pH-Dependent Foam Formation Using Amphoteric Colloidal Polymer Particles
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
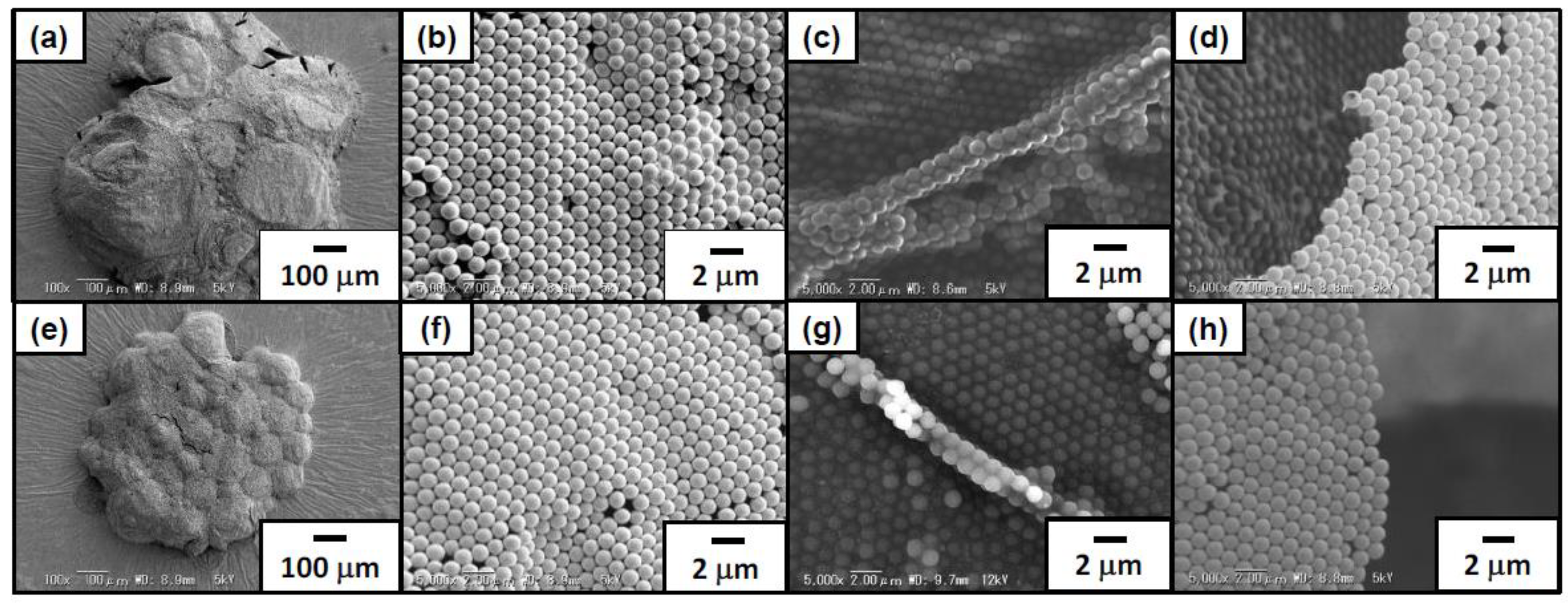
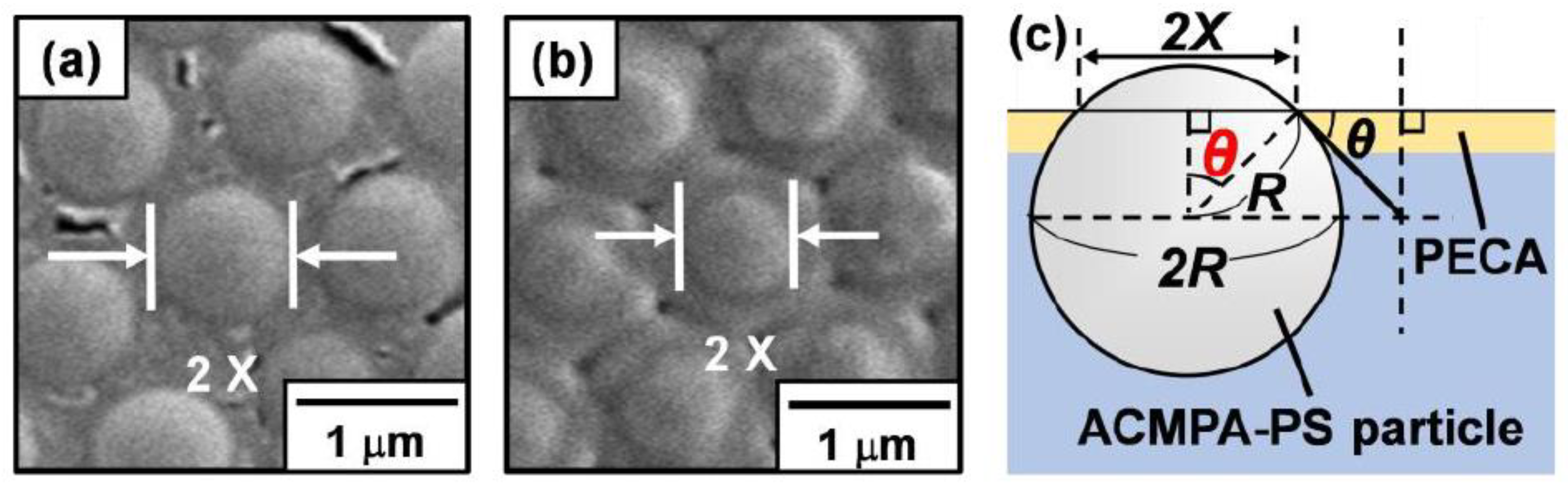
3.1. Synthesis and Characterization of Amphoteric Polystyrene (PS) Particles
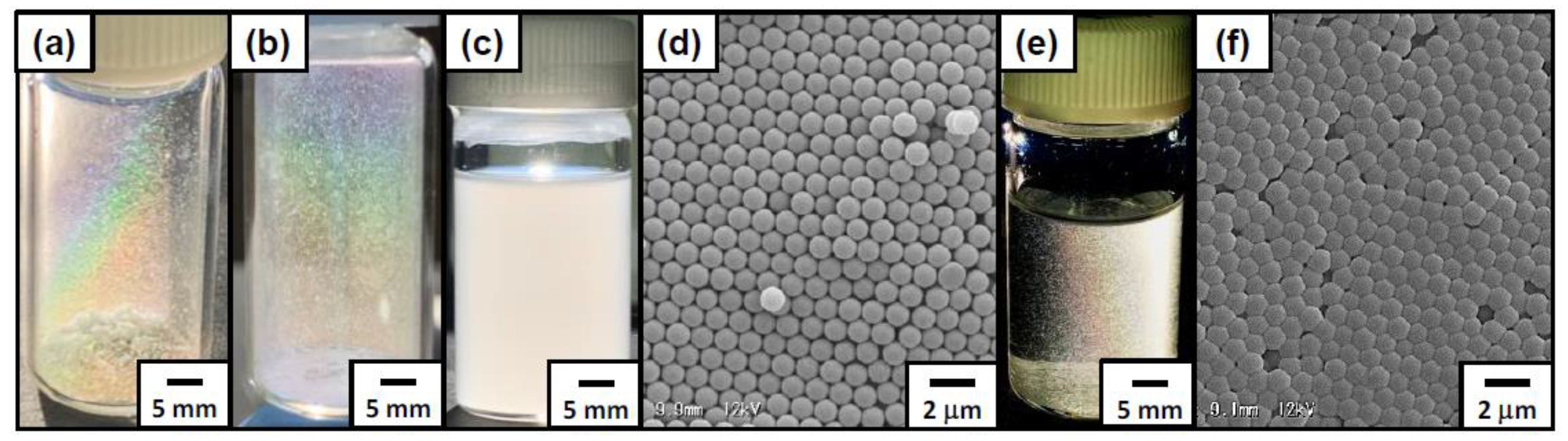
3.2. Foams Stabilized with Amphoteric PS Particles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ramsden, W. Separation of solids in the surface-layers of solutions and ‘suspensions’ (Observations on surface-membranes, bubbles, emulsions, and mechanical coagulation)-Preliminary account. Proc. R. Soc. 1903, 72, 156–164. [Google Scholar]
- Binks, B.P. Colloidal Particles at Liquid Interfaces; Horozov, T.S., Ed.; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Studart, A.R.; Gonzenbach, U.T.; Tervoort, E.; Gauckler, L.J. Processing routes to macroporous ceramics: A review. J. Am. Ceram. Soc. 2006, 89, 1771–1789. [Google Scholar] [CrossRef]
- Hunter, T.N.; Pugh, R.J.; Franks, G.V.; Jameson, G.J. The role of particles in stabilising foams and emulsions. Adv. Colloid Interface Sci. 2008, 137, 57–81. [Google Scholar] [CrossRef]
- Fujii, S.; Murakami, R. Smart particles as foam and liquid marble stabilizers. KONA Powder Part. J. 2008, 26, 153–166. [Google Scholar] [CrossRef]
- Dickinson, E.; Ettelaie, R.; Kostakis, T.; Murray, B.S. Factors controlling the formation and stability of air bubbles stabilized by partially hydrophobic silica nanoparticles. Langmuir 2004, 20, 8517–8525. [Google Scholar] [CrossRef]
- Binks, B.P.; Horozov, T.S. Aqueous foams stabilized solely by silica nanoparticles. Angew. Chem. Int. Ed. 2005, 44, 3722–3725. [Google Scholar] [CrossRef]
- Gonzenbach, U.T.; Studart, A.R.; Tervoort, E.; Gauckler, L.J. Ultrastable particle stabilized foams. Angew. Chem. Int. Ed. 2006, 45, 3526–3530. [Google Scholar] [CrossRef]
- Jorba, D.A.; Thomas, E.L. Foams of Graphene, Method of Making and Materials Made Thereof. The International Patent System WO2012174360A1, 20 December 2012. [Google Scholar]
- Subramaniam, A.B.; Abkarian, M.; Stone, H.A. Controlled assembly of jammed colloidal shells on fluid droplets. Nat. Mater. 2005, 4, 553–556. [Google Scholar] [CrossRef]
- Fujii, S.; Ryan, A.J.; Armes, S.P. Long-range structural order, moiré patterns, and iridescence in latex-stabilized foams. J. Am. Chem. Soc. 2006, 128, 7882–7886. [Google Scholar] [CrossRef]
- Fujii, S.; Iddon, P.D.; Ryan, A.J.; Armes, S.P. Aqueous particulate foams stabilized solely with polymer latex particles. Langmuir 2006, 22, 7512–7520. [Google Scholar] [CrossRef]
- Kettlewell, S.L.; Schmid, A.; Fujii, S.; Dupin, D.; Armes, S.P. Is latex surface charge an important parameter for foam stabilization. Langmuir 2007, 23, 11381–11386. [Google Scholar] [CrossRef]
- Alargova, R.G.; Warhadpande, D.S.; Paunov, V.N.; Velev, O.D. Foam superstabilization by polymer microrods. Langmuir 2004, 20, 10371–10374. [Google Scholar] [CrossRef]
- Iqbal, S.H.; Webster, J. The trapping of aquatic hyphomycete spores by air bubbles. Trans. Br. Mycol. Soc. 1973, 60, 37–48. [Google Scholar] [CrossRef]
- Dognon, A. Concentration et separation des molecular et separation des molecular et des particules par le method des mousses. Rev. Sci. (Fr.) 1941, 79, 613–619. [Google Scholar]
- Fameau, A.-L.; Adrian, C.; Saint-Jalmes, A.; Klitzing, R.V. Responsive aqueous foams. Chem. Phys. Chem. 2015, 16, 66–75. [Google Scholar] [CrossRef]
- Fujii, S.; Nakamura, Y. Stimuli-responsive bubbles and foams stabilized with solid particles. Langmuir 2017, 33, 7365–7379. [Google Scholar] [CrossRef]
- Fujii, S. Stimulus-responsive soft dispersed systems developed based on functional polymer particles: Bubbles and liquid marbles. Polym. J. 2019, 51, 1081–1101. [Google Scholar] [CrossRef]
- Fujii, S.; Akiyama, K.; Nakayama, S.; Hamasaki, S.; Yusa, S.; Nakamura, Y. pH- and temperature-responsive aqueous foams stabilized by hairy latex particles. Soft Matter 2015, 11, 572–579. [Google Scholar] [CrossRef]
- Fujii, S.; Mochizuki, M.; Aono, K.; Hamasaki, S.; Murakami, R.; Nakamura, Y. pH-Responsive aqueous foams stabilized by hairy latex particles. Langmuir 2011, 27, 12902–12909. [Google Scholar] [CrossRef]
- Nakayama, S.; Yusa, S.; Nakamura, Y.; Fujii, S. Aqueous foams stabilized by temperature-sensitive hairy polymer particles. Soft Matter 2015, 11, 9099–9106. [Google Scholar] [CrossRef]
- Nakayama, S.; Hamasaki, S.; Ueno, S.; Mochizuki, M.; Yusa, S.; Nakamura, Y.; Fujii, S. Foams stabilized with solid particles carrying stimuli-responsive polymer hairs. Soft Matter 2016, 12, 4794–4804. [Google Scholar] [CrossRef]
- Ito, M.; Takano, K.; Hanochi, H.; Yusa, S.; Nakamura, Y.; Fujii, S. pH-Responsive aqueous bubbles stabilized with polymer particles carrying poly(4-vinylpyridine) colloidal stabilizer. Front. Chem. 2018, 6, 269. [Google Scholar] [CrossRef]
- Binks, B.P.; Murakami, R.; Armes, S.P.; Fujii, S.; Schmid, A. pH-Responsive aqueous foams stabilized by ionisable latex particles. Langmuir 2007, 23, 8691–8694. [Google Scholar] [CrossRef]
- Gu, S.; Inukai, S.; Konno, M. Preparation of monodisperse, micron-sized polystyrene particles with an amphoteric initiator in soapfree polymerization. J. Chem. Eng. Jpn. 2002, 35, 977–981. [Google Scholar] [CrossRef]
- Gu, S.; Inukai, S.; Konno, M. Soapfree Synthesis of monodisperse, micron-sized polystyrene particles in aqueous media. J. Chem. Eng. Jpn. 2003, 36, 1231–1235. [Google Scholar] [CrossRef]
- Yamada, Y.; Sakamoto, T.; Gu, S.; Konno, M. Soap-free synthesis for producing highly monodisperse, micrometer-sized polystyrene particles up to 6 μm. J. Colloid Interface Sci. 2005, 281, 249–252. [Google Scholar] [CrossRef]
- Nagao, D.; Sakamoto, T.; Konno, H.; Gu, S.; Konno, M. Preparation of micrometer-sized polymer particles with control of Initiator dissociation during soap-free emulsion polymerization. Langmuir 2006, 22, 10958–10962. [Google Scholar] [CrossRef]
- Vogel, N.; Ally, J.; Bley, K.; Kappl, M.; Landfester, K.; Weiss, C.K. Direct visualization of the interfacial position of colloidal particles and their assemblies. Nanoscale 2014, 6, 6879–6885. [Google Scholar] [CrossRef]
- Fukuoka, K.; Tomikawa, A.; Nakamura, Y.; Fujii, S. Aqueous foams stabilized with several tens of micrometer-sized polymer particles: Effects of surface hydrophilic-hydrophobic balance on foamability and foam stability. Chem. Lett. 2016, 45, 667–669. [Google Scholar] [CrossRef]
- Sekido, T.; Kappl, M.; Butt, H.-J.; Yusa, S.; Nakamura, Y.; Fujii, S. Effects of pH on structure and mechanical properties of dried pH-responsive latex particles. Soft Matter 2017, 13, 7562–7570. [Google Scholar] [CrossRef]
- Sekido, T.; Wooh, S.; Fuchs, R.; Kappl, M.; Nakamura, Y.; Butt, H.-J.; Fujii, S. Controlling the structure of supraballs by pH-responsive particle assembly. Langmuir 2017, 33, 1995–2002. [Google Scholar] [CrossRef]
- Asaumi, Y.; Rey, M.; Vogel, N.; Nakamura, Y.; Fujii, S. Particle monolayer-stabilized light-sensitive liquid marbles from polypyrrole-coated microparticles. Langmuir 2020. [Google Scholar] [CrossRef]
- Fang, S.; Fujimoto, K.; Kondo, S.; Shiraki, K.; Kawaguchi, H. Emulsifier-free emulsion copolymerizartion of styrene and acrylamide using an amphoteric initiator. Colloid Polym. Sci. 2000, 278, 864–871. [Google Scholar] [CrossRef]
- Beattie, J.K.; Djordjev, A.M. The pristine oil/water interface: Surfactant-free hydroxide-charged emulsions. Angew. Chem. Int. Ed. 2004, 43, 3568–3571. [Google Scholar] [CrossRef]
- Roger, K.; Cabane, B. Why are hydrophobic/water interfaces negatively charged? Angew. Chem. Int. Ed. 2012, 51, 5625–5628. [Google Scholar] [CrossRef]
- Mills, P.; Snabre, P. Settling of a suspension of hard spheres. Europhys. Lett. 1994, 25, 651–656. [Google Scholar] [CrossRef]
- Kobayashi, M.; Juillerat, F.; Galletto, P.; Bowen, P.; Borkovec, M. Aggregation and charging of colloidal silica particles: Effect of particle size. Langmuir 2005, 21, 5761–5769. [Google Scholar] [CrossRef]
- Takahashi, M. Potential of microbubbles in aqueous solutions: Electrical properties of the gas—Water Interface. J. Phys. Chem. B 2005, 109, 21858–21864. [Google Scholar] [CrossRef]
- Jackson, J.D. Classical Electrodynamics, 3rd ed.; Wiley: New York, NY, USA, 1998; Volume 4, pp. 145–173. [Google Scholar]
- Yim, H.; Kent, M.S.; Matheson, A.; Stevens, M.J.; Ivkov, R.; Satija, S.; Majewski, J.; Smith, G.S. Adsorption of sodium poly(styrenesulfonate) to the air surface of water by Neutron and X-ray reflectivity and surface tension measurements: Polymer concentration dependence. Macromolecules 2002, 35, 9737–9747. [Google Scholar] [CrossRef]
- Ahrens, H.; Baltes, H.; Schmitt, J.; Möwald, H.; Helm, C. Polyelectrolyte adsorption onto insoluble monolayers at the air/water interface. Macromolecules 2001, 34, 4504–4512. [Google Scholar] [CrossRef]
- Matsuoka, H.; Maeda, S.; Kaewsaiha, P.; Matsumoto, K. Micellization of non-surface-active diblock copolymers in water. Special characteristics of Poly (styrene)-block-poly (styrenesulfonate). Langmuir 2004, 20, 7412–7421. [Google Scholar] [CrossRef]
- Fujii, S.; Kappl, M.; Butt, H.-J.; Sugimoto, T.; Nakamura, Y. Soft Janus colloidal crystal film. Angew. Chem. Int. Ed. 2012, 51, 9809–9813. [Google Scholar] [CrossRef]
- Levine, S.; Bowen, B.; Partridge, S.J. Stabilization of emulsions by fine particles I. Partitioning of particles between continuous phase and oil/water interface. Colloids Surf. 1989, 38, 325–343. [Google Scholar] [CrossRef]
- Rayleigh, L., XII. On the Manufacture and Theory of Diffraction Gratings. Philos. Mag. 1874, 47, 81–93. [Google Scholar] [CrossRef]
- Rayleigh, L., XXV. On the Manufacture and Theory of Diffraction Gratings. Philos. Mag. 1874, 47, 193–205. [Google Scholar] [CrossRef]
- Hayashi, S.; Seo, T.; Hata, H.; Hirai, T. Lens functions of polymer micro-particle arrays. Kobunshi Ronbunshu 1994, 51, 623–630. [Google Scholar] [CrossRef]
- Lazarov, G.S.; Denkov, N.D.; Velev, O.D.; Kralchevsky, P.A.; Nagayama, K.J. Formation of two-dimensional structures from colloidal particles on fluorinated oil substrate. Chem. Soc. Faraday Trans. 1994, 90, 2077–2083. [Google Scholar] [CrossRef]
- Hirai, T.; Hayashi, S. Lens functions of polymer microparticle arrays. Colloids Surf. A 1999, 153, 503–513. [Google Scholar] [CrossRef]
- Velev, O.D.; Lenhoff, A.M.; Kaler, E.W. A class of microstructured particles through colloidal crystallization. Science 2000, 287, 2240–2243. [Google Scholar] [CrossRef]
- Fudouzi, H.; Xia, Y. Colloidal crystals with tunable colors and their use as photonic papers. Langmuir 2003, 19, 9653–9660. [Google Scholar] [CrossRef]
- Okubo, T. Polymer colloidal crystals. Prog. Polym. Sci. 1993, 18, 481–517. [Google Scholar] [CrossRef]
- Takeoka, Y. Stimuli-responsive opals: Colloidal crystals and colloidal amorphous arrays for use in functional structurally colored materials. J. Mater. Chem. C 2013, 1, 6059–6074. [Google Scholar] [CrossRef]
- Kawamura, A.; Kohri, M.; Yoshioka, S.; Taniguchi, T.; Kishikawa, K. Structural color tuning: Mixing melanin-like particles with different diameters to create neutral colors. Langmuir 2017, 33, 3824–3830. [Google Scholar] [CrossRef]
- Lenzmann, F.; Li, K.; Kitai, A.H.; Stöver, H.D.H. Thin-film micropatterning using polymer microspheres. Chem. Mater. 1994, 6, 156–159. [Google Scholar] [CrossRef]
- Prevo, B.G.; Velev, O.D. Controlled, rapid deposition of structured coatings from micro- and nanoparticle suspensions. Langmuir 2004, 20, 2099–2107. [Google Scholar] [CrossRef]
- Ito, M.; Mayama, H.; Asaumi, Y.; Nakamura, Y.; Fujii, S. Light-driven locomotion of bubbles. Langmuir 2020. [Google Scholar] [CrossRef]
- Chen, S.; Fei, L.; Ge, F.; Liu, J.; Yin, Y.; Wang, C. A versatile and recycled pigment foam coloring approach for natural and synthetic fibers with nearly-zero pollutant discharge. J. Clean. Prod. 2020, 243, 118504. [Google Scholar] [CrossRef]
- Tang, C.; Xiao, E.; Sinko, P.J.; Szekely, Z.; Prud’homme, R.K. Responsive foams for nanoparticle delivery. Colloids Surf. B Biointerfaces 2015, 133, 81–87. [Google Scholar] [CrossRef]
- Singh, R.; Panthi, K.; Weerasooriya, U.; Mohanty, K.K. Multistimuli-responsive foams using an anionic surfactant. Langmuir 2018, 34, 11010–11020. [Google Scholar] [CrossRef]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fukui, S.; Hirai, T.; Nakamura, Y.; Fujii, S. pH-Dependent Foam Formation Using Amphoteric Colloidal Polymer Particles. Polymers 2020, 12, 511. https://doi.org/10.3390/polym12030511
Fukui S, Hirai T, Nakamura Y, Fujii S. pH-Dependent Foam Formation Using Amphoteric Colloidal Polymer Particles. Polymers. 2020; 12(3):511. https://doi.org/10.3390/polym12030511
Chicago/Turabian StyleFukui, Sayaka, Tomoyasu Hirai, Yoshinobu Nakamura, and Syuji Fujii. 2020. "pH-Dependent Foam Formation Using Amphoteric Colloidal Polymer Particles" Polymers 12, no. 3: 511. https://doi.org/10.3390/polym12030511
APA StyleFukui, S., Hirai, T., Nakamura, Y., & Fujii, S. (2020). pH-Dependent Foam Formation Using Amphoteric Colloidal Polymer Particles. Polymers, 12(3), 511. https://doi.org/10.3390/polym12030511




