Microscopic Techniques for the Analysis of Micro and Nanostructures of Biopolymers and Their Derivatives
Abstract
1. Introduction
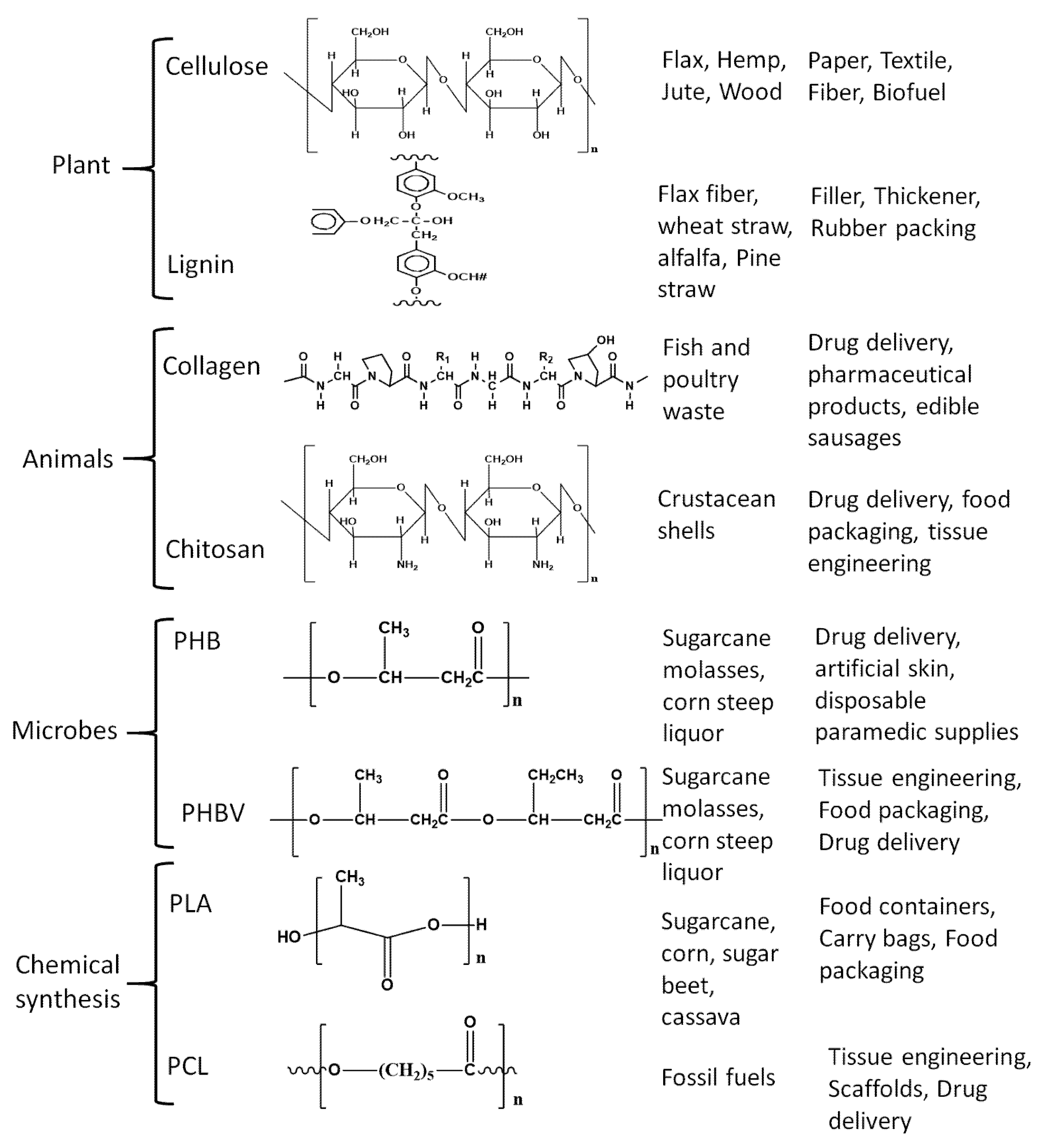
2. Biopolymers
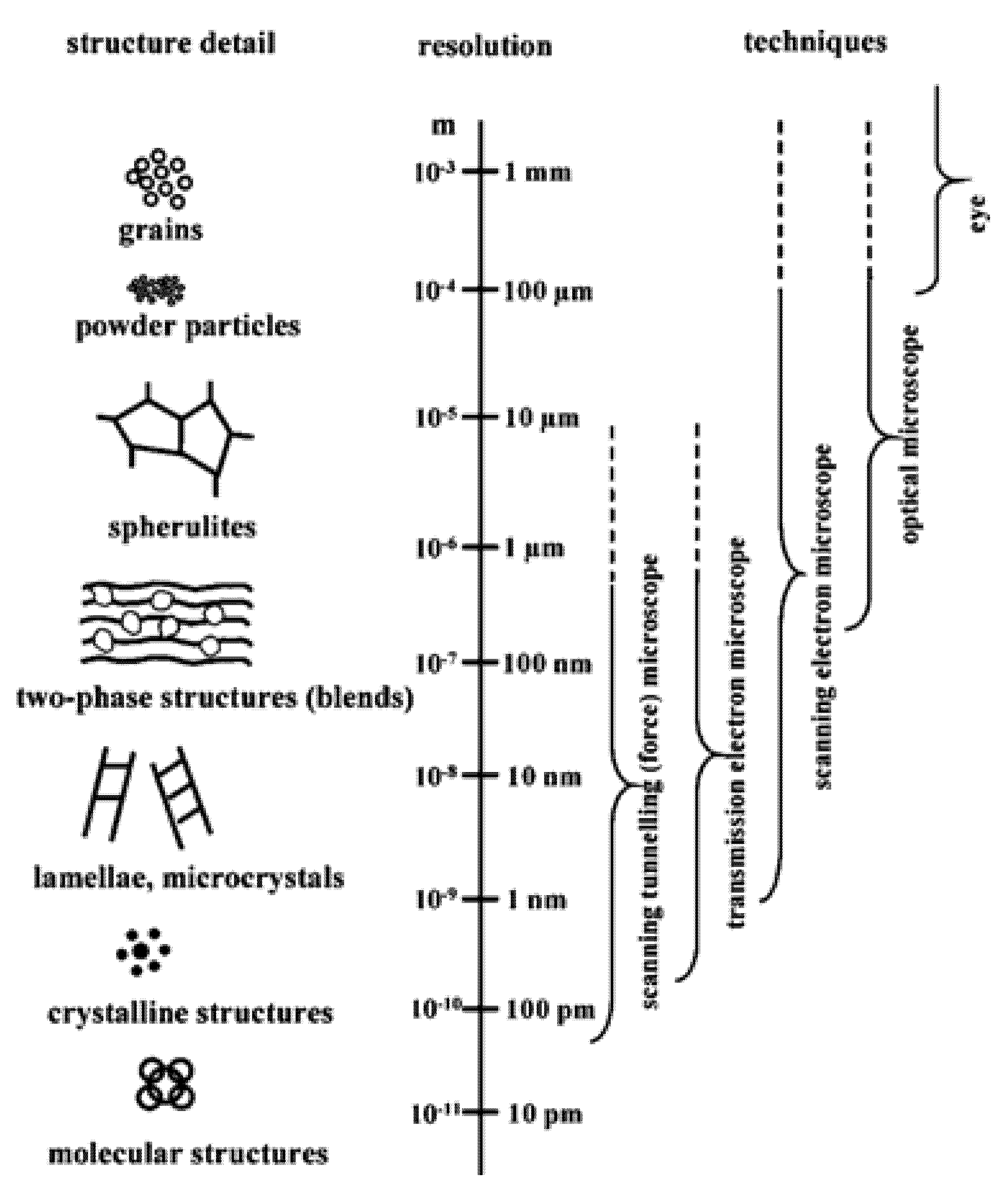
3. Microscopic Techniques
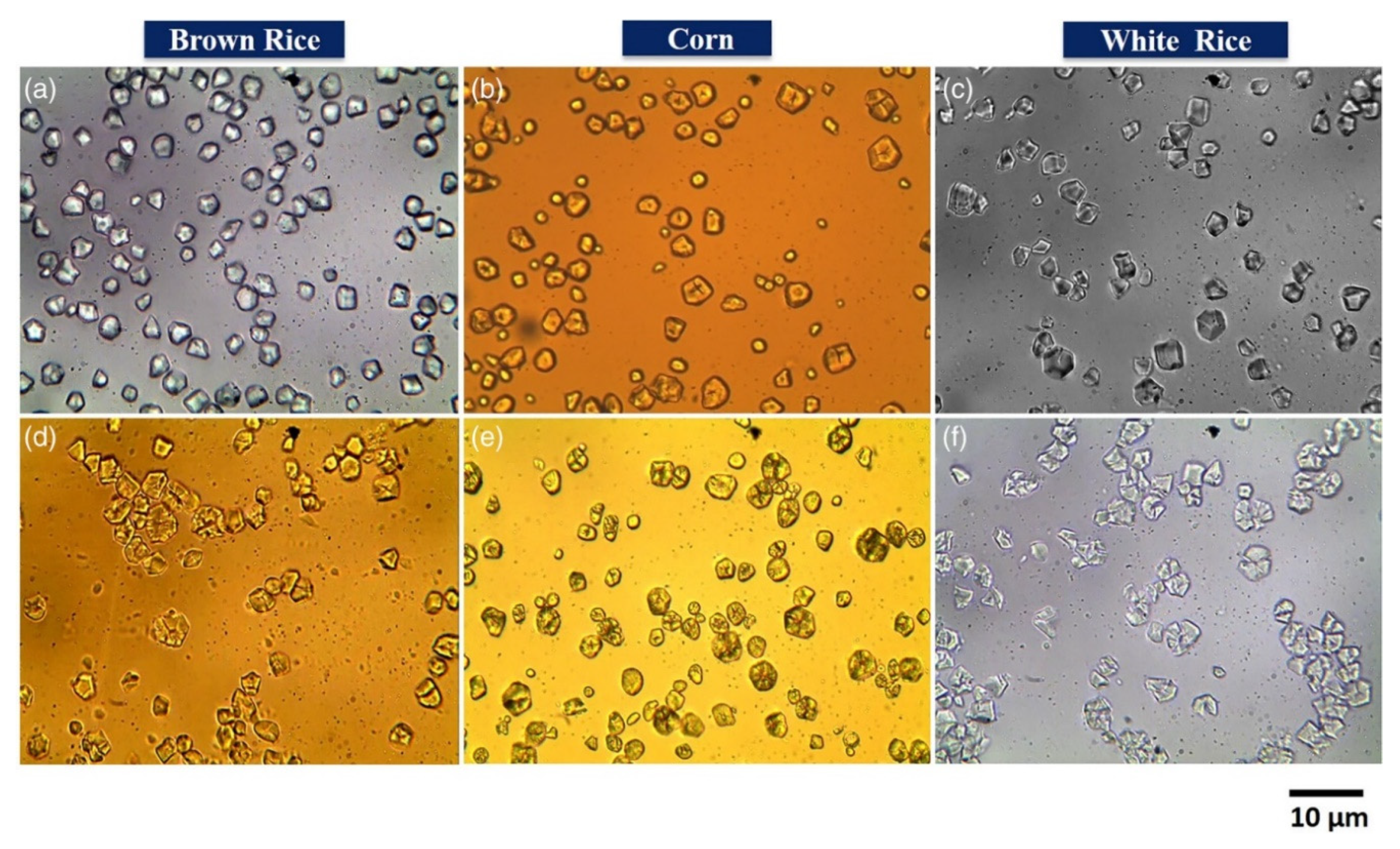
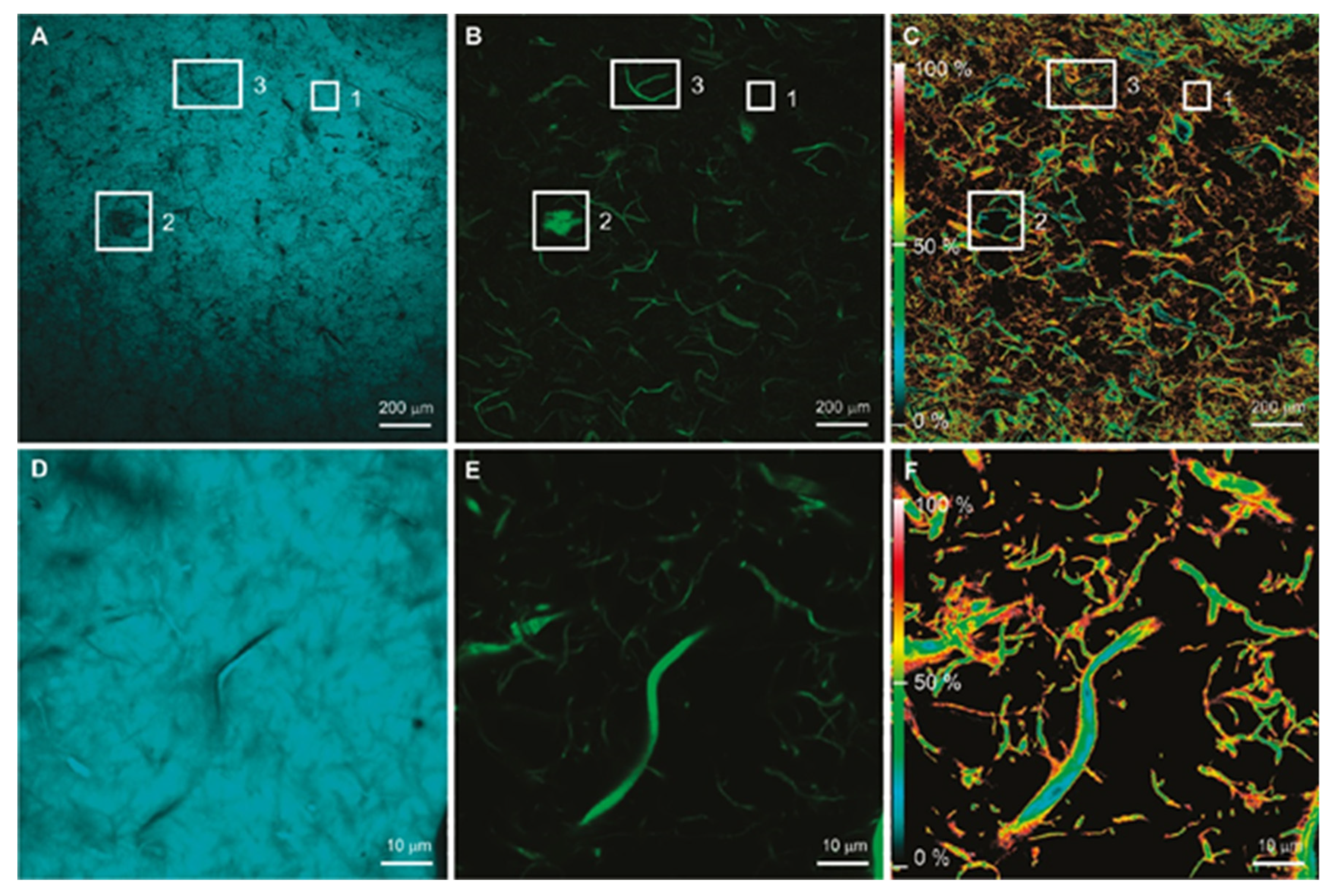
3.1. Optical Microscopy
3.2. Scanning Electron Microscopy
3.3. Transmission Electron Microscopy
3.4. Scanning Probe Microscopy
3.5. Scanning Tunneling Microscopy
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bassas-Galià, M. Rediscovering Biopolymers. In Consequences of Microbial Interactions with Hydrocarbons, Oils, and Lipids: Production of Fuels and Chemicals; Springer International Publishing: Cham, Switzerland, 2017; pp. 529–550. [Google Scholar]
- Hernández, N.; Williams, R.C.; Cochran, E.W. The battle for the “green” polymer. Different approaches for biopolymer synthesis: Bioadvantaged vs. bioreplacement. Org. Biomol. Chem. 2014, 12, 2834–2849. [Google Scholar] [CrossRef] [PubMed]
- Cacciotti, I.; Mori, S.; Cherubini, V.; Nanni, F. Eco-sustainable systems based on poly(lactic acid), diatomite and coffee grounds extract for food packaging. Int. J. Biol. Macromol. 2018, 112, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Garavand, F.; Rouhi, M.; Razavi, S.H.; Cacciotti, I.; Mohammadi, R. Improving the integrity of natural biopolymer films used in food packaging by crosslinking approach: A review. Int. J. Biol. Macromol. 2017, 104, 687–707. [Google Scholar] [CrossRef]
- Venkateshaiah, A.; Cheong, J.Y.; Habel, C.; Wacławek, S.; Lederer, T.; Černík, M.; Kim, I.-D.; Padil, V.V.T.; Agarwal, S. Tree Gum–Graphene Oxide Nanocomposite Films as Gas Barriers. ACS Appl. Nano Mater. 2020, 3, 633–640. [Google Scholar] [CrossRef]
- Babu, R.P.; O’Connor, K.; Seeram, R. Current progress on bio-based polymers and their future trends. Prog. Biomater. 2013, 2, 8. [Google Scholar] [CrossRef]
- He, M.; Lu, A.; Zhang, L. Advances in Cellulose Hydrophobicity Improvement; American Chemical Society: Washington, DC, USA, 2014. [Google Scholar]
- Sajid, M.A.; Shahzad, S.A.; Hussain, F.; Skene, W.G.; Khan, Z.A.; Yar, M. Synthetic modifications of chitin and chitosan as multipurpose biopolymers: A review. Synth. Commun. 2018, 48, 1893–1908. [Google Scholar] [CrossRef]
- Abraham, A.; Soloman, P.A.; Rejini, V.O. Preparation of Chitosan-Polyvinyl Alcohol Blends and Studies on Thermal and Mechanical Properties. Procedia Technol. 2016, 24, 741–748. [Google Scholar] [CrossRef]
- Raj, A.; Prashantha, K.; Samuel, C. Compatibility in biobased poly(L-lactide)/polyamide binary blends: From melt-state interfacial tensions to (thermo)mechanical properties. J. Appl. Polym. Sci. 2020, 137, 48440. [Google Scholar] [CrossRef]
- Resano-Goizueta, I.; Ashokan, B.K.; Trezza, T.A.; Padua, G.W. Effect of Nano-Fillers on Tensile Properties of Biopolymer Films. J. Polym. Environ. 2018, 26, 3817–3823. [Google Scholar] [CrossRef]
- Agusnar, H.; Wirjosentono, B.; Rihayat, T. Improving the quality of biopolymer (poly lactic acid) with the addition of bentonite as filler. IOP Conf. Ser. Mater. Sci. Eng. 2017, 222, 012008. [Google Scholar]
- Mekonnen, T.; Mussone, P.; Khalil, H.; Bressler, D. Progress in bio-based plastics and plasticizing modifications. J. Mater. Chem. A 2013, 1, 13379–13398. [Google Scholar] [CrossRef]
- Dufresne, A.; Thomas, S.; Pothen, L.A. Biopolymer Nanocomposites; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013. [Google Scholar]
- Okamoto, M.; John, B. Synthetic biopolymer nanocomposites for tissue engineering scaffolds. Prog. Polym. Sci. 2013, 38, 1487–1503. [Google Scholar] [CrossRef]
- Jacob, J.; Haponiuk, J.T.; Thomas, S.; Gopi, S. Biopolymer based nanomaterials in drug delivery systems: A review. Mater. Today Chem. 2018, 9, 43–55. [Google Scholar] [CrossRef]
- Joye, I.J.; McClements, D.J. Biopolymer-based nanoparticles and microparticles: Fabrication, characterization, and application. Curr. Opin. Colloid Interface Sci. 2014, 19, 417–427. [Google Scholar] [CrossRef]
- Gupta, B.; Tummalapalli, M.; Deopura, B.L.; Alam, M.S. Preparation and characterization of in-situ crosslinked pectin–gelatin hydrogels. Carbohydr. Polym. 2014, 106, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Padil, V.V.T.; Senan, C.; Wacſawek, S.; Ŀerník, M. Electrospun fibers based on Arabic, karaya and kondagogu gums. Int. J. Biol. Macromol. 2016, 91, 299–309. [Google Scholar] [CrossRef]
- Silvestri, D.; Mikšíček, J.; Wacławek, S.; Torres-Mendieta, R.; Padil, V.V.; Černík, M. Production of electrospun nanofibers based on graphene oxide/gum Arabic. Int. J. Biol. Macromol. 2019, 124, 396–402. [Google Scholar] [CrossRef]
- Vinod, V.T.P.; Saravanan, P.; Sreedhar, B.; Devi, D.K.; Sashidhar, R.B. A facile synthesis and characterization of Ag, Au and Pt nanoparticles using a natural hydrocolloid gum kondagogu (Cochlospermum gossypium). Colloids Surf. B Biointerfaces 2011, 83, 291–298. [Google Scholar] [CrossRef]
- Thekkae Padil, V.V.; Černík, M. Green synthesis of copper oxide nanoparticles using gum karaya as a biotemplate and their antibacterial application. Int. J. Nanomed. 2013, 8, 889–898. [Google Scholar]
- Silvestri, D.; Wacławek, S.; Sobel, B.; Torres-Mendieta, R.; Novotný, V.; Nguyen, N.H.; Ševců, A.; Padil, V.V.; Müllerová, J.; Stuchlík, M.; et al. A poly(3-hydroxybutyrate)–chitosan polymer conjugate for the synthesis of safer gold nanoparticles and their applications. Green Chem. 2018, 20, 4975–4982. [Google Scholar] [CrossRef]
- Venkateshaiah, A.; Silvestri, D.; Ramakrishnan, R.K.; Wacławek, S.; Padil, V.V.; Černík, M.; Varma, R.S. Gum kondagoagu/reduced graphene oxide framed platinum nanoparticles and their catalytic role. Molecules 2019, 24, 3643. [Google Scholar] [CrossRef] [PubMed]
- Padil, V.V.T.; Wacławek, S.; Černík, M.; Varma, R.S. Tree gum-based renewable materials: Sustainable applications in nanotechnology, biomedical and environmental fields. Biotechnol. Adv. 2018, 36, 1984–2016. [Google Scholar] [CrossRef] [PubMed]
- Mülhaupt, R. Green Polymer Chemistry and Bio-based Plastics: Dreams and Reality. Macromol. Chem. Phys. 2013, 214, 159–174. [Google Scholar] [CrossRef]
- Hu, B. Biopolymer-Based Lightweight Materials for Packaging Applications. ACS Symposium Series, Am. Chem. Soc. 2014, 1175, 239–255. [Google Scholar]
- Yadav, P.; Yadav, H.; Shah, V.G.; Shah, G.; Dhaka, G. Biomedical biopolymers, their origin and evolution in biomedical sciences: A systematic review. J. Clin. Diagn. Res. 2015, 9, 21–25. [Google Scholar] [CrossRef]
- Cao, Y.; Wu, J.; Zhang, J.; Li, H.; Zhang, Y.; He, J. Room temperature ionic liquids (RTILs): A new and versatile platform for cellulose processing and derivatization. Chem. Eng. J. 2009, 147, 13–21. [Google Scholar] [CrossRef]
- Rajinipriya, M.; Nagalakshmaiah, M.; Astruc, J.; Robert, M.; Elkoun, S. Single stage purification of flax, hemp, and milkweed stem and their physical and morphological properties. Int. J. Polym. Anal. Charact. 2018, 23, 78–88. [Google Scholar] [CrossRef]
- Heinze, T.; El Seoud, O.A.; Koschella, A. Production and Characteristics of Cellulose from Different Sources; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Gellerstedt, G. Softwood kraft lignin: Raw material for the future. Ind. Crops Prod. 2015, 77, 845–854. [Google Scholar] [CrossRef]
- Iravani, S.; Varma, R.S. Greener synthesis of lignin nanoparticles and their applications. Green Chem. 2020, 22, 612–636. [Google Scholar]
- Agrawal, A.; Kaushik, N.; Biswas, S. Derivatives and Applications of Lignin: An Insight. Scitech J. 2014, 1, 30–36. [Google Scholar]
- Bertoft, E. Understanding Starch Structure: Recent Progress. Agronomy 2017, 7, 56. [Google Scholar] [CrossRef]
- Ogunsona, E.; Ojogbo, E.; Mekonnen, T. Advanced material applications of starch and its derivatives. Eur. Polym. J. 2018, 108, 570–581. [Google Scholar] [CrossRef]
- Stamov, D.R.; Pompe, T. Structure and function of ECM-inspired composite collagen type i scaffolds. Soft Matter 2012, 8, 10200–10212. [Google Scholar] [CrossRef]
- Sionkowska, A.; Skrzyński, S.; Śmiechowski, K.; Kołodziejczak, A. The review of versatile application of collagen. Polym. Adv. Technol. 2017, 28, 4–9. [Google Scholar] [CrossRef]
- Silvestri, D.; Wacławek, S.; KRamakrishnan, R.; Venkateshaiah, A.; Krawczyk, K.; Padil, V.V.; Sobel, B.; Černík, M. The Use of a Biopolymer Conjugate for an Eco-Friendly One-Pot Synthesis of Palladium-Platinum Alloys. Polymers Basel 2019, 11, 1948. [Google Scholar] [CrossRef]
- Crini, G. Historical review on chitin and chitosan biopolymers. Environ. Chem. Lett. 2019, 17, 1623–1643. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Lichtfouse, E.; Torri, G.; Crini, G. Fundamentals and Applications of Chitosan. In Sustainable Agriculture Reviews; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Zhao, D.; Yu, S.; Sun, B.; Gao, S.; Guo, S.; Zhao, K. Biomedical applications of chitosan and its derivative nanoparticles. Polymers Basel 2018, 10, 462. [Google Scholar] [CrossRef]
- Shariatinia, Z. Pharmaceutical applications of chitosan. Adv. Colloid Interface Sci. 2019, 263, 131–194. [Google Scholar] [CrossRef]
- Vahedikia, N.; Garavand, F.; Tajeddin, B.; Cacciotti, I.; Jafari, S.M.; Omidi, T.; Zahedi, Z. Biodegradable zein film composites reinforced with chitosan nanoparticles and cinnamon essential oil: Physical, mechanical, structural and antimicrobial attributes. Colloids Surf. B Biointerfaces 2019, 177, 25–32. [Google Scholar] [CrossRef]
- Cacciotti, I.; Lombardelli, C.; Benucci, I.; Esti, M. Clay/chitosan biocomposite systems as novel green carriers for covalent immobilization of food enzymes. J. Mater. Res. Technol. 2019, 8, 3644–3652. [Google Scholar] [CrossRef]
- Li, Z.; Yang, J.; Loh, X.J. Polyhydroxyalkanoates: Opening doors for a sustainable future. NPG Asia Mater. 2016, 8, e265. [Google Scholar] [CrossRef]
- Winnacker, M. Polyhydroxyalkanoates: Recent Advances in Their Synthesis and Applications. Eur. J. Lipid Sci. Technol. 2019, 121, 1900101. [Google Scholar] [CrossRef]
- Możejko-Ciesielska, J.; Kiewisz, R. Bacterial polyhydroxyalkanoates: Still fabulous? Microbiol. Res. 2016, 192, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Balaji, S.; Gopi, K.; Muthuvelan, B. A review on production of poly β hydroxybutyrates from cyanobacteria for the production of bio plastics. Algal Res. 2013, 2, 278–285. [Google Scholar] [CrossRef]
- Ray, S.; Kalia, V.C. Biomedical Applications of Polyhydroxyalkanoates. Indian J. Microbiol. 2017, 57, 261–269. [Google Scholar] [CrossRef]
- Yeo, J.C.C.; Muiruri, J.K.; Thitsartarn, W.; Li, Z.; He, C. Recent advances in the development of biodegradable PHB-based toughening materials: Approaches, advantages and applications. Mater. Sci. Eng. C 2018, 92, 1092–1116. [Google Scholar] [CrossRef]
- Bianco, A.; Calderone, M.; Cacciotti, I. Electrospun PHBV/PEO co-solution blends: Microstructure, thermal and mechanical properties. Mater. Sci. Eng. C 2013, 33, 1067–1077. [Google Scholar] [CrossRef]
- Cacciotti, I.; Calderone, M.; Bianco, A. Tailoring the properties of electrospun PHBV mats: Co-solution blending and selective removal of PEO. Eur. Polym. J. 2013, 49, 3210–3222. [Google Scholar] [CrossRef]
- Mehta, R.; Kumar, V.; Bhunia, H.; Upadhyay, S.N. Synthesis of Poly(Lactic Acid): A Review. J. Macromol. Sci. Part C Polym. Rev. 2005, 45, 325–349. [Google Scholar] [CrossRef]
- Singhvi, M.S.; Zinjarde, S.S.; Gokhale, D.V. Polylactic acid: Synthesis and biomedical applications. J. Appl. Microbiol. 2019, 127, 1612–1626. [Google Scholar] [CrossRef]
- Hu, Y.; Daoud, W.; Cheuk, K.; Lin, C. Newly Developed Techniques on Polycondensation, Ring-Opening Polymerization and Polymer Modification: Focus on Poly(Lactic Acid). Materials Basel 2016, 9, 133. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Geever, L.M.; Killion, J.A.; Lyons, J.G.; Higginbotham, C.L.; Devine, D.M. Review of Multifarious Applications of Poly (Lactic Acid). Polym. Plast. Technol. Eng. 2016, 55, 1057–1075. [Google Scholar] [CrossRef]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef] [PubMed]
- Sisson, A.L.; Ekinci, D.; Lendlein, A. The contemporary role of ε-caprolactone chemistry to create advanced polymer architectures. Polymer 2013, 54, 4333–4350. [Google Scholar] [CrossRef]
- Fuoco, T.; Finne-Wistrand, A. Enhancing the Properties of Poly(ε-caprolactone) by Simple and Effective Random Copolymerization of ε-Caprolactone with p -Dioxanone. Biomacromolecules 2019, 20, 3171–3180. [Google Scholar] [CrossRef]
- Malikmammadov, E.; Tanir, T.E.; Kiziltay, A.; Hasirci, V.; Hasirci, N. PCL and PCL-based materials in biomedical applications. J. Biomater. Sci. Polym. Ed. 2018, 29, 863–893. [Google Scholar] [CrossRef]
- Espinoza, S.M.; Patil, H.I.; San Martin Martinez, E.; Casañas Pimentel, R.; Ige, P.P. Poly-ε-caprolactone (PCL), a promising polymer for pharmaceutical and biomedical applications: Focus on nanomedicine in cancer. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 85–126. [Google Scholar] [CrossRef]
- Bianco, A.; Di Federico, E.; Cacciotti, I. Electrospun poly(ε-caprolactone)-based composites using synthesized β-tricalcium phosphate. Polym. Adv. Technol. 2011, 22, 1832–1841. [Google Scholar] [CrossRef]
- Michler, G.H. Overview of Techniques; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Malheiro, V.N.; Caridade, S.G.; Alves, N.M.; Mano, J.F. New poly(ε-caprolactone)/chitosan blend fibers for tissue engineering applications. Acta Biomater. 2010, 6, 418–428. [Google Scholar] [CrossRef]
- Khalid, S.; Yu, L.; Meng, L.; Liu, H.; Ali, A.; Chen, L. Poly(lactic acid)/starch composites: Effect of microstructure and morphology of starch granules on performance. J. Appl. Polym. Sci. 2017, 134, 45504. [Google Scholar] [CrossRef]
- Govindaraju, I.; Pallen, S.; Umashankar, S.; Mal, S.S.; Kaniyala Melanthota, S.; Mahato, D.R.; Zhuo, G.Y.; Mahato, K.K.; Mazumder, N. Microscopic and spectroscopic characterization of rice and corn starch. Microsc. Res. Tech. 2020, 1–9. [Google Scholar] [CrossRef]
- Vigneshwaran, N.; Ammayappan, L.; Huang, Q. Effect of Gum arabic on distribution behavior of nanocellulose fillers in starch film. Appl. Nanosci. 2011, 1, 137–142. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Y.; Xi, X.; Shrestha, S.; Jiang, P.; Zhang, W.; Gao, C. Amino acid-modified chitosan nanoparticles for Cu2+ chelation to suppress CuO nanoparticle cytotoxicity. J. Mater. Chem. B 2017, 5, 3521–3530. [Google Scholar] [CrossRef]
- Majoinen, J.; Kontturi, E.; Ikkala, O.; Gray, D.G. SEM imaging of chiral nematic films cast from cellulose nanocrystal suspensions. Cellulose 2012, 19, 1599–1605. [Google Scholar] [CrossRef]
- Rizzieri, R.; Baker, F.S.; Donald, A.M. A study of the large strain deformation and failure behaviour of mixed biopolymer gels via in situ ESEM. Polymer 2003, 44, 5927–5935. [Google Scholar] [CrossRef]
- Karhale, S.; Bhenki, C.; Rashinkar, G.; Helavi, V. Covalently anchored sulfamic acid on cellulose as heterogeneous solid acid catalyst for the synthesis of structurally symmetrical and unsymmetrical 1,4-dihydropyridine derivatives. New J. Chem. 2017, 41, 5133–5141. [Google Scholar] [CrossRef]
- Shankar, S.; Oun, A.A.; Rhim, J.W. Preparation of antimicrobial hybrid nano-materials using regenerated cellulose and metallic nanoparticles. Int. J. Biol. Macromol. 2018, 107, 17–27. [Google Scholar] [CrossRef]
- Gopiraman, M.; Deng, D.; Saravanamoorthy, S.; Chung, I.M.; Kim, I.S. Gold, silver and nickel nanoparticle anchored cellulose nanofiber composites as highly active catalysts for the rapid and selective reduction of nitrophenols in water. RSC Adv. 2018, 8, 3014–3023. [Google Scholar] [CrossRef]
- Pakravan, M.; Heuzey, M.-C.; Ajji, A. Core–Shell Structured PEO-Chitosan Nanofibers by Coaxial Electrospinning. Biomacromolecules 2012, 13, 412–421. [Google Scholar] [CrossRef]
- Camesano, T.A.; Wilkinson, K.J. Single Molecule Study of Xanthan Conformation Using Atomic Force Microscopy. Biomacromolecules 2001, 2, 1184–1191. [Google Scholar]
- Teckentrup, J.; Al-Hammood, O.; Steffens, T.; Bednarz, H.; Walhorn, V.; Niehaus, K.; Anselmetti, D. Comparative analysis of different xanthan samples by atomic force microscopy. J. Biotechnol. 2017, 257, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Moffat, J.; Morris, V.J.; Al-Assaf, S.; Gunning, A.P. Visualisation of xanthan conformation by atomic force microscopy. Carbohydr. Polym. 2016, 148, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Lahiji, R.R.; Xu, X.; Reifenberger, R.; Raman, A.; Rudie, A.; Moon, R.J. Atomic Force Microscopy Characterization of Cellulose Nanocrystals. Langmuir 2010, 26, 4480–4488. [Google Scholar] [CrossRef] [PubMed]
- Robert, R.W.; Raman, J.M.A. Mechanical properties of cellulose nanomaterials studied by contact resonance atomic force microscopy. Cellulose 2016, 23. [Google Scholar]
- Nagalakshmaiah, M.; kissi NEl Mortha, G.; Dufresne, A. Structural investigation of cellulose nanocrystals extracted from chili leftover and their reinforcement in cariflex-IR rubber latex. Carbohydr. Polym. 2016, 136, 945–954. [Google Scholar] [CrossRef] [PubMed]
- Haugstad, K.E.; Håti, A.G.; Nordgård, C.T.; Adl, P.S.; Maurstad, G.; Sletmoen, M.; Draget, K.I.; Dias, R.S.; Stokke, B.T. Direct Determination of Chitosan–Mucin Interactions Using a Single-Molecule Strategy: Comparison to Alginate–Mucin Interactions. Polymers Basel 2015, 7, 161–185. [Google Scholar] [CrossRef]
- Jaiswal, M.K.; Banerjee, R.; Pradhan, P.; Bahadur, D. Thermal behavior of magnetically modalized poly(N-isopropylacrylamide)-chitosan based nanohydrogel. Colloids Surf. B Biointerfaces 2010, 81, 185–194. [Google Scholar] [CrossRef]
- Gunning, A.P.; McMaster, T.J.; Morris, V.J. Scanning tunnelling microscopy of xanthan gum. Carbohydr. Polym. 1993, 21, 47–51. [Google Scholar] [CrossRef]
- Nakajima, K.; Ikehara, T.; Nishi, T. Observation of gellan gum by scanning tunneling microscopy. Carbohydr. Polym. 1996, 30, 77–81. [Google Scholar] [CrossRef]
- Zhang, Y.Z. Size and arrangement of elementary fibrils in crystalline cellulose studied with scanning tunneling microscopy. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. 1997, 15, 1502. [Google Scholar] [CrossRef]
- Abdullah, O.G.; Aziz, B.K.; Aziz, S.B.; Suhail, M.H. Surfaces modification of methylcellulose: Cobalt nitrate polymer electrolyte by sulfurated hydrogen gas treatment. J. Appl. Polym. Sci. 2018, 135, 46676. [Google Scholar] [CrossRef]
- Weisenburger, S.; Sandoghdar, V. Light microscopy: An ongoing contemporary revolution. Contemp. Phys. 2015, 56, 123–143. [Google Scholar] [CrossRef]
- Chen, N.; Rehman, S.; Sheppard, C.J.R. Recent advances in optical microscopy methods for subcellular imaging of thick biological tissues. Crit. Rev. Biomed. Eng. 2013, 41, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Agocs, E.; Attota, R.K. Enhancing optical microscopy illumination to enable quantitative imaging. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Coceancigh, H.; Higgins, D.A.; Ito, T. Optical Microscopic Techniques for Synthetic Polymer Characterization. Anal. Chem. 2019, 91, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wang, X.; Liu, H.; Wei, L.; Xiao, L. Recent advances in optical microscopic methods for single-particle tracking in biological samples. Anal. Bioanal. Chem. 2019, 411, 4445–4463. [Google Scholar] [CrossRef]
- Murphy, D.B.; Davidson, M.W. Fundamentals of Light Microscopy and Electronic Imaging, 2nd ed.; John Wiley and Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Vielreicher, M.; Schürmann, S.; Detsch, R.; Schmidt, M.A.; Buttgereit, A.; Boccaccini, A.; Friedrich, O. Taking a deep look: Modern microscopy technologies to optimize the design and functionality of biocompatible scaffolds for tissue engineering in regenerative medicine. J. R. Soc. Interface 2013, 10, 20130263. [Google Scholar] [CrossRef][Green Version]
- Hubbe, M.A.; Chandra, R.P.; Dogu, D.; Van Velzen, S.T.J. Analytical Staining of Cellulosic Materials: A Review. BioRes. 2019, 14, 7387–7464. [Google Scholar]
- Daemen, S.; van Zandvoort, M.A.M.J.; Parekh, S.H.; Hesselink, M.K.C. Microscopy tools for the investigation of intracellular lipid storage and dynamics. Mol. Metab. 2016, 5, 153–163. [Google Scholar] [CrossRef]
- Westphal, V.; Hell, S.W. Nanoscale resolution in the focal plane of an optical microscope. Phys. Rev. Lett. 2005, 94, 143903. [Google Scholar] [CrossRef]
- Hayes, B.S.; Gammon, L.M. Optical Microscopy of Fiber Reinforced Composites; ASM International: Materials Park, OH, USA, 2010. [Google Scholar]
- Vanderghem, C.; Jacquet, N.; Danthine, S.; Blecker, C.; Paquot, M. Effect of Physicochemical Characteristics of Cellulosic Substrates on Enzymatic Hydrolysis by Means of a Multi-Stage Process for Cellobiose Production. Appl. Biochem. Biotechnol. 2012, 166, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, T.J.; Pérez, E.; Guzmán, R.; Tapia, M.S.; Famá, L. Physicochemical and Functional Properties of Native and Modified by Crosslinking, Dark-Cush-Cush Yam (Dioscorea Trifida) and Cassava (Manihot Esculenta) Starch. J. Polym. Biopolym. Phys. Chem. 2014, 2, 1–5. [Google Scholar]
- Gao, C.; Bao, X.; Yu, L.; Liu, H.; Simon, G.P.; Chen, L.; Liu, X. Thermal properties and miscibility of semi-crystalline and amorphous PLA blends. J. Appl. Polym. Sci. 2014, 131, 41205. [Google Scholar] [CrossRef]
- Gao, M.; Ren, Z.; Yan, S.; Sun, J.; Chen, X. An optical microscopy study on the phase structure of poly(L-lactide acid)/poly(propylene carbonate) blends. J. Phys. Chem. B 2012, 116, 9832–9837. [Google Scholar] [CrossRef]
- Sokhal, K.S.; Dasaroju, G.; Bulasara, V.K. Formation, stability and comparison of water/oil emulsion using gum arabic and guar gum and effect of aging of polymers on drag reduction percentage in water/oil flow. Vacuum 2018, 159, 247–253. [Google Scholar] [CrossRef]
- Rousi, Z.; Malhiac, C.; Fatouros, D.G.; Paraskevopoulou, A. Complex coacervates formation between gelatin and gum Arabic with different arabinogalactan protein fraction content and their characterization. Food Hydrocoll. 2019, 96, 577–588. [Google Scholar] [CrossRef]
- Lippincott-Schwartz, J.; Manley, S. Putting super-resolution fluorescence microscopy to work. Nat. Methods 2009, 6, 21–23. [Google Scholar] [CrossRef]
- Thorley, J.A.; Pike, J.; Rappoport, J.Z. Super-Resolution Microscopy: A Comparison of Commercially Available Options; Elsevier Inc.: Philadelphia, PA, USA, 2014. [Google Scholar]
- Jia, S.; Han, B.; Kutz, J.N. Example-Based Super-Resolution Fluorescence Microscopy. Sci. Rep. 2018, 8, 1–8. [Google Scholar] [CrossRef]
- Abitbol, T.; Palermo, A.; Moran-Mirabal, J.M.; Cranston, E.D. Fluorescent Labeling and Characterization of Cellulose Nanocrystals with Varying Charge Contents. Biomacromolecules 2013, 14, 3278–3284. [Google Scholar] [CrossRef]
- Shazali, N.A.H.; Zaidi, N.E.; Ariffin, H.; Abdullah, L.C.; Ghaemi, F.; Abdullah, J.M.; Takashima, I.; Rahman, N.A.; Afizan, N.M. Characterization and cellular internalization of Spherical Cellulose Nanocrystals (CNC) into normal and cancerous fibroblasts. Materials Basel 2019, 12, 3251. [Google Scholar] [CrossRef]
- Ur-Rehman, A.; Khan, N.M.; Ali, F.; Khan, H.; Khan, Z.U.; Jan, A.K.; Khan, G.S.; Ahmad, S. Kinetics Study of Biopolymers Mixture with the Help of Confocal Laser Scanning Microscopy. J. Food Process Eng. 2016, 39, 533–541. [Google Scholar] [CrossRef]
- Zammarano, M.; Maupin, P.H.; Sung, L.P.; Gilman, J.W.; McCarthy, E.D.; Kim, Y.S.; Fox, D.M. Revealing the Interface in Polymer Nanocomposites. ACS Nano 2011, 5, 3391–3399. [Google Scholar] [CrossRef] [PubMed]
- Spence, J.C.H. High-Resolution Electron Microscopy; Oxford University Press: New York, NY, USA, 2013. [Google Scholar]
- Yu, X.; Arey, B.; Chatterjee, S.; Chun, J. Improving in situ liquid SEM imaging of particles. Surf. Interface Anal. 2019, 51, 1325–1331. [Google Scholar] [CrossRef]
- Nguyen, J.N.T.; Harbison, A.M. Scanning Electron Microscopy Sample Preparation and Imaging; Humana Press Inc.: Totowa, NJ, USA, 2017; Volume 1606. [Google Scholar]
- Shekarforoush, E.; Mirhosseini, H.; Amid, B.T.; Ghazali, H.; Muhammad, K.; Sarker, M.Z.I.; Paykary, M. Rheological Properties and Emulsifying Activity of Gum Karaya (Sterculia Urens) in Aqueous System and Oil in Water Emulsion: Heat Treatment and Microwave Modification. Int. J. Food Prop. 2016, 19, 662–679. [Google Scholar] [CrossRef]
- Pang, J.; Liu, X.; Zhang, X.; Wu, Y.; Sun, R. Fabrication of Cellulose Film with Enhanced Mechanical Properties in Ionic Liquid 1-Allyl-3-methylimidaxolium Chloride (AmimCl). Materials Basel 2013, 6, 1270–1284. [Google Scholar] [CrossRef] [PubMed]
- Phinichka, N.; Kaenthong, S. Regenerated cellulose from high alpha cellulose pulp of steam-exploded sugarcane bagasse. J. Mater. Res. Technol. 2018, 7, 55–65. [Google Scholar] [CrossRef]
- İlgü, H.; Turan, T.; Şanli-Mohamed, G. Preparation, Characterization and Optimization of Chitosan Nanoparticles as Carrier for Immobilization of Thermophilic Recombinant Esterase. J. Macromol. Sci. Part A 2011, 48, 713–721. [Google Scholar]
- Saari, H.; Fuentes, C.; Sjöö, M.; Rayner, M.; Wahlgren, M. Production of starch nanoparticles by dissolution and non-solvent precipitation for use in food-grade Pickering emulsions. Carbohydr. Polym. 2017, 157, 558–566. [Google Scholar] [CrossRef]
- Mun, S.; Kim, H.C.; Yadave, M.; Kim, J. Graphene oxide–gellan gum–sodium alginate nanocomposites: Synthesis, characterization, and mechanical behavior. Compos. Interfaces 2015, 22, 249–263. [Google Scholar] [CrossRef]
- Wittmar, A.; Vorat, D.; Ulbricht, M. Two step and one step preparation of porous nanocomposite cellulose membranes doped with TiO 2. RSC Adv. 2015, 5, 88070–88078. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, D. Synthesis and characterization of a chitosan based nanocomposite injectable hydrogel. Carbohydr. Polym. 2016, 136, 1228–1237. [Google Scholar] [CrossRef] [PubMed]
- Bagheri-Khoulenjani, S.; Mirzadeh, H.; Etrati-Khosroshahi, M.; Ali Shokrgozar, M. Particle size modeling and morphology study of chitosan/gelatin/nanohydroxyapatite nanocomposite microspheres for bone tissue engineering. J. Biomed. Mater. Res. Part A 2013, 101, 1758–1767. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, S.A.; Weese, E.; Nakamatsu, J.; Torres, F. Development of Biopolymer Nanocomposites Based on Polysaccharides Obtained from Red Algae Chondracanthus chamissoi Reinforced with Chitin Whiskers and Montmorillonite. Polym.-Plast. Technol. Eng. 2016, 55, 1557–1564. [Google Scholar] [CrossRef]
- Tarus, B.; Fadel, N.; Al-Oufy, A.; El-Messiry, M. Effect of polymer concentration on the morphology and mechanical characteristics of electrospun cellulose acetate and poly (vinyl chloride) nanofiber mats. Alexandria Eng. J. 2016, 55, 2975–2984. [Google Scholar] [CrossRef]
- Diantoro, M.; Kusumaatmaja, A.; Triyana, K. Preparation of PVA/Chitosan/TiO 2 nanofibers using electrospinning method. AIP Conf. Proc. 2016, 1755, 150002. [Google Scholar]
- Qasim, S.B.; Najeeb, S.; Delaine-Smith, R.M.; Rawlinson, A.; Ur Rehman, I. Potential of electrospun chitosan fibers as a surface layer in functionally graded GTR membrane for periodontal regeneration. Dent. Mater. 2017, 33, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Padil, V.V.; Senan, C.; Wacławek, S.; Cerník, M.; Agarwal, S.; Varma, R.S. Bioplastic Fibers from Gum Arabic for Greener Food Wrapping Applications. ACS Sustain. Chem. Eng. 2019, 7, 5900–5911. [Google Scholar] [CrossRef]
- Fazeli, M.; Florez, J.P.; Simão, R.A. Improvement in adhesion of cellulose fibers to the thermoplastic starch matrix by plasma treatment modification. Compos. Part B Eng. 2019, 163, 207–216. [Google Scholar] [CrossRef]
- Anžlovar, A.; Kunaver, M.; Krajnc, A.; Žagar, E. Nanocomposites of LLDPE and Surface-Modified Cellulose Nanocrystals Prepared by Melt Processing. Molecules 2018, 23, 1782. [Google Scholar] [CrossRef]
- Naranda, J.; Sušec, M.; Maver, U.; Gradišnik, L.; Gorenjak, M.; Vukasović, A.; Ivković, A.; Rupnik, M.S.; Vogrin, M.; Krajnc, P. Polyester type polyHIPE scaffolds with an interconnected porous structure for cartilage regeneration. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef]
- Zhou, F.L.; Parker, G.J.M.; Eichhorn, S.J.; Hubbard Cristinacce, P.L. Production and cross-sectional characterization of aligned co-electrospun hollow microfibrous bulk assemblies. Mater. Charact. 2015, 109, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.M.; Qian, J.; Zhang, J.; Lin, Z.F.; Li, J.S.; Xu, J.Z.; Li, Z.M. Engineering porous poly(lactic acid) scaffolds with high mechanical performance via a solid state extrusion/porogen leaching approach. Polymers Basel 2016, 8, 213. [Google Scholar] [CrossRef]
- Mafirad, S.; Mehrnia, M.R.; Zahedi, P.; Hosseini, S.-N. Chitosan-based nanocomposite membranes with improved properties: Effect of cellulose acetate blending and TiO2 nanoparticles incorporation. Polym. Compos. 2017, 39, 4452–4466. [Google Scholar] [CrossRef]
- Zhang, Z.-H.; Han, Z.; Zeng, X.-A.; Xiong, X.-Y.; Liu, Y.-J. Enhancing mechanical properties of chitosan films via modification with vanillin. Int. J. Biol. Macromol. 2015, 81, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Nanjunda, R.B.; Venkata, L.V.; Vishnu, M.K.; Mylarappa, M.; Raghavendra, N.; Venkatesh, T. Preparation of chitosan/different organomodified clay polymer nanocomposites: Studies on morphological, swelling, thermal stability and anti-bacterial properties. Nanosyst. Phys. Chem. Math. 2016, 7, 667–674. [Google Scholar]
- Kumar, P.; Sandeep, K.P.; Alavi, S.; Truong, V.D.; Gorga, R.E. Effect of Type and Content of Modified Montmorillonite on the Structure and Properties of Bio-Nanocomposite Films Based on Soy Protein Isolate and Montmorillonite. J. Food Sci. 2010, 75, N46–N56. [Google Scholar] [CrossRef]
- Jain, R.; Mahto, V.; Mahto, T.K. Study of the Effect of Xanthan Gum Based Graft Copolymer on Water Based Drilling Fluid. J. Macromol. Sci. Part A 2014, 51, 976–982. [Google Scholar] [CrossRef]
- Abdullah, N.H.; Shameli, K.; Nia, P.M.; Etesami, M.; Abdullah, E.C.; Abdullah, L.C. Electrocatalytic activity of starch/Fe3O4/zeolite bionanocomposite for oxygen reduction reaction. Arab. J. Chem. 2017, 13, 1297–1308. [Google Scholar] [CrossRef]
- Yusof, Y.M.; Shukur, M.F.; Illias, H.A.; Kadir, M.F.Z. Conductivity and electrical properties of corn starch-chitosan blend biopolymer electrolyte incorporated with ammonium iodide. R. Swedish Acad. Sci. Phys. Scr. Phys. Scr 2014, 89, 10. [Google Scholar] [CrossRef]
- Rajisha, K.R.; Maria, H.J.; Pothan, L.A.; Ahmad, Z.; Thomas, S. Preparation and characterization of potato starch nanocrystal reinforced natural rubber nanocomposites. Int. J. Biol. Macromol. 2014, 67, 147–153. [Google Scholar] [CrossRef]
- Daio, T.; Bayer, T.; Ikuta, T.; Nishiyama, T.; Takahashi, K.; Takata, Y.; Sasaki, K.; Lyth, S.M. In-Situ ESEM and EELS Observation of Water Uptake and Ice Formation in Multilayer Graphene Oxide. Sci. Rep. 2015, 5, 11807. [Google Scholar] [CrossRef]
- Jenkins, L.M.; Donald, A.M. Use of the environmental scanning electron microscope for the observation of the swelling behaviour of cellulosic fibres. Scanning 2006, 19, 92–97. [Google Scholar] [CrossRef]
- Podor, R.; Ravaux, J.; Brau, H.-P. In Situ Experiments in the Scanning Electron Microscope Chamber, Scanning electron Microscopy; IntechOpen: London, UK, 2012. [Google Scholar]
- Jansson, A.; Nafari, A.; Sanz-Velasco, A.; Svensson, K.; Gustafsson, S.; Hermansson, A.M.; Olsson, E. Novel Method for Controlled Wetting of Materials in the Environmental Scanning Electron Microscope. Microsc. Microanal. 2013, 19, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Girão, A.V.; Caputo, G.; Ferro, M.C. Application of Scanning Electron Microscopy–Energy Dispersive X-Ray Spectroscopy (SEM-EDS). Compr. Anal. Chem. 2017, 75, 153–168. [Google Scholar]
- Chauhan, K.; Priya, V.; Singh, P.; Chauhan, G.S.; Kumari, S.; Singhal, R.K. A green and highly efficient sulfur functionalization of starch. RSC Adv. 2015, 5, 51762–51772. [Google Scholar] [CrossRef]
- Anjum, F.; Bukhari, S.A.; Siddique, M.; Shahid, M.; Potgieter, J.H.; Jaafar, H.Z.; Ercisli, S.; Zia-Ul-Haq, M. Microwave Irradiated Copolymerization of Xanthan Gum with Acrylamide for Colonic Drug Delivery. BioResources 2015, 10, 1434–1451. [Google Scholar] [CrossRef]
- Ghannam, H.E.; STalab, A.; VDolgano, N.; MSHusse, A.; Abdelmagui, N.M. Characterization of Chitosan Extracted from Different Crustacean Shell Wastes. J. Appl. Sci. 2016, 16, 454–461. [Google Scholar]
- Sofla, M.R.K.; Brown, R.J.; Tsuzuki, T.; Rainey, T.J. A comparison of cellulose nanocrystals and cellulose nanofibres extracted from bagasse using acid and ball milling methods. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 035004. [Google Scholar] [CrossRef]
- Singh, P.; Chauhan, K.; Priya, V.; Singhal, R.K. A greener approach for impressive removal of As(iii)/As(v) from an ultra-low concentration using a highly efficient chitosan thiomer as a new adsorbent. RSC Adv. 2016, 6, 64946–64961. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, X.; Jin, X. Preparation and Properties of Minocycline-Loaded Carboxymethyl Chitosan Gel/Alginate Nonwovens Composite Wound Dressings. Mar. Drugs 2019, 17, 575. [Google Scholar] [CrossRef]
- Kuei, B.; Aplan, M.P.; Litofsky, J.H.; Gomez, E.D. New opportunities in transmission electron microscopy of polymers. Mater. Sci. Eng. R Rep. 2020, 139, 100516. [Google Scholar] [CrossRef]
- Libera, M.R.; Egerton, R.F. Advances in the Transmission Electron Microscopy of Polymers. Polym. Rev. 2010, 50, 321–339. [Google Scholar] [CrossRef]
- Winey, M.; Meehl, J.B.; O’Toole, E.T.; Giddings, T.H. Conventional transmission electron microscopy. Mol. Biol. Cell 2014, 25, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Rice, S.B.; Chan, C.; Brown, S.C.; Eschbach, P.; Han, L.; Ensor, D.S.; Stefaniak, A.B.; Bonevich, J.; Vladár, A.E.; Walker, A.R.H. Particle size distributions by transmission electron microscopy: an interlaboratory comparison case study. Metrologia 2013, 50, 663. [Google Scholar] [CrossRef] [PubMed]
- Mielańczyk, Ł.; Matysiak, N.; Klymenko, O.; Wojnicz, R. Transmission Electron Microscopy of Biological Samples; IntechOpen: Vienna, Austria, 2015. [Google Scholar]
- Tang, C.Y.; Yang, Z. Transmission Electron Microscopy (TEM); Elsevier Inc.: Philadelphia, PA, USA, 2017. [Google Scholar]
- Kirkland, A.I.; Chang, S.L.Y.; Hutchison, J.L. Springer Handbooks; Springer: Berlin/Heidelberg, Germany, 2019; pp. 3–47. [Google Scholar]
- Alnarabiji, M.S.; Yahya, N.; Hamed, Y.; Ardakani, S.E.M.; Azizi, K.; Klemeš, J.J.; Abdullaha, B.; Tasfyd, S.F.H.; Hamide, S.B.A.; Nashed, O. Scalable bio-friendly method for production of homogeneous metal oxide nanoparticles using green bovine skin gelatin. J. Clean. Prod. 2017, 162, 186–194. [Google Scholar] [CrossRef]
- Santos, E.D.B.; Lima, E.C.N.L.; Oliveira, C.S.; De Sigoli, F.A.; Mazali, I.O. Fast detection of paracetamol on a gold nanoparticle-chitosan substrate by SERS. Anal. Methods 2014, 6, 3564–3568. [Google Scholar] [CrossRef]
- Bhagyaraj, S.; Krupa, I. Alginate-Mediated Synthesis of Hetero-Shaped Silver Nanoparticles and Their Hydrogen Peroxide Sensing Ability. Molecules 2020, 25, 435. [Google Scholar] [CrossRef]
- Josefsson, G.; Tanem, B.S.; Li, Y.; Vullum, P.E.; Gamstedt, E.K. Prediction of elastic properties of nanofibrillated cellulose from micromechanical modeling and nano-structure characterization by transmission electron microscopy. Cellulose 2013, 20, 761–770. [Google Scholar] [CrossRef]
- Ramesh, S.; Sivasamy, A.; Kim, H.S.; Kim, J.H. High-performance N-doped MWCNT/GO/cellulose hybrid composites for supercapacitor electrodes. RSC Adv. 2017, 7, 49799–49809. [Google Scholar] [CrossRef]
- Qin, X.; Zhang, H.; Wang, Z.; Jin, Y. Magnetic chitosan/graphene oxide composite loaded with novel photosensitizer for enhanced photodynamic therapy. RSC Adv. 2018, 8, 10376–10388. [Google Scholar] [CrossRef]
- Kondo, T.; Kasai, W.; Brown, R.M. Formation of nematic ordered cellulose and chitin. Cellulose 2004, 11, 463–474. [Google Scholar] [CrossRef]
- Danev, R.; Yanagisawa, H.; Kikkawa, M. Cryo-Electron Microscopy Methodology: Current Aspects and Future Directions. Trends Biochem. Sci. 2019, 44, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Frank, J. Story in a sample-the potential (and limitations) of cryo-electron microscopy applied to molecular machines. Biopolymers 2013, 99, 832–836. [Google Scholar] [CrossRef]
- Majoinen, J.; Haataja, J.S.; Appelhans, D.; Lederer, A.; Olszewska, A.; Seitsonen, J.; Aseyev, V.; Kontturi, E.; Rosilo, H.; Österberg, M.; et al. Supracolloidal Multivalent Interactions and Wrapping of Dendronized Glycopolymers on Native Cellulose Nanocrystals. J. Am. Chem. Soc. 2014, 136, 866–869. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, M.; Basu, K.; Benoit, C.; Cirtiu, C.M.; Vali, H.; Moores, A. Cellulose Nanocrystals as Chiral Inducers: Enantioselective Catalysis and Transmission Electron Microscopy 3D Characterization. J. Am. Chem. Soc. 2015, 137, 6124–6127. [Google Scholar] [CrossRef] [PubMed]
- Pennycook, S.J.; Lupini, A.R.; Varela, M.; Borisevich, A.; Peng, Y.; Oxley, M.P.; Van Benthem, K.; Chisholm, M.F. Scanning transmission electron microscopy for nanostructure characterization. In Scanning Microscopy for Nanotechnology: Techniques and Applications; Springer New York: New York, NY, USA, 2007. [Google Scholar]
- Nellist, P.D. Springer Handbooks; Springer: Berlin/Heidelberg, Germany, 2019; pp. 49–99. [Google Scholar]
- Ponce, A.; Mejía-Rosales, S.; José-Yacamán, M. Scanning transmission electron microscopy methods for the analysis of nanoparticles. Methods Mol. Biol. 2012, 906, 453–471. [Google Scholar]
- Rodríguez-Argüelles, M.C.; Sieiro, C.; Cao, R.; Nasi, L. Chitosan and silver nanoparticles as pudding with raisins with antimicrobial properties. J. Colloid Interface Sci. 2011, 364, 80–84. [Google Scholar] [CrossRef]
- Teodoro, K.B.R.; Migliorini, F.L.; Facure, M.H.M.; Correa, D.S. Conductive electrospun nanofibers containing cellulose nanowhiskers and reduced graphene oxide for the electrochemical detection of mercury(II). Carbohydr. Polym. 2019, 207, 747–754. [Google Scholar] [CrossRef]
- Liu, K.; Nasrallah, J.; Chen, L.; Huang, L.; Ni, Y. Preparation of CNC-dispersed Fe3O4 nanoparticles and their application in conductive paper. Carbohydr. Polym. 2015, 126, 175–178. [Google Scholar] [CrossRef]
- Karakus, S.; Ilgar, M.; Kahyaoglu, I.M.; Kilislioglu, A. Influence of ultrasound irradiation on the intrinsic viscosity of guar gum–PEG/rosin glycerol ester nanoparticles. Int. J. Biol. Macromol. 2019, 141, 1118–1127. [Google Scholar] [CrossRef]
- Motahharifar, N.; Nasrollahzadeh, M.; Taheri-Kafrani, A.; Varma, R.S.; Shokouhimehr, M. Magnetic chitosan-copper nanocomposite: A plant assembled catalyst for the synthesis of amino- and N-sulfonyl tetrazoles in eco-friendly media. Carbohydr. Polym. 2020, 232, 115819. [Google Scholar] [CrossRef] [PubMed]
- Torres-Martínez, N.E.; Garza-Navarro, M.A.; García-Gutiérrez, D.; González-González, V.A.; Torres-Castro, A.; Ortiz-Méndez, U. Hybrid nanostructured materials with tunable magnetic characteristics. J. Nanoparticle Res. 2014, 16, 1–12. [Google Scholar] [CrossRef]
- Teixeira ED, M.; Lotti, C.; Corrêa, A.C.; Teodoro, K.B.; Marconcini, J.M.; Mattoso, L.H. Thermoplastic corn starch reinforced with cotton cellulose nanofibers. J. Appl. Polym. Sci. 2011, 120, 2428–2433. [Google Scholar] [CrossRef]
- Omerzu, A.; Saric, I.; Piltaver, I.K.; Petravic, M.; Kapun, T.; Zule, J.; Stifter, S.; Salamon, K. Prevention of spontaneous combustion of cellulose with a thin protective Al2O3 coating formed by atomic layer deposition. Surf. Coat. Technol. 2018, 333, 81–86. [Google Scholar] [CrossRef]
- Song, S.; Zhao, Y.; Yuan, X.; Zhang, J. β-Chitin nanofiber hydrogel as a scaffold to in situ fabricate monodispersed ultra-small silver nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2019, 574, 36–43. [Google Scholar] [CrossRef]
- Liu, K.; Liang, H.; Nasrallah, J.; Chen, L.; Huang, L.; Ni, Y. Preparation of the CNC/Ag/beeswax composites for enhancing antibacterial and water resistance properties of paper. Carbohydr. Polym. 2016, 142, 183–188. [Google Scholar] [CrossRef]
- Sosiati, H.; Wijayanti, D.A.; Triyana, K.; Kamiel, B. Morphology and crystallinity of sisal nanocellulose after sonication. AIP Conf. Proc. 2017, 1755, 20029. [Google Scholar]
- Gupta, K.; Kaushik, A.; Tikoo, K.B.; Kumar, V.; Singhal, S. Enhanced catalytic activity of composites of NiFe2O4 and nano cellulose derived from waste biomass for the mitigation of organic pollutants. Arab. J. Chem. 2017, 13, 783–798. [Google Scholar] [CrossRef]
- Celebi, H.; Kurt, A. Effects of processing on the properties of chitosan/cellulose nanocrystal films. Carbohydr. Polym. 2015, 133, 284–293. [Google Scholar] [CrossRef]
- Guirguis, O.; Abdelzaher, N.; El-Bassyouni, G.; Moselhey, M. Structural, Thermal and Optical Modifications of Chitosan due to UV-Ozone Irradiation. Egypt. J. Chem. 2018, 61, 350–360. [Google Scholar] [CrossRef]
- Anandan, M.; Gurumallesh Prabu, H. Dodonaea viscosa Leaf Extract Assisted Synthesis of Gold Nanoparticles: Characterization and Cytotoxicity against A549 NSCLC Cancer Cells. J. Inorg. Organomet. Polym. Mater. 2018, 28, 932–941. [Google Scholar] [CrossRef]
- Yulizar, Y.; Utari, T.; Ariyanta, H.A.; Maulina, D. Green Method for Synthesis of Gold Nanoparticles Using Polyscias scutellaria Leaf Extract under UV Light and Their Catalytic Activity to Reduce Methylene Blue. J. Nanomater. 2017, 2017, 1–6. [Google Scholar] [CrossRef]
- Kiruba Daniel, S.C.G.; Vinothini, G.; Subramanian, N.; Nehru, K.; Sivakumar, M. Biosynthesis of Cu, ZVI, and Ag nanoparticles using Dodonaea viscosa extract for antibacterial activity against human pathogens. J. Nanoparticle Res. 2013, 15, 1319. [Google Scholar] [CrossRef]
- Guidelli, E.J.; Ramos, A.P.; Zaniquelli, M.E.D.; Baffa, O. Green synthesis of colloidal silver nanoparticles using natural rubber latex extracted from Hevea brasiliensis. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 82, 140–145. [Google Scholar] [CrossRef]
- Vanamudan, A.; Sudhakar, P.P. Biopolymer capped silver nanoparticles with potential for multifaceted applications. Int. J. Biol. Macromol. 2016, 86, 262–268. [Google Scholar] [CrossRef]
- Karoutsos, V. Scanning probe microscopy: Instrumentation and applications on thin films and magnetic multilayers. J. Nanosci. Nanotechnol. 2009, 9, 6783–6798. [Google Scholar] [CrossRef]
- Huey, B.D.; Luria, J.; Bonnell, D.A. Springer Handbooks; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1239–1277. [Google Scholar]
- Wallace, A.F. Scanning Probe Microscopy, Analytical Geomicrobiology; Cambridge University Press: Cambridge, UK, 2019. [Google Scholar]
- Raigoza, A.F.; Dugger, J.W.; Webb, L.J. Review: Recent Advances and Current Challenges in Scanning Probe Microscopy of Biomolecular Surfaces and Interfaces. ACS Appl. Mater. Interfaces 2013, 5, 9249–9261. [Google Scholar] [CrossRef]
- Cohen, S.H.; Bray, M.T.; Lightbody, M.L. Atomic Force Microscopy/Scanning Tunneling Microscopy; Springer: Boston, MA, USA, 1994. [Google Scholar]
- Binnig, G.; Quate, C.F.; Gerber, C. Atomic Force Microscope. Phys. Rev. Lett. 1986, 56, 930–933. [Google Scholar] [CrossRef]
- Hansma, P.K.; Elings, V.B.; Marti, O.; Bracker, C.E. Scanning tunneling microscopy and atomic force microscopy: Application to biology and technology. Science 1988, 242, 209–216. [Google Scholar] [CrossRef]
- Santos, N.C.; Castanho, M.A.R.B. An overview of the biophysical applications of atomic force microscopy. Biophys. Chem. 2004, 107, 133–149. [Google Scholar] [CrossRef]
- Giessibl, F.J. Advances in atomic force microscopy. Rev. Mod. Phys. 2003, 75, 949–983. [Google Scholar] [CrossRef]
- Ando, T.; Uchihashi, T.; Kodera, N.; Yamamoto, D.; Miyagi, A.; Taniguchi, M.; Yamashita, H. High-speed AFM and nano-visualization of biomolecular processes. Pflüg. Arch. Eur. J. Physiol. 2008, 456, 211–225. [Google Scholar] [CrossRef] [PubMed]
- Alsteens, D.; Dupres, V.; Dague, E.; Verbelen, C.; André, G.; Francius, G.; Dufrêne, Y.F. Imaging Chemical Groups and Molecular Recognition Sites on Live Cells Using AFM; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Barish, J.A.; Goddard, J.M. Topographical and chemical characterization of polymer surfaces modified by physical and chemical processes. J. Appl. Polym. Sci. 2011, 120, 2863–2871. [Google Scholar] [CrossRef]
- Nnebe, I.; Schneider, J.W. Characterization of distance-dependent damping in tapping-mode atomic force microscopy force measurements in liquid. Langmuir 2004, 20, 3195–3201. [Google Scholar] [CrossRef]
- Gunning, A.P.; Kirby, A.R.; Ridout, M.J.; Brownsey, G.J.; Morris, V.J. Investigation of Gellan Networks and Gels by Atomic Force Microscopy. Macromolecules 1996, 29, 6791–6796. [Google Scholar] [CrossRef]
- Kirby, A.R.; Gunning, A.P.; Waldron, K.W.; Morris, V.J.; Ng, A. Visualization of plant cell walls by atomic force microscopy. Biophys. J. 1996, 70, 1138–1143. [Google Scholar] [CrossRef]
- Hansma, H.G.; Hoh, J.H. Biomolecular Imaging with the Atomic Force Microscope. Ann. Rev. Biophys. Biomol. Struct. 1994, 23, 115–140. [Google Scholar] [CrossRef]
- Kirby, A.R.; Gunning, A.P.; Morris, V.J. Imaging polysaccharides by atomic force microscopy. Biopolymers 1998, 38, 355–366. [Google Scholar] [CrossRef]
- Patrick Gunning, A.; Kirby, A.R.; Morris, V.J. Imaging xanthan gum in air by ac “tapping” mode atomic force microscopy. Ultramicroscopy 1996, 63, 1–3. [Google Scholar] [CrossRef]
- McIntire, T.M.; Penner, R.M.; Brant, D.A. Observations of a circular, triple-helical polysaccharide using noncontact atomic force microscopy. Macromolecules 1995, 28, 6375–6377. [Google Scholar] [CrossRef]
- Uricanu, V.I.; Duits, M.H.G.; Nelissen, R.M.F.; MLBennink, A.; Mellema, J. Local Structure and Elasticity of Soft Gelatin Gels Studied with Atomic Force Microscopy. Langmuir 2003, 19, 8182–8194. [Google Scholar] [CrossRef]
- Iijima, M.; Shinozaki, M.; Hatakeyama, T.; Takahashi, M.; Hatakeyama, H. AFM studies on gelation mechanism of xanthan gum hydrogels. Carbohydr. Polym. 2007, 68, 701–707. [Google Scholar] [CrossRef]
- Kocun, M.; Grandbois, M.; Cuccia, L.A. Single molecule atomic force microscopy and force spectroscopy of chitosan. Colloids Surf. B Biointerfaces 2011, 82, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Maver, U.; Maver, T.; Persin, Z.; Mozetic, M.; Vesel, A.; Gaberšček, M.; Stana-Kleinschek, K. Polymer Characterization with the Atomic Force Microscope. In Polymer Science; IntechOpen: London, UK, 2013. [Google Scholar]
- Vezenov, D.V.; Noy, A.; Ashby, P. Chemical force microscopy: Probing chemical origin of interfacial forces and adhesion. J. Adhes. Sci. Technol. 2005, 19, 313–364. [Google Scholar] [CrossRef]
- Steffens, C.; Leite, F.L.; Bueno, C.C.; Manzoli, A.; Herrmann, P.S.D.P. Atomic Force Microscopy as a Tool Applied to Nano/Biosensors. Sensors 2012, 12, 8278–8300. [Google Scholar] [CrossRef]
- Ito, T.; Ibrahim, S.; Grabowska, I. Chemical-force microscopy for materials characterization. TrAC Trends Anal. Chem. 2010, 29, 225–233. [Google Scholar] [CrossRef]
- Arslan, B.; Ju, X.; Zhang, X.; Abu-Lail, N.I. Heterogeneity and Specificity of Nanoscale Adhesion Forces Measured between Self-Assembled Monolayers and Lignocellulosic Substrates: A Chemical Force Microscopy Study. Langmuir 2015, 31, 10233–10245. [Google Scholar] [CrossRef]
- Le Troëdec, M.; Rachini, A.; Peyratout, C.; Rossignol, S.; Max, E.; Kaftan, O.; Fery, A.; Smith, A. Influence of chemical treatments on adhesion properties of hemp fibres. J. Colloid Interface Sci. 2011, 356, 303–310. [Google Scholar] [CrossRef]
- Boland, T.; Ratner, B.D. Direct measurement of hydrogen bonding in DNA nucleotide bases by atomic force microscopy. Proc. Natl. Acad. Sci. USA. 1995, 92, 5297–5301. [Google Scholar] [CrossRef]
- Lee, I.; Evans, B.R.; Foston, M.; Ragauskas, A.J. Silicon cantilever functionalization for cellulose-specific chemical force imaging of switchgrass. Anal. Methods 2015, 7, 4541–4545. [Google Scholar] [CrossRef]
- Passeri, D.; Angeloni, L.; Reggente, M.; Rossi, M. Magnetic force microscopy, Magnetic Characterization Techniques for Nanomaterials; Springer: Berlin/Heidelberg, Germany, 2017; pp. 209–259. [Google Scholar]
- Kazakova, O.; Puttock, R.; Barton, C.; Corte-León, H.; Jaafar, M.; Neu, V.; Asenjo, A. Frontiers of magnetic force microscopy. J. Appl. Phys. 2019, 125, 060901. [Google Scholar] [CrossRef]
- Torre, B.; Bertoni, G.; Fragouli, D.; Falqui, A.; Salerno, M.; Diaspro, A.; Cingolani, R.; Athanassiou, A. Magnetic force microscopy and energy loss imaging of superparamagnetic iron oxide nanoparticles. Sci. Rep. 2011, 1, 202. [Google Scholar] [CrossRef] [PubMed]
- Passeri, D.; Dong, C.; Reggente, M.; Angeloni, L.; Barteri, M.; Scaramuzzo, F.A.; Angelis, F.D.; Marinelli, F.; Antonelli, F.; Rinaldi, F.; et al. Magnetic force microscopy: Quantitative issues in biomaterials. Biomatter 2014, 4, e29507. [Google Scholar] [CrossRef] [PubMed]
- Nisticò, R. Magnetic materials and water treatments for a sustainable future. Res. Chem. Intermed. 2017, 43, 6911–6949. [Google Scholar] [CrossRef]
- Marín, T.; Montoya, P.; Arnache, O.; Pinal, R.; Calderón, J. Development of magnetite nanoparticles/gelatin composite films for triggering drug release by an external magnetic field. Mater. Des. 2018, 152, 78–87. [Google Scholar] [CrossRef]
- Nisticò, R.; Franzoso, F.; Cesano, F.; Scarano, D.; Magnacca, G.; Parolo, M.E.; Carlos, L. Chitosan-Derived Iron Oxide Systems for Magnetically Guided and Efficient Water Purification Processes from Polycyclic Aromatic Hydrocarbons. ACS Sustain. Chem. Eng. 2017, 5, 793–801. [Google Scholar]
- Cesano, F.; Fenoglio, G.; Carlos, L.; Nisticò, R. One-step synthesis of magnetic chitosan polymer composite films. Appl. Surf. Sci. 2015, 345, 175–181. [Google Scholar] [CrossRef]
- Lewandowska-Łańcucka, J.; Staszewska, M.; Szuwarzyński, M.; Kępczyński, M.; Romek, M.; Tokarz, W.; Szpak, A.; Kania, G.; Nowakowska, M. Synthesis and characterization of the superparamagnetic iron oxide nanoparticles modified with cationic chitosan and coated with silica shell. J. Alloys Compd. 2014, 586, 45–51. [Google Scholar]
- Zasadzinski, J.A.; Schneir, J.; Gurley, J.; Elings, V.; Hansma, P.K. Scanning tunneling microscopy of freeze-fracture replicas of biomembranes. Science 1988, 239, 1013–1015. [Google Scholar] [CrossRef]
- Marti, O.; Ribi, H.O.; Drake, B.; Albrecht, T.R.; Quate, C.F.; Hansma, P.K. Atomic force microscopy of an organic monolayer. Science 1988, 239, 50–52. [Google Scholar] [CrossRef]
- Travaglini, G.; Rohrer, H.; Amrein, M.; Gross, H. Scanning tunneling microscopy on biological matter. Surf. Sci. 1987, 181, 380–390. [Google Scholar] [CrossRef]
- Sonnenfeld, R.; Hansma, P.K.; Gross, H.; Stoll, E.; Travaglini, G. Atomic-resolution microscopy in water. Science 1986, 232, 211–213. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Kareem, O.; Abdel-Rahim, H.; Ezzat, I.; Essa, D.M. Evaluating the use of chitosan coated Ag nano-SeO 2 composite in consolidation of Funeral Shroud from the Egyptian Museum of Cairo. J. Cult. Herit. 2015, 16, 486–495. [Google Scholar] [CrossRef]
- Hameroff, S.R.; Simić-Krstić, J.; Kelley, M.F.; Voelker, M.A.; He, J.D.; Dereniak, E.L.; McCuskey, R.S.; Schneiker, C.W. Scanning tunneling microscopy of biopolymers: Conditions for microtubule stabilization. J. Vac. Sci. Technol. A Vac. Surf. Film. 1989, 7, 2890–2894. [Google Scholar] [CrossRef]
- Golovnya, R.V.; Terenina, M.B.; Krikunova, N.I.; Yuryev, V.P.; Misharina, T.A. Formation of Supramolecular Structures of Aroma Compounds with Polysaccharides of Corn Starch Cryotextures. Starch Stärke 2001, 53, 269–277. [Google Scholar] [CrossRef]
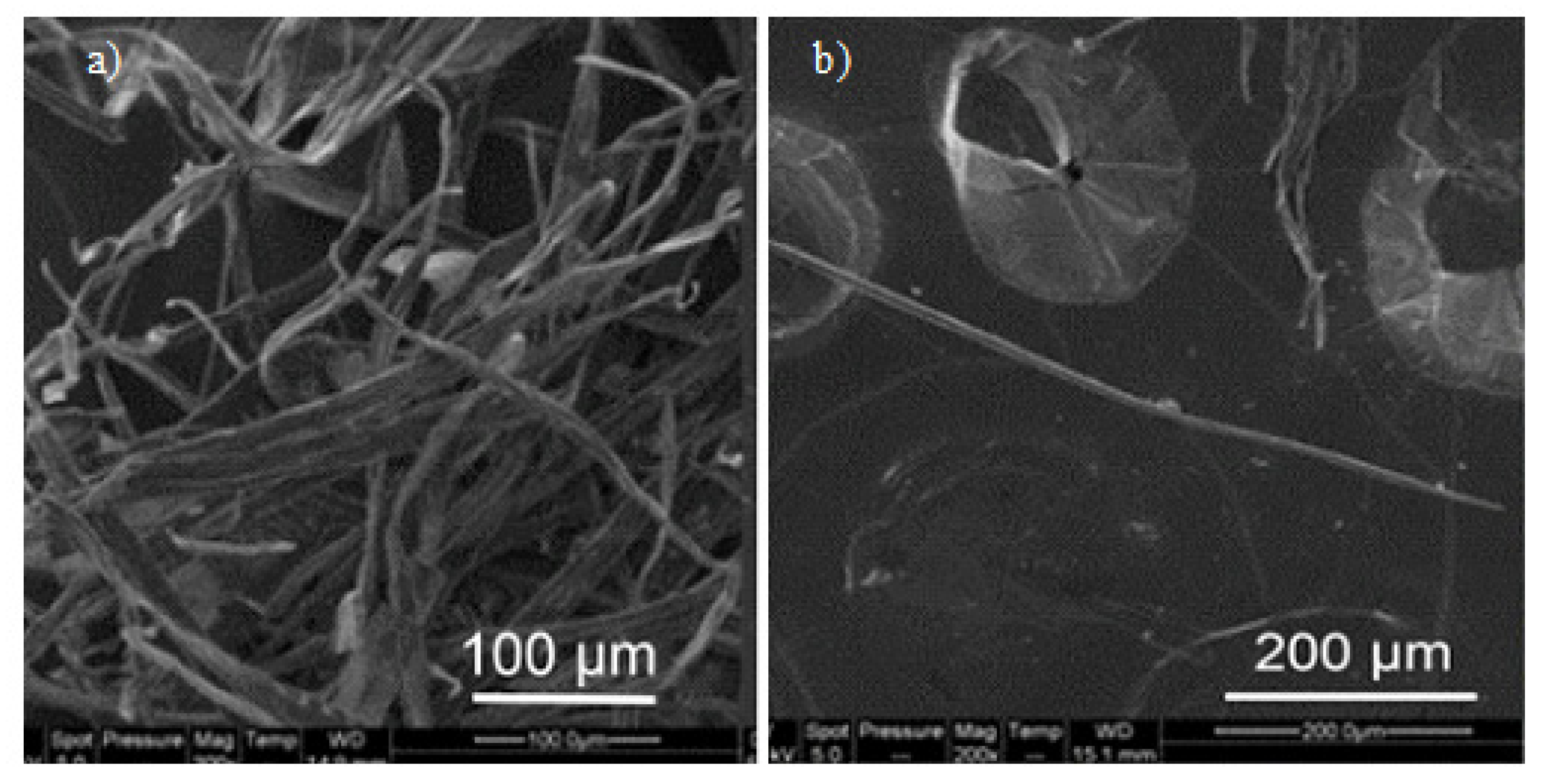
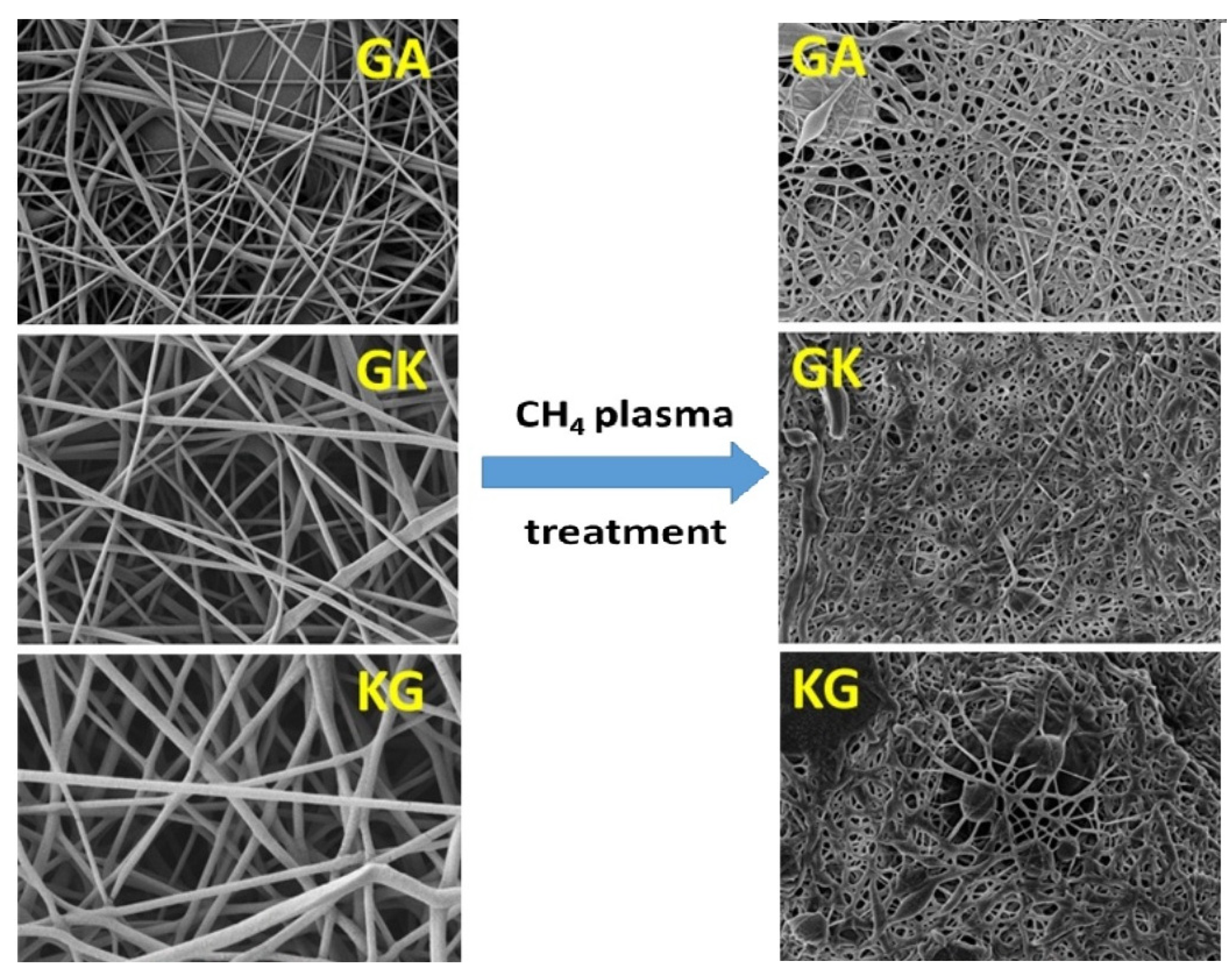
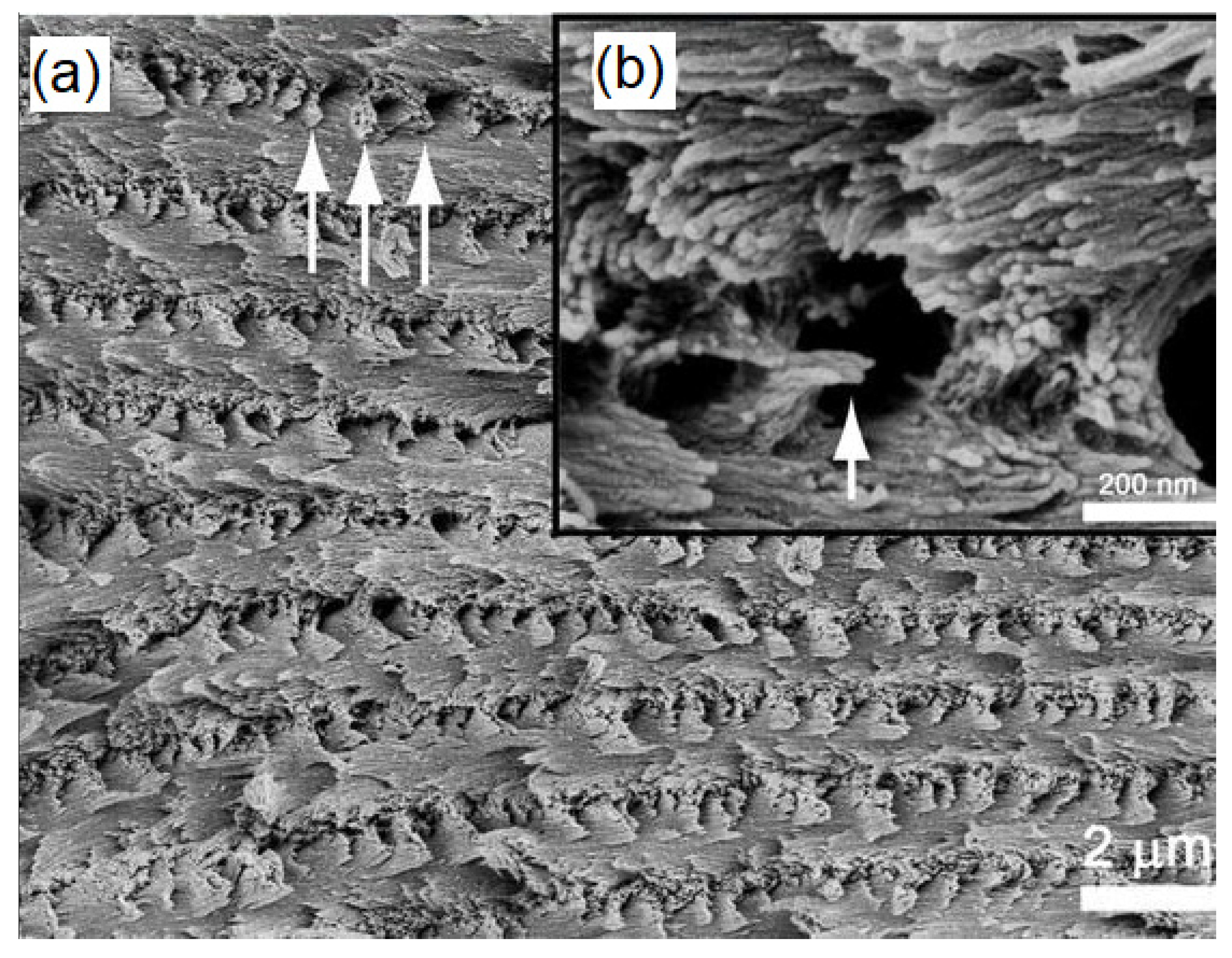
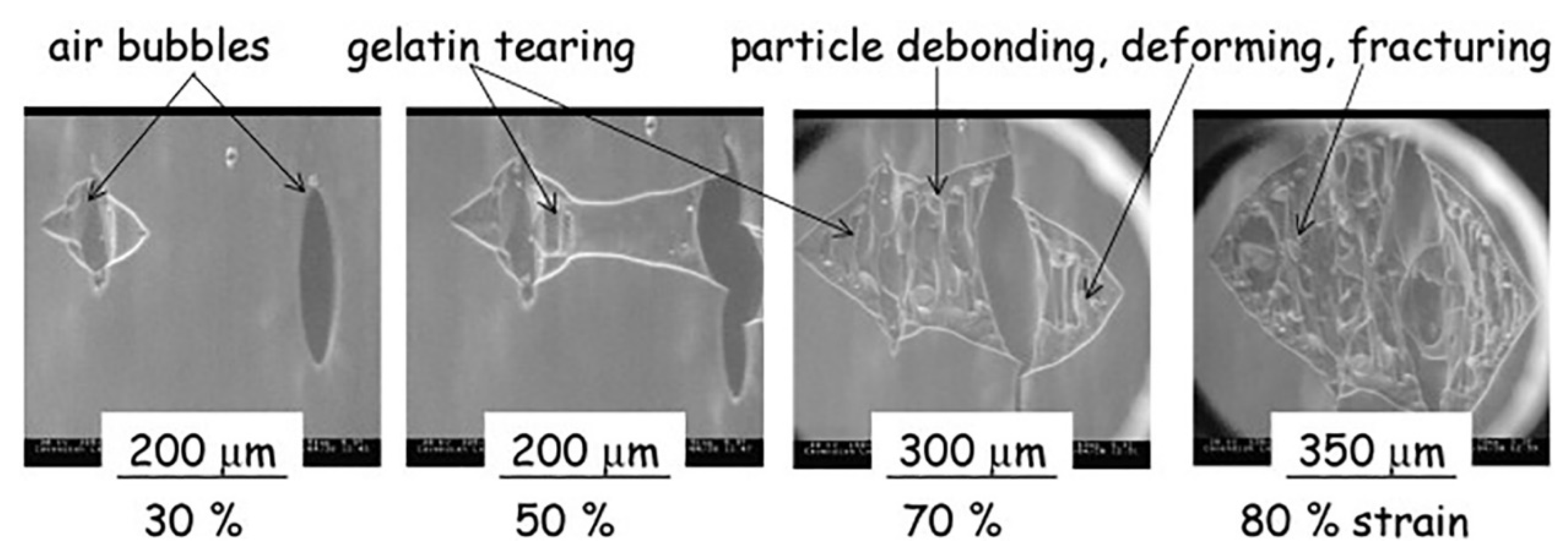


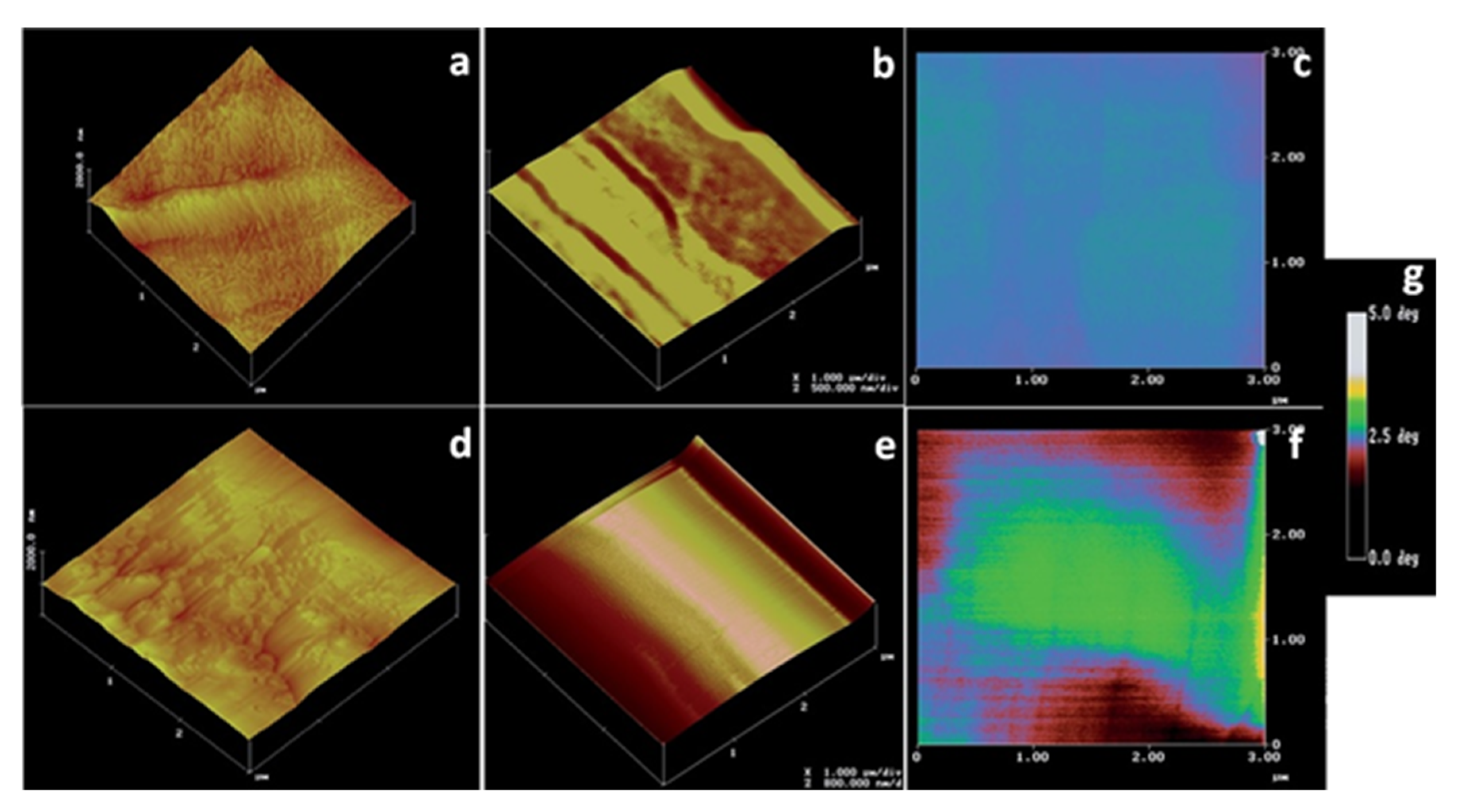
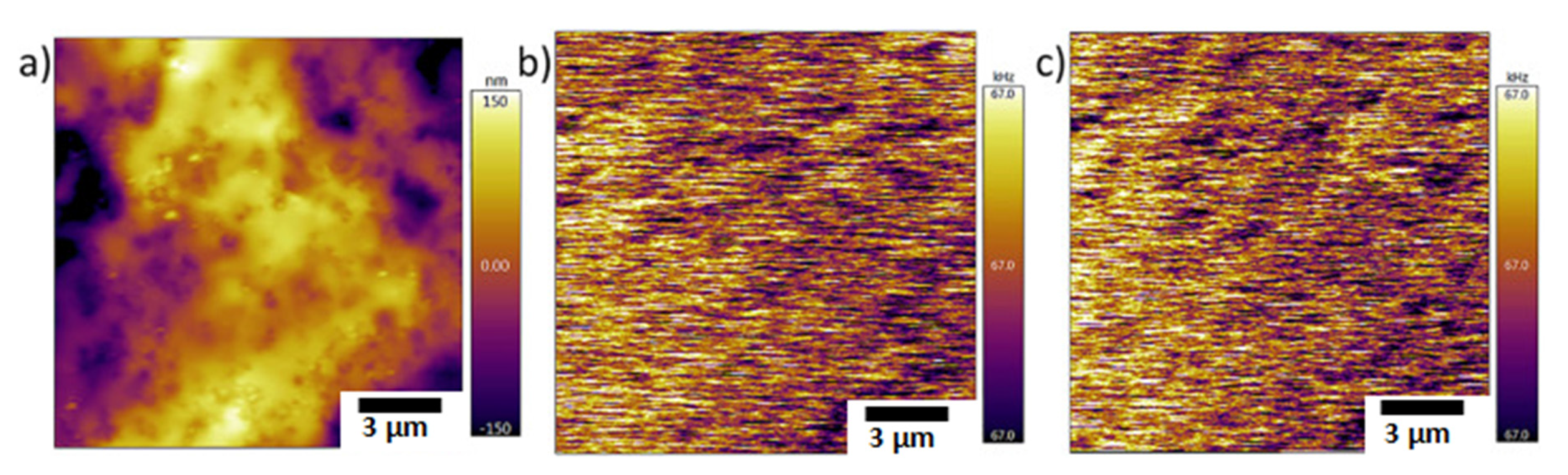
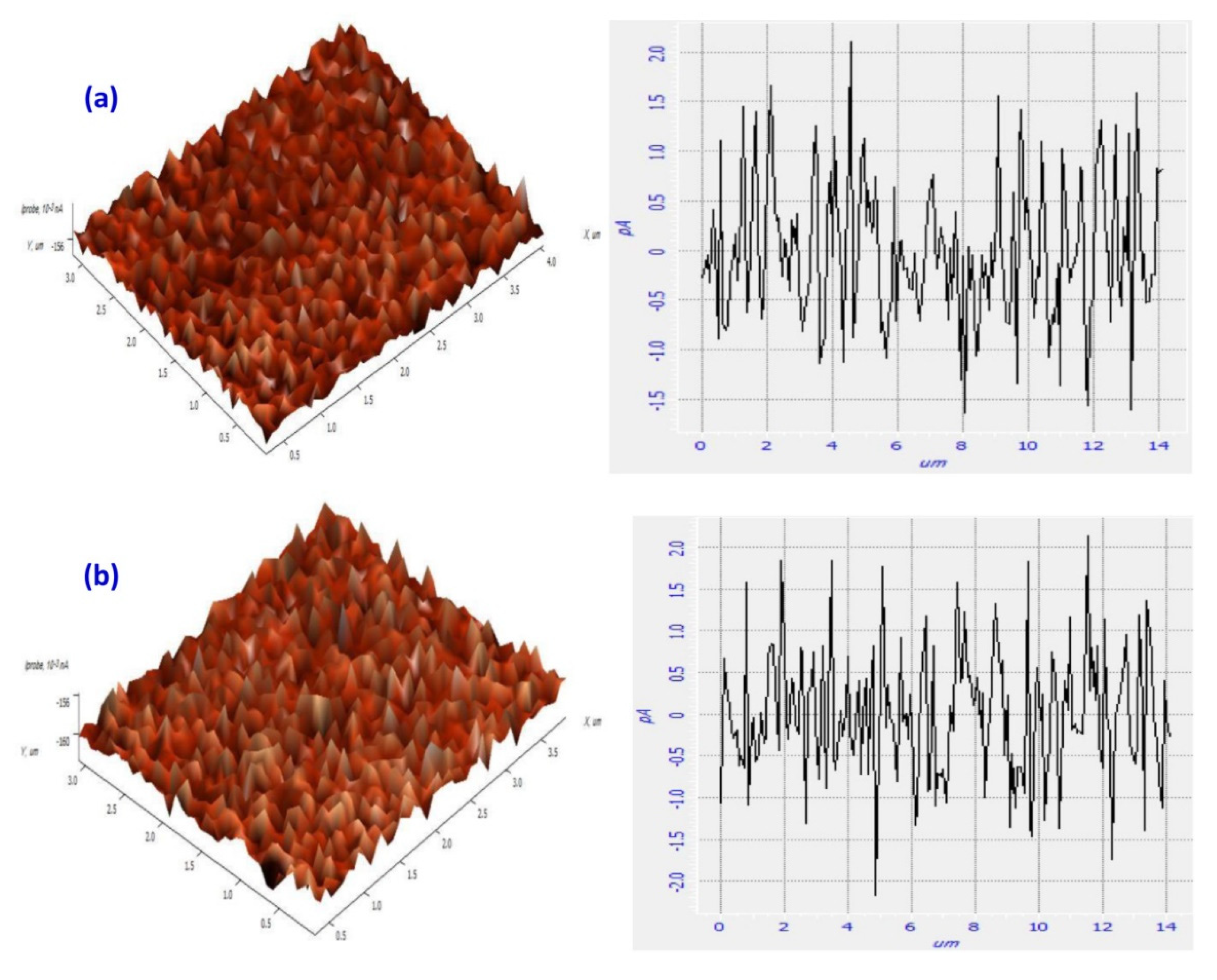
| Technique | Application | Biopolymer | References |
|---|---|---|---|
| Optical Microscopy | Fiber diameter | Poly(ε-caprolactone)/chitosan blend | [65] |
| Size and ahape | Starch granules | [66,67] | |
| Filler dispersion | starch/ Gum Arabic/nanocellulose | [68] | |
| Scanning electron microscopy (SEM) | Particle size | Chitosan | [69] |
| Particle shape | Starch granules | [66] | |
| Fiber diameter and surface modification | Gum Arabic, Gum Karaya, Kondagogu gum | [19] | |
| Crystal alignment | Cellulose nanocrystals | [70] | |
| Failure behavior | Gelatin/maltodextrin | [71] | |
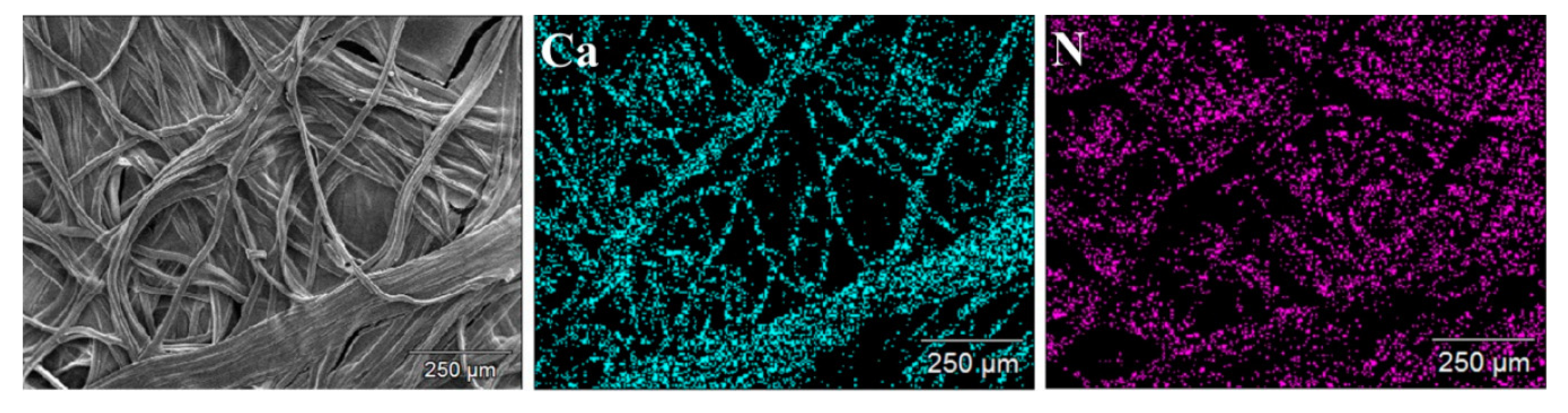
| SEM + energy-dispersive X-ray spectroscopy | Elemental composition | Cellulose | [72,73] |
| Transmission electron microscopy (TEM) | Particle dispersion | Cellulose nanofiber | [74] |
| Particle Size | Kondagogu gum biopolymer assisted Pt nanoparticles | [24] | |
| Core shell structure | Chitosan/PEO | [75] | |
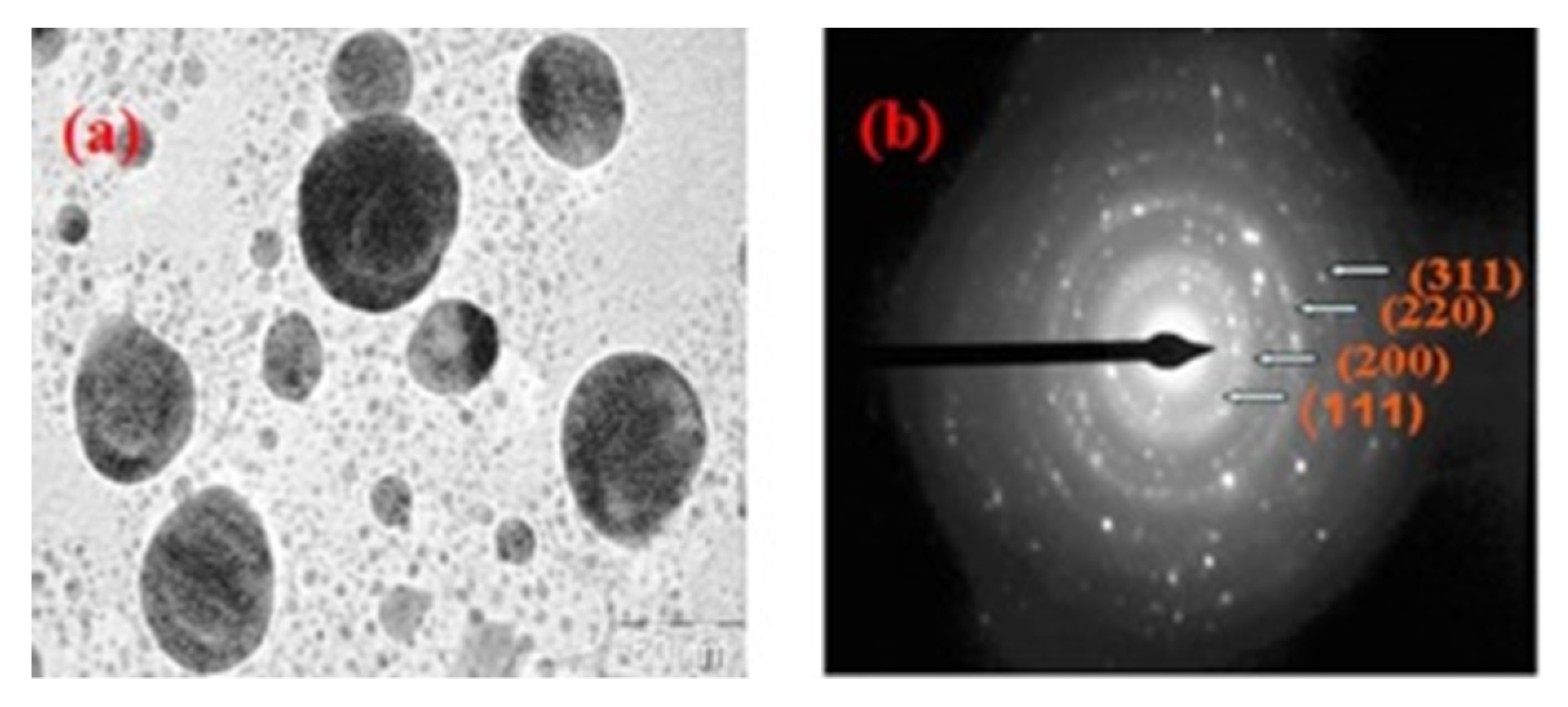
| TEM + selected area electron diffraction | Crystallographic analysis | Biopolymer assisted nanoparticles | [21,24] |
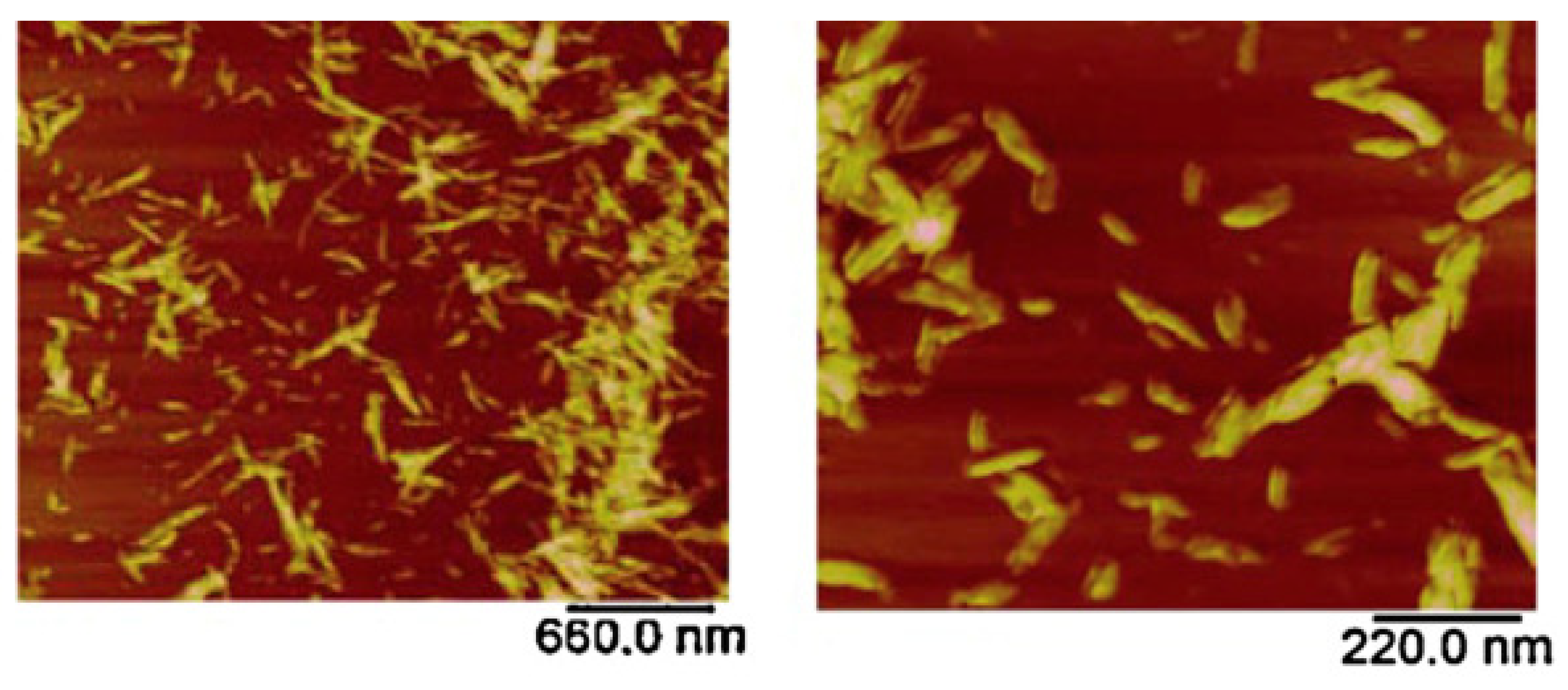
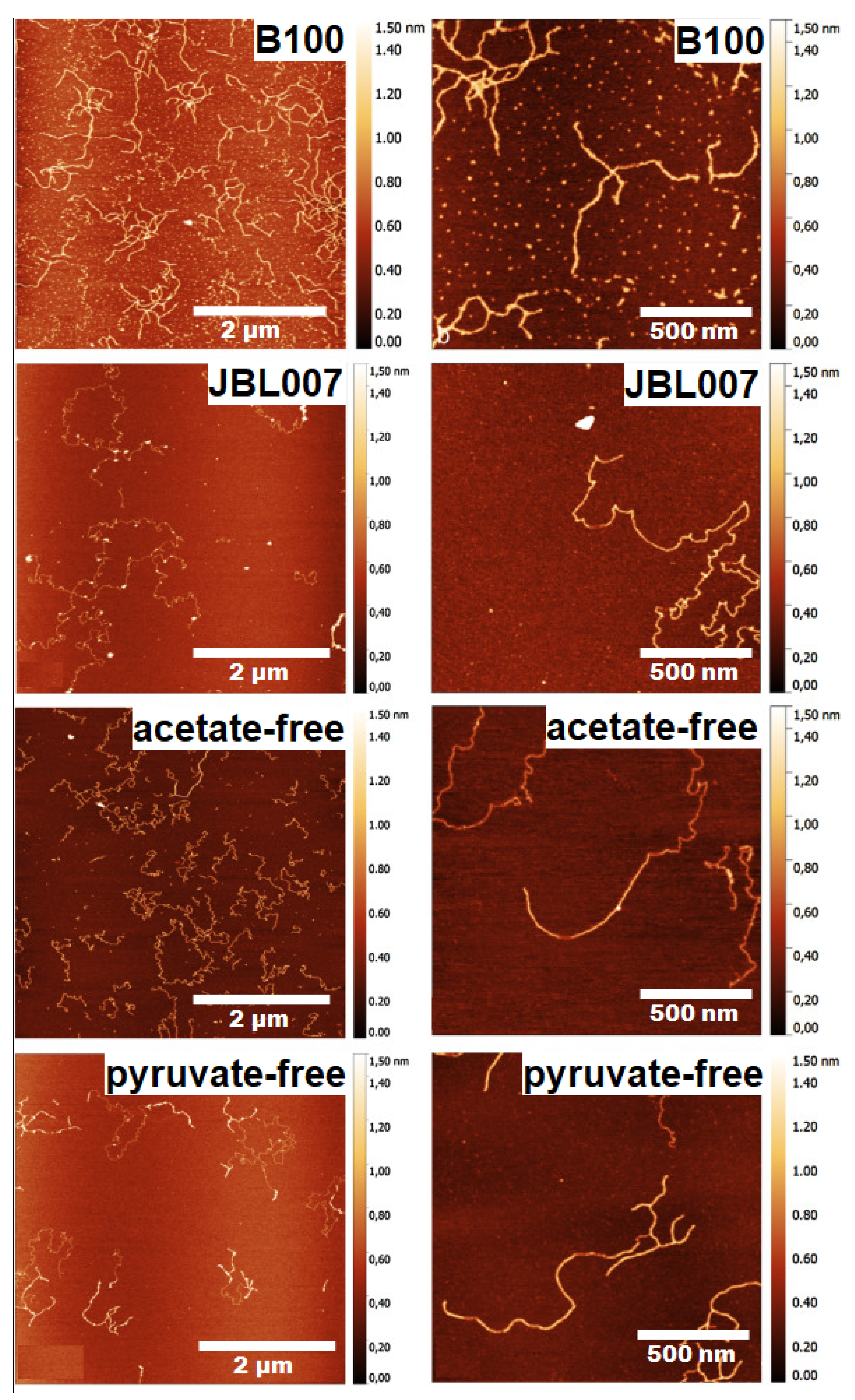
| Atomic force microscopy | Molecular structure and conformation | Xanthan gum | [76,77,78] |
| Nanomaterial topography | Nanocellulose | [79,80] | |
| Particle size and shape | Nanocellulose | [81] | |
| Chemical force microscopy | Chemical interactions | Chitosan | [82] |
| Magnetic force microscopy | Magnetic properties | Chitosan based magnetic nanohydrogels | [83] |
| Scanning tunneling microscopy | Molecular structure Particle Size Surface modification | Bacterial polysaccharides Cellulose Cellulose | [84,85] [86] [87] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venkateshaiah, A.; Padil, V.V.T.; Nagalakshmaiah, M.; Waclawek, S.; Černík, M.; Varma, R.S. Microscopic Techniques for the Analysis of Micro and Nanostructures of Biopolymers and Their Derivatives. Polymers 2020, 12, 512. https://doi.org/10.3390/polym12030512
Venkateshaiah A, Padil VVT, Nagalakshmaiah M, Waclawek S, Černík M, Varma RS. Microscopic Techniques for the Analysis of Micro and Nanostructures of Biopolymers and Their Derivatives. Polymers. 2020; 12(3):512. https://doi.org/10.3390/polym12030512
Chicago/Turabian StyleVenkateshaiah, Abhilash, Vinod V.T. Padil, Malladi Nagalakshmaiah, Stanisław Waclawek, Miroslav Černík, and Rajender S. Varma. 2020. "Microscopic Techniques for the Analysis of Micro and Nanostructures of Biopolymers and Their Derivatives" Polymers 12, no. 3: 512. https://doi.org/10.3390/polym12030512
APA StyleVenkateshaiah, A., Padil, V. V. T., Nagalakshmaiah, M., Waclawek, S., Černík, M., & Varma, R. S. (2020). Microscopic Techniques for the Analysis of Micro and Nanostructures of Biopolymers and Their Derivatives. Polymers, 12(3), 512. https://doi.org/10.3390/polym12030512










