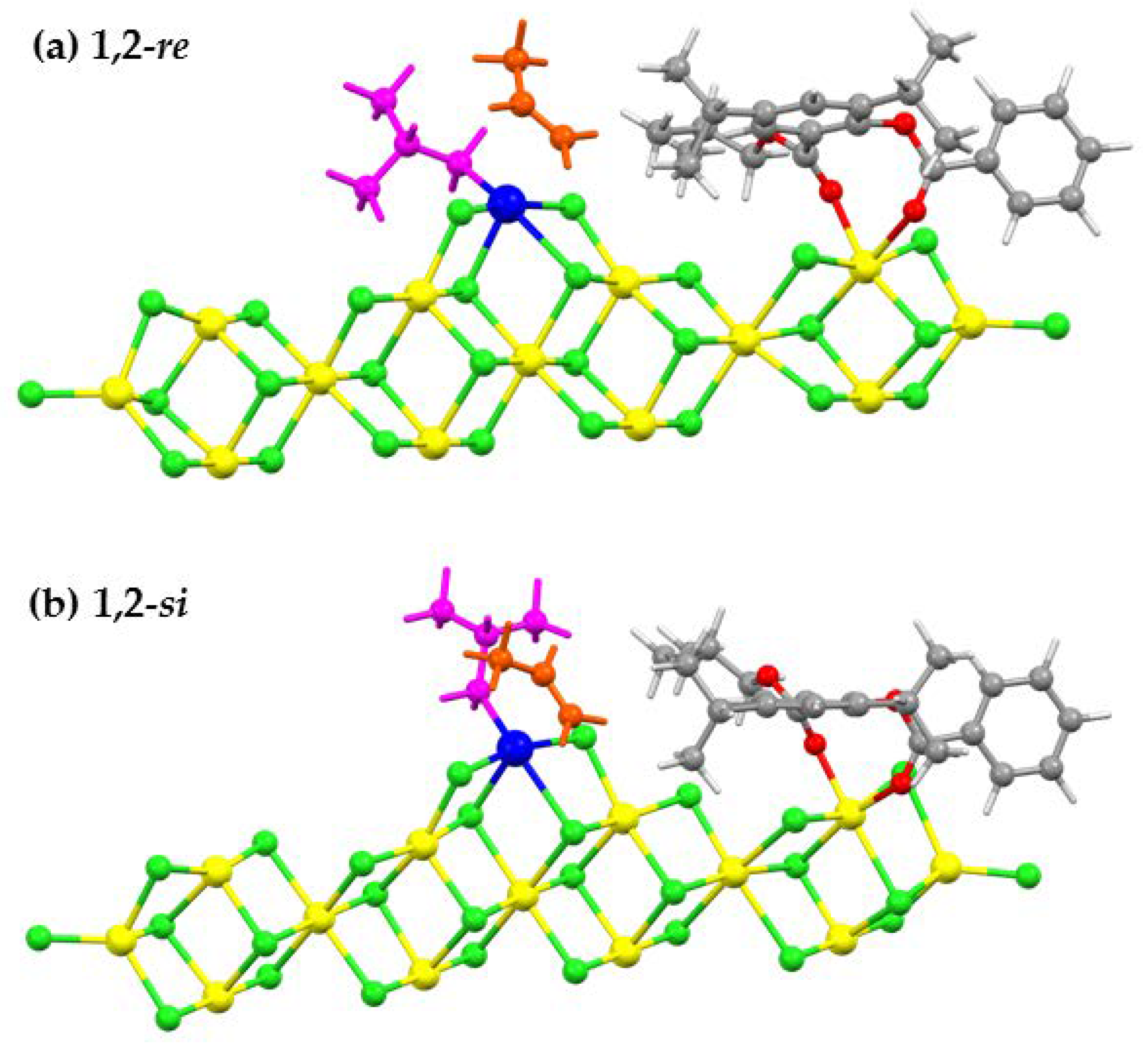
3.1. Preferred Adsorption Modes of Salicylate Donors
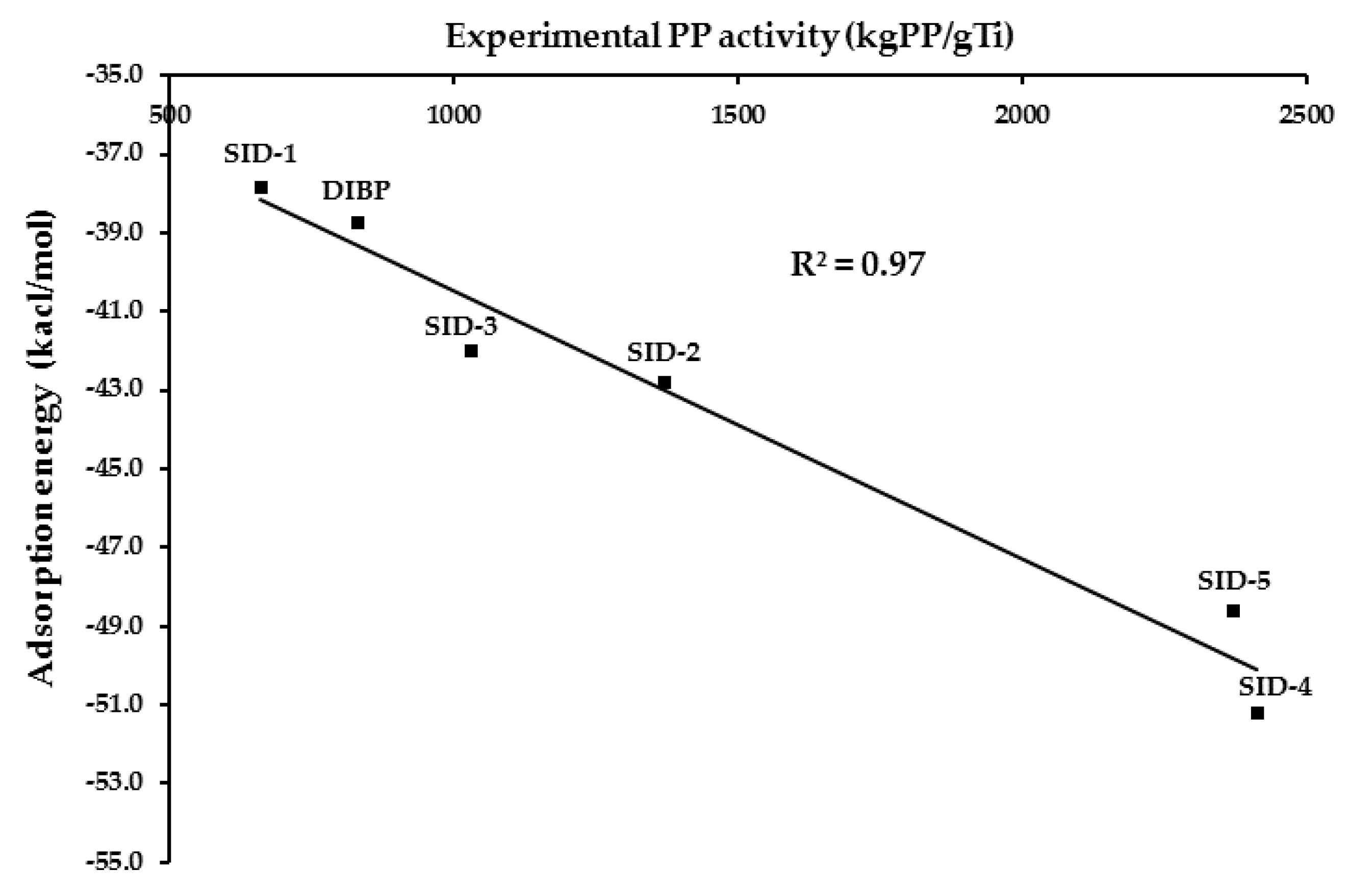
3.2. Adsorption Energies of Five Salicylate Donors
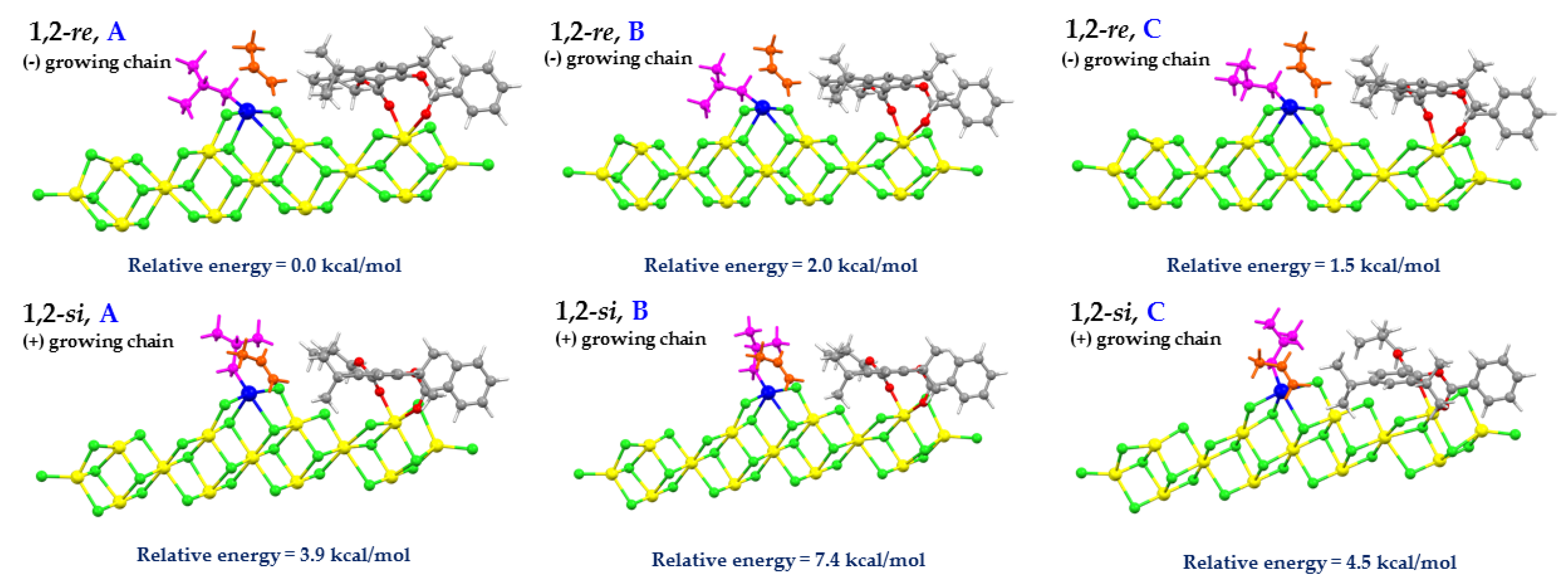
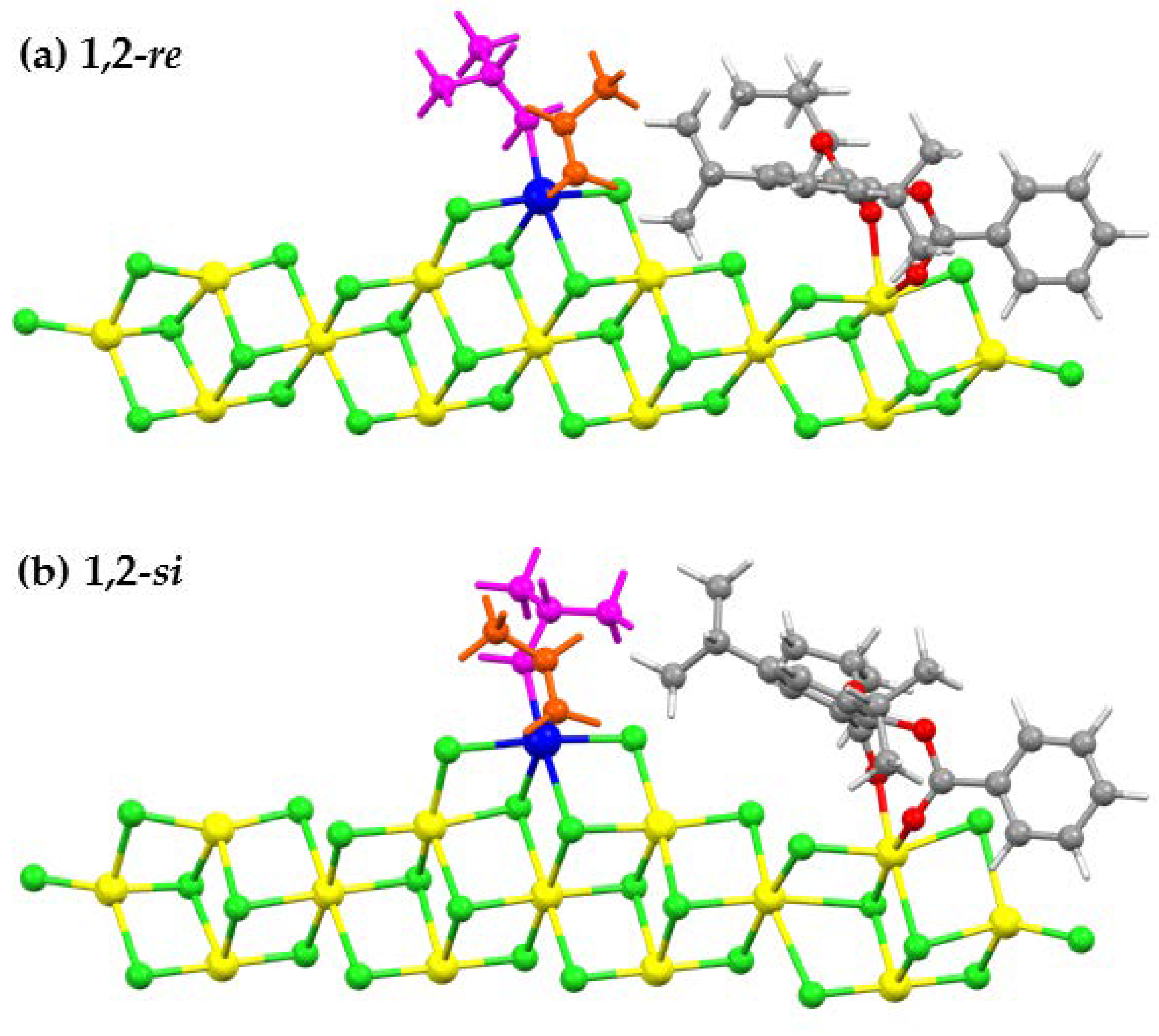
3.3. Activation Energies and Stereoselectivities
3.4. Comparing with Other Internal Electron Donors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yao, Z.-J.; Deng, W. Half-sandwich late transition metal complexes based on functionalized carborane ligands. Coord. Chem. Rev. 2016, 309, 21–35. [Google Scholar] [CrossRef]
- Shamiri, A.; Chakrabarti, M.; Jahan, S.; Hussain, M.; Kaminsky, W.; Aravind, P.; Yehye, W. The Influence of Ziegler-Natta and Metallocene Catalysts on Polyolefin Structure, Properties, and Processing Ability. Materials 2014, 7, 5069–5108. [Google Scholar] [CrossRef]
- Maddah, H.A. Polypropylene as a Promising Plastic: A Review. Am. J. Polym. Sci. 2016, 6, 1–11. [Google Scholar]
- Shumilo, O.N.; Bulgakov, N.N.; Likholobov, V.A. On the mechanism of ethylene and propylene insertion into metal-hydroxo bonds. Reacti. Kineti. Catal. Lett 1983, 22, 87–93. [Google Scholar] [CrossRef]
- Sacchi, M.C.; Tritto, I.; Shan, C.; Mendichi, R.; Noristi, L. Role of the pair of internal and external donors in magnesium chloride-supported Ziegler-Natta catalysts. Macromolecules 1991, 24, 6823–6826. [Google Scholar] [CrossRef]
- Soga, K.; Shiono, T.; Doi, Y. Influence of internal and external donors on activity and stereospecificity of ziegler-natta catalysts. Makromol. Chem. 1988, 189, 1531–1541. [Google Scholar] [CrossRef]
- Ferreira, M.a.L.; Damiani, D.E. Effect of different donors on kinetics of Zn catalysts and molecular weight of the obtained polypropylene. J. Mol. Catal. Chem. 1999, 150, 53–69. [Google Scholar] [CrossRef]
- Morini, G.; Albizzati, E.; Balbontin, G.; Mingozzi, I.; Sacchi, M.C.; Forlini, F.; Tritto, I. Microstructure Distribution of Polypropylenes Obtained in the Presence of Traditional Phthalate/Silane and Novel Diether Donors: A Tool for Understanding the Role of Electron Donors in MgCl2-Supported Ziegler−Natta Catalysts. Macromolecules 1996, 29, 5770–5776. [Google Scholar] [CrossRef]
- Singh, G.; Kaur, S.; Makwana, U.; Patankar, R.B.; Gupta, V.K. Influence of Internal Donors on the Performance and Structure of MgCl2 Supported Titanium Catalysts for Propylene Polymerization. Macromol. Chem. Phys. 2009, 210, 69–76. [Google Scholar] [CrossRef]
- Guo, J.; Hu, G.; Chen, Z. Synthesis of novel electron donors and their application to propylene polymerization. Trans. Tianjin Univ. 2012, 18, 8–14. [Google Scholar] [CrossRef]
- Cui, N.; Ke, Y.; Li, H.; Zhang, Z.; Guo, C.; Lv, Z.; Hu, Y. Effect of diether as internal donor on MgCl2-supported Ziegler–Natta catalyst for propylene polymerization. J. Appl. Polym. Sci 2006, 99, 1399–1404. [Google Scholar] [CrossRef]
- Brambilla, L.; Zerbi, G.; Piemontesi, F.; Nascetti, S.; Morini, G. Structure of Donor Molecule 9,9-Bis(Methoxymethyl)-Fluorene in Ziegler-Natta Catalyst by Infrared Spectroscopy and Quantum Chemical Calculation. J. Phys. Chem. C 2010, 114, 11475–11484. [Google Scholar] [CrossRef]
- Tanase, S.; Katayama, K.; Yabunouchi, N.; Sadashima, T.; Tomotsu, N.; Ishihara, N. Design of novel malonates as internal donors for MgCl2-supported TiCl4 type polypropylene catalysts and their mechanistic aspects, Part 1. J. Mol. Catal. A 2007, 273, 211–217. [Google Scholar] [CrossRef]
- Liu, B.; Cheng, R.; Liu, Z.; Qiu, P.; Zhang, S.; Taniike, T.; Terano, M.; Tashino, K.; Fujita, T. Experimental and Computational Approaches on the Isospecific Role of Monoester-Type Internal Electron Donor for TiCl4/MgCl2 Ziegler-Natta Catalysts. Macromol. Symp. 2007, 260, 42–48. [Google Scholar] [CrossRef]
- Marques, M.d.F.V.; Cardoso, R.d.S.; da Silva, M.G. Preparation of MgCl2-supported Ziegler–Natta catalyst systems with new electron donors. Appl. Catal. A 2010, 374, 65–70. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, A.; Li, H.; Luo, Z.; Zheng, T.; Zhang, L.; Hu, Y. Microstructure of polypropylene and active center in Ziegler-Natta catalyst: Effect of novel salicylate internal donor. RSC Adv. 2016, 6, 75023–75031. [Google Scholar] [CrossRef]
- Ratanasak, M.; Parasuk, V. Roles of malonate donor on activity and stereoselectivity of Ziegler–Natta catalyzed propylene polymerization. J. Organomet. Chem. 2015, 775, 6–11. [Google Scholar] [CrossRef]
- Cavallo, L.; Del Piero, S.; Ducéré, J.-M.; Fedele, R.; Melchior, A.; Morini, G.; Piemontesi, F.; Tolazzi, M. Key Interactions in Heterogeneous Ziegler−Natta Catalytic Systems: Structure and Energetics of TiCl4−Lewis Base Complexes. J. Phys. Chem. C 2007, 111, 4412–4419. [Google Scholar] [CrossRef]
- Correa, A.; Credendino, R.; Pater, J.T.M.; Morini, G.; Cavallo, L. Theoretical Investigation of Active Sites at the Corners of MgCl2 Crystallites in Supported Ziegler–Natta Catalysts. Macromolecules 2012, 45, 3695–3701. [Google Scholar] [CrossRef]
- Lee, J.W.; Jo, W.H. Chemical structure–stereospecificity relationship of internal donor in heterogeneous Ziegler–Natta catalyst for propylene polymerization by DFT and MM calculations. J. Organomet. Chem. 2009, 694, 3076–3083. [Google Scholar] [CrossRef]
- Toto, M.; Morini, G.; Guerra, G.; Corradini, P.; Cavallo, L. Influence of 1,3-Diethers on the Stereospecificity of Propene Polymerization by Supported Ziegler−Natta Catalysts. A Theoretical Investigation on Their Adsorption on (110) and (100) Lateral Cuts of MgCl2 Platelets. Macromolecules 2000, 33, 1134–1140. [Google Scholar] [CrossRef]
- Vanka, K.; Singh, G.; Iyer, D.; Gupta, V.K. DFT Study of Lewis Base Interactions with the MgCl2 Surface in the Ziegler−Natta Catalytic System: Expanding the Role of the Donors. J. Phys. Chem. C 2010, 114, 15771–15781. [Google Scholar] [CrossRef]
- Ratanasak, M.; Parasuk, V. Understanding the roles of novel electron donors in Ziegler-Natta catalyzed propylene polymerization. RSC Adv. 2016, 6, 112776–112783. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Kulkarni, S.A.; Bhaduri, S. Density functional study on the role of electron donors in propylene polymerization using Ziegler–Natta catalyst. J. Organomet. Chem. 2005, 690, 1356–1365. [Google Scholar] [CrossRef]
- Taniike, T.; Terano, M. Coadsorption and Support-Mediated Interaction of Ti Species with Ethyl Benzoate in MgCl2-Supported Heterogeneous Ziegler-Natta Catalysts Studied by Density Functional Calculations. Macromol. Rapid Commun. 2007, 28, 1918–1922. [Google Scholar] [CrossRef]
- Taniike, T.; Terano, M. Coadsorption model for first-principle description of roles of donors in heterogeneous Ziegler–Natta propylene polymerization. J. Catal. 2012, 293, 39–50. [Google Scholar] [CrossRef]
- Shen, X.-R.; Fu, Z.-S.; Hu, J.; Wang, Q.; Fan, Z.-Q. Mechanism of Propylene Polymerization with MgCl2-Supported Ziegler–Natta Catalysts Based on Counting of Active Centers: The Role of External Electron Donor. J. Phys. Chem. C 2013, 117, 15174–15182. [Google Scholar] [CrossRef]
- Wondimagegn, T.; Ziegler, T. The Role of External Alkoxysilane Donors on Stereoselectivity and Molecular Weight in MgCl2-Supported Ziegler–Natta Propylene Polymerization: A Density Functional Theory Study. J. Phys. Chem. C 2012, 116, 1027–1033. [Google Scholar] [CrossRef]
- Credendino, R.; Pater, J.T.M.; Liguori, D.; Morini, G.; Cavallo, L. Investigating Alkoxysilane Coverage and Dynamics on the (104) and (110) Surfaces of MgCl2-Supported Ziegler–Natta Catalysts. J. Phys. Chem. C 2012, 116, 22980–22986. [Google Scholar] [CrossRef]
- Busico, V.; Cipullo, R.; Pellecchia, R.; Ronca, S.; Roviello, G.; Talarico, G. Design of stereoselective Ziegler–Natta propene polymerization catalysts. Proc. Natl. Acad. Sci. USA 2006, 103, 15321–15326. [Google Scholar] [CrossRef]
- Sakai, S. Ab initio studies on the Ziegler–Natta polymerization mechanisms of ethylene and propylene. Role of cocatalysis and stereoregulation. Int. J. Quantum Chem. 1997, 65, 739–747. [Google Scholar] [CrossRef]
- Seth, M.; Ziegler, T. Theoretical Study of the Copolymerization of Ethylene and Propylene by a Heterogeneous Ziegler−Natta Catalyst. Macromolecules 2004, 37, 9191–9200. [Google Scholar] [CrossRef]
- Cossee, P. Ziegler-Natta catalysis I. Mechanism of polymerization of α-olefins with Ziegler-Natta catalysts. J. Catal. 1964, 3, 80–88. [Google Scholar] [CrossRef]
- Arlman, E.J. Ziegler-Natta catalysis II. Surface structure of layer-lattice transition metal chlorides. J. Catal. 1964, 3, 89–98. [Google Scholar] [CrossRef]
- Arlman, E.J.; Cossee, P. Ziegler-Natta catalysis III. Stereospecific polymerization of propene with the catalyst system TiCl3-AlEt3. J. Catal. 1964, 3, 99–104. [Google Scholar] [CrossRef]
- Brookhart, M.; Green, M.L.H. Carbon-hydrogen-transition metal bonds. J. Organomet. Chem. 1983, 250, 395–408. [Google Scholar] [CrossRef]
- Doi, Y.; Suzuki, S.; Nozawa, F.; Soga, K.; Keii, T. Structure and Reactivity of “Living” Polypropylene. In Studies in Surface Science and Catalysis; Keii, T., Soga, K., Eds.; Elsevier: Amsterdam, Netherlands, 1986; Volume 25, pp. 257–270. [Google Scholar]
- Brintzinger, H.H.; Fischer, D.; Mülhaupt, R.; Rieger, B.; Waymouth, R.M. Stereospecific Olefin Polymerization with Chiral Metallocene Catalysts. Angew. Chem. Int. Ed. Engl. 1995, 34, 1143–1170. [Google Scholar] [CrossRef]
- Correa, A.; Piemontesi, F.; Morini, G.; Cavallo, L. Key Elements in the Structure and Function Relationship of the MgCl2/TiCl4/Lewis Base Ziegler−Natta Catalytic System. Macromolecules 2007, 40, 9181–9189. [Google Scholar] [CrossRef]
- Xie, K.; Zhu, B.; Xu, R.; Xu, J.; Liu, P. Periodic DFT study of the donor interactions with the MgCl2 surface in the Ziegler-Natta catalytic system. RSC Adv. 2016, 6, 13137–13144. [Google Scholar] [CrossRef]
- Shetty, S. Synergistic, reconstruction and bonding effects during the adsorption of internal electron donors and TiCl4 on MgCl2 surface: A periodic-DFT investigation. Surf. Sci. 2016, 653, 55–65. [Google Scholar] [CrossRef]
- Stukalov, D.V.; Zakharov, V.A.; Zilberberg, I.L. Adsorption Species of Ethyl Benzoate in MgCl2-Supported Ziegler−Natta Catalysts. A Density Functional Theory Study. J. Phys. Chem. C 2010, 114, 429–435. [Google Scholar] [CrossRef]
- D’Amore, M.; Credendino, R.; Budzelaar, P.H.M.; Causá, M.; Busico, V. A periodic hybrid DFT approach (including dispersion) to MgCl2-supported Ziegler–Natta catalysts—1: TiCl4 adsorption on MgCl2 crystal surfaces. J. Catal. 2012, 286, 103–110. [Google Scholar] [CrossRef]
- Andoni, A.; Chadwick, J.C.; Niemantsverdriet, H.J.W.; Thüne, P.C. The role of electron donors on lateral surfaces of MgCl2-supported Ziegler–Natta catalysts: Observation by AFM and SEM. J. Catal. 2008, 257, 81–86. [Google Scholar] [CrossRef]
- Brambilla, L.; Zerbi, G.; Piemontesi, F.; Nascetti, S.; Morini, G. Structure of MgCl2–TiCl4 complex in co-milled Ziegler–Natta catalyst precursors with different TiCl4 content: Experimental and theoretical vibrational spectra. J. Mol. Catal. A 2007, 263, 103–111. [Google Scholar] [CrossRef]
- Grimme, S. Density functional theory with London dispersion corrections. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2011, 1, 211–228. [Google Scholar] [CrossRef]
- Credendino, R.; Minenkov, Y.; Liguori, D.; Piemontesi, F.; Melchior, A.; Morini, G.; Tolazzi, M.; Cavallo, L. Accurate experimental and theoretical enthalpies of association of TiCl4 with typical Lewis bases used in heterogeneous Ziegler-Natta catalysis. Phys. Chem. Chem. Phys. 2017, 19, 26996–27006. [Google Scholar] [CrossRef]
- Dolg, M.; Wedig, U.; Stoll, H.; Preuss, H. Energy-adjusted abinitio pseudopotentials for the first row transition elements. J. Chem. Phys. 1987, 86, 866–872. [Google Scholar] [CrossRef]
- Partin, D.E.; O’Keeffe, M. The structures and crystal chemistry of magnesium chloride and cadmium chloride. J. Solid State Chem. 1991, 95, 176–183. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision B.01.; Gaussian, Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Corradini, P.; Guerra, G. Models for the stereospecificity in homogeneous and heterogeneous Ziegler-Natta polymerizations. Prog. Polym. Sci. 1991, 16, 239–257. [Google Scholar] [CrossRef]
- Corradini, P.; Guerra, G.; Cavallo, L. Do New Century Catalysts Unravel the Mechanism of Stereocontrol of Old Ziegler−Natta Catalysts? Acc. Chem. Res. 2004, 37, 231–241. [Google Scholar] [CrossRef]
- Busico, V.; Corradini, P.; De Martino, L.; Proto, A.; Savino, V.; Albizzati, E. Polymerization of propene in the presence of MgCl2-supported Ziegler-Natta catalysts, 1. The role of ethyl benzoate as “internal” and “external” base. Makromol. Chem. 1985, 186, 1279–1288. [Google Scholar] [CrossRef]
- Credendino, R.; Busico, V.; Causà, M.; Barone, V.; Budzelaar, P.H.M.; Zicovich-Wilson, C. Periodic DFT modeling of bulk and surface properties of MgCl2. Phys. Chem. Chem. Phys. 2009, 11, 6525–6532. [Google Scholar] [CrossRef] [PubMed]
- Breuza, E.; Antinucci, G.; Budzelaar, P.H.M.; Busico, V.; Correa, A.; Ehm, C. MgCl2-Supported Ziegler–Natta Catalysts: A DFT-D “Flexible-Cluster” Approach to Internal Donor Adducts. J. Phys. Chem. C 2018, 122, 9046–9053. [Google Scholar] [CrossRef]
- Ratanasak, M.; Rungrotmongkol, T.; Saengsawang, O.; Hannongbua, S.; Parasuk, V. Towards the design of new electron donors for Ziegler–Natta catalyzed propylene polymerization using QSPR modeling. Polymer 2015, 56, 340–345. [Google Scholar] [CrossRef]
- Kim, G.-H.; Um, B.-H.; Son, K.-C.; Oh, K.; Koh, H.-L. MgCl2-supported Ziegler–Natta catalyst containing dibenzoyl sulfide donor for propylene polymerization. J. Appl. Polym. Sci. 2014, 131, 40743–40748. [Google Scholar] [CrossRef]
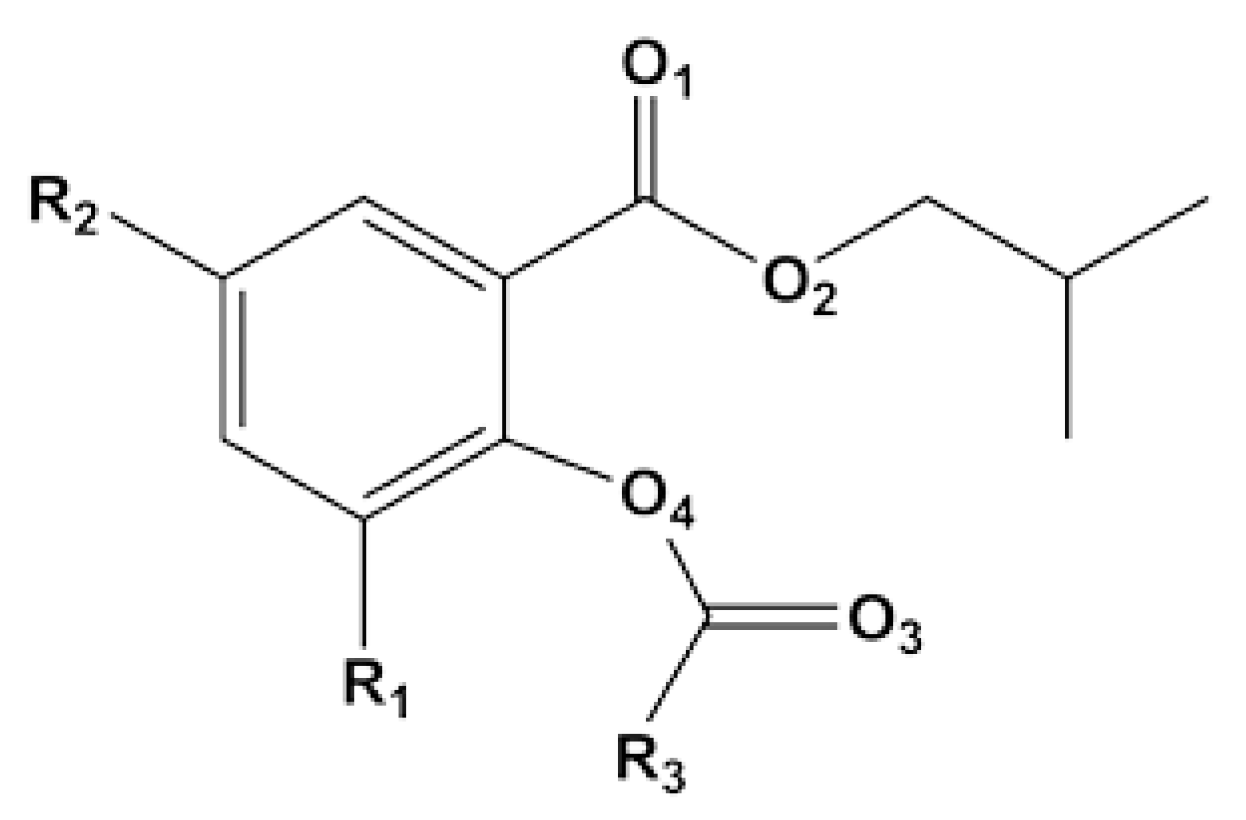

| SID | R1 | R2 | R3 | Activity (kgPP gTi−1) | %mm a | %I.I. b |
|---|---|---|---|---|---|---|
| SID-1 | H | H | Ph | 660 | 85.5 | 96.3 |
| SID-2 | Me | H | tBu | 1370 | 88.1 | 96.9 |
| SID-3 | Me | H | Ph | 1030 | 89.6 | 98.0 |
| SID-4 | iPr | iPr | Ph | 2410 | 91.0 | 98.6 |
| SID-5 | tBu | tBu | Ph | 2370 | 88.9 | 97.7 |
| Adsorption Mode | Eads (kcal/mol) | Distance (Å) | |||
|---|---|---|---|---|---|
| O1-Mg | O2-Mg | O3-Mg | O4-Mg | ||
| Mono | −30.2 | 1.99 | 4.04 | 4.49 | 4.41 |
| Chelate | −51.2 | 2.03 | 4.17 | 2.09 | 3.58 |
| Bridge | −45.9 | 2.03 | 3.74 | 2.06 | 3.24 |
| Zip | −42.0 | 5.58 | 4.06 | 1.98 | 4.17 |
| Chelate Mode | Eads (kcal/mol) | Distance (Å) | |||
|---|---|---|---|---|---|
| O1-Mg | O2-Mg | O3-Mg | O4-Mg | ||
| SID-1 | −37.8 | 2.04 | 4.18 | 2.08 | 3.64 |
| SID-2 | −42.8 | 2.05 | 4.18 | 2.10 | 3.61 |
| SID-3 | −42.0 | 2.04 | 4.18 | 2.09 | 3.58 |
| SID-4 | −51.2 | 2.03 | 4.17 | 2.09 | 3.58 |
| SID-5 | −48.6 | 2.02 | 4.17 | 2.09 | 3.67 |
| DIBP | −38.7 | 2.03 | 3.78 | 2.07 | 4.01 |
| SID | Insertion | ΔEπ (kcal/mol) | Ea (kcal/mol) | Ea(app) (kcal/mol) | Rel. (kcal/mol) | ν (cm−1) |
|---|---|---|---|---|---|---|
| 1 | 1,2-si | −59.5 | 6.4 | −53.1 | 1.1 | −340i |
| 1,2-re | −63.1 | 9.0 | −54.1 | −339i | ||
| 2 | 1,2-si | −58.0 | 5.1 | −52.9 | 3.5 | −344i |
| 1,2-re | −62.6 | 6.1 | −56.5 | −354i | ||
| 3 | 1,2-si | −58.1 | 5.7 | −52.4 | 3.2 | −331i |
| 1,2-re | −63.5 | 7.9 | −55.6 | −345i | ||
| 4 | 1,2-si | −59.2 | 3.9 | −55.3 | 3.9 | −332i |
| 1,2-re | −63.2 | 4.6 | −58.6 | −340i | ||
| 5 | 1,2-si | −58.8 | 4.4 | −54.5 | 3.6 | −362i |
| 1,2-re | −62.7 | 4.8 | −57.9 | −375i |
| 1,2-re Insertion | Theoretical Predictions | Ref. | Experiments | Ref. | ||
|---|---|---|---|---|---|---|
| Ea(app) (kcal/mol) | Rel. (kcal/mol) | Activity (kg-PP/gCat) | %Isotacticity (%mmmm) | |||
| w/o donor | −26.6 | 0.2 | [17] | - | - | |
| Malonate i | −38.5 ac | −1.5 | [17] | 25 | 97.5 | [13] |
| Sulfide ii | −58.2 | 11.9 | [23] | 40 | 91.7 | [57] |
| Phthalate iii | −37.0 | 4.0 | [23] | 22 | 88.7 | [57] |
| 830 b | 91.7 c | [16] | ||||
| Salicylate iv | −58.6 | 3.9 | 2410 b | 91.0 c | [16] | |
| Electron Donor | ΔΔEπ (kcal/mol) | ΔEa (kcal/mol) | Rel. (kcal/mol) |
|---|---|---|---|
| Malonate | 1.6 | −3.1 | −1.5 |
| Sulfide | 14.0 | −2.1 | 11.9 |
| Phthalate | 4.3 | −0.3 | 4.0 |
| Salicylate | 3.9 | −0.7 | 3.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ratanasak, M.; Hasegawa, J.-y.; Parasuk, V. Roles of Salicylate Donors in Enhancement of Productivity and Isotacticity of Ziegler–Natta Catalyzed Propylene Polymerization. Polymers 2020, 12, 883. https://doi.org/10.3390/polym12040883
Ratanasak M, Hasegawa J-y, Parasuk V. Roles of Salicylate Donors in Enhancement of Productivity and Isotacticity of Ziegler–Natta Catalyzed Propylene Polymerization. Polymers. 2020; 12(4):883. https://doi.org/10.3390/polym12040883
Chicago/Turabian StyleRatanasak, Manussada, Jun-ya Hasegawa, and Vudhichai Parasuk. 2020. "Roles of Salicylate Donors in Enhancement of Productivity and Isotacticity of Ziegler–Natta Catalyzed Propylene Polymerization" Polymers 12, no. 4: 883. https://doi.org/10.3390/polym12040883
APA StyleRatanasak, M., Hasegawa, J.-y., & Parasuk, V. (2020). Roles of Salicylate Donors in Enhancement of Productivity and Isotacticity of Ziegler–Natta Catalyzed Propylene Polymerization. Polymers, 12(4), 883. https://doi.org/10.3390/polym12040883






