Ammonium Polyphosphate with High Specific Surface Area by Assembling Zeolite Imidazole Framework in EVA Resin: Significant Mechanical Properties, Migration Resistance, and Flame Retardancy
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of ZIF-67 and APP-ZIFs
2.3. Preparation of Flame-Retardant EVA Composites
2.4. Characterization
3. Results and Discussion
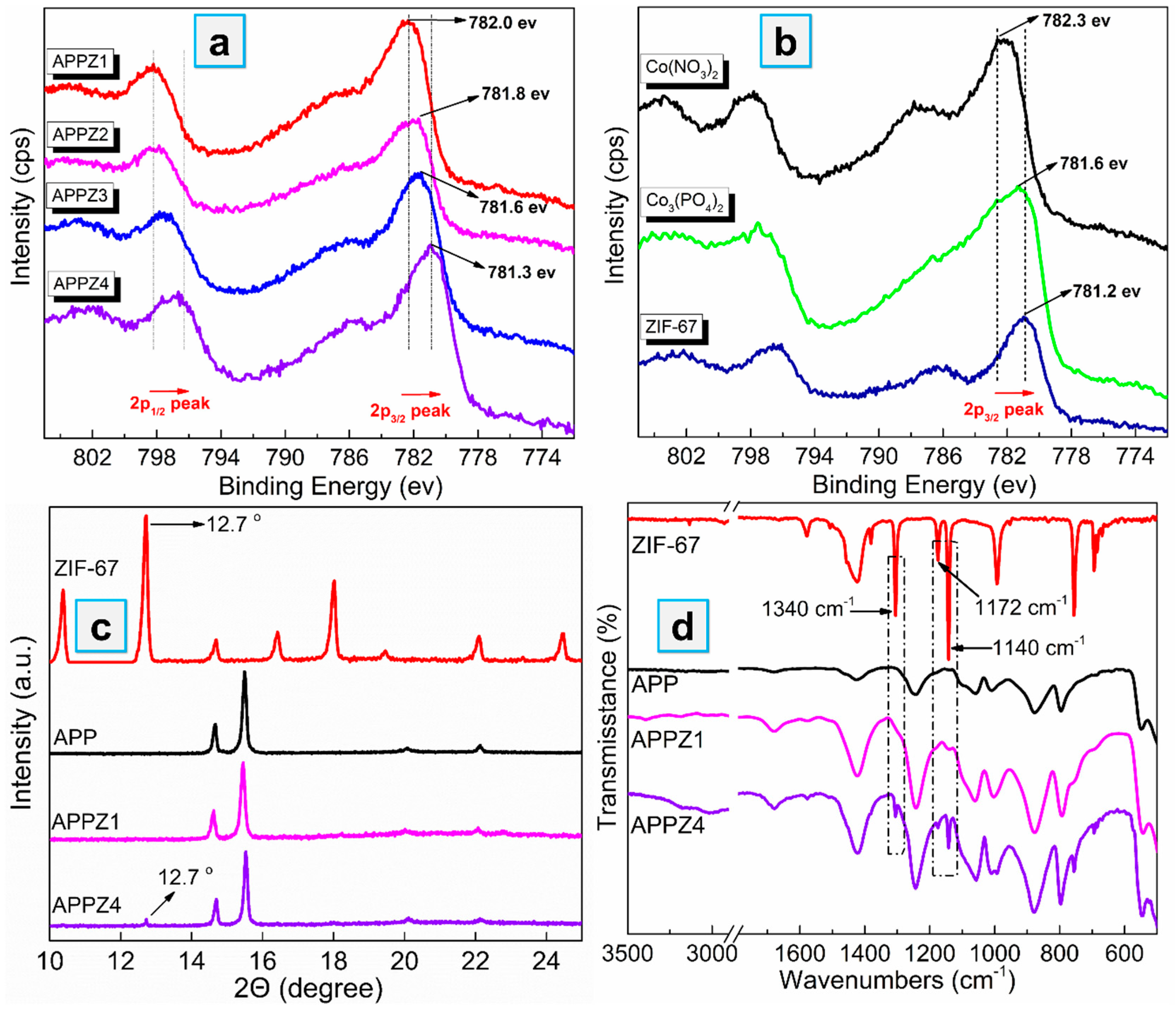
3.1. Assembly Mechanism and Structure Characterization of APP-ZIFs
3.1.1. Assembly Mechanism and Structure Characterization of APP-ZIFs
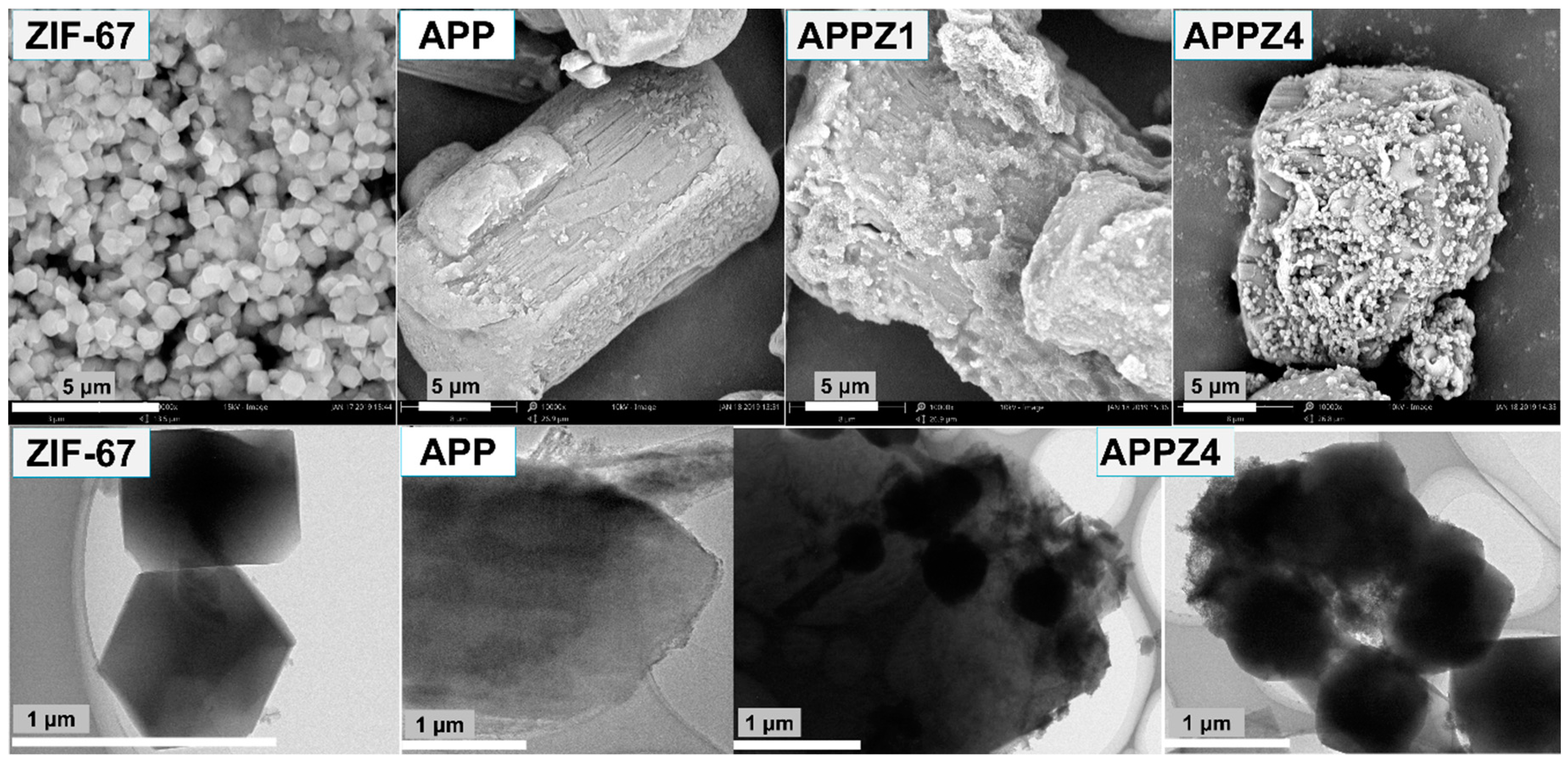
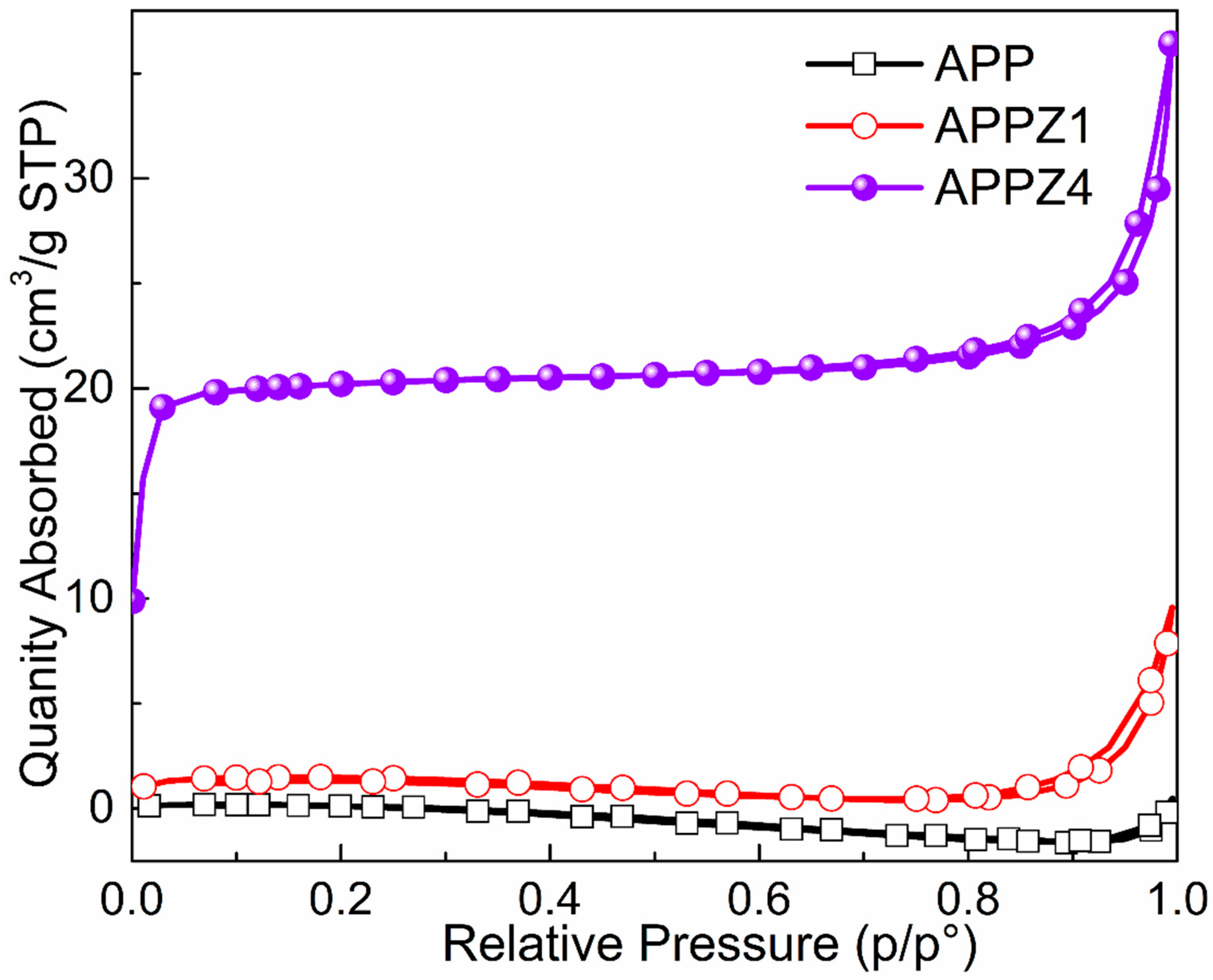
3.1.2. Morphology and Specific Surface Area Analysis
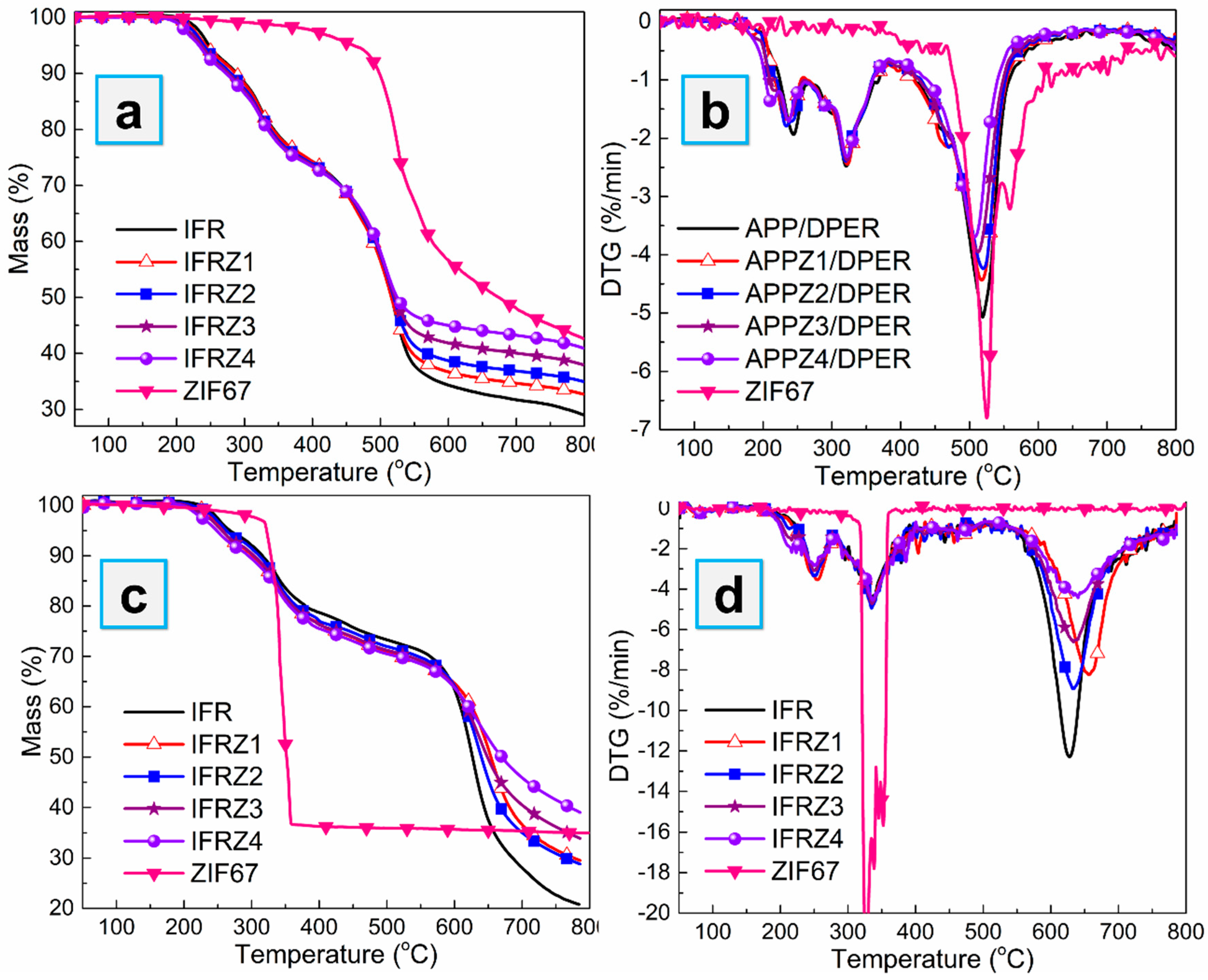
3.2. Thermal Degradation Behaviors of APP-ZIFs with DPER as an IFR System
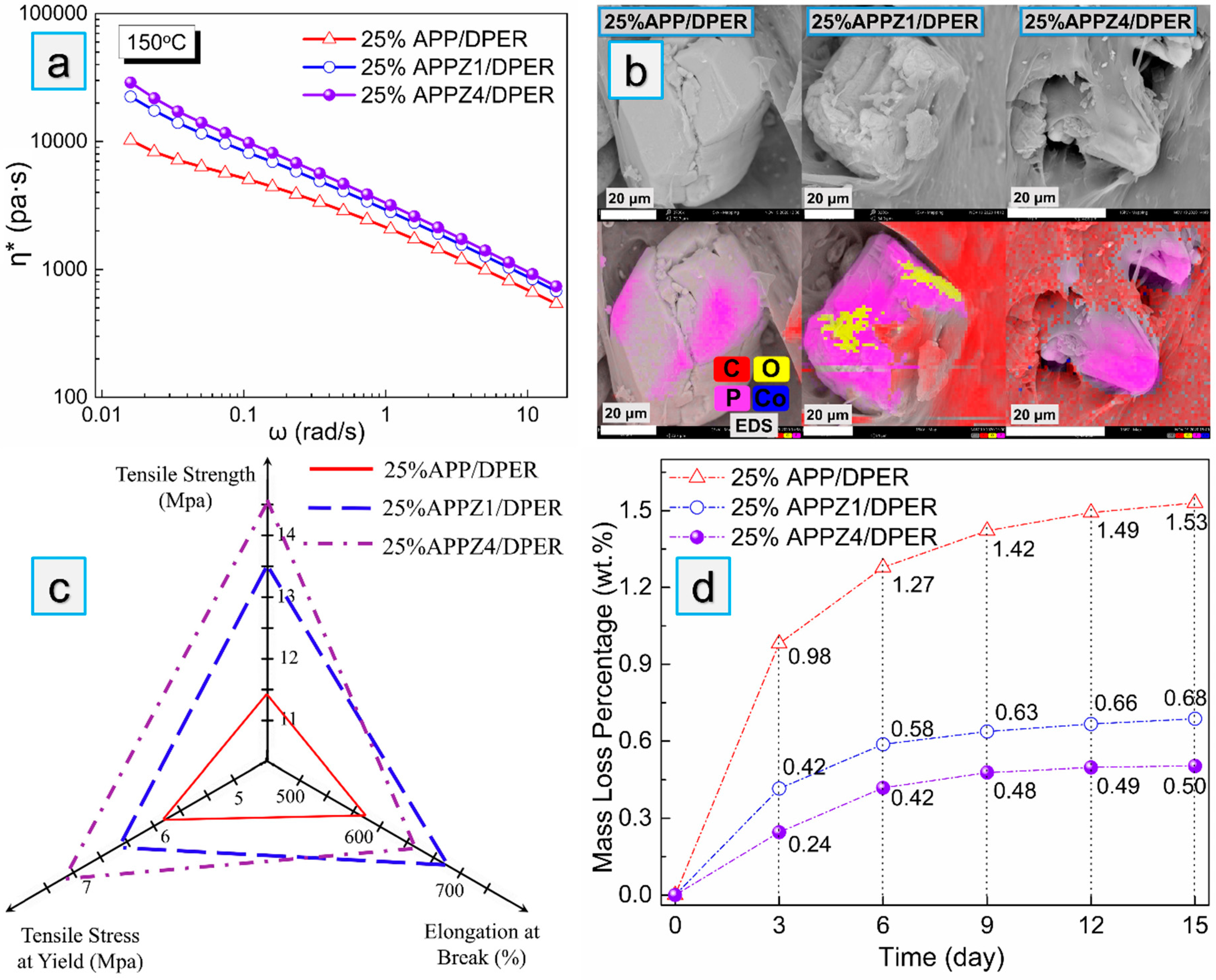
3.3. Interfacial Interaction, Mechanical Properties, and Migration Resistance
3.4. Flame Retardancy and Smoke Suppression
3.4.1. LOI and UL 94 Test
3.4.2. CCT
3.5. Thermal Stability and Decomposing Volatiles of EVA Composites
3.6. Micromorphology and Chemical Structure of the Char Residues
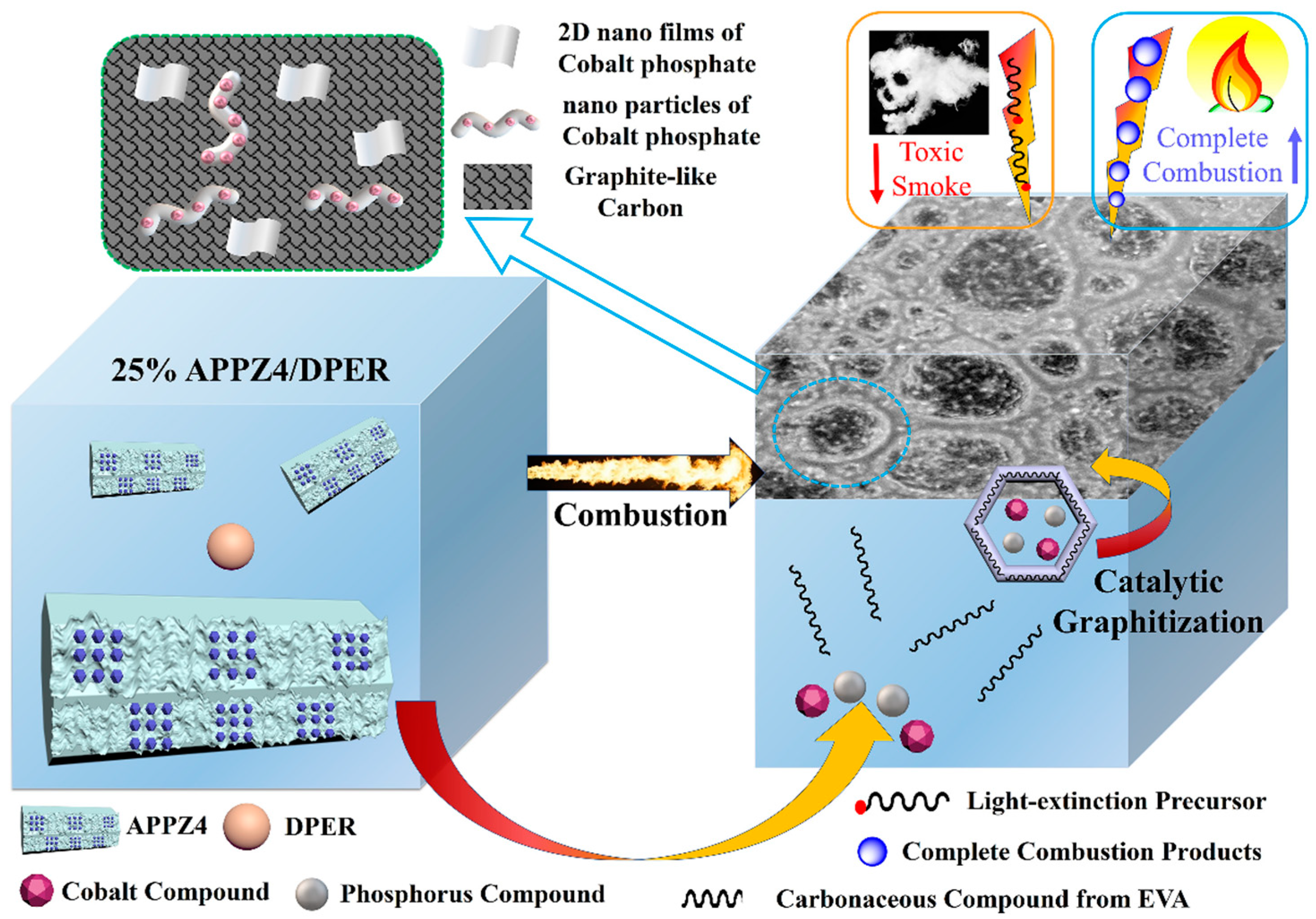
3.7. Flame-Retardant and Smoke Suppression Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Schneider, C.; Langer, R.; Loveday, D.; Hair, D. Applications of ethylene vinyl acetate copolymers (EVA) in drug delivery systems. J. Control. Release 2017, 262, 284–295. [Google Scholar] [CrossRef]
- Osman, A.F.; Fitri, T.F.M.; Rakibuddin, M.; Hashim, F.; Johari, S.A.T.T.; Ananthakrishnan, R.; Ramli, R. Pre-dispersed organo-montmorillonite (organo-MMT) nanofiller: Morphology, cytocompatibility and impact on flexibility, toughness and biostability of biomedical ethyl vinyl acetate (EVA) copolymer. Mat. Sci. Eng. C-Mater. 2017, 74, 194–206. [Google Scholar] [CrossRef] [PubMed]
- Mysiukiewicz, O.; Gospodarek, B.; Lawniczak, P.; Sterzyński, T. Influence of the conductive network creation on electrical, rheological, and mechanical properties of composites based on LDPE and EVA matrices. Adv. Polym. Technol. 2018, 37, 3542–3551. [Google Scholar] [CrossRef]
- Li, Y.M.; Deng, C.; Shi, X.H.; Xu, B.R.; Chen, H.; Wang, Y.Z. Simultaneously improved flame retardance and ceramifiable properties of polymer-based composites via the formed crystalline phase at high temperature. ACS Appl. Mater. Interfaces 2019, 11, 7459–7471. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.B.; Tang, Q.B.; Hong, N.N.; Song, L.; Wang, L.; Shi, Y.Q.; Hu, Y. Effect of cellulose acetate butyrate microencapsulated ammonium polyphosphate on the flame retardancy, mechanical, electrical, and thermal properties of intumescent flame-retardant ethylene–vinyl acetate copolymer/microencapsulated ammonium polyphosphate/polyamide-6 blends. ACS Appl. Mater. Interfaces 2011, 3, 3754–3761. [Google Scholar] [PubMed]
- Xu, B.; Ma, W.; Bi, X.L.; Shao, L.S.; Qian, L.J. synergistic effects of nano-zinc oxide on improving the flame retardancy of EVA composites with an efficient triazine-based charring agent. J. Polym. Environ. 2019, 27, 1127–1140. [Google Scholar] [CrossRef]
- Tan, Y.; Wachtendorf, V.; Klack, P.; Kukofka, T.; Ruder, J.; Schartel, B. Durability of the flame retardance of ethylene-vinyl acetate copolymer cables: Comparing different flame retardants exposed to different weathering conditions. J. Appl. Polym. Sci. 2020, 137, 47548. [Google Scholar] [CrossRef]
- Dong, L.P.; Deng, C.; Li, R.M.; Cao, Z.J.; Lin, L.; Chen, L.; Wang, Y.Z. Poly(piperazinyl phosphamide): A novel highly-efficient charring agent for an EVA/APP intumescent flame retardant system. RSC Adv. 2016, 6, 30436–30444. [Google Scholar] [CrossRef]
- Wang, W.; Wang, X.; Pan, Y.; Liew, K.M.; Mohamed, O.A.; Song, L.; Hu, Y. Synthesis of phosphorylated graphene oxide based multilayer coating: Self-assembly method and application for improving the fire safety of cotton fabrics. Ind. Eng. Chem. Res. 2017, 56, 6664–6670. [Google Scholar] [CrossRef]
- Wang, W.; Kan, Y.C.; Pan, H.F.; Pan, Y.; Li, B.G.; Liew, K.M.; Hu, Y. Phosphorylated cellulose applied for the exfoliation of LDH: An advanced reinforcement for polyvinyl alcohol. Compos. Part A-Appl. Sci. 2016, 94, 170–177. [Google Scholar] [CrossRef]
- Lou, F.P.; Wu, K.; Wang, Q.; Qian, Z.Y.; Li, S.J.; Guo, W.H. Improved flame-retardant and ceramifiable properties of EVA composites by combination of ammonium polyphosphate and aluminum hydroxide. Polymers 2019, 11, 125. [Google Scholar] [CrossRef] [PubMed]
- Ran, G.W.; Liu, X.D.; Guo, J.; Sun, J.; Li, H.F.; Gu, X.Y.; Zhang, S. Improving the flame retardancy and water resistance of polylactic acid by introducing polyborosiloxane microencapsulated ammonium polyphosphate. Compos. Part B-Eng. 2019, 173, 106772. [Google Scholar] [CrossRef]
- Jung, D.; Persi, I.; Bhattacharyya, D. Synergistic effects of feather fibers and phosphorus compound on chemically modified chicken feather/polypropylene composites. ACS Sustain. Chem. Eng. 2019, 7, 19072–19080. [Google Scholar] [CrossRef]
- Huang, J.G.; Liang, M.Y.; Feng, C.M.; Liu, H.B. Synergistic effects of 4A zeolite on the flame-retardant properties and thermal stability of an efficient halogen-free flame-retardant EVA composite. Polym. Eng. Sci. 2016, 56, 380–387. [Google Scholar] [CrossRef]
- Qin, Z.L.; Yang, R.J.; Zhang, W.C.; Li, D.H.; Jiao, Q.J. Synergistic barrier effect of aluminum phosphate on flame retardant polypropylene based on ammonium polyphosphate/dipentaerythritol system. Mater. Des. 2019, 181, 107913. [Google Scholar] [CrossRef]
- Liu, J.J.; Zhou, K.Q.; Tang, G.; Wang, B.B.; Gui, Z.; Yuen, R.K.K.; Hu, Y. Synthesis of Co3(HPO4)2(OH)2 nanosheets and its synergistic effect with intumescent flame retardants in ethylene-vinyl acetate copolymer. Polym. Compos. 2018, 39, 238–246. [Google Scholar] [CrossRef]
- Pi, L.J.; Li, L.; Liu, K.L.; Zhang, Q.F.; Li, H.Q.; Zhai, T.Y. Recent progress on 2D noble-transition-metal dichalcogenides. Adv. Funct. Mater. 2019, 29, 1904932. [Google Scholar] [CrossRef]
- Nie, S.; Hu, Y.; Song, L.; He, S.Q.; Yang, D.D. Study on a novel and efficient flame retardant synergist-nanoporous nickel phosphates VSB-1 with intumescent flame retardants in polypropylene. Polym. Adv. Technol. 2008, 19, 489–495. [Google Scholar] [CrossRef]
- Zhang, P.; Song, L.; Lu, H.D.; Hu, Y.; Xing, W.Y.; Ni, J.X.; Wang, J. Synergistic Effect of nanoflaky manganese phosphate on thermal degradation and flame retardant properties of intumescent flame retardant polypropylene system. Polym. Degrad. Stab. 2009, 94, 201–207. [Google Scholar] [CrossRef]
- Hou, Y.; Hu, W.; Gui, Z.; Hu, Y. A Novel Co(II)-based metal-organic framework with phosphorus-containing structure: Build for enhancing fire safety of epoxy. Compos. Sci. Technol. 2017, 152, 231–242. [Google Scholar] [CrossRef]
- Liao, Q.; He, M.; Zhou, Y.M.; Nie, S.X.; Wang, Y.J.; Hu, S.C.; Yang, H.Y.; Li, H.F.; Tong, Y. Highly cuboid-shaped heterobimetallic metal-organic frameworks derived from porous Co/ZnO/C microrods with improved electromagnetic wave absorption capabilities. ACS Appl. Mater. Interfaces 2018, 10, 29136–29144. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.H.; Yang, C.C.; Lin, C.H.; Jiang, H.L. Metal-organic-framework-derived hollow N-doped porous carbon with ultrahigh concentrations of single Zn atoms for efficient carbon dioxide conversion. Angew. Chem. Int. Ed. 2019, 58, 3511–3515. [Google Scholar] [CrossRef] [PubMed]
- He, Y.B.; Chang, Z.; Wu, S.C.; Qiao, Y.; Bai, S.Y.; Jiang, K.Z.; He, P.; Zhou, H.S. Simultaneously inhibiting lithium dendrites growth and polysulfides shuttle by a flexible MOF-based membrane in Li-S batteries. Adv. Energy Mater. 2018, 8, 1802130. [Google Scholar] [CrossRef]
- Yang, Z.H.; Lv, H.L.; Wu, R.B. Rational construction of graphene oxide with MOF-derived porous NiFe@C nanocubes for high-performance microwave attenuation. Nano Res. 2016, 9, 3671–3682. [Google Scholar] [CrossRef]
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; Van Duyne, P.V.; Hupp, J.T. Metal-organic framework materials as chemical sensors. Chem. Rev. 2012, 112, 1105–1125. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.M.; Kim, W.S.; Huh, S. Size-dependent catalysis by DABCO-functionalized Zn-MOF with one-dimensional channels. Dalton Trans. 2011, 40, 10826–10829. [Google Scholar] [CrossRef]
- Li, S.M.; Yang, K.; Ya, P.; Ma, K.R.; Zhang, Z.; Huang, Q. Three-dimensional porous Carbon/Co3O4 composites derived from Graphene/Co-MOF for high performance supercapacitor electrodes. Appl. Surf. Sci. 2020, 503, 144090. [Google Scholar] [CrossRef]
- Panella, B.; Hirscher, M.; Pütter, H.; Muller, U. Hydrogen adsorption in metal-organic frameworks: Cu-MOFs and Zn-MOFs compared. Adv. Funct. Mater. 2006, 16, 520–524. [Google Scholar] [CrossRef]
- Huang, L.N.; Chen, C.G.; Huang, X.Y.; Ruan, S.C.; Zeng, Y.J. Enhanced electromagnetic absorbing performance of MOF-derived Ni/NiO/Cu@C composites. Compos. Part B-Eng. 2019, 164, 583–589. [Google Scholar] [CrossRef]
- Hou, Y.B.; Hu, W.Z.; Gui, Z.; Hu, Y. Preparation of metal-organic frameworks and their application as flame retardants for polystyrene. Ind. Eng. Chem. Res. 2017, 56, 2036–2045. [Google Scholar] [CrossRef]
- Shi, X.W.; Dai, X.; Cao, Y.; Li, J.W.; Huo, C.G.; Wang, X.L. Degradable poly(lactic acid)/metal−organic framework nanocomposites exhibiting good mechanical, flame retardant, and dielectric properties for the fabrication of disposable electronics. Ind. Eng. Chem. Res. 2017, 56, 3887–3894. [Google Scholar] [CrossRef]
- Hou, Y.B.; Liu, L.X.; Qiu, S.L.; Zhou, X.; Gui, Z.; Hu, Y. DOPO-modified two-dimensional Co-based metal-organic framework: Preparation and application for enhancing fire safety of poly(lactic acid). ACS Appl. Mater. Interfaces 2018, 10, 8274–8286. [Google Scholar] [CrossRef] [PubMed]
- Li, A.J.; Xu, W.Z.; Chen, R.; Liu, Y.C.; Li, W. Fabrication of zeolitic imidazolate frameworks on layered double hydroxide nanosheets to improve the fire safety of epoxy resin. Compos. Part A-Appl. S. 2018, 112, 558–571. [Google Scholar] [CrossRef]
- Zhou, X.; Mu, X.W.; Cai, W.; Wang, J.L.; Chu, F.K.; Xu, Z.M.; Song, L.; Xing, W.Y.; Hu, Y. Design of hierarchical NiCo-LDH@PZS hollow dodecahedron architecture and application in high-performance epoxy resin with excellent fire safety. ACS Appl. Mater. Interfaces 2019, 11, 41736–41749. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.Y.; Qin, C.; Wang, X.L.; Yang, G.S.; Shao, K.Z.; Lan, Y.Q.; Su, Z.M.; Huang, P.; Wang, C.G.; Wang, E.B. Zeolitic imidazolate framework-8 as efficient PH-sensitive drug delivery vehicle. Dalton Trans. 2012, 41, 6906–6909. [Google Scholar] [CrossRef]
- Zheng, H.; Zhang, Y.; Liu, L.; Wan, W.; Guo, P.; Nyström, A.M.; Zou, X. One-pot synthesis of metal–organic frameworks with encapsulated target molecules and their applications for controlled drug delivery. J. Am. Chem. Soc. 2016, 138, 962–968. [Google Scholar] [CrossRef]
- Pan, Y.T.; Zhang, L.; Zhao, X.M.; Wang, D.Y. Interfacial engineering of renewable metal organic framework derived honeycomb-like nanoporous aluminum hydroxide with tunable porosity. Chem. Sci. 2017, 8, 3399–3409. [Google Scholar] [CrossRef]
- Wang, Y.L.; Li, Z.P.; Li, Y.Y.; Wang, J.Y.; Liu, X.; Song, T.Y.; Yang, X.M.; Hao, J.W. Spray-drying-assisted layer-by-layer assembly of alginate, 3-aminopropyltriethoxysilane, and magnesium hydroxide flame retardant and its catalytic graphitization in ethylene−vinyl acetate resin. ACS Appl Mater Inter. 2018, 10, 10490–10500. [Google Scholar] [CrossRef]






| Samples | XPS (ac.%) | ICP-MS (wt %) | ||
|---|---|---|---|---|
| P | N | Co | Co | |
| APP | 21.8 | 78.2 | 0.0 | -- |
| APPZ1 | 31.6 | 43.4 | 25.0 | 0.76 |
| APPZ2 | 31.2 | 47.6 | 21.2 | 1.15 |
| APPZ3 | 20.0 | 50.3 | 29.7 | 1.52 |
| APPZ4 | 13.8 | 52.6 | 33.6 | 1.82 |
| Samples | N2 Atmosphere | Air Atmosphere | ||||
|---|---|---|---|---|---|---|
| aR 800°C (%) | b ΔR (%) | c ΔR/Mco (%/%) | R 800°C (%) | ΔR (%) | ΔR/Mco (%/%) | |
| APP/DPER | 29.0 | -- | -- | 20.8 | -- | -- |
| APPZ1/DPER | 32.7 | 3.7 | 4.9 | 29.5 | 8.7 | 11.4 |
| APPZ2/DPER | 35.0 | 6.0 | 5.2 | 28.8 | 8.0 | 7.0 |
| APPZ3/DPER | 38.0 | 9.0 | 5.9 | 33.9 | 13.1 | 8.6 |
| APPZ4/DPER | 41.0 | 12.0 | 6.6 | 39.0 | 18.2 | 10.0 |
| ZIF-67 | 42.7 | -- | 35.0 | -- | ||
| Samples | LOI (%) | UL 94 Rating (3.2 mm) | t1/t2# (s) | Dripping |
|---|---|---|---|---|
| EVA | 19.0 | NR | -- * | Y |
| 25% APP/DPER | 26.2 | V-2 | 0.8/12.4 | Y |
| 28% APP/DPER | 28.2 | V-2 | 0.9/4.1 | Y |
| 30% APP/DPER | 29.3 | V-0 | 0.7/2.1 | N |
| 25% APPZ1/DPER | 28.4 | V-0 | 0.7/4.7 | N |
| 25% APPZ4/DPER | 29.4 | V-0 | 0.7/4.3 | N |
| Samples | PHRR (kw/m2) | THR (MJ/m2) | PSPR (10−3 m2/s) | TSP (m2) | av-EHC (MJ/kg) | PCOP (10−3 m2/s) |
|---|---|---|---|---|---|---|
| EVA | 2037 ± 35 | 145.9 ± 6.2 | 159 ± 7 | 18.5 ± 0.9 | 48.0 ± 1.3 | 17.6 ± 0.9 |
| 25% APP/DPER | 568 ± 22 | 90.5 ± 2.1 | 123 ± 4 | 20.2 ± 1.0 | 30.9 ± 0.5 | 14.2 ± 0.7 |
| 25% APPZ1/DPER | 464 ± 20 | 85.9 ± 2.2 | 96 ± 3 | 17.7 ± 0.6 | 31.5 ± 0.4 | 12.2 ± 0.6 |
| 25% APPZ4/DPER | 371 ± 18 | 80.2 ± 1.9 | 75 ± 3 | 15.6 ± 0.5 | 33.3 ± 0.5 | 8.7 ± 0.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Shi, H.; Zhu, P.; Wei, Y.; Hao, J. Ammonium Polyphosphate with High Specific Surface Area by Assembling Zeolite Imidazole Framework in EVA Resin: Significant Mechanical Properties, Migration Resistance, and Flame Retardancy. Polymers 2020, 12, 534. https://doi.org/10.3390/polym12030534
Wang J, Shi H, Zhu P, Wei Y, Hao J. Ammonium Polyphosphate with High Specific Surface Area by Assembling Zeolite Imidazole Framework in EVA Resin: Significant Mechanical Properties, Migration Resistance, and Flame Retardancy. Polymers. 2020; 12(3):534. https://doi.org/10.3390/polym12030534
Chicago/Turabian StyleWang, Jingyu, Hui Shi, Pinlie Zhu, Yuanjie Wei, and Jianwei Hao. 2020. "Ammonium Polyphosphate with High Specific Surface Area by Assembling Zeolite Imidazole Framework in EVA Resin: Significant Mechanical Properties, Migration Resistance, and Flame Retardancy" Polymers 12, no. 3: 534. https://doi.org/10.3390/polym12030534
APA StyleWang, J., Shi, H., Zhu, P., Wei, Y., & Hao, J. (2020). Ammonium Polyphosphate with High Specific Surface Area by Assembling Zeolite Imidazole Framework in EVA Resin: Significant Mechanical Properties, Migration Resistance, and Flame Retardancy. Polymers, 12(3), 534. https://doi.org/10.3390/polym12030534






