Methylene-Bridged Tridentate Salicylaldiminato Binuclear Titanium Complexes as Copolymerization Catalysts for the Preparation of LLDPE through [Fe]/[Ti] Tandem Catalysis
Abstract
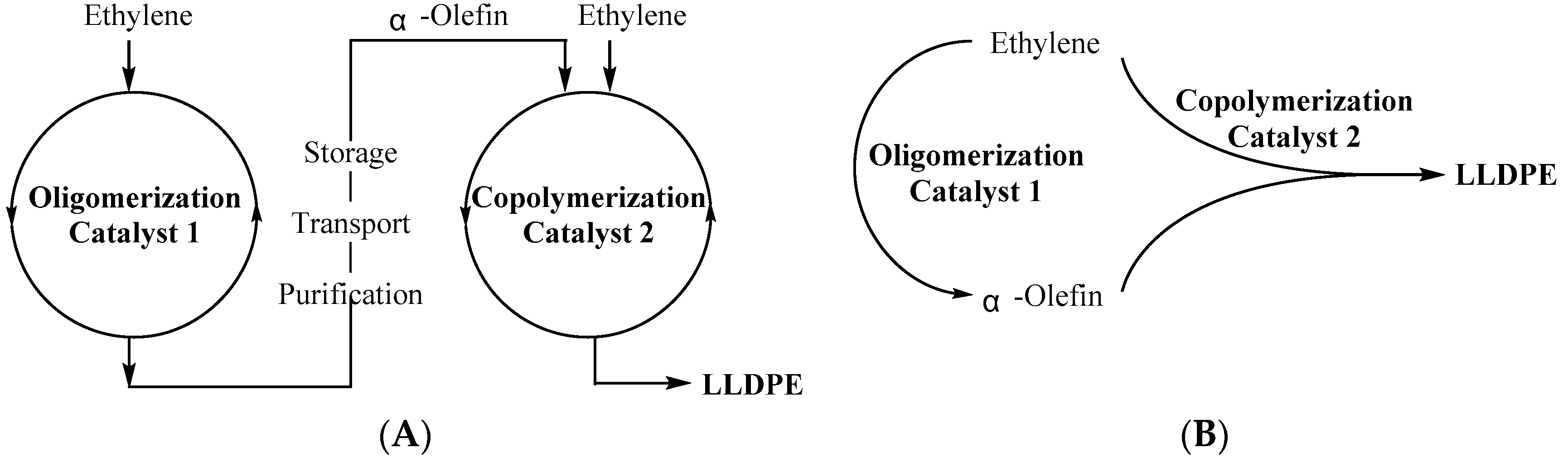
:1. Introduction
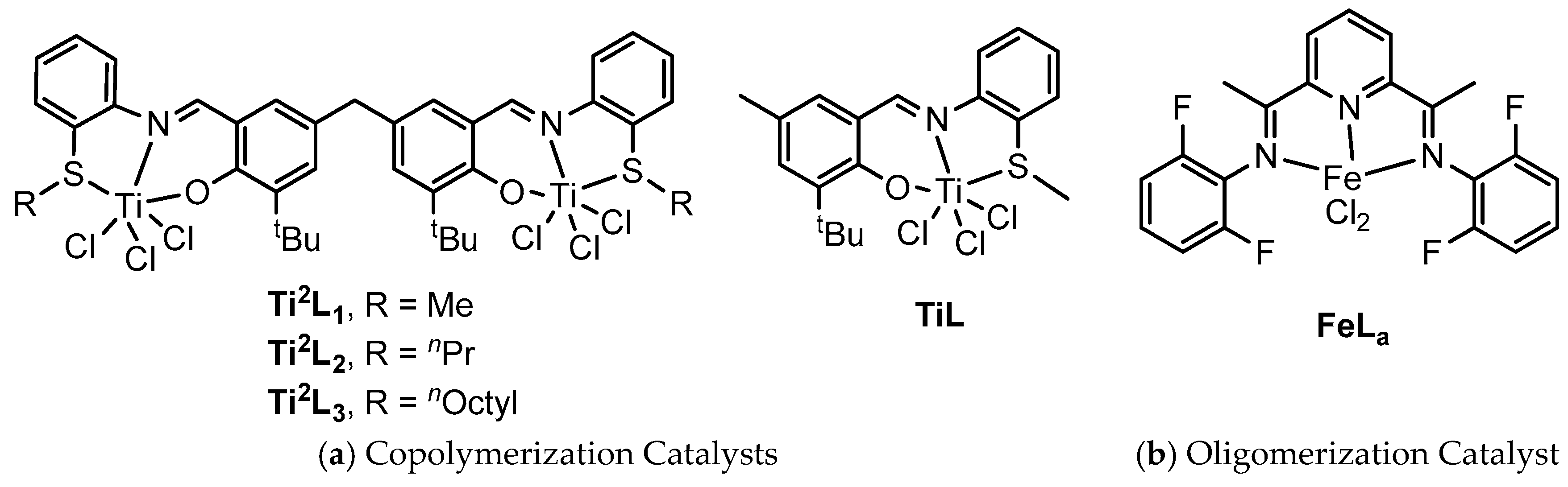
2. Materials and Methods
2.1. Materials
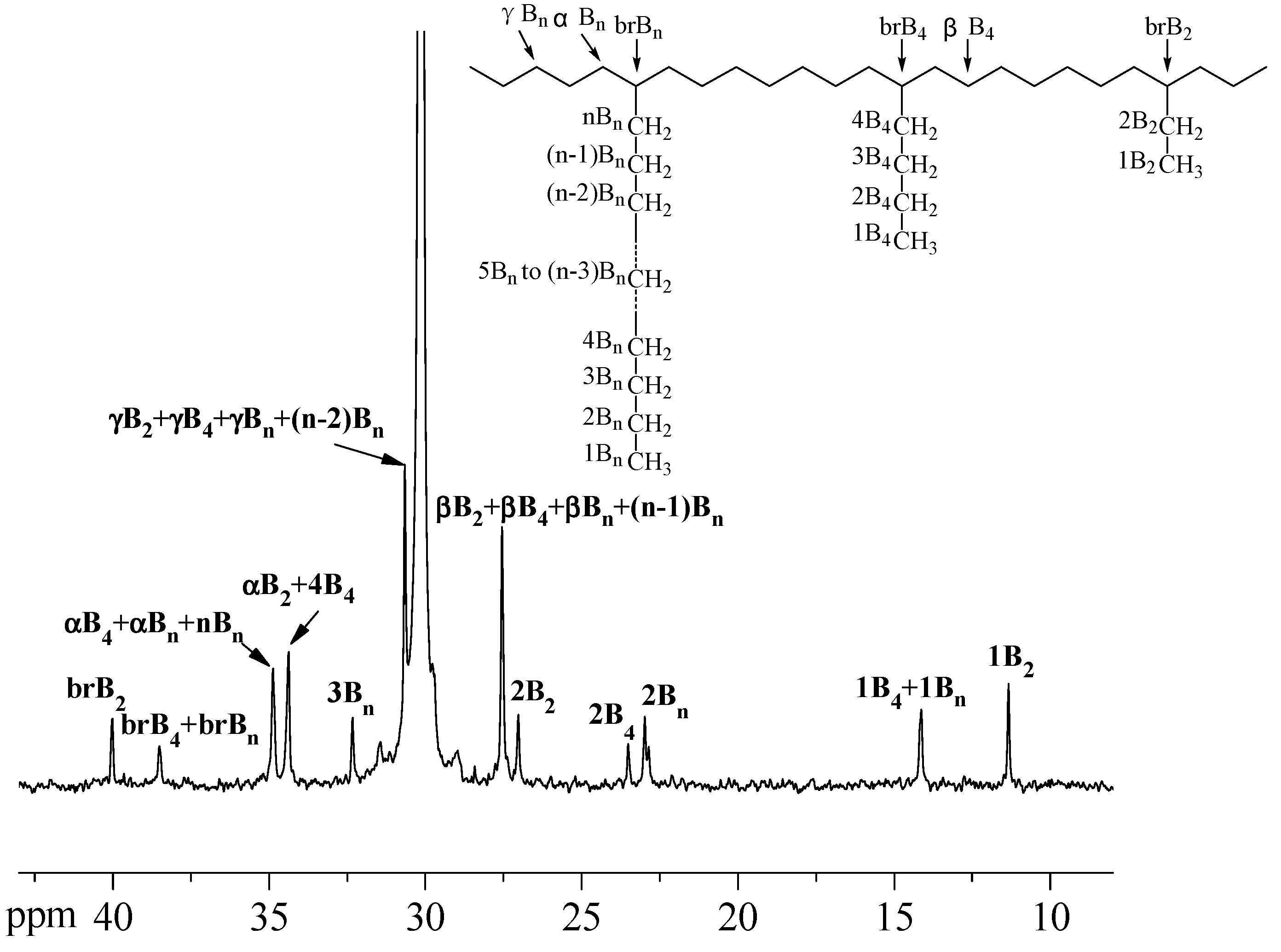
2.2. Characterization
2.3. Ethylene In-Situ Copolymerization
3. Results and Discussion
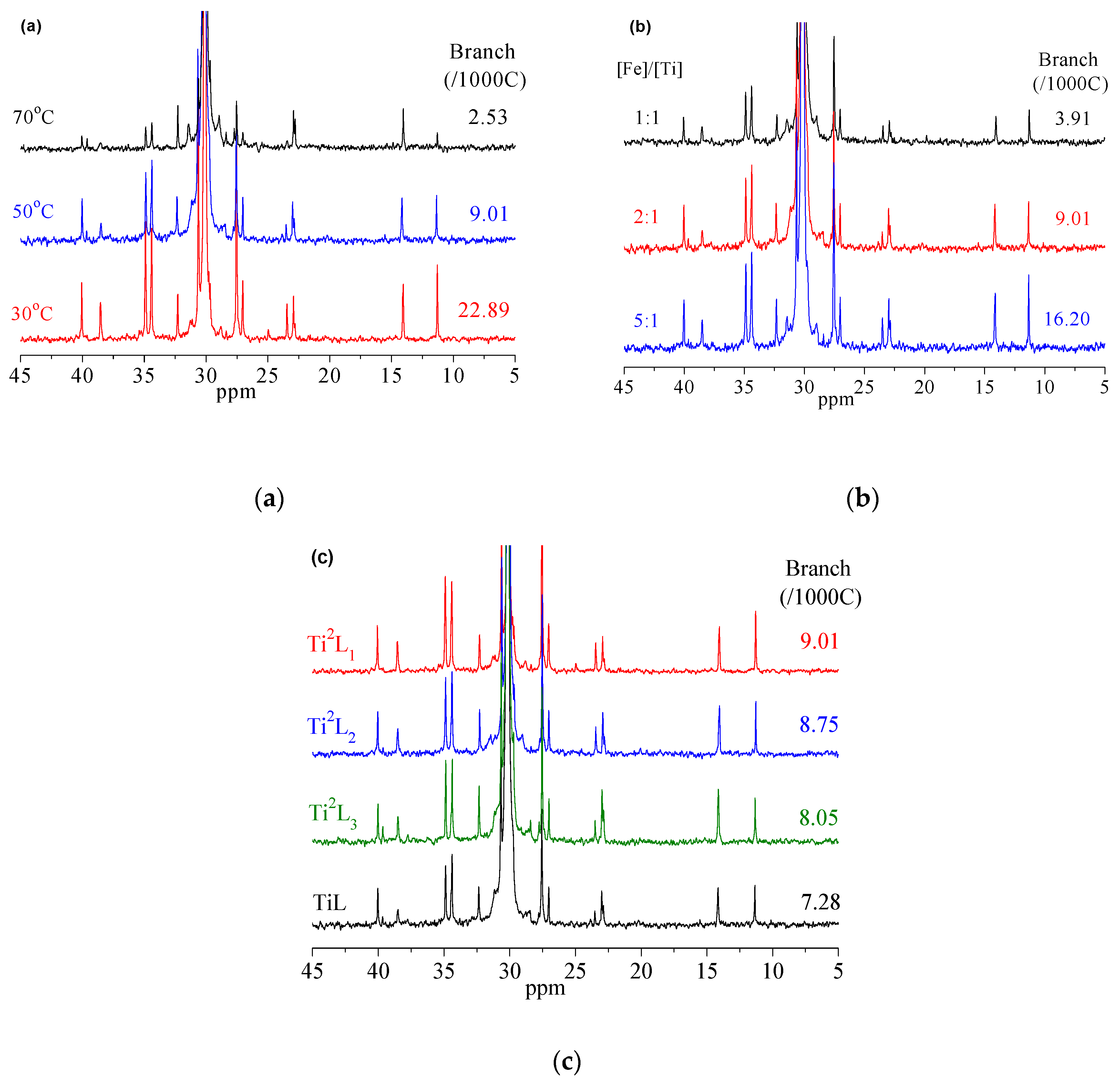
3.1. Influence of Reaction Temperature
3.2. Influence of Fe/Ti Molar Ratio
3.3. Influence of the Structure of Ti Complexes
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Beach, D.L.; Kissin, Y.V. Dual functional catalysis for ethylene polymerization to branched polyethylene. I. Evaluation of catalytic systems. J. Polym. Sci. Polym. Chem. Part A 1984, 22, 3027–3042. [Google Scholar] [CrossRef]
- Kissin, Y.V.; Beach, D.L. Dual-functional catalysis for ethylene polymerization to branched polyethylene. II. Kinetics of ethylene polymerization with a mixed homogeneous-heterogeneous Ziegler-Natta catalyst system. J. Polym. Sci. Polym. Chem. Part A 1986, 24, 1069–1084. [Google Scholar] [CrossRef]
- Xie, G.H.; Fang, Y.Q. A study of ethylene dimerization and copolymerization using a dual catalyst system. Petrochem. Technol. (China) 1994, 23, 491–497. [Google Scholar]
- Benham, E.A.; Smith, P.D.; McDaniel, M.P. A process for the simultaneous oligomerization and copolymerization of ethylene. Polym. Eng. Sci. 1988, 28, 1469–1472. [Google Scholar] [CrossRef]
- Zhu, B.; Guo, C.; Liu, Z.; Yin, Y. In situ copolymerization of ethylene to produce linear low-density polyethylene by Ti(OBu)4/AlEt3-MAO/SiO2/Et(Ind)2ZrCl2. J. Appl. Polym. Sci. 2004, 94, 2451–2455. [Google Scholar] [CrossRef]
- Zhang, Z.; Lu, Z.; Chen, S.; Li, H.; Zhang, X.; Lu, Y.; Hu, Y. Synthesis of branched polyethylene from ethylene stock by an interference-free tandem catalysis of TiCl4/MgCl2 and iron catalyst. J. Mol. Catal. Chem. A 2005, 236, 87–93. [Google Scholar] [CrossRef]
- Lu, Z.; Zhang, Z.; Li, Y.; Wu, C.; Hu, Y. Synthesis of branched polyethylene by in situ polymerization of ethylene with combined iron catalyst and Ziegler-Natta catalyst. J. Appl. Polym. Sci. 2006, 99, 2898–2903. [Google Scholar] [CrossRef]
- Yang, M.; Yan, W.; Hao, X.; Liu, B.; Wen, L.; Liu, P. Tandem catalytic systems: One catalyst combined with two different activators for preparing branched polyethylene with ethylene as single monomer. Macromolecules 2009, 42, 905–907. [Google Scholar] [CrossRef]
- Yang, M.; Li, Q.; Li, X.; Hu, B.; Dong, X.; Qu, J.; Liu, B.; Wang, X.; Hao, X. Tandem catalysis of one metallocene catalyst combined with two different cocatalysts for preparing branched polyethylene. Chin. J. Polym. Sci. 2016, 34, 298–306. [Google Scholar] [CrossRef]
- Sperber, O.; Kaminsky, W. Synthesis of long-chain branched comp-structured polyethylene from ethylene by tandem action of two single-site catalysts. Macromolecules 2003, 36, 9014–9019. [Google Scholar] [CrossRef]
- Ye, Z.; Al Obaidi, F.; Zhu, S. A tandem catalytic system for the synthesis of ethylene hex-1-ene copolymers from ethylene stock. Macromol. Rapid Commun. 2004, 25, 647–652. [Google Scholar] [CrossRef]
- Alobaidi, F.; Ye, Z.; Zhu, S. Direct synthesis of linear low-density polyethylene of ethylene/1-hexene from ethylene with a tandem catalytic system in a single reactor. J. Polym. Sci. Polym. Chem. Part A 2004, 42, 4327–4336. [Google Scholar] [CrossRef]
- Komon, Z.J.A.; Bu, X.; Bazan, G.C. Synthesis of butene-ethylene and hexene-butene-ethylene copolymers from ethylene via tandem action of well-defined homogeneous catalysts. J. Am. Chem. Soc. 2000, 122, 1830–1831. [Google Scholar] [CrossRef]
- Komon, Z.J.A.; Diamond, G.M.; Leclerc, M.K.; Murphy, V.; Okazaki, M.; Bazan, G.C. Triple tandem catalyst mixtures for the synthesis of polyethylenes with varying structures. J. Am. Chem. Soc. 2002, 124, 15280–15285. [Google Scholar] [CrossRef] [PubMed]
- Quijada, R.; Rojas, R.; Bazan, G.; Komon, Z.J.A.; Mauler, R.S.; Galland, G.B. Synthesis of branched polyethylene from ethylene by tandem action of iron and zirconium single site catalysts. Macromolecules 2001, 34, 2411–2417. [Google Scholar] [CrossRef]
- Wet-Roos, D.D.; Dixon, J.T. Homogeneous tandem catalysis of Bis(2-decylthioethyl)amine-chromium trimerization catalyst in combination with metallocene catalysts. Macromolecules 2004, 37, 9314–9320. [Google Scholar] [CrossRef]
- Bianchini, C.; Frediani, M.; Giambastiani, G.; Kaminsky, W.; Meli, A.; Passaglia, E. Amorphous polyethylene by tandem action of cobalt and titanium single-site catalysts. Macromol. Rapid Commun. 2005, 26, 1218–1223. [Google Scholar] [CrossRef]
- Zhang, Z.; Cui, N.; Lu, Y.; Ke, Y.; Hu, Y. Preparation of linear low-density polyethylene by the in situ copolymerization of ethylene with an iron oligomerization catalyst and rac-ethylene bis(indenyl) zirconium (IV) dichloride. J. Polym. Sci. Polym. Chem. Part A 2005, 43, 984–993. [Google Scholar] [CrossRef]
- Wet-Roos, D.D.; Toit, A.D.; Joubert, D.J. Homogeneous tandem catalysis of the bis-(diphenylphosphino)-amine/chromium tetramerization catalyst with metallocene catalysts. J. Polym. Sci. Polym. Chem. Part A 2006, 44, 6847–6856. [Google Scholar] [CrossRef]
- Sémeril, D.; Lejeune, M.; Matt, D. Calix[4]arene-derived nickel diphosphine complexes for LLDPE synthesis via orthogonal tandem and one-pot catalysis. New J. Chem. 2007, 31, 502–505. [Google Scholar] [CrossRef]
- Zhang, J.; Li, B.; Fan, H.; Zhu, S. Synthesis of ethylene-1-hexene copolymers from ethylene stock by tandem action of bis(2-dodecylsulfanyl-ethyl)amine-CrCl3 and Et(Ind)2ZrCl2. J. Polym. Sci. Polym. Chem. Part A 2007, 45, 3562–3569. [Google Scholar] [CrossRef]
- Xu, H.; Guo, C.; Zhang, M.; Yang, H.; Dong, J.; Yuan, G. In situ copolymerization of ethylene to linear low-density polyethylene (LLDPE) with calcosilicate (CAS-1) supported dual-functional catalytic system. Catal. Commun. 2007, 8, 2143–2149. [Google Scholar] [CrossRef]
- Milani, M.A.; De Souza, M.O.; De Souza, R.F. NiP^O and [Cp2ZrCl2/MAO] as a versatile dual-function catalyst system for in situ polymerization of ethylene to linear low-density polyethylene (LLDPE). Catal. Commun. 2010, 11, 1094–1097. [Google Scholar] [CrossRef]
- Schwerdtfeger, E.D.; Price, C.J.; Chai, J.; Miller, S.A. Tandem catalyst system for linear low-density polyethylene with short and long branching. Macromolecules 2010, 43, 4838–4842. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, P.; Wang, W.-J.; Li, B.-G.; Liu, W.; Zhu, S. Preparation of comb-shaped polyolefin elastomers having ethylene/1-octene copolymer backbone and long chain polyethylene branches via a tandem metallocene catalyst system. Macromolecules 2018, 51, 8790–8799. [Google Scholar] [CrossRef]
- Pinheiro, A.C.; Casagrande, A.C.A.; Casagrande, O.L. Linear low-density polyethylene nanocomposites by in situ polymerization using a zirconium-nickel tandem catalyst system. J. Polym. Sci. Polym. Chem. Part A 2014, 52, 3506–3512. [Google Scholar]
- Guo, S.; Fan, H.; Bu, Z.; Li, B.-G.; Zhu, S. High temperature high pressure tandem polymerization of ethylene for synthesis of ethylene-1-hexene copolymers from single reactor with SNS-Cr and CGC-Ti catalysts. Macromol. React. Eng. 2015, 9, 32–39. [Google Scholar] [CrossRef]
- Karbach, F.F.; Macko, T.; Duchateau, R. Preparation of ethylene/1-hexene copolymers from ethylene using a fully silica-supported tandem catalyst system. Macromolecules 2016, 49, 1229–1241. [Google Scholar] [CrossRef]
- Aluthge, D.C.; Sattler, A.; Al-Harthi, M.A.; Labinger, J.A.; Bercaw, J.E. Cosupported tandem catalysts for production of linear low-density polyethylene from an ethylene-only feed. ACS Catal. 2016, 6, 6581–6584. [Google Scholar] [CrossRef]
- Gibson, V.C.; Spitzmesser, S.K. Advances in non-metallocene olefin polymerization catalysis. Chem. Rev. 2003, 103, 283–315. [Google Scholar] [CrossRef]
- Makio, H.; Terao, H.; Iwashita, A.; Fujita, T. FI catalysts for olefin polymerization a comprehensive treatment. Chem. Rev. 2011, 111, 2363–2449. [Google Scholar] [CrossRef] [PubMed]
- Collins, S. Polymerization catalysis with transition metal amidinate and related complexes. Coord. Chem. Rev. 2011, 255, 118–138. [Google Scholar] [CrossRef]
- Redshaw, C.; Tang, Y. Tridentate ligands and beyond in group IV metal a-olefin homo-/co-polymerization catalysis. Chem. Soc. Rev. 2012, 41, 4484–4510. [Google Scholar] [CrossRef] [PubMed]
- Pei, L.; He, S.; Gao, J.; Liao, H.; Gao, H. Homo- and copolymerizations of ethylene and norbornene using bis(β-ketoamino) titanium catalysts containing pyrazolone rings. Polymers 2017, 9, 262. [Google Scholar] [CrossRef] [PubMed]
- Tuskaev, V.A.; Gagieva, S.C.; Kurmaev, D.A.; Kolosov, N.A.; Mikhaylik, E.S.; Golubev, E.K.; Sizov, A.I.; Zubkevich, S.V.; Vasil’ev, V.G.; Nikiforova, G.G.; et al. Titanium(III, IV)-containing catalytic systems for production of ultrahigh molecular weight polyethylene nascent reactor powders, suitable for solventless processing—Impact of oxidation states of transition metal. Polymers 2018, 10, 2. [Google Scholar] [CrossRef] [PubMed]
- Delferro, M.; Marks, T.J. Multinuclear olefin polymerization catalysts. Chem. Rev. 2011, 111, 2450–2485. [Google Scholar] [CrossRef]
- McInnis, J.P.; Delferro, M.; Marks, T.J. Multinuclear group 4 catalysis: Olefin polymerization pathways modified by strong metal metal cooperative effects. Acc. Chem. Res. 2014, 47, 2545–2557. [Google Scholar] [CrossRef]
- Salata, M.R.; Marks, T.J. Synthesis, characterization, and marked polymerization selectivity characteristics of binuclear phenoxyiminato organozirconium catalysts. J. Am. Chem. Soc. 2008, 130, 12–13. [Google Scholar] [CrossRef]
- Salata, M.R.; Marks, T.J. Catalyst nuclearity effects in olefin polymerization. Enhanced activity and comonomer enchainment in ethylene + olefin copolymerizations mediated by bimetallic group 4 phenoxyiminato catalysts. Macromolecules 2009, 42, 1920–1933. [Google Scholar] [CrossRef]
- Buchwalter, P.; Rosé, J.; Braunstein, P. Multimetallic catalysis based on heterometallic complexes and clusters. Chem. Rev. 2015, 115, 28–126. [Google Scholar] [CrossRef]
- Bratko, I.; Gómez, M. Polymetallic complexes linked to a single-frame ligand: Cooperative effects in catalysis. Dalton Trans. 2013, 42, 10664–10681. [Google Scholar] [CrossRef] [PubMed]
- Radlauer, M.R.; Buckley, A.K.; Henling, L.M.; Agapie, T. Bimetallic coordination insertion polymerization of unprotected polar monomers: Copolymerization of amino olefins and ethylene by dinickel bisphenoxyiminato catalysts. J. Am. Chem. Soc. 2013, 135, 3784–3787. [Google Scholar] [CrossRef] [PubMed]
- Radlauer, M.R.; Day, M.W.; Agapie, T. Bimetallic effects on ethylene polymerization in the presence of amines: Inhibition of the deactivation by lewis bases. J. Am. Chem. Soc. 2012, 134, 1478–1481. [Google Scholar] [CrossRef] [PubMed]
- Han, S.L.; Yao, E.D.; Qin, W.; Zhang, S.F.; Ma, Y.G. Binuclear heteroligated titanium catalyst based on phenoxyimine ligands: Synthesis, characterization, and ethylene (Co)polymerization. Macromolecules 2012, 45, 4054–4059. [Google Scholar] [CrossRef]
- Rong, C.; Wang, F.; Li, W.; Chen, M. Ethylene polymerization by dinuclear xanthene-bridged imino- and aminopyridyl nickel complexes. Organometallics 2017, 36, 4458–4464. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, X.; Gong, X.; Xu, D.; Ma, Y. Macrocyclic trinuclear nickel phenoxyimine catalysts for hightemperature polymerization of ethylene and isospecific polymerization of propylene. Macromolecules 2017, 50, 6561–6568. [Google Scholar] [CrossRef]
- Ji, P.; Solomon, J.B.; Lin, Z.; Johnson, A.; Jordan, R.F.; Lin, W. Transformation of metal organic framework secondary building units into hexanuclear Zr-alkyl catalysts for ethylene polymerization. J. Am. Chem. Soc. 2017, 139, 11325–11328. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Ye, Z. “Living” polymerization of ethylene and 1-hexene using novel binuclear Pd–diimine catalysts. Polymers 2017, 9, 282. [Google Scholar]
- Xie, G.; Qian, C. Dramatic electronic effect of fluoro substituents on the olefin polymerization activity of mono β-diiminato titanium complexes. J. Polym. Sci. Polym. Chem. Part A 2008, 46, 211–217. [Google Scholar] [CrossRef]
- Xie, G.; Li, Y.; Sun, J.; Qian, C. Titanium complexes with β-ketoiminate chelate ligands for ethylene polymerization: The significant influence of substituents on structures and catalytic activities. Inorg. Chem. Commun. 2009, 12, 796–799. [Google Scholar] [CrossRef]
- Xie, G.; Li, T.; Zhang, A. Highly active and selective ethylene oligomerization catalysts: Asymmetric 2,6-bis(imino)pyridyl iron (II) complexes with alkyl and halogen substitutients. Inorg. Chem. Commun. 2010, 13, 1199–1202. [Google Scholar] [CrossRef]
- Xie, G.; Song, W.; Li, T.; Xu, X.; Lan, Z.; Li, Y.; Zhang, A. Synthesis, characterization, and catalytic behavior of copper complexes with fluorosubstituted β-ketoimine ligands. J. Appl. Polym. Sci. 2014, 131, 41178. [Google Scholar] [CrossRef]
- Li, T.; Song, W.; Ai, H.; You, Q.; Zhang, A.; Xie, G. β-diiminato titanium complexes with varying fluorine substitution patterns on the N-aryl moiety: Probing the effect of ligand substitution on ethylene polymerization. J. Polym. Res. 2015, 22, 631. [Google Scholar] [CrossRef]
- Xiao, X.; Wen, Y.; Wang, X.; Lei, L.; Xia, P.; Li, T.; Zhang, A.; Xie, G. Synthesis and structural characterization of trifluoromethyl-substituted β-ketoimino copper(II) complex and its catalytic behavior for methyl acrylate polymerization. Russ. J. Coord. Chem. 2015, 41, 543–548. [Google Scholar] [CrossRef]
- Wang, L.; You, X.; You, Q.; Li, T.; Zhang, A.; Xie, G. Synthesis and structures of mono(β-diiminato) copper complexes and their catalytic performances for homo- and copolymerizations of methyl acrylate. Transit. Metal Chem. 2016, 41, 857–866. [Google Scholar] [CrossRef]
- You, X.; Wang, L.; You, Q.; Li, T.; Zhang, A.; Xie, G. Synthesis, crystal structure and catalytic performances of trifluoro-substituted mono(β-diiminato) copper(II) complex. Chin. J. Struct. Chem. 2017, 36, 107–112. [Google Scholar]
- Xie, G.; Liu, G.; Li, L.; Li, T.; Zhang, A.; Feng, J. Tandem catalysis of iron and titanium non-metallocene catalysts for the production of branched polyethylene. Catal. Commun. 2014, 45, 7–10. [Google Scholar] [CrossRef]
- Xie, G.; Zhang, X.; Li, T.; Li, L.; Liu, G.; Zhang, A. Preparation of linear low-density polyethylene from ethylene by tandem catalysis of iron and titanium non-metallocene catalysts. J. Mol. Catal. Chem. A 2014, 383–384, 121–127. [Google Scholar] [CrossRef]
- Li, T.; Lan, Z.; Xie, G.; Luo, D.; Li, L.; Xiong, S.; Zhang, L.; Ouyang, L.; Zhang, A. Binuclear titanium catalysts based on methylene-bridged tridentate salicylaldiminato ligands for ethylene homo- and copolymerization. Catal. Lett. 2017, 147, 996–1005. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, X.; Xiao, X.; Luo, D.; Zeng, Y.; Li, T.; Li, X.; Zhang, A.; Xie, G. A novel tridentate [ONS] binuclear titanium complex bearing oxo-bridged macrocyclic structure for ethylene polymerization. J. Organomet. Chem. 2018, 856, 50–55. [Google Scholar] [CrossRef]
- Luo, D.; Zeng, Y.; Chen, X.; Xia, P.; Xie, G.; You, Q.; Zhang, L.; Li, T.; Li, X.; Zhang, A. Synthesis, characterization and olefin polymerization behaviors of phenylene-bridged bis-β-carbonylenamine binuclear titanium complexes. RSC Adv. 2018, 8, 6954–6964. [Google Scholar] [CrossRef]
- Chen, X.; Zeng, Y.; Lan, Z.; You, Q.; Li, T.; Li, X.; Zhang, D.; Xie, G. A methylene-bridged salicylaldiminato tridentate [ONS] binuclear titanium complex for ethylene-norbornene copolymerization. J. Macromol. Sci. Pure Appl. Chem. Part A 2018, 55, 489–495. [Google Scholar] [CrossRef]
- Galland, G.B.; Quijada, R.; Rojas, R.; Bazan, G.; Komon, Z.J.A. NMR study of branched polyethylenes obtained with combined Fe and Zr catalysts. Macromolecules 2002, 35, 339–345. [Google Scholar] [CrossRef]
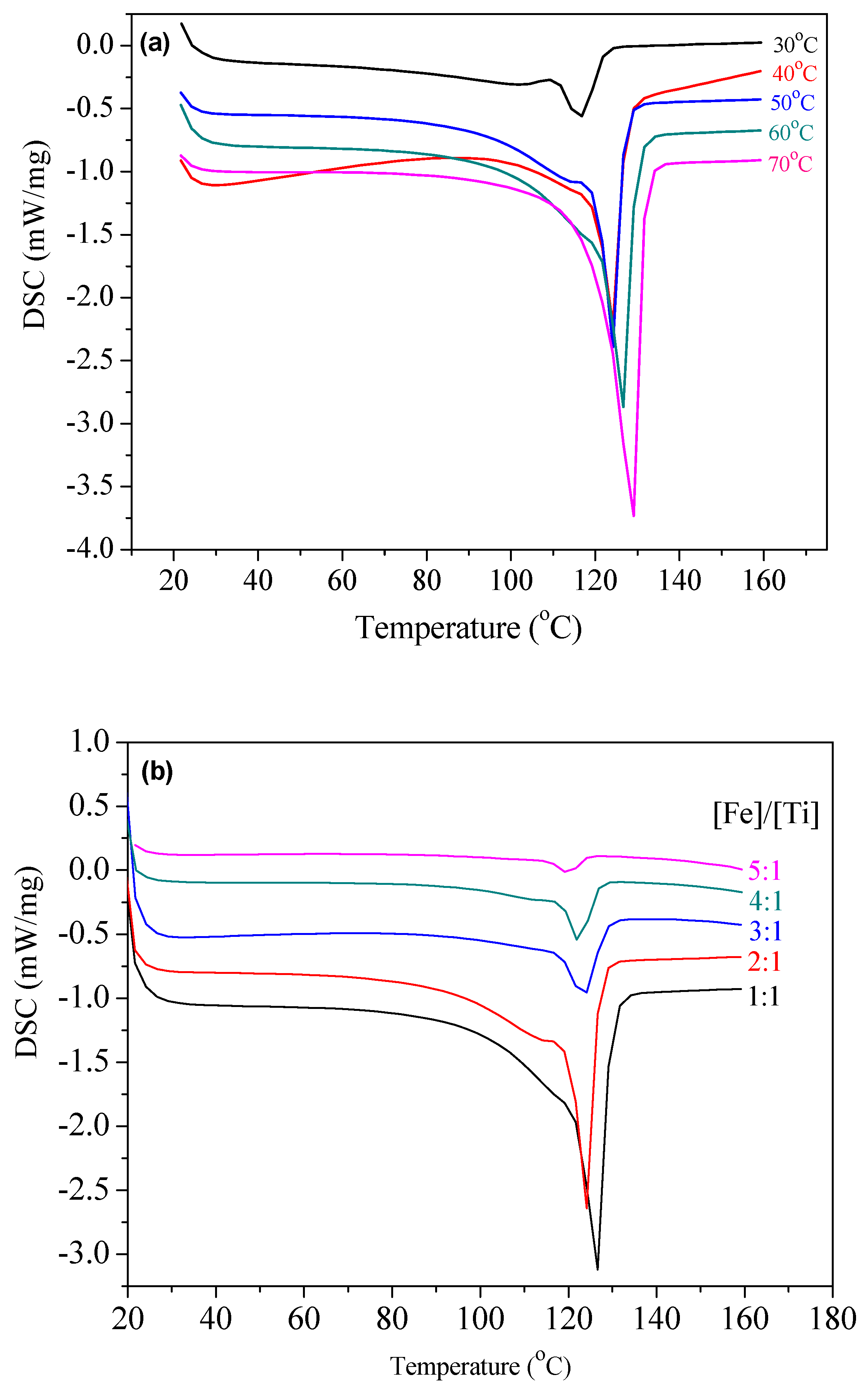
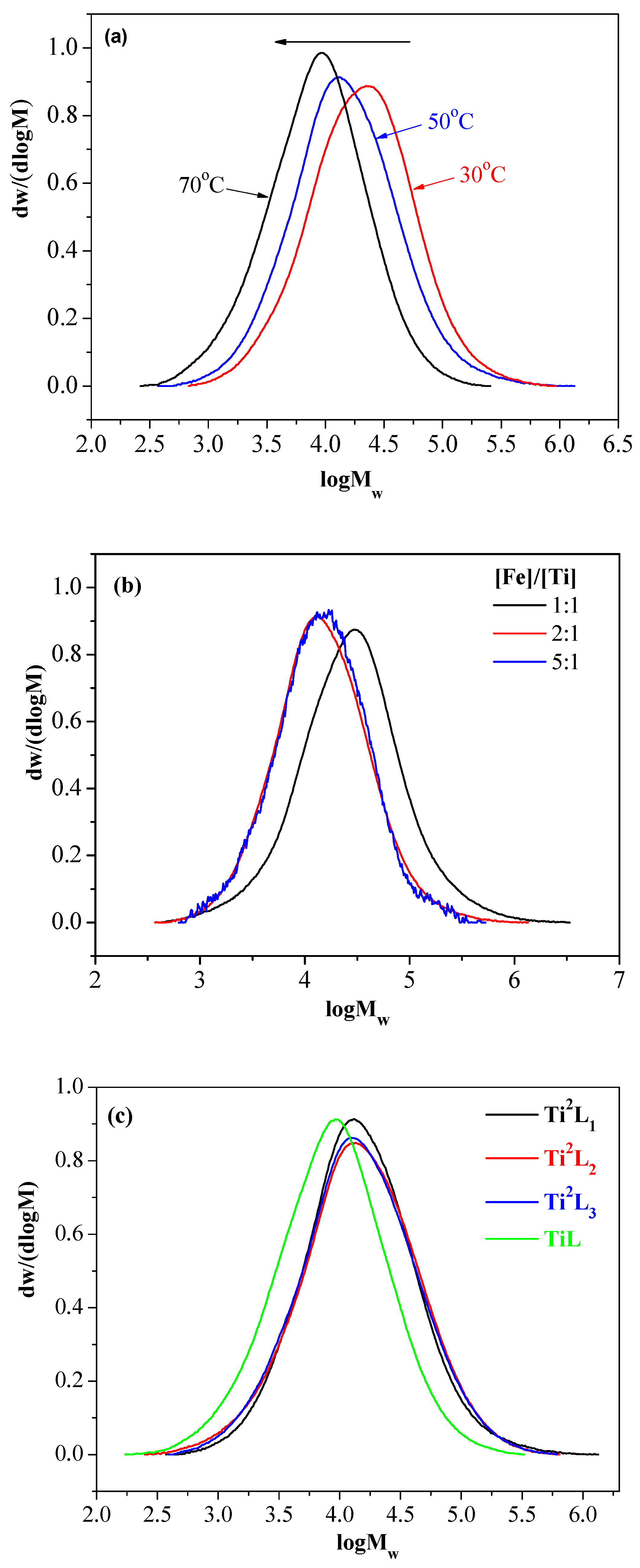
| Entry | Ti Complex | [Fe]/[Ti] | T (°C) | Act.b | Tm c (°C) | Xcc (%) | Branch d (1000 C) | Mw e | Mw/Mn |
|---|---|---|---|---|---|---|---|---|---|
| 1 f | Ti2L1 | 1:0 | 50 | 0.78 | |||||
| 2 | Ti2L1 | 0:1 | 50 | 0.73 | 136.4 | 60.3 | 0 | n.d. | n.d. |
| 3 | Ti2L1 | 1:1 | 50 | 0.72 | 126.3 | 46.1 | 3.91 | 5.41 | 4.18 |
| 4 | Ti2L1 | 2:1 | 30 | 0.75 | 115.7 | 2.8 | 22.89 | 3.37 | 2.95 |
| 5 | Ti2L1 | 2:1 | 40 | 0.95 | 123.9 | 37.5 | n.d. | n.d. | n.d. |
| 6 | Ti2L1 | 2:1 | 50 | 1.18 | 124.2 | 40.1 | 9.01 | 2.57 | 3.08 |
| 7 | Ti2L1 | 2:1 | 60 | 1.20 | 126.4 | 47.5 | n.d. | n.d. | n.d. |
| 8 | Ti2L1 | 2:1 | 70 | 0.69 | 128.7 | 53.6 | 2.53 | 1.30 | 2.62 |
| 9 | Ti2L1 | 3:1 | 50 | 1.43 | 123.3 | 14.0 | n.d. | n.d. | n.d. |
| 10 | Ti2L1 | 4:1 | 50 | 3.22 | 122.3 | 10.7 | n.d. | n.d. | n.d. |
| 11 | Ti2L1 | 5:1 | 50 | 8.95 | 119.5 | 7.3 | 16.20 | 2.34 | 2.78 |
| 12 | Ti2L2 | 2:1 | 50 | 1.16 | 124.4 | 40.7 | 8.75 | 2.54 | 3.44 |
| 13 | Ti2L3 | 2:1 | 50 | 0.97 | 124.7 | 42.8 | 8.05 | 2.50 | 3.26 |
| 14 g | TiL | 2:1 | 50 | 1.84 | 125.2 | 44.4 | 7.28 | 1.42 | 3.01 |
| Entry | Branching with Respect to Total | Branching with Respect to Total [E] Units | Branch/ 1000C | |||||
|---|---|---|---|---|---|---|---|---|
| NE’(%) | NB’(%) | NL’(%) | NE’’(%) | NB’’(%) | NL’’(%) | R (%) | ||
| 4 | 55.45 | 27.84 | 16.71 | 2.54 | 1.27 | 0.76 | 4.58 | 22.89 |
| 6 | 30.03 | 12.87 | 1.03 | 0.54 | 0.23 | 1.80 | 9.01 | |
| 8 | 80.12 | 15.66 | 4.22 | 0.41 | 0.08 | 0.02 | 0.51 | 2.53 |
| 3 | 72.15 | 21.94 | 5.91 | 0.56 | 0.17 | 0.05 | 0.78 | 3.91 |
| 11 | 57.87 | 30.96 | 11.17 | 1.87 | 1.00 | 0.36 | 3.24 | 16.20 |
| 12 | 51.61 | 26.73 | 21.66 | 0.90 | 0.47 | 0.38 | 1.75 | 8.75 |
| 13 | 62.35 | 19.14 | 18.52 | 1.00 | 0.31 | 0.30 | 1.61 | 8.05 |
| 14 | 62.38 | 29.47 | 8.15 | 0.91 | 0.43 | 0.12 | 1.46 | 7.28 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Y.; Li, J.; Luo, D.; You, Q.; Yang, Z.; Li, T.; Li, X.; Xie, G. Methylene-Bridged Tridentate Salicylaldiminato Binuclear Titanium Complexes as Copolymerization Catalysts for the Preparation of LLDPE through [Fe]/[Ti] Tandem Catalysis. Polymers 2019, 11, 1114. https://doi.org/10.3390/polym11071114
Luo Y, Li J, Luo D, You Q, Yang Z, Li T, Li X, Xie G. Methylene-Bridged Tridentate Salicylaldiminato Binuclear Titanium Complexes as Copolymerization Catalysts for the Preparation of LLDPE through [Fe]/[Ti] Tandem Catalysis. Polymers. 2019; 11(7):1114. https://doi.org/10.3390/polym11071114
Chicago/Turabian StyleLuo, Yani, Jian Li, Derong Luo, Qingliang You, Zifeng Yang, Tingcheng Li, Xiandan Li, and Guangyong Xie. 2019. "Methylene-Bridged Tridentate Salicylaldiminato Binuclear Titanium Complexes as Copolymerization Catalysts for the Preparation of LLDPE through [Fe]/[Ti] Tandem Catalysis" Polymers 11, no. 7: 1114. https://doi.org/10.3390/polym11071114
APA StyleLuo, Y., Li, J., Luo, D., You, Q., Yang, Z., Li, T., Li, X., & Xie, G. (2019). Methylene-Bridged Tridentate Salicylaldiminato Binuclear Titanium Complexes as Copolymerization Catalysts for the Preparation of LLDPE through [Fe]/[Ti] Tandem Catalysis. Polymers, 11(7), 1114. https://doi.org/10.3390/polym11071114






