Destruction of Polyelectrolyte Microcapsules Formed on CaCO3 Microparticles and the Release of a Protein Included by the Adsorption Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Fluorescently Labeled PAH and BSA
2.3. Preparation of CaCO3 Microspherulites
2.4. Preparation of Polyelectrolyte Microcapsules
2.5. Inclusion of Protein in the Microcapsule by the Adsorption Method
2.6. Registration of the Dissociation of the Polyelectrolyte Microcapsules and the Yield of Protein from Polyelectrolyte Microcapsules
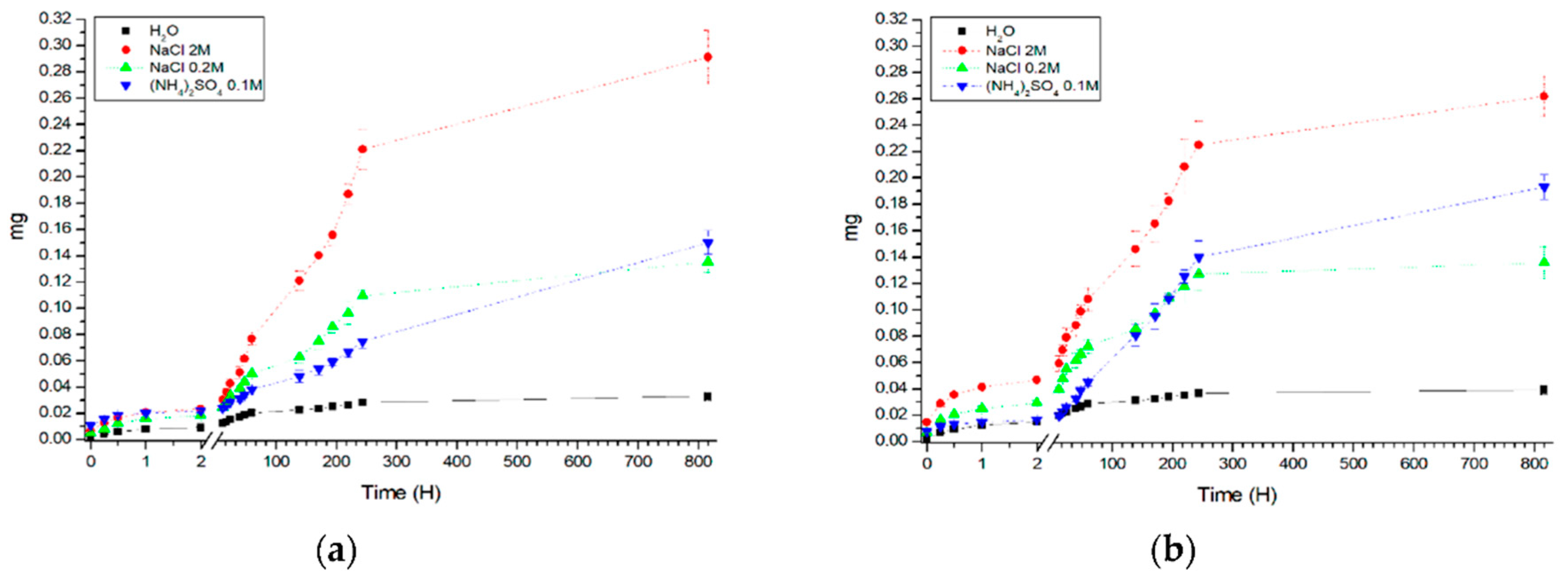
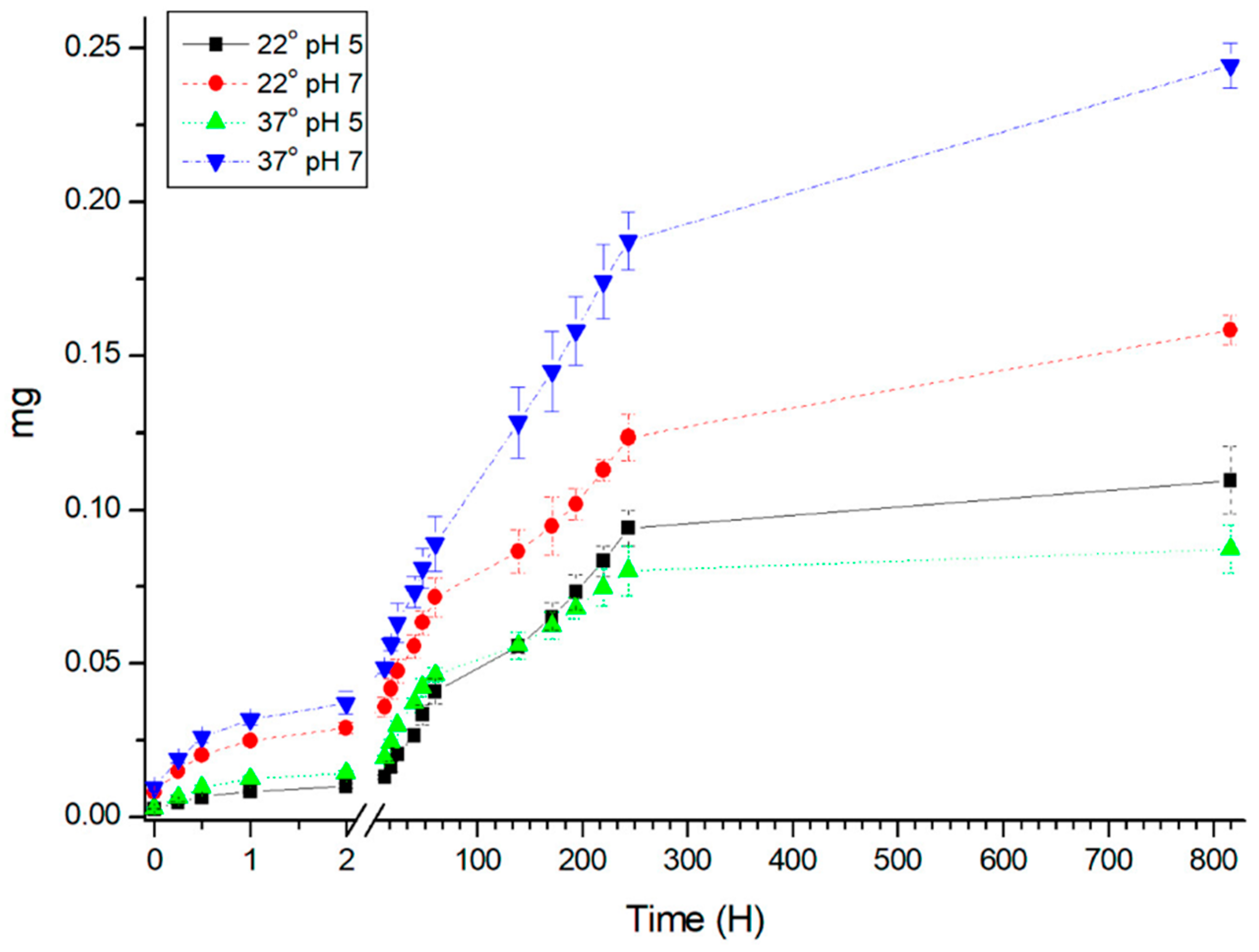
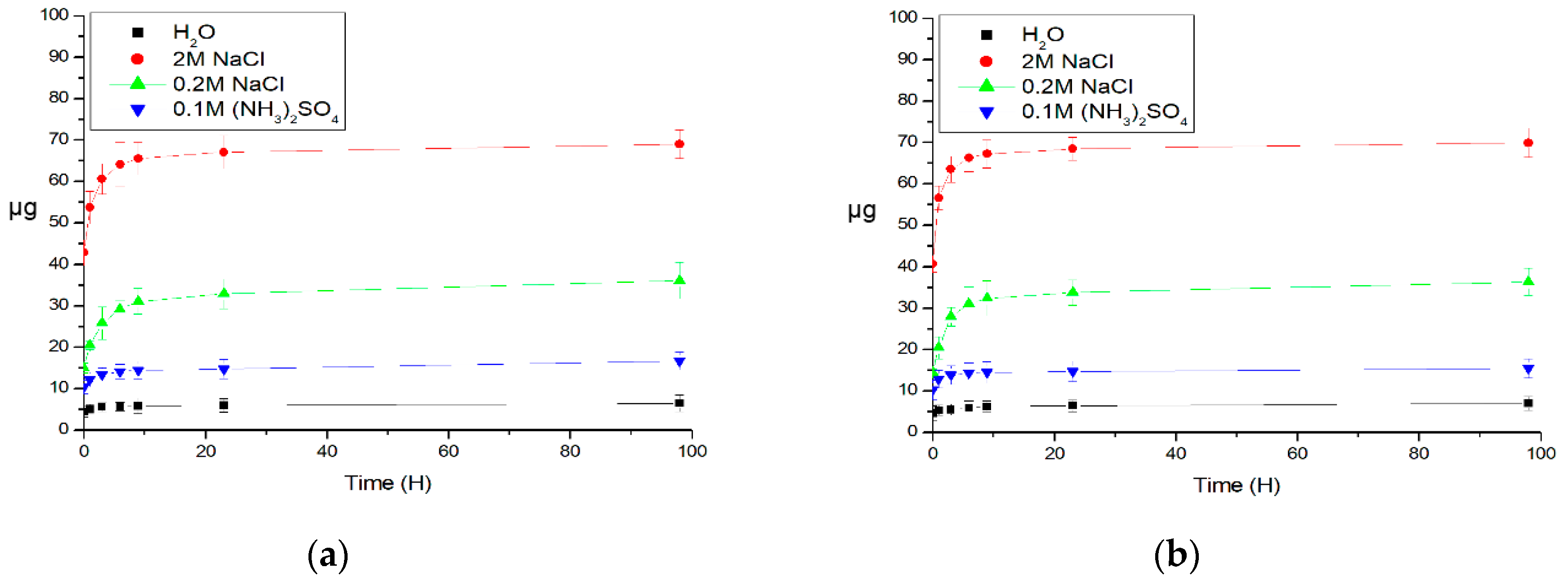
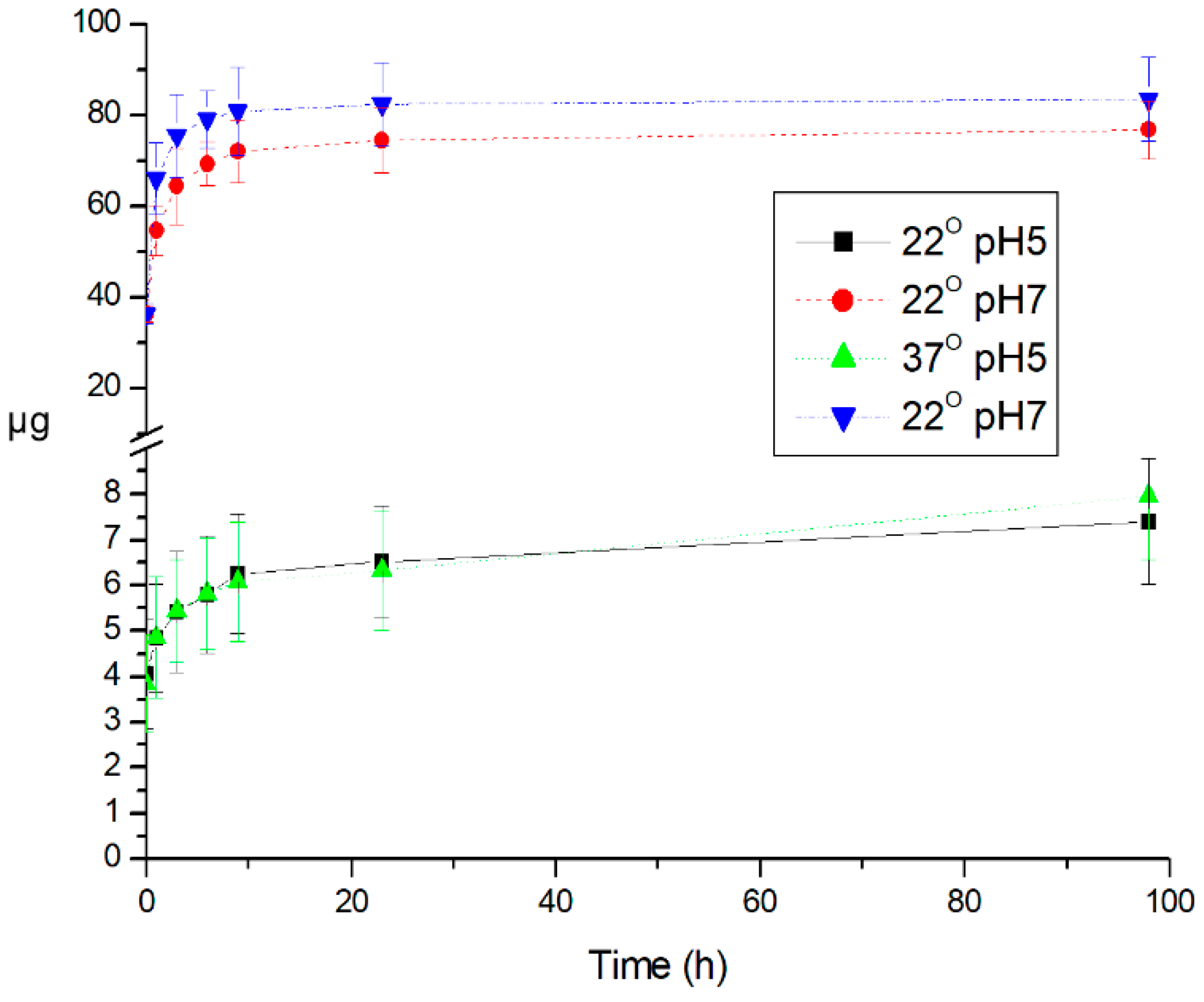
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Donath, E.; Sukhorukov, G.B.; Caruso, F.; Davis, S.A.; Möhwald, H. Novel Hollow Polymer Shells by Colloid-Templated Assembly of Polyelectrolytes. Angew. Chem. Int. Ed. 1998, 37, 2201–2205. [Google Scholar] [CrossRef]
- Decher, G.; Schlenoff, J.B. Multilayer Thin Films: Sequential Assembly of Nanocomposite Materials, 2nd ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012; ISBN 9783527316489. [Google Scholar]
- Sukhorukov, G.; Fery, A.; Möhwald, H. Intelligent micro- and nanocapsules. Prog. Polym. Sci. 2005, 30, 885–897. [Google Scholar] [CrossRef]
- Sukhorukov, G.B.; Antipov, A.A.; Voigt, A.; Donath, E.; Möhwald, H. pH-controlled macromolecule encapsulation in and release from polyelectrolyte multilayer nanocapsules. Macromol. Rapid Commun. 2001, 22, 44–46. [Google Scholar] [CrossRef]
- Kharlampieva, E.; Koziovskaya, V.; Sukhishvili, S.A. Layer-by-layer hydrogen-bonded polymer films: From fundamentals to applications. Adv. Mater. 2009, 21, 3053–3065. [Google Scholar] [CrossRef]
- Kazakova, L.I.; Shabarchina, L.I.; Anastasova, S.; Pavlov, A.M.; Vadgama, P.; Skirtach, A.G.; Sukhorukov, G.B. Chemosensors and biosensors based on polyelectrolyte microcapsules containing fluorescent dyes and enzymes. Anal. Bioanal. Chem. 2013, 405, 1559–1568. [Google Scholar] [CrossRef] [PubMed]
- Kazakova, L.I.; Shabarchina, L.I.; Sukhorukov, G.B. Co-encapsulation of enzyme and sensitive dye as a tool for fabrication of microcapsule based sensor for urea measuring. Phys. Chem. Chem. Phys. 2011, 13, 11110–11117. [Google Scholar] [CrossRef]
- Shchukin, D.G.; Ustinovich, E.; Sviridov, D.V.; Lvov, Y.M.; Sukhorukov, G.B. Photocatalytic microreactors based on TiO2-modified polyelectrolyte multilayer capsules. Photochem. Photobiol. Sci. 2003, 2, 975–977. [Google Scholar] [CrossRef]
- Shchukin, D.G.; Radtchenko, I.L.; Sukhorukov, G.B. Micron-scale hollow polyelectrolyte capsules with nanosized magnetic Fe3O4 inside. Mater. Lett. 2003, 57, 1743–1747. [Google Scholar] [CrossRef]
- Antipov, A.A.; Sukhorukov, G.B. Polyelectrolyte multilayer capsules as vehicles with tunable permeability. Adv. Colloid Interface Sci. 2004, 111, 49–61. [Google Scholar] [CrossRef]
- She, Z.; Wang, C.; Li, J.; Sukhorukov, G.B.; Antipina, M.N. Encapsulation of basic fibroblast growth factor by polyelectrolyte multilayer microcapsules and its controlled release for enhancing cell proliferation. Biomacromolecules 2012, 13, 2174–2180. [Google Scholar] [CrossRef]
- Pavlov, A.M.; Saez, V.; Cobley, A.; Graves, J.; Sukhorukov, G.B.; Mason, T.J. Controlled protein release from microcapsules with composite shells using high frequency ultrasound-Potential for in vivo medical use. Soft Matter 2011, 7, 4341–4347. [Google Scholar] [CrossRef]
- Reibetanz, U.; Claus, C.; Typlt, E.; Hofmann, J.; Donath, E. Defoliation and plasmid delivery with layer-by-layer coated colloids. Macromol. Biosci. 2006, 6, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Javiern, A.M.; Del Pino, P.; Bedard, M.F.; Ho, D.; Skirtach, A.G.; Sukhorukov, G.B.; Plank, C.; Parak, W.J. Photoactivated release of cargo from the cavity of polyelectrolyte capsules to the cytosol of cells. Langmuir 2008, 14, 12517–12520. [Google Scholar] [CrossRef] [PubMed]
- Kozlovskaya, V.; Liu, F.; Yang, Y.; Ingle, K.; Qian, S.; Halade, G.V.; Urban, V.S.; Kharlampieva, E. Temperature-Responsive Polymersomes of Poly(3-methyl-N-vinylcaprolactam)-block-poly(N-vinylpyrrolidone) To Decrease Doxorubicin-Induced Cardiotoxicity. Biomacromolecules 2019, 20, 3989–4000. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Leporatti, S.; Donath, E.; Möhwald, H. Studies on the drug release properties of polysaccharide multilayers encapsulated ibuprofen microparticles. Langmuir 2001, 17, 5375–5380. [Google Scholar] [CrossRef]
- Pargaonkar, N.; Lvov, Y.M.; Li, N.; Steenekamp, J.H.; De Villiers, M.M. Controlled release of dexamethasone from microcapsules produced by polyelectrolyte layer-by-layer nanoassembly. Pharm. Res. 2005, 22, 826–835. [Google Scholar] [CrossRef]
- Ye, S.; Wang, C.; Liu, X.; Tong, Z.; Ren, B.; Zeng, F. New loading process and release properties of insulin from polysaccharide microcapsules fabricated through layer-by-layer assembly. J. Control. Release 2006, 112, 79–87. [Google Scholar] [CrossRef]
- Kozlovskaya, V.; Chen, J.; Zavgorodnya, O.; Hasan, M.B.; Kharlampieva, E. Multilayer Hydrogel Capsules of Interpenetrated Network for Encapsulation of Small Molecules. Langmuir 2018, 34, 11832–11842. [Google Scholar] [CrossRef]
- Zheng, J.; Yue, X.; Dai, Z.; Wang, Y.; Liu, S.; Yan, X. Novel iron-polysaccharide multilayered microcapsules for controlled insulin release. Acta Biomater. 2009, 5, 1499–1507. [Google Scholar] [CrossRef]
- Ai, H.; Jones, S.A.; De Villiers, M.M.; Lvov, Y.M. Nano-encapsulation of furosemide microcrystals for controlled drug release. J. Control. Release 2003, 86, 59–68. [Google Scholar] [CrossRef]
- Hiller, S.; Leporatti, S.; Schnäckel, A.; Typlt, E.; Donath, E. Protamine assembled in multilayers on colloidal particles can be exchanged and released. Biomacromolecules 2004, 5, 1580–1587. [Google Scholar] [CrossRef]
- She, Z.; Antipina, M.N.; Li, J.; Sukhorukov, G.B. Mechanism of protein release from polyelectrolyte multilayer microcapsules. Biomacromolecules 2010, 11, 1241–1247. [Google Scholar] [CrossRef]
- Yang, M.; Shi, J.; Schlenoff, J.B. Control of Dynamics in Polyelectrolyte Complexes by Temperature and Salt. Macromolecules 2019, 52, 1930–1941. [Google Scholar] [CrossRef]
- Sill, A.; Nestler, P.; Azinfar, A.; Helm, C.A. Tailorable Polyanion Diffusion Coefficient in LbL Films: The Role of Polycation Molecular Weight and Polymer Conformation. Macromolecules 2019, 52, 9045–9052. [Google Scholar] [CrossRef]
- Kienle, D.F.; Schwartz, D.K. Complex Salt Dependence of Polymer Diffusion in Polyelectrolyte Multilayers. J. Phys. Chem. Lett. 2019, 10, 987–992. [Google Scholar] [CrossRef]
- Dubrovskii, A.V.; Kochetkova, O.Y.; Kim, A.L.; Musin, E.V.; Seraya, O.Y.; Tikhonenko, S.A. Destruction of shells and release of a protein from microcapsules consisting of non-biodegradable polyelectrolytes. Int. J. Polym. Mater. Polym. Biomater. 2019, 68, 160–164. [Google Scholar] [CrossRef]
- Kazakova, L.I.; Dubrovskiĭ, A.V.; Moshkov, D.A.; Shabarchina, L.I.; Sukhorukov, B.I. An electron microscopy study of the structure of polyelectrolyte microcapsules containing protein containing no protein. Biofizika 2007, 52, 850–854. [Google Scholar]
- Tikhonenko, S.A.; Saburova, E.A.; Durdenko, E.N.; Sukhorukov, B.I. Enzyme-polyelectrolyte complex: Salt effects on the reaction of urease with polyallylamine. Russ. J. Phys. Chem. A 2009, 83, 1781–1788. [Google Scholar] [CrossRef]
- Saburova, E.A.; Tikhonenko, S.A.; Dybovskaya, Y.N.; Sukhorukov, B.I. Changes in the activity and structure of urease in the interaction with polyelectrolytes. Russ. J. Phys. Chem. A 2008, 82, 468–474. [Google Scholar]
- Dubrovskii, A.V.; Shabarchina, L.I.; Kim, Y.A.; Sukhorukov, B.I. Influence of the temperature on polyelectrolyte microcapsules: Light scattering and confocal microscopy data. Russ. J. Phys. Chem. A 2006, 80, 1703–1707. [Google Scholar] [CrossRef]
- Goicoechea, J.; Arregui, F.J.; Corres, J.M.; Matias, I.R. Study and Optimization of Self-Assembled Polymeric Multilayer Structures with Neutral Red for pH Sensing Applications. J. Sens. 2008, 2008, 1–7. [Google Scholar] [CrossRef]
- Dickhaus, B.N.; Priefer, R. Determination of polyelectrolyte pKa values using surface-to-air tension measurements. Colloids Surfaces A Physicochem. Eng. Asp. 2016, 488, 15–19. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musin, E.V.; Kim, A.L.; Tikhonenko, S.A. Destruction of Polyelectrolyte Microcapsules Formed on CaCO3 Microparticles and the Release of a Protein Included by the Adsorption Method. Polymers 2020, 12, 520. https://doi.org/10.3390/polym12030520
Musin EV, Kim AL, Tikhonenko SA. Destruction of Polyelectrolyte Microcapsules Formed on CaCO3 Microparticles and the Release of a Protein Included by the Adsorption Method. Polymers. 2020; 12(3):520. https://doi.org/10.3390/polym12030520
Chicago/Turabian StyleMusin, Egor V., Aleksandr L. Kim, and Sergey A. Tikhonenko. 2020. "Destruction of Polyelectrolyte Microcapsules Formed on CaCO3 Microparticles and the Release of a Protein Included by the Adsorption Method" Polymers 12, no. 3: 520. https://doi.org/10.3390/polym12030520
APA StyleMusin, E. V., Kim, A. L., & Tikhonenko, S. A. (2020). Destruction of Polyelectrolyte Microcapsules Formed on CaCO3 Microparticles and the Release of a Protein Included by the Adsorption Method. Polymers, 12(3), 520. https://doi.org/10.3390/polym12030520




