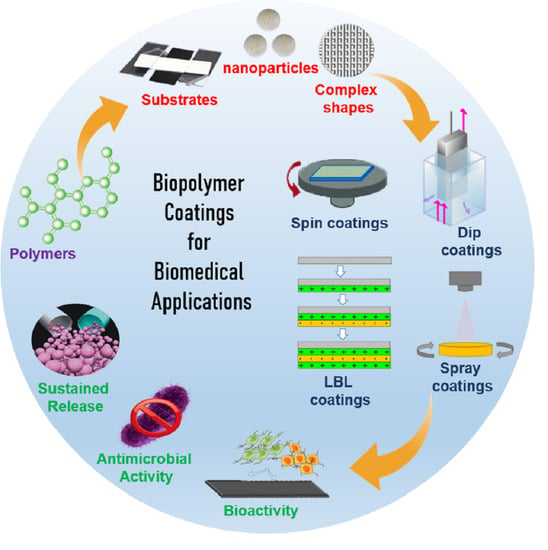
Biopolymer Coatings for Biomedical Applications
Abstract
:1. Introduction
2. Polymer Coatings and Films
3. Biopolymer Coating Methods
4. Biopolymer Coatings on Metal Implants
5. Biopolymer Coatings for Surface Modification
5.1. Polyvinylidene Fluoride (PVDF)
5.2. Polymethyl Methacrylate (PMMA)
5.3. Polypropylene (PP)
5.4. Polydimethylsiloxane (PDMS)
5.5. Polyurethane (PU)
6. Other Biopolymer Coatings
7. Biopolymer Coatings on Nanoparticles
8. Conclusions and Future Prospects
Funding
Conflicts of Interest
References
- Makhlouf, A.S.H.; Perez, A.; Guerrero, E. Recent trends in smart polymeric coatings in biomedicine and drug delivery applications. In Advances in Smart Coatings and Thin Films for Future Industrial and Biomedical Engineering Applications; Elsevier Inc.: Amsterdam, The Netherland, 2019; pp. 359–381. ISBN 9780128498705. [Google Scholar] [CrossRef]
- Augello, C.; Liu, H. Surface modification of magnesium by functional polymer coatings for neural applications. In Surface Modification of Magnesium and its Alloys for Biomedical Applications; Elsevier Ltd.: Amsterdam, The Netherlands, 2015; Volume 2, pp. 335–353. ISBN 9781782420835. [Google Scholar] [CrossRef]
- Landry, M.J.; Rollet, F.G.; Kennedy, T.E.; Barrett, C.J. Layers and Multilayers of Self-Assembled Polymers: Tunable Engineered Extracellular Matrix Coatings for Neural Cell Growth. Langmuir 2018, 34, 8709–8730. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Han, U.; Choi, D.; Hong, J. Layer-by-layer assembled polymeric thin films as prospective drug delivery carriers: Design and applications. Biomater. Res. 2018, 22, 29. [Google Scholar] [CrossRef] [PubMed]
- Leontidis, E. Langmuir-Blodgett Films: Sensor and Biomedical Applications and Comparisons with the Layer-by-Layer Method. In Surface Treatments for Biological, Chemical and Physical Applications; Gursoy, M., Karaman, M., Eds.; Wiley-VCH Verlag: Weinheim, Germany, 2016; pp. 181–208. [Google Scholar] [CrossRef]
- Hussain, S.A.; Dey, B.; Bhattacharjee, D.; Mehta, N. Unique supramolecular assembly through Langmuir—Blodgett (LB) technique. Heliyon 2018, 4, e01038. [Google Scholar] [CrossRef] [Green Version]
- Kim, W.; Jung, J. Polymer brush: A promising grafting approach to scaffolds for tissue engineering. BMB Rep. 2016, 49, 655–661. [Google Scholar] [CrossRef] [Green Version]
- Krishnamoorthy, M.; Hakobyan, S.; Ramstedt, M.; Gautrot, J.E. Surface-initiated polymer brushes in the biomedical field: Applications in membrane science, biosensing, cell culture, regenerative medicine and antibacterial coatings. Chem. Rev. 2014, 114, 10976–11026. [Google Scholar] [CrossRef]
- Ma, S.; Zhang, X.; Yu, B.; Zhou, F. Brushing up functional materials. NPG Asia Mater. 2019, 11, 24. [Google Scholar] [CrossRef]
- Ramkumar, M.C.; Cools, P.; Arunkumar, A.; De Geyter, N.; Morent, R.; Kumar, V.; Udaykumar, S.; Gopinath, P.; Jaganathan, S.K.; Pandiyaraj, K.N. Polymer coatings for biocompatibility and reduced nonspecific adsorption. In Functionalised Cardiovascular Stents; Elsevier Ltd.: Amsterdam, The Netherland, 2018; pp. 155–198. ISBN 9780081004968. [Google Scholar] [CrossRef]
- Khelifa, F.; Ershov, S.; Habibi, Y.; Snyders, R.; Dubois, P. Free-Radical-Induced Grafting from Plasma Polymer Surfaces. Chem. Rev. 2016, 116, 3975–4005. [Google Scholar] [CrossRef]
- Anand, V.; Thomas, R.; Thulasi Raman, K.H.; Gowravaram, M.R. Plasma-Induced Polymeric Coatings. In Non-Thermal Plasma Technology for Polymeric Materials; Elsevier Inc.: Amsterdam, The Netherland, 2019; pp. 129–157. ISBN 9780128131527. [Google Scholar] [CrossRef]
- Song, J.; Winkeljann, B.; Lieleg, O. Biopolymer-Based Coatings: Promising Strategies to Improve the Biocompatibility and Functionality of Materials Used in Biomedical Engineering. Adv. Mater. Interfaces 2020, 7, 2000850. [Google Scholar] [CrossRef]
- Reggente, M.; Natali, M.; Passeri, D.; Lucci, M.; Davoli, I.; Pourroy, G.; Masson, P.; Palkowski, H.; Hangen, U.; Carradò, A.; et al. Multiscale mechanical characterization of hybrid Ti/PMMA layered materials. Colloids Surf. A Physicochem. Eng. Asp. 2017, 532, 244–251. [Google Scholar] [CrossRef]
- Liu, S.; Chen, C.; Chen, L.; Zhu, H.; Zhang, C.; Wang, Y. Pseudopeptide polymer coating for improving biocompatibility and corrosion resistance of 316L stainless steel. RSC Adv. 2015, 5, 98456–98466. [Google Scholar] [CrossRef]
- Gnedenkov, S.V.; Sinebryukhov, S.L.; Mashtalyar, D.V.; Egorkin, V.S.; Sidorova, M.V.; Gnedenkov, A.S. Composite polymer-containing protective coatings on magnesium alloy MA8. Corros. Sci. 2014, 85, 52–59. [Google Scholar] [CrossRef]
- Gnedenkov, S.V.; Sinebryukhov, S.L.; Zavidnaya, A.G.; Egorkin, V.S.; Puz’, A.V.; Mashtalyar, D.V.; Sergienko, V.I.; Yerokhin, A.L.; Matthews, A. Composite hydroxyapatite-PTFE coatings on Mg-Mn-Ce alloy for resorbable implant applications via a plasma electrolytic oxidation-based route. J. Taiwan Inst. Chem. Eng. 2014, 45, 3104–3109. [Google Scholar] [CrossRef]
- Oosterbeek, R.N.; Seal, C.K.; Seitz, J.M.; Hyland, M.M. Polymer-bioceramic composite coatings on magnesium for biomaterial applications. Surf. Coat. Technol. 2013, 236, 420–428. [Google Scholar] [CrossRef]
- Guo, Y.; Su, Y.; Gu, R.; Zhang, Z.; Li, G.; Lian, J.; Ren, L. Enhanced corrosion resistance and biocompatibility of biodegradable magnesium alloy modified by calcium phosphate/collagen coating. Surf. Coat. Technol. 2020, 401, 126318. [Google Scholar] [CrossRef]
- Klinge, U.; Klosterhalfen, B.; Öttinger, A.P.; Junge, K.; Schumpelick, V. PVDF as a new polymer for the construction of surgical meshes. Biomaterials 2002, 23, 3487–3493. [Google Scholar] [CrossRef]
- Chiu, Y.-Y.; Lin, W.-Y.; Wang, H.-Y.; Huang, S.-B.; Wu, M.-H. Development of a piezoelectric polyvinylidene fluoride (PVDF) polymer-based sensor patch for simultaneous heartbeat and respiration monitoring. Sens. Actuators A Phys. 2013, 189, 328–334. [Google Scholar] [CrossRef]
- Sheikh, F.A.; Beigh, M.A.; Qadir, A.S.; Qureshi, S.H.; Kim, H. Hydrophilically modified poly(vinylidene fluoride) nanofibers incorporating cellulose acetate fabricated by colloidal electrospinning for future tissue-regeneration applications. Polym. Compos. 2019, 40, 1619–1630. [Google Scholar] [CrossRef]
- Azimi, B.; Sorayani Bafqi, M.S.; Fusco, A.; Ricci, C.; Gallone, G.; Bagherzadeh, R.; Donnarumma, G.; Uddin, M.J.; Latifi, M.; Lazzeri, A.; et al. Electrospun ZnO/Poly(Vinylidene Fluoride-Trifluoroethylene) Scaffolds for Lung Tissue Engineering. Tissue Eng. Part A 2020. [Google Scholar] [CrossRef]
- Nunes-Pereira, J.; Ribeiro, S.; Ribeiro, C.; Gombek, C.J.; Gama, F.M.; Gomes, A.C.; Patterson, D.A.; Lanceros-Méndez, S. Poly(vinylidene fluoride) and copolymers as porous membranes for tissue engineering applications. Polym. Test. 2015, 44, 234–241. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, C.; Sencadas, V.; Correia, D.M.; Lanceros-Méndez, S. Piezoelectric polymers as biomaterials for tissue engineering applications. Colloids Surf. B Biointerfaces 2015, 136, 46–55. [Google Scholar] [CrossRef] [Green Version]
- Rajabi, A.H.; Jaffe, M.; Arinzeh, T.L. Piezoelectric materials for tissue regeneration: A review. Acta Biomater. 2015, 24, 12–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Liao, C.; Tjong, S.C. Electrospun polyvinylidene fluoride-based fibrous scaffolds with piezoelectric characteristics for bone and neural tissue engineering. Nanomaterials 2019, 9, 952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tien, N.T.; Jeon, S.; Kim, D.-I.; Trung, T.Q.; Jang, M.; Hwang, B.-U.; Byun, K.-E.; Bae, J.; Lee, E.; Tok, J.B.-H.; et al. A flexible bimodal sensor array for simultaneous sensing of pressure and temperature. Adv. Mater. 2014, 26, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Khadtare, S.; Ko, E.J.; Kim, Y.H.; Lee, H.S.; Moon, D.K. A flexible piezoelectric nanogenerator using conducting polymer and silver nanowire hybrid electrodes for its application in real-time muscular monitoring system. Sens. Actuators A Phys. 2019, 299, 111575. [Google Scholar] [CrossRef]
- Yu, Y.; Sun, H.; Orbay, H.; Chen, F.; England, C.G.; Cai, W.; Wang, X. Biocompatibility and in vivo operation of implantable mesoporous PVDF-based nanogenerators. Nano Energy 2016, 27, 275–281. [Google Scholar] [CrossRef] [Green Version]
- Shin, K.Y.; Lee, J.S.; Jang, J. Highly sensitive, wearable and wireless pressure sensor using free-standing ZnO nanoneedle/PVDF hybrid thin film for heart rate monitoring. Nano Energy 2016, 22, 95–104. [Google Scholar] [CrossRef]
- Han, X.; Chen, X.; Tang, X.; Chen, Y.L.; Liu, J.H.; Shen, Q.D. Flexible Polymer Transducers for Dynamic Recognizing Physiological Signals. Adv. Funct. Mater. 2016, 26, 3640–3648. [Google Scholar] [CrossRef]
- Shen, X.; Zhao, Y.; Chen, L. Polycation-grafted poly(vinylidene fluoride) membrane with biofouling resistance. Chem. Eng. Technol. 2015, 38, 859–866. [Google Scholar] [CrossRef]
- Koh, E.; Lee, Y.T. Antimicrobial activity and fouling resistance of a polyvinylidene fluoride (PVDF) hollow-fiber membrane. J. Ind. Eng. Chem. 2017, 47, 260–271. [Google Scholar] [CrossRef]
- Shen, X.; Liu, P.; Xia, S.; Liu, J.; Wang, R.; Zhao, H.; Liu, Q.; Xu, J.; Wang, F. Anti-fouling and anti-bacterial modification of poly(vinylidene fluoride) membrane by blending with the capsaicin-based copolymer. Polymers 2019, 11, 323. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Ding, X.; Jiang, Y.; Guan, Y.; Wei, D.; Zheng, A.; Xu, X. Permanent Antimicrobial Poly(vinylidene fluoride) Prepared by Chemical Bonding with Poly(hexamethylene guanidine). ACS Omega 2020, 5, 10481–10488. [Google Scholar] [CrossRef] [PubMed]
- Tavakolmoghadam, M.; Mohammadi, T. Application of Colloidal Precipitation Method Using Sodium Polymethacrylate as Dispersant for TiO2/PVDF Membrane Preparation and Its Antifouling Properties. Polym. Eng. Sci. 2019, 59, E422–E434. [Google Scholar] [CrossRef]
- Rajavel, K.; Shen, S.; Ke, T.; Lin, D. Achieving high bactericidal and antibiofouling activities of 2D titanium carbide (Ti3C2Tx) by delamination and intercalation. 2D Mater. 2019, 6, 35040. [Google Scholar] [CrossRef]
- Yin, Z.; Tian, B.; Zhu, Q.; Duan, C. Characterization and application of PVDF and its copolymer films prepared by spin-coating and langmuir-blodgett method. Polymers 2019, 11, 2033. [Google Scholar] [CrossRef] [Green Version]
- Trung, T.Q.; Ramasundaram, S.; Hong, S.W.; Lee, N.E. Flexible and transparent nanocomposite of reduced graphene oxide and P(VDF-TrFE) copolymer for high thermal responsivity in a field-effect transistor. Adv. Funct. Mater. 2014, 24, 3438–3445. [Google Scholar] [CrossRef]
- Sorayani Bafqi, M.S.; Bagherzadeh, R.; Latifi, M. Fabrication of composite PVDF-ZnO nanofiber mats by electrospinning for energy scavenging application with enhanced efficiency. J. Polym. Res. 2015, 22, 130. [Google Scholar] [CrossRef]
- Elnabawy, E.; Hassanain, A.H.; Shehata, N.; Popelka, A.; Nair, R.; Yousef, S.; Kandas, I. Piezoelastic PVDF/TPU nanofibrous composite membrane: Fabrication and characterization. Polymers 2019, 11, 1634. [Google Scholar] [CrossRef] [Green Version]
- Aliahmadipoor, P.; Ghazanfari, D.; Gohari, R.J.; Akhgar, M.R. Preparation of PVDF/FMBO composite electrospun nanofiber for effective arsenate removal from water. RSC Adv. 2020, 10, 24653–24662. [Google Scholar] [CrossRef]
- Hu, X.; Yan, X.; Gong, L.; Wang, F.; Xu, Y.; Feng, L.; Zhang, D.; Jiang, Y. Improved Piezoelectric Sensing Performance of P(VDF-TrFE) Nanofibers by Utilizing BTO Nanoparticles and Penetrated Electrodes. ACS Appl. Mater. Interfaces 2019, 11, 7379–7386. [Google Scholar] [CrossRef]
- Maity, K.; Garain, S.; Henkel, K.; Schmeißer, D.; Mandal, D. Self-Powered Human-Health Monitoring through Aligned PVDF Nanofibers Interfaced Skin-Interactive Piezoelectric Sensor. ACS Appl. Polym. Mater. 2020, 2, 862–878. [Google Scholar] [CrossRef]
- Haddadi, S.A.; Ghaderi, S.; Amini, M.; Ramazani, S.A.A. Mechanical and piezoelectric characterizations of electrospun PVDF-nanosilica fibrous scaffolds for biomedical applications. Mater. Today Proc. 2018, 5, 15710–15716. [Google Scholar] [CrossRef]
- Sengupta, P.; Ghosh, A.; Bose, N.; Mukherjee, S.; Roy Chowdhury, A.; Datta, P. A comparative assessment of poly(vinylidene fluoride)/conducting polymer electrospun nanofiber membranes for biomedical applications. J. Appl. Polym. Sci. 2020, 137, e49115. [Google Scholar] [CrossRef]
- Wang, A.; Liu, Z.; Hu, M.; Wang, C.; Zhang, X.; Shi, B.; Fan, Y.; Cui, Y.; Li, Z.; Ren, K. Piezoelectric nanofibrous scaffolds as in vivo energy harvesters for modifying fibroblast alignment and proliferation in wound healing. Nano Energy 2018, 43, 63–71. [Google Scholar] [CrossRef]
- Manoukian, O.S.; Sardashti, N.; Stedman, T.; Gailiunas, K.; Ojha, A.; Penalosa, A.; Mancuso, C.; Hobert, M.; Kumbar, S.G. Biomaterials for tissue engineering and regenerative medicine. In Encyclopedia of Biomedical Engineering; Elsevier Inc.: Amsterdam, The Netherland, 2018; Volume 1–3, pp. 462–482. ISBN 9780128051443. [Google Scholar] [CrossRef]
- Saboktakin, M. Medical Applications of Poly Methyl Methacrylate Nanocomposites. JSMC Nanotechnol. Nanomed. 2019, 3, 7. [Google Scholar]
- Singh Jessy, R.; Hakimi Ibrahim, M. Biodegradability and Biocompatibility of Polymers with Emphasis on Bone Scaffolding: A Brief Review. Int. J. Sci. Res. Publ. 2014, 4, 7–9. [Google Scholar]
- Reggente, M.; Masson, P.; Dollinger, C.; Palkowski, H.; Zafeiratos, S.; Jacomine, L.; Passeri, D.; Rossi, M.; Vrana, N.E.; Pourroy, G.; et al. Novel Alkali Activation of Titanium Substrates to Grow Thick and Covalently Bound PMMA Layers. ACS Appl. Mater. Interfaces 2018, 10, 5967–5977. [Google Scholar] [CrossRef] [PubMed]
- Reggente, M.; Kriegel, S.; He, W.; Masson, P.; Pourroy, G.; Mura, F.; Faerber, J.; Passeri, D.; Rossi, M.; Palkowski, H.; et al. How alkali-activated Ti surfaces affect the growth of tethered PMMA chains: A close-up study on the PMMA thickness and surface morphology. Pure Appl. Chem. 2019, 91, 1687–1694. [Google Scholar] [CrossRef]
- Sun, A.; Ashammakhi, N.; Dokmeci, M.R. Methacrylate coatings for titanium surfaces to optimize biocompatibility. Micromachines 2020, 11, 87. [Google Scholar] [CrossRef] [Green Version]
- Galvão, C.N.; Sanches, L.M.; Mathiazzi, B.I.; Ribeiro, R.T.; Petri, D.F.S.; Carmona-Ribeiro, A.M. Antimicrobial coatings from hybrid nanoparticles of biocompatible and antimicrobial polymers. Int. J. Mol. Sci. 2018, 19, 2965. [Google Scholar] [CrossRef] [Green Version]
- Suteewong, T.; Wongpreecha, J.; Polpanich, D.; Jangpatarapongsa, K.; Kaewsaneha, C.; Tangboriboonrat, P. PMMA particles coated with chitosan-silver nanoparticles as a dual antibacterial modifier for natural rubber latex films. Colloids Surf. B Biointerfaces 2019, 174, 544–552. [Google Scholar] [CrossRef]
- Scheidbach, H.; Tamme, C.; Tannapfel, A.; Lippert, H.; Köckerling, F. In vivo studies comparing the biocompatibility of various polypropylene meshes and their handling properties during endoscopic total extraperitoneal (TEP) patchplasty: An experimental study in pigs. Surg. Endosc. Other Interv. Tech. 2004, 18, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Saitaer, X.; Sanbhal, N.; Qiao, Y.; Li, Y.; Gao, J.; Brochu, G.; Guidoin, R.; Khatri, A.; Wang, L. Polydopamine-inspired surface modification of polypropylene hernia mesh devices via cold oxygen plasma: Antibacterial and drug release properties. Coatings 2019, 9, 164. [Google Scholar] [CrossRef] [Green Version]
- Teo, A.J.T.; Mishra, A.; Park, I.; Kim, Y.J.; Park, W.T.; Yoon, Y.J. Polymeric Biomaterials for Medical Implants and Devices. ACS Biomater. Sci. Eng. 2016, 2, 454–472. [Google Scholar] [CrossRef]
- Hachim, D.; Brown, B.N. Surface modification of polypropylene for enhanced layer-by-layer deposition of polyelectrolytes. J. Biomed. Mater. Res. Part A 2018, 106, 2078–2085. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.; Choi, H.; Jeong, H.; Baek, S.; Han, S.; Chung, D.J.; Lee, H.S. Thermally Crosslinked Biocompatible Hydrophilic Polyvinylpyrrolidone Coatings on Polypropylene with Enhanced Mechanical and Adhesion Properties. Macromol. Res. 2018, 26, 151–156. [Google Scholar] [CrossRef]
- Steinmetz, H.P.; Sason, E.; Lublin-Tennenbaum, T.; Margel, S. Engineering of new durable cross-linked poly(styryl bisphosphonate) thin coatings onto polypropylene films for biomedical applications. Appl. Surf. Sci. 2020, 508, 145171. [Google Scholar] [CrossRef]
- Gehlen, D.B.; De Lencastre Novaes, L.C.; Long, W.; Ruff, A.J.; Jakob, F.; Haraszti, T.; Chandorkar, Y.; Yang, L.; Van Rijn, P.; Schwaneberg, U.; et al. Rapid and Robust Coating Method to Render Polydimethylsiloxane Surfaces Cell-Adhesive. ACS Appl. Mater. Interfaces 2019, 11, 41091–41099. [Google Scholar] [CrossRef]
- Zhang, H.; Chiao, M. Anti-fouling coatings of poly(dimethylsiloxane) devices for biological and biomedical applications. J. Med. Biol. Eng. 2015, 35, 143–155. [Google Scholar] [CrossRef] [Green Version]
- Rudy, A.; Kuliasha, C.; Uruena, J.; Rex, J.; Schulze, K.D.; Stewart, D.; Angelini, T.; Sawyer, W.G.; Perry, S.S. Lubricous Hydrogel Surface Coatings on Polydimethylsiloxane (PDMS). Tribol. Lett. 2017, 65, 3. [Google Scholar] [CrossRef]
- Mai, H.N.; Hyun, D.C.; Park, J.H.; Kim, D.Y.; Lee, S.M.; Lee, D.H. Antibacterial drug-release polydimethylsiloxane coating for 3d-printing dental polymer: Surface alterations and antimicrobial effects. Pharmaceuticals 2020, 13, 304. [Google Scholar] [CrossRef]
- Xue, P.; Li, Q.; Li, Y.; Sun, L.; Zhang, L.; Xu, Z.; Kang, Y. Surface modification of poly(dimethylsiloxane) with polydopamine and hyaluronic acid to enhance hemocompatibility for potential applications in medical implants or devices. ACS Appl. Mater. Interfaces 2017, 9, 33632–33644. [Google Scholar] [CrossRef] [PubMed]
- Gowa Oyama, T.; Barba, B.J.D.; Hosaka, Y.; Taguchi, M. Single-step fabrication of polydimethylsiloxane microwell arrays with long-lasting hydrophilic inner surfaces. Appl. Phys. Lett. 2018, 112, 213704. [Google Scholar] [CrossRef]
- Mahmoodi, Z.; Mohammadnejad, J.; Razavi Bazaz, S.; Abouei Mehrizi, A.; Ghiass, M.A.; Saidijam, M.; Dinarvand, R.; Ebrahimi Warkiani, M.; Soleimani, M. A simple coating method of PDMS microchip with PTFE for synthesis of dexamethasone-encapsulated PLGA nanoparticles. Drug Deliv. Transl. Res. 2019, 9, 707–720. [Google Scholar] [CrossRef] [PubMed]
- Barrioni, B.R.; De Carvalho, S.M.; Oréfice, R.L.; De Oliveira, A.A.R.; Pereira, M.D.M. Synthesis and characterization of biodegradable polyurethane films based on HDI with hydrolyzable crosslinked bonds and a homogeneous structure for biomedical applications. Mater. Sci. Eng. C 2015, 52, 22–30. [Google Scholar] [CrossRef]
- Davis, F.J.; Mitchell, G.R. Polyurethane based materials with applications in medical devices. In Bio-Materials and Prototyping Applications in Medicine; Bartolo, P., Bidanda, B., Eds.; Springer: Berlin, Germany, 2008; pp. 27–48. ISBN 9780387476827. [Google Scholar] [CrossRef]
- Tatai, L.; Moore, T.G.; Adhikari, R.; Malherbe, F.; Jayasekara, R.; Griffiths, I.; Gunatillake, P.A. Thermoplastic biodegradable polyurethanes: The effect of chain extender structure on properties and in-vitro degradation. Biomaterials 2007, 28, 5407–5417. [Google Scholar] [CrossRef]
- Guelcher, S.A.; Gallagher, K.M.; Didier, J.E.; Klinedinst, D.B.; Doctor, J.S.; Goldstein, A.S.; Wilkes, G.L.; Beckman, E.J.; Hollinger, J.O. Synthesis of biocompatible segmented polyurethanes from aliphatic diisocyanates and diurea diol chain extenders. Acta Biomater. 2005, 1, 471–484. [Google Scholar] [CrossRef]
- Da Silva, G.R.; da Silva-Cunha, A.; Behar-Cohen, F.; Ayres, E.; Oréfice, R.L. Biodegradation of polyurethanes and nanocomposites to non-cytotoxic degradation products. Polym. Degrad. Stab. 2010, 95, 491–499. [Google Scholar] [CrossRef]
- Joseph, J.; Patel, R.M.; Wenham, A.; Smith, J.R. Biomedical applications of polyurethane materials and coatings. Trans. Inst. Met. Finish. 2018, 96, 121–129. [Google Scholar] [CrossRef] [Green Version]
- Roohpour, N.; Moshaverinia, A.; Wasikiewicz, J.M.; Paul, D.; Wilks, M.; Millar, M.; Vadgama, P. Development of bacterially resistant polyurethane for coating medical devices. Biomed. Mater. 2012, 7, 015007. [Google Scholar] [CrossRef]
- Roohpour, N.; Wasikiewicz, J.M.; Moshaverinia, A.; Paul, D.; Grahn, M.F.; Rehman, I.U.; Vadgama, P. Polyurethane membranes modified with isopropyl myristate as a potential candidate for encapsulating electronic implants: A study of biocompatibility and water permeability. Polymers 2010, 2, 102–119. [Google Scholar] [CrossRef] [Green Version]
- Griffin, M.; Castro, N.; Bas, O.; Saifzadeh, S.; Butler, P.; Hutmacher, D.W. The Current Versatility of Polyurethane Three-Dimensional Printing for Biomedical Applications. Tissue Eng. Part B Rev. 2020, 26, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Wendels, S.; Avérous, L. Biobased polyurethanes for biomedical applications. Bioact. Mater. 2021, 6, 1083–1106. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, S.; Solouk, A.; Mirzadeh, H.; Seifalian, A.M. Electroconductive polyurethane/graphene nanocomposite for biomedical applications. Compos. Part B Eng. 2019, 168, 421–431. [Google Scholar] [CrossRef]
- Mousa, H.M.; Abdal-Hay, A.; Bartnikowski, M.; Mohamed, I.M.A.; Yasin, A.S.; Ivanovski, S.; Park, C.H.; Kim, C.S. A Multifunctional Zinc Oxide/Poly(Lactic Acid) Nanocomposite Layer Coated on Magnesium Alloys for Controlled Degradation and Antibacterial Function. ACS Biomater. Sci. Eng. 2018, 4, 2169–2180. [Google Scholar] [CrossRef]
- Zykova, Y.; Kudryavtseva, V.; Gai, M.; Kozelskaya, A.; Frueh, J.; Sukhorukov, G.; Tverdokhlebov, S. Free-standing microchamber arrays as a biodegradable drug depot system for implant coatings. Eur. Polym. J. 2019, 114, 72–80. [Google Scholar] [CrossRef]
- Kim, Y.K.; Lee, K.B.; Kim, S.Y.; Jang, Y.S.; Kim, J.H.; Lee, M.H. Improvement of osteogenesis by a uniform PCL coating on a magnesium screw for biodegradable applications. Sci. Rep. 2018, 8, 13264. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Hamzah, E.; Kasiri-Asgarani, M.; Jabbarzare, S.; Iqbal, N.; Abdul Kadir, M.R. Deposition of nanostructured fluorine-doped hydroxyapatite-polycaprolactone duplex coating to enhance the mechanical properties and corrosion resistance of Mg alloy for biomedical applications. Mater. Sci. Eng. C 2016, 60, 526–537. [Google Scholar] [CrossRef]
- Zhang, T.; Yu, Q.; Fang, L.; Wang, J.; Wu, T.; Song, P. All-Organic Multilayer Coatings for Advanced Poly(lactic acid) Films with High Oxygen Barrier and Excellent Antifogging Properties. ACS Appl. Polym. Mater. 2019, 1, 3470–3476. [Google Scholar] [CrossRef]
- Lv, H.; Tang, D.; Sun, Z.; Gao, J.; Yang, X.; Jia, S.; Peng, J. Electrospun PCL-based polyurethane/HA microfibers as drug carrier of dexamethasone with enhanced biodegradability and shape memory performances. Colloid Polym. Sci. 2020, 298, 103–111. [Google Scholar] [CrossRef]
- Farrokhi-Rad, M.; Shahrabi, T.; Mahmoodi, S.; Khanmohammadi, S. Electrophoretic deposition of hydroxyapatite-chitosan-CNTs nanocomposite coatings. Ceram. Int. 2017, 43, 4663–4669. [Google Scholar] [CrossRef]
- Clavijo, S.; Membrives, F.; Quiroga, G.; Boccaccini, A.R.; Santillán, M.J. Electrophoretic deposition of chitosan/Bioglass® and chitosan/Bioglass®/TiO2 composite coatings for bioimplants. Ceram. Int. 2016, 42, 14206–14213. [Google Scholar] [CrossRef]
- Avcu, E.; Baştan, F.E.; Abdullah, H.Z.; Rehman, M.A.U.; Avcu, Y.Y.; Boccaccini, A.R. Electrophoretic deposition of chitosan-based composite coatings for biomedical applications: A review. Prog. Mater. Sci. 2019, 103, 69–108. [Google Scholar] [CrossRef]
- Frank, L.A.; Onzi, G.R.; Morawski, A.S.; Pohlmann, A.R.; Guterres, S.S.; Contri, R.V. Chitosan as a coating material for nanoparticles intended for biomedical applications. React. Funct. Polym. 2020, 147, 104459. [Google Scholar] [CrossRef]
- Ramshaw, J.A.M.; Glattauer, V. Coating of biomedical materials with collagen. Biophys. Chem. Prop. Collagen Biomed. Appl. 2019, 10–17. [Google Scholar] [CrossRef]
- Somaiah, C.; Kumar, A.; Mawrie, D.; Sharma, A.; Patil, S.D.; Bhattacharyya, J.; Swaminathan, R.; Jaganathan, B.G. Collagen promotes higher adhesion, survival and proliferation of mesenchymal stem cells. PLoS ONE 2015, 10, e0145068. [Google Scholar] [CrossRef] [Green Version]
- Stanton, A.E.; Tong, X.; Yang, F. Varying solvent type modulates collagen coating and stem cell mechanotransduction on hydrogel substrates. APL Bioeng. 2019, 3, 036108. [Google Scholar] [CrossRef]
- Ragetly, G.; Griffon, D.J.; Chung, Y.S. The effect of type II collagen coating of chitosan fibrous scaffolds on mesenchymal stem cell adhesion and chondrogenesis. Acta Biomater. 2010, 6, 3988–3997. [Google Scholar] [CrossRef]
- Wei, Q.; Haag, R. Universal polymer coatings and their representative biomedical applications. Mater. Horiz. 2015, 2, 567–577. [Google Scholar] [CrossRef] [Green Version]
- Pereira, V.D.A.; Ribeiro, I.S.; Paula, H.C.B.; de Paula, R.C.M.; Sommer, R.L.; Rodriguez, R.J.S.; Abreu, F.O.M.S. Chitosan-based hydrogel for magnetic particle coating. React. Funct. Polym. 2020, 146, 104431. [Google Scholar] [CrossRef]
- Marins, J.A.; Montagnon, T.; Ezzaier, H.; Hurel, C.; Sandre, O.; Baltrunas, D.; Mazeika, K.; Petrov, A.; Kuzhir, P. Colloidal Stability of Aqueous Suspensions of Polymer-Coated Iron Oxide Nanorods: Implications for Biomedical Applications. ACS Appl. Nano Mater. 2018, 1, 6760–6772. [Google Scholar] [CrossRef] [Green Version]
- Qiao, R.; Fu, C.; Li, Y.; Qi, X.; Ni, D.; Nandakumar, A.; Siddiqui, G.; Wang, H.; Zhang, Z.; Wu, T.; et al. Sulfoxide-Containing Polymer-Coated Nanoparticles Demonstrate Minimal Protein Fouling and Improved Blood Circulation. Adv. Sci. 2020, 7, 2000406. [Google Scholar] [CrossRef] [PubMed]
- Pon-On, W.; Tithito, T.; Maneeprakorn, W.; Phenrat, T.; Tang, I.M. Investigation of magnetic silica with thermoresponsive chitosan coating for drug controlled release and magnetic hyperthermia application. Mater. Sci. Eng. C 2019, 97, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Mngadi, S.; Mokhosi, S.; Singh, M.; Mdlalose, W. Chitosan-Functionalized Mg0.5Co0.5Fe2O4 Magnetic Nanoparticles Enhance Delivery of 5-Fluorouracil. Coatings 2020, 10, 446. [Google Scholar] [CrossRef]
- Zarouni, M.; Salehi, R.; Akbarzadeh, A.; Samadi, N.; Davaran, S.; Ramezani, F.; Dariushnejad, H. Biocompatible Polymer Coated Paramagnetic Nanoparticles for Doxorubicin Delivery: Synthesis and Anticancer Effects Against Human Breast Cancer Cells. Int. J. Polym. Mater. Polym. Biomater. 2015, 64, 718–726. [Google Scholar] [CrossRef]
- Labala, S.; Mandapalli, P.K.; Kurumaddali, A.; Venuganti, V.V.K. Layer-by-layer polymer coated gold nanoparticles for topical delivery of imatinib mesylate to treat melanoma. Mol. Pharm. 2015, 12, 878–888. [Google Scholar] [CrossRef]
- Fuller, M.A.; Köper, I. Biomedical applications of polyelectrolyte coated spherical gold nanoparticles. Nano Converg. 2019, 6, 11. [Google Scholar] [CrossRef]
- Abedin, M.R.; Umapathi, S.; Mahendrakar, H.; Laemthong, T.; Coleman, H.; Muchangi, D.; Santra, S.; Nath, M.; Barua, S. Polymer coated gold-ferric oxide superparamagnetic nanoparticles for theranostic applications. J. Nanobiotechnol. 2018, 16, 80. [Google Scholar] [CrossRef] [Green Version]








| Coatings Material | Coating Method | Substrate/Nanoparticle | Applications Area | Refs. |
|---|---|---|---|---|
| poly(2-methyl-2-oxazoline) (PMOXA) | Electrochemical non brush bionic coating | 316L stainless steel | bioactivity, antifouling properties, prevent late stent thrombosis and in-stent restenosis | [15] |
| Polytetrafluoroethylene | PEO coating | magnesium alloy MA8 | protective and antifriction properties | [16] |
| hydroxyapatite- polytetrafluoroethylene | PEO coating | Mg–Mn–Ce alloys | corrosion resistance and impart bioactivity | [17] |
| 1. CaP coating 2. polylactic acid | immersion dip coating | magnesium | corrosion resistance and elongation of degradation time | [18] |
| phosphate/collagen (CaP/Col) composite coatings | Chemical conversion and dip coating | Magnesium alloys | corrosion resistance and inducing bioactivity | [19] |
| PVDF | Spin coating | Free standing ZnO grown film | wearable and wireless pressure sensor for heart rate monitoring | [31] |
| R-GO/P(VDF-TrFE) | liquid phase blending Spin coating | Flexible and glass substrate | flexible, optically transparent, and highly responsive temperature sensor | [40] |
| polyaniline-coated PVDF | Electrospinning in situ conversion | Aluminum foil | Human health monitoring | [45] |
| PVDF-nanosilica | Electrospinning | Aluminum foil | Increased piezoelectric property for biomedical application | [46] |
| PVDF/conducting polymer | Electrospinning | aluminum foil | Electrical conductivity and bioactivity | [47] |
| P(VDF-TrFE) | Electrospinning | aluminum foil | implanted energy harvester, bioactivity | [48] |
| PMMA | Alkali activation and surface-initiated atom transfer radical polymerization | Titanium | hybrid prosthesis, bioactivity | [52,53] |
| methacrylate | modelling | Titanium | less susceptible biofilm formation coating, bioactivity | [54] |
| PMMA/PDDA | spin coating or casting and drying | Si, glass, or polystyrene sheets | Antimicrobial coating | [55] |
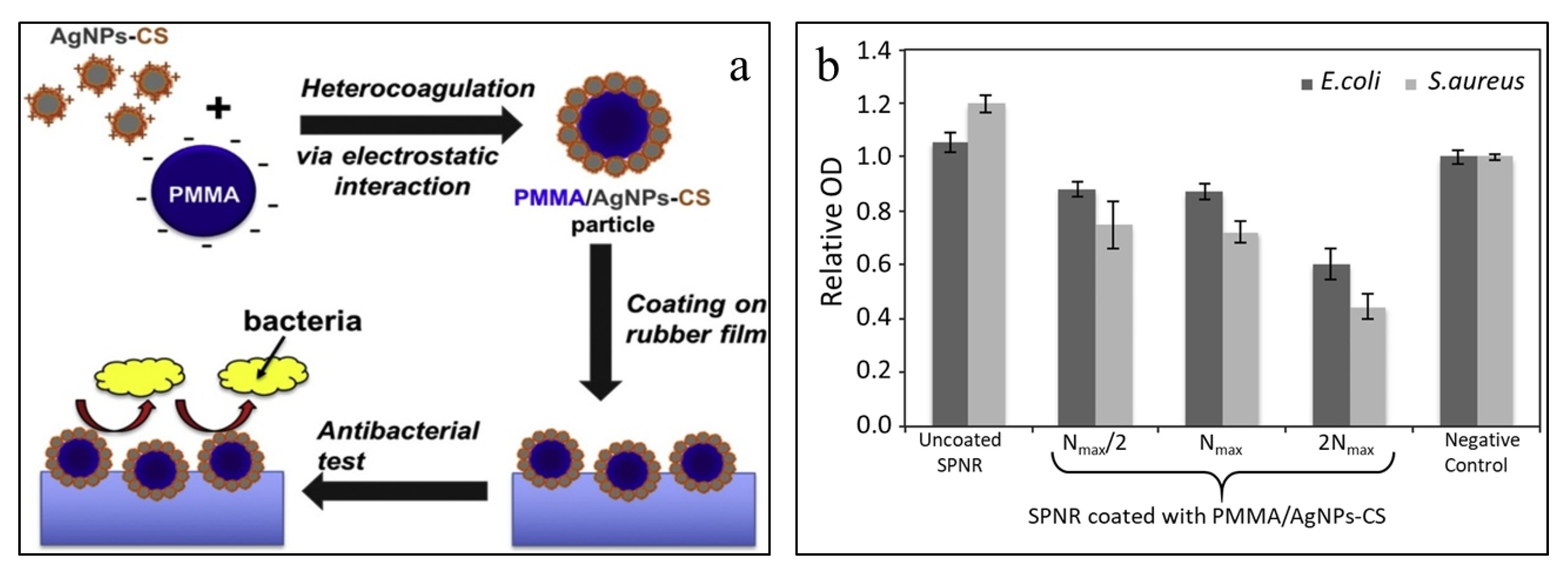
| PMMA/AgNPs-CS | immersion method | Soft rubber | Antimicrobial coating | [56] |
| PDA | cold oxygen plasma | PP hernia mesh | drug absorption and longer release, antibacterial properties | [58] |
| PVP:PEGDA | Cross-linking | PP | hydrophilicity and bioactivity | [61] |
| Poly(StBP) | Spreading and curing with UV | PP | Bone tissue engineering | [62] |
| cell-adhesive peptide | Dip coating | PDMS | functionalize biomedical devices with sensitive and complex components | [63] |
| poly(acrylamide–acrylic acid) | Chemical bonding | PDMS | Ultralow friction coatings | [65] |
| chlorhexidine (CHX)-loaded PDMS | oxygen plasma and heat treatment | 3D-printed dental polymer | induce surface wettability, microstructure, and antibacterial activity | [66] |
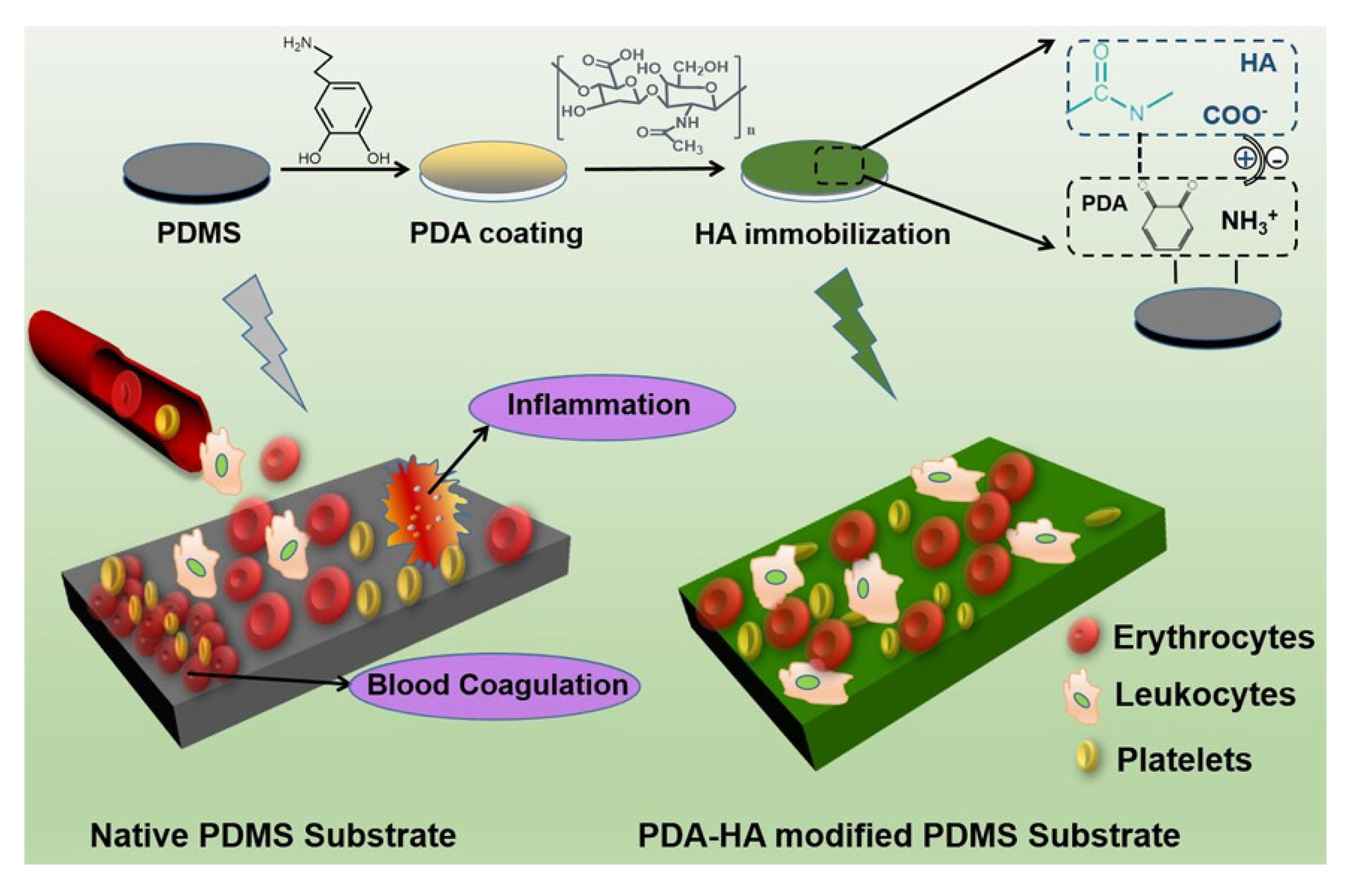
| PDA and hyaluronic acid | drop casting | PDMS | Hemocompatible medical device and implant | [67] |
| PDMS | low-energy electron beam irradiation | PDMS | long-lasting hydrophilic surface | [68] |
| PTFE | Printing/Solution injection and curing | PDMS | To encapsulate anti-inflammatory drugs, super hydrophobicity | [69] |
| PU | casting | Freestanding films | Biodegradable material for biomedical application | [70] |
| PU/Ag | end-capped with functional groups | freestanding | developing medical device coatings and associated applications | [76] |
| Isopropyl Myristate | casting | PU | bioactivity and low water permeability | [77] |
| PU/graphene | electrospinning | Aluminum foil | electroconductivity, bioactivity and mechanical properties | [80] |
| PLA/ZnO | Dip coating | Mg alloy (AZ31) | Reduced Mg degradation rate | [81] |
| PLLA | Dip coating | PDMS stamp | Drug delivery application | [82] |
| PCL | PEO and dip coating | Mg screw | Bone forming ability and osteogenesis | [83] |
| PCL/FHA composite duplex coating | Dip coatings | Mg alloy | bioactivity and controlled Mg degradation | [84] |
| PCL/PU/apatite | Electrospinning | Aluminum foil | controlled drug delivery | [86] |
| Different polymer coatings | Polymer adsorption | Iron oxide nanorods | perfectly stabile colloidal nanoparticle for medical application | [97] |
| PMSEA | RAFT polymerization | iron oxide nanoparticles | extended blood circulation time and reduced accumulation | [90] |
| Chitosan | encapsulation | magnetic silica nanoparticles | pH/thermos-chemotherapy using an AMF drug delivery system | [99] |
| Chitosan | adsorption | Mg0.5Co0.5Fe2O4 | Drug delivery | [100] |
| Chitosan -PMAA | in situ polymerization | Fe3O4 MNPs | DOX delivery in breast cancer treatment | [101] |
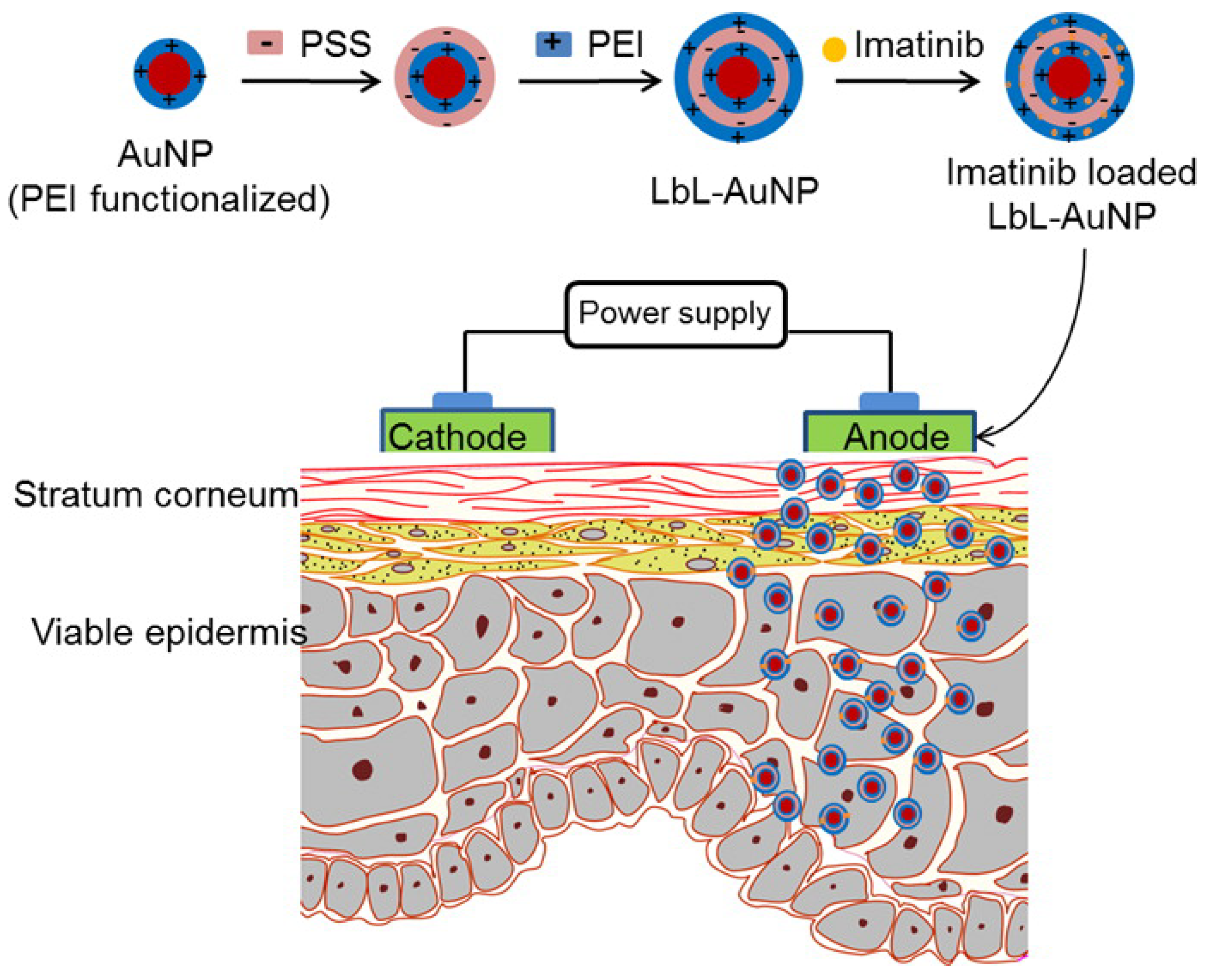
| PSS/PEI | LBL | AuNPs | Drug delivery to the layers of skin in melanoma treatment | [102] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nathanael, A.J.; Oh, T.H. Biopolymer Coatings for Biomedical Applications. Polymers 2020, 12, 3061. https://doi.org/10.3390/polym12123061
Nathanael AJ, Oh TH. Biopolymer Coatings for Biomedical Applications. Polymers. 2020; 12(12):3061. https://doi.org/10.3390/polym12123061
Chicago/Turabian StyleNathanael, A. Joseph, and Tae Hwan Oh. 2020. "Biopolymer Coatings for Biomedical Applications" Polymers 12, no. 12: 3061. https://doi.org/10.3390/polym12123061
APA StyleNathanael, A. J., & Oh, T. H. (2020). Biopolymer Coatings for Biomedical Applications. Polymers, 12(12), 3061. https://doi.org/10.3390/polym12123061