Chitosan–Hydroxyapatite Composite Layers Generated in Radio Frequency Magnetron Sputtering Discharge: From Plasma to Structural and Morphological Analysis of Layers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Chitosan–Hydroxyapatite Composites
2.3. Synthesis of Chitosan–Hydroxyapatite Layers
2.4. Characterization Methods
3. Results
3.1. Plasma Characterization by Langmuir Probe Electrical Measurements
3.2. Mass Spectra Analysis of Synthesis Plasma
3.3. XPS Analysis of Hydroxyapatite–Chitosan Coatings
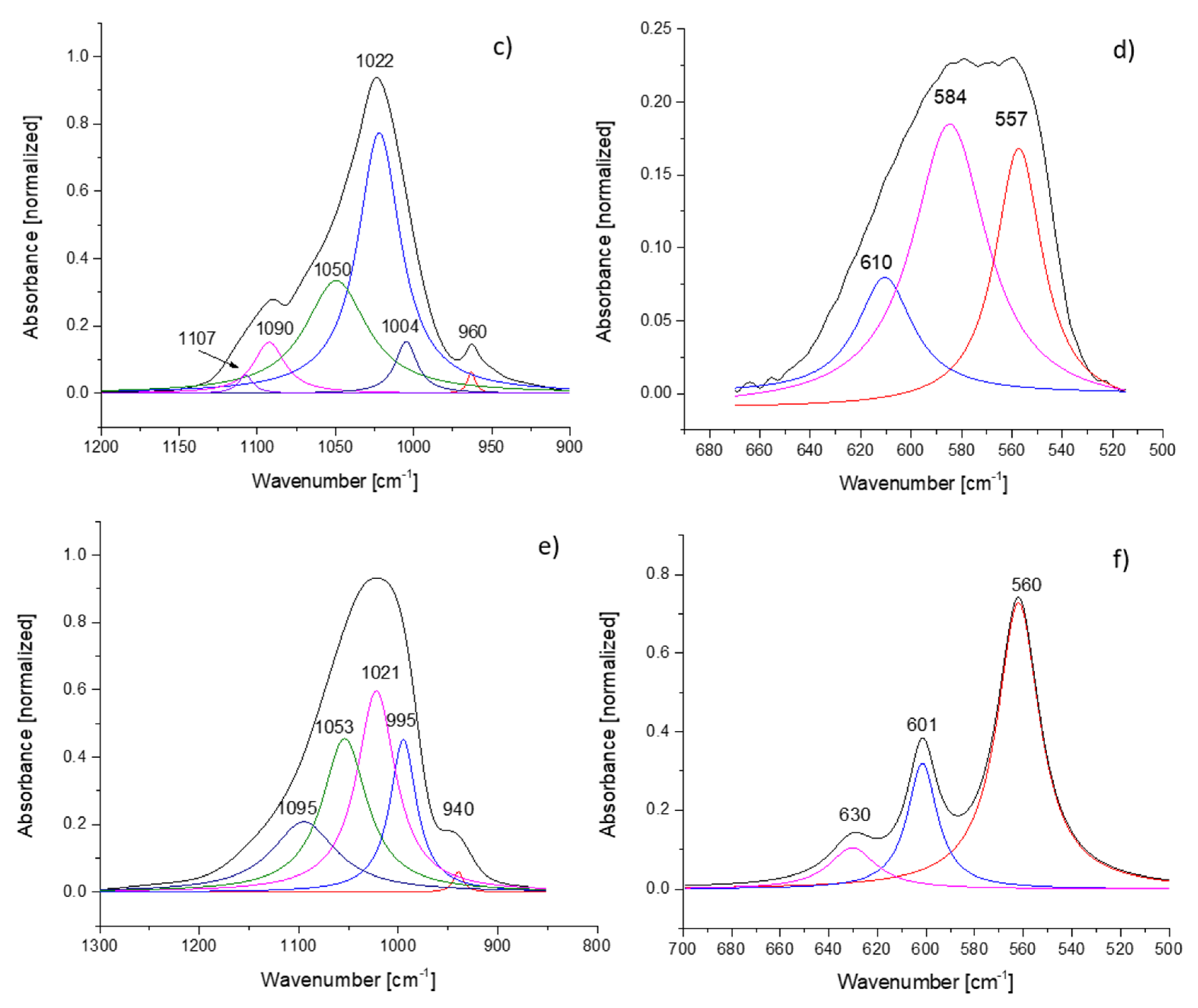
3.4. Fourier Transform Infrared Spectroscopy Analyses of Chitosan–Hydroxyapatite Composite Coatings
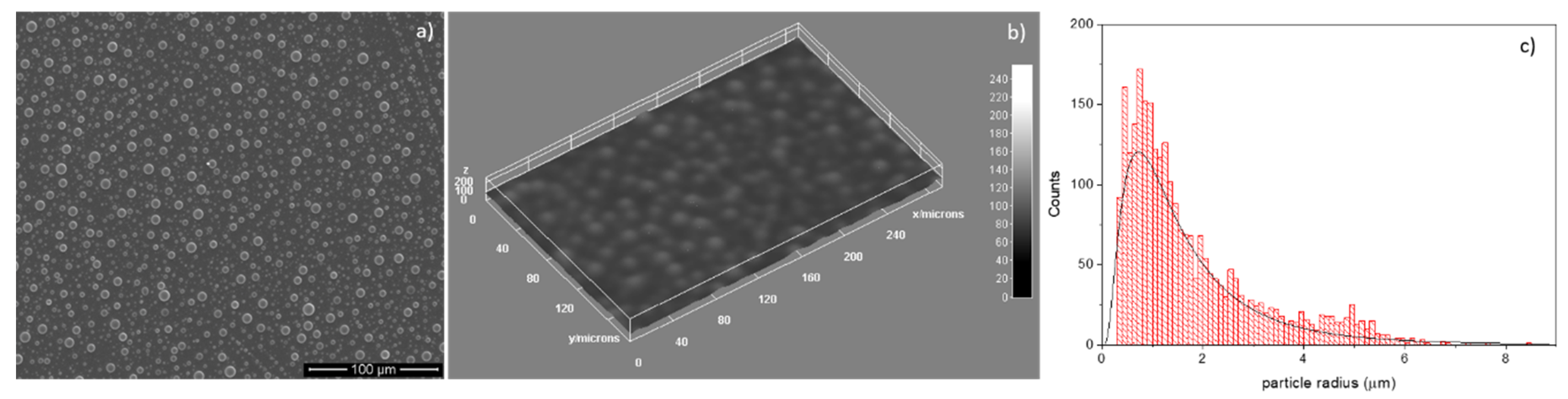
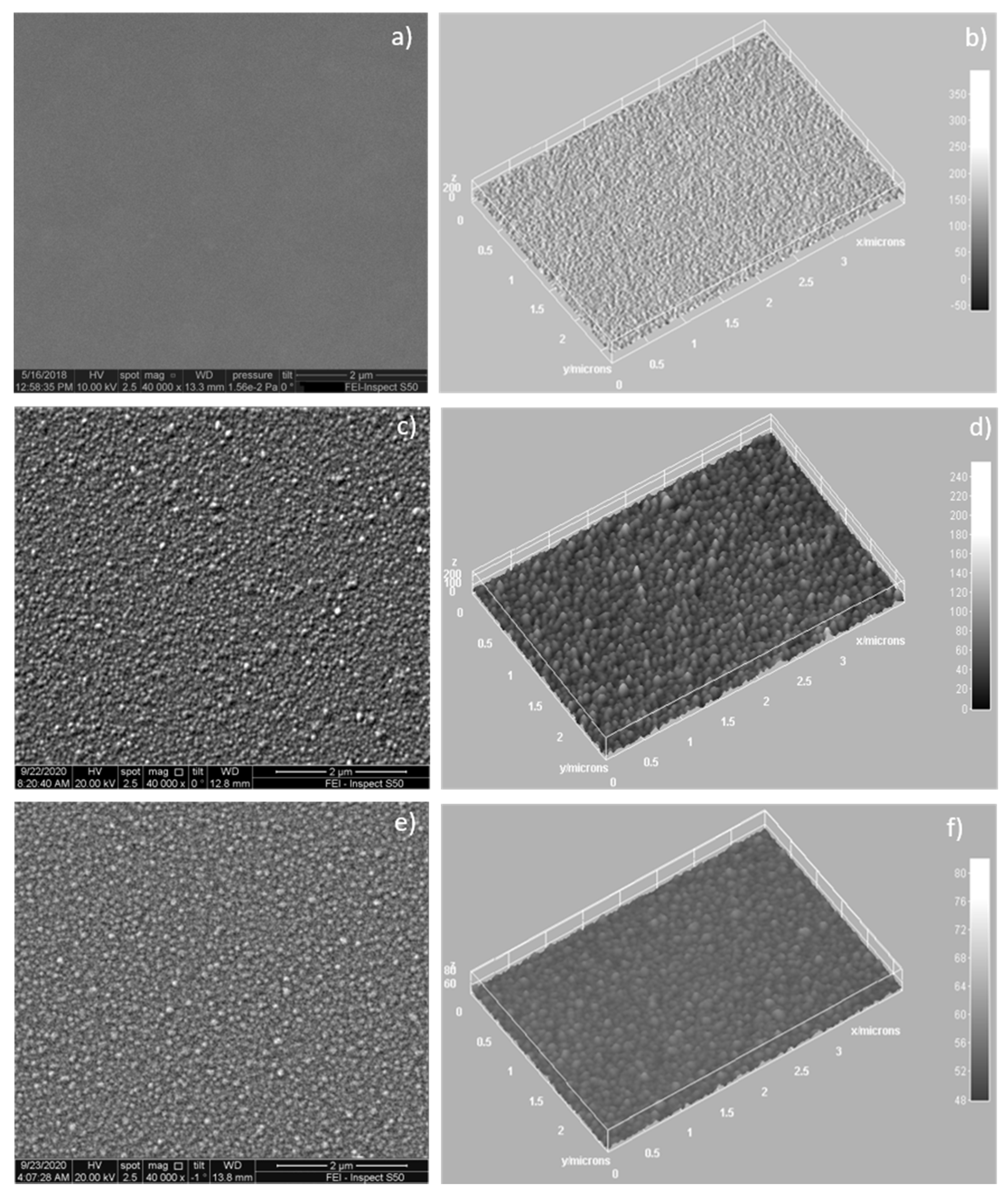
3.5. SEM Analysis of HAp–Chitosan Coatings
3.6. EDS Analysis of HApCs Coatings
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Surmenev, R.; Vladescu, A.; Surmeneva, M.; Ivanova, M.B.A.; Grubova, I.; Cotrut, C.M. Radio Frequency Magnetron Sputter Deposition as a Tool for Surface Modification of Medical Implants. In Modern Technologies for Creating the Thin-film Systems and Coatings; IntechOpen: London, UK, 2017. [Google Scholar]
- Teh, S.J.; Lai, C.W. Carbon nanotubes for dental implants. In Applications of Nanocomposite Materials in Dentistry; Elsevier BV: Amsterdam, The Netherlands, 2019; pp. 93–105. [Google Scholar]
- Surmenev, R.A.; Surmenev, R.A.; Grubova, I.Y.; Chernozem, R.V.; Krause, B.; Baumbach, T.; Loza, K.; Epple, M. RF magnetron sputtering of a hydroxyapatite target: A comparison study on polytetrafluorethylene and titanium substrates. Appl. Surf. Sci. 2017, 414, 335–344. [Google Scholar] [CrossRef]
- Cord-Landwehr, S.; Ihmor, P.; Niehues, A.; Luftmann, H.; Moerschbacher, B.M.; Mormann, M. Quantitative Mass-Spectrometric Sequencing of Chitosan Oligomers Revealing Cleavage Sites of Chitosan Hydrolases. Anal. Chem. 2017, 89, 2893–2900. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, A.G.; Prokhorov, E.; Bárcenas, G.L.; Mora-García, A.G.; Kovalenko, Y.; Rivera-Muñoz, E.M.; Raucci, M.G.; Buonocore, G.; Miñoz, E.M.R. Chitosan-hydroxyapatite nanocomposites: Effect of interfacial layer on mechanical and dielectric properties. Mater. Chem. Phys. 2018, 217, 151–159. [Google Scholar] [CrossRef]
- Arizmendi-Morquecho, A.M.; Chávez-Valdez, A.; Navarro, C.; Moreno, K.J. Performance evaluation of chitosan/hydroxyapatite composite coating on ultrahigh molecular weight polyethylene. Polym. Test. 2013, 32, 32–37. [Google Scholar] [CrossRef]
- Li, B.; Xia, X.; Guo, M.; Jiang, Y.; Li, Y.; Zhang, Z.; Liu, S.; Li, H.; Liang, C.; Wang, H. Biological and antibacterial properties of the micro-nanostructured hydroxyapatite/chitosan coating on titanium. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.; Liang, J.; Cui, Y.; Xu, S.; Zhao, N. Fabrication of novel bioactive hydroxyapatite-chitosan-silica hybrid scaffolds: Combined the sol-gel method with 3D plotting technique. Carbohydr. Polym. 2018, 197, 183–193. [Google Scholar] [CrossRef]
- Sorkhi, L.; Farrokhi-Rad, M.; Shahrabi, T. Electrophoretic Deposition of Hydroxyapatite–Chitosan–Titania on Stainless Steel 316 L. Surfaces 2019, 2, 458–467. [Google Scholar] [CrossRef] [Green Version]
- Groza, A.; Dreghici, D.B.; Ganciu, M. Calcium Phosphate Layers Deposited on Thermal Sensitive Polymer Substrates in Radio Frequency Magnetron Plasma Discharge. Coatings 2019, 9, 709. [Google Scholar] [CrossRef] [Green Version]
- Iconaru, S.L.; Groza, A.; Stan, G.E.; Predoi, D.; Gaiaschi, S.; Truşcă, R.-D.; Chifiriuc, M.C.; Măruţescu, L.; Tite, T.; Stanciu, G.A.; et al. Preparations of Silver/Montmorillonite Biocomposite Multilayers and Their Antifungal Activity. Coatings 2019, 9, 817. [Google Scholar] [CrossRef] [Green Version]
- Predoi, D.; Iconaru, S.L.; Predoi, D.; Groza, A.; Gaiaschi, S.; Rokosz, K.; Raaen, S.; Negrila, C.; Prodan, A.M.; Costescu, A.; et al. Development of Cerium-Doped Hydroxyapatite Coatings with Antimicrobial Properties for Biomedical Applications. Coatings 2020, 10, 516. [Google Scholar] [CrossRef]
- Choukourov, A.; Pleskunov, P.; Nikitin, D.; Titov, V.; Shelemin, A.; Vaidulych, M.; Kuzminova, A.; Solař, P.; Hanuš, J.; Kousal, J.; et al. Advances and challenges in the field of plasma polymer nanoparticles. Beilstein J. Nanotechnol. 2017, 8, 2002–2014. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, J. Mechanisms of Plasma Polymerization-Reviewed from a Chemical Point of View. Plasma Process. Polym. 2011, 8, 783–802. [Google Scholar] [CrossRef]
- Chen, F.; Wang, Z.C.; Lin, C. Preparation and characterization of nano-sized hydroxyapatite particles and hydroxyapatite/chitosan nano-composite for use in biomedical materials. Mater. Lett. 2002, 57, 858–861. [Google Scholar] [CrossRef]
- Ganguli, A.; Sahu, B.B.; Tarey, R.D. A new structure for RF-compensated Langmuir probes with external filters tunable in the absence of plasma. Plasma Sources Sci. Technol. 2008, 17, 015003. [Google Scholar] [CrossRef]
- López, E.O.; Mello, A.; Sendão, H.; Costa, L.T.; Rossi, A.L.; Ospina, R.O.; Borghi, F.F.; Filho, J.G.S.; Rossi, A.M. Growth of Crystalline Hydroxyapatite Thin Films at Room Temperature by Tuning the Energy of the RF-Magnetron Sputtering Plasma. ACS Appl. Mater. Interfaces 2013, 5, 9435–9445. [Google Scholar] [CrossRef]
- Groza, A.; Surmeian, A. Characterization of the Oxides Present in a Polydimethylsiloxane Layer Obtained by Polymerisation of Its Liquid Precursor in Corona Discharge. J. Nanomater. 2015, 2015, 1–8. [Google Scholar] [CrossRef]
- Surmenev, R.A.; Surmenev, R.A.; Evdokimov, K.E.; Pichugin, V.; Peitsch, T.; Epple, M. The influence of the deposition parameters on the properties of an rf-magnetron-deposited nanostructured calcium phosphate coating and a possible growth mechanism. Surf. Coat. Technol. 2011, 205, 3600–3606. [Google Scholar] [CrossRef]
- Stevenson, D.P. The Strengths of Chemical Bonds. J. Am. Chem. Soc. 1955, 77, 2350. [Google Scholar] [CrossRef]
- Thiry, D.; Konstantinidis, S.; Cornil, J.; Snyders, R. Plasma diagnostics for the low-pressure plasma polymerization process: A critical review. Thin Solid Films 2016, 606, 19–44. [Google Scholar] [CrossRef]
- Liu, H.; Yoo, H.J.; Håkansson, K. Characterization of Phosphate-Containing Metabolites by Calcium Adduction and Electron Capture Dissociation. J. Am. Soc. Mass Spectrom. 2008, 19, 799–808. [Google Scholar] [CrossRef] [Green Version]
- Ghumman, C.A.A.; Moutinho, A.; Santos, A.; Tolstogouzov, A.; Teodoro, O.M.N.D. TOF-SIMS VG Ionex IX23LS: Upgrade and application for the urinary stones analysis. Surf. Interface Anal. 2012, 45, 532–536. [Google Scholar] [CrossRef]
- Ghumman, C.; Moutinho, A.; Santos, A.; Teodoro, O.M.N.D.; Tolstogouzov, A. An upgraded TOF-SIMS VG Ionex IX23LS: Study on the negative secondary ion emission of III–V compound semiconductors with prior neutral cesium deposition. Appl. Surf. Sci. 2012, 258, 2490–2497. [Google Scholar] [CrossRef]
- Ghumman, C.A.A.; Moutinho, A.; Santos, A.; Tolstogouzov, A.; Teodoro, O.M.N.D. TOF-SIMS study of cystine and cholesterol stones. J. Mass Spectrom. 2012, 47, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Ghumman, C.A.A.; Moutinho, A.M.C.; Tolstogouzov, A.; Teodoro, O.M.N.D. Time-of-flight secondary ion mass spectrometric identification of calcium formate Ca(HCO2 )2 and metabolite of vitamin B6 in human stones. Rapid Commun. Mass Spectrom. 2011, 25, 997–999. [Google Scholar] [CrossRef] [PubMed]
- Ghumman, C.A.A.; Carreira, O.M.T.; Moutinho, A.M.C.; Tolstogouzov, A.; Vassilenko, V.; Teodoro, O.M.N.D. Identification of human calculi with time-of-flight secondary ion mass spectrometry. Rapid Commun. Mass Spectrom. 2010, 24, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Briggs, D. Handbook of X-ray Photoelectron Spectroscopy Wanger, C.D.; Riggs, W.M.; Davis, L.E.; Moulder, J.F.; and Muilenberg, G.E. Perkin-Elmer Corp., Physical Electronics Division, Eden Prairie, Minnesota, USA, 1979. 190 pp. $195. Surf. Interface Anal. 1981, 3. [Google Scholar] [CrossRef]
- Maachou, H.; Genet, M.J.; Aliouche, D.; Dupont-Gillain, C.C.; Rouxhet, P.G. XPS analysis of chitosan-hydroxyapatite biomaterials: From elements to compounds. Surf. Interface Anal. 2013, 45, 1088–1097. [Google Scholar] [CrossRef]
- Nageswaran, G.; Jothi, L.; Jagannathan, S. Plasma Assisted Polymer Modifications. In Non-Thermal Plasma Technology for Polymeric Materials; Elsevier BV: Amsterdam, The Netherlands, 2019; pp. 95–127. [Google Scholar]
- Berzina-Cimdina, L.; Borodajenko, N. Research of Calcium Phosphates Using Fourier Transform Infrared Spectroscopy; IntechOpen: London, UK, 2012. [Google Scholar]
- Coates, J. Interpretation of Infrared Spectra, A Practical Approach. In Encyclopedia of Analytical Chemistry; John Wiley & Sons Ltd.: Chichester, UK, 2000; pp. 10815–10837. [Google Scholar]
- Kumirska, J.; Czerwicka, M.; Kaczyński, Z.; Bychowska, A.; Brzozowski, K.; Thöming, J.; Stepnowski, P. Application of Spectroscopic Methods for Structural Analysis of Chitin and Chitosan. Mar. Drugs 2010, 8, 1567–1636. [Google Scholar] [CrossRef] [Green Version]
- Monsees, T.K.; Azem, F.A.; Cotrut, C.M.; Braic, M.; Abdulgader, R.; Pana, I.; Birlik, I.; Kiss, A.; Booysen, R.; Vladescu, A. Biodegradable Ceramics Consisting of Hydroxyapatite for Orthopaedic Implants. Coatings 2017, 7, 184. [Google Scholar] [CrossRef] [Green Version]
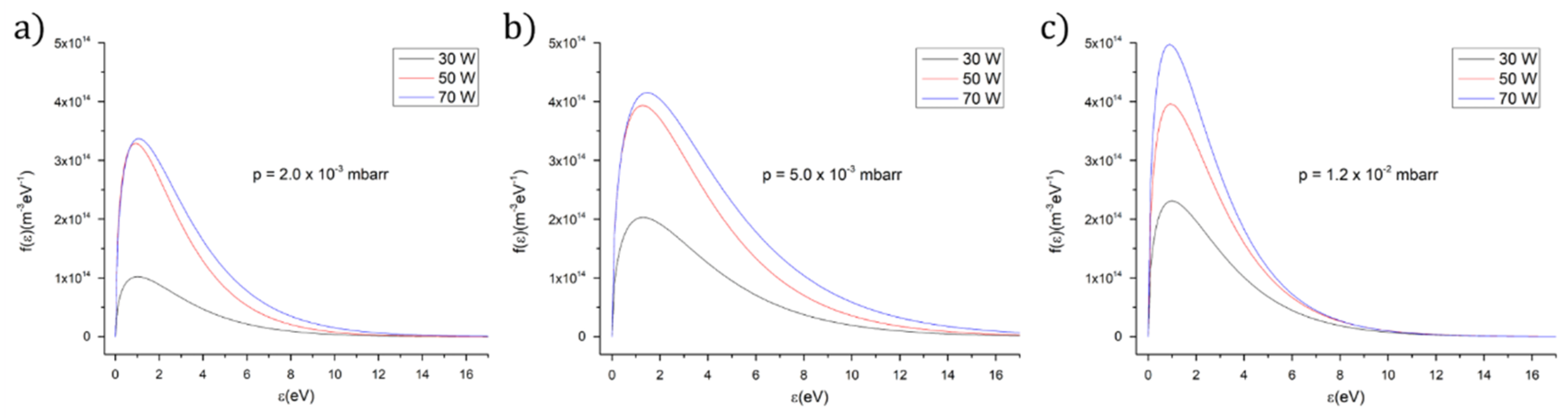
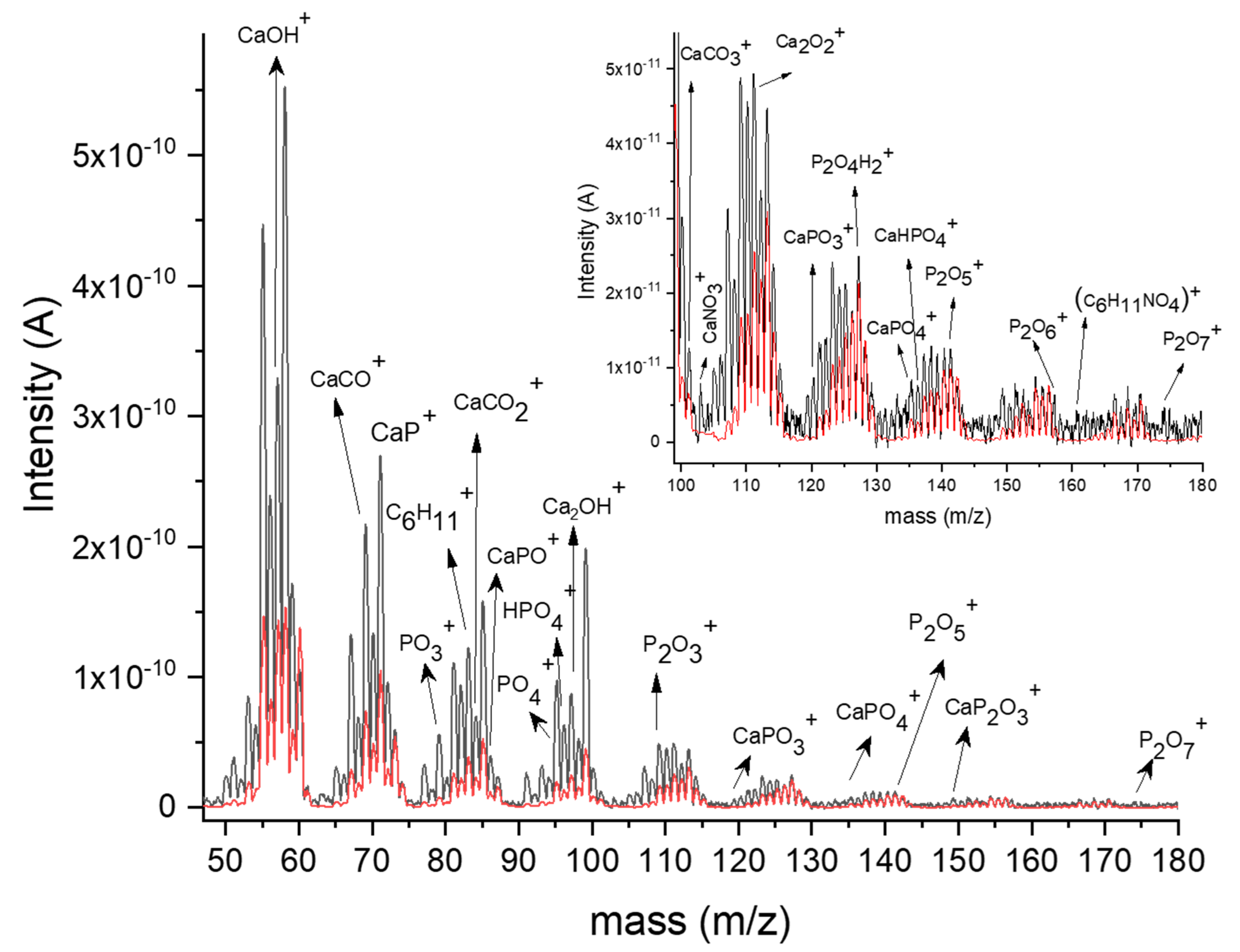
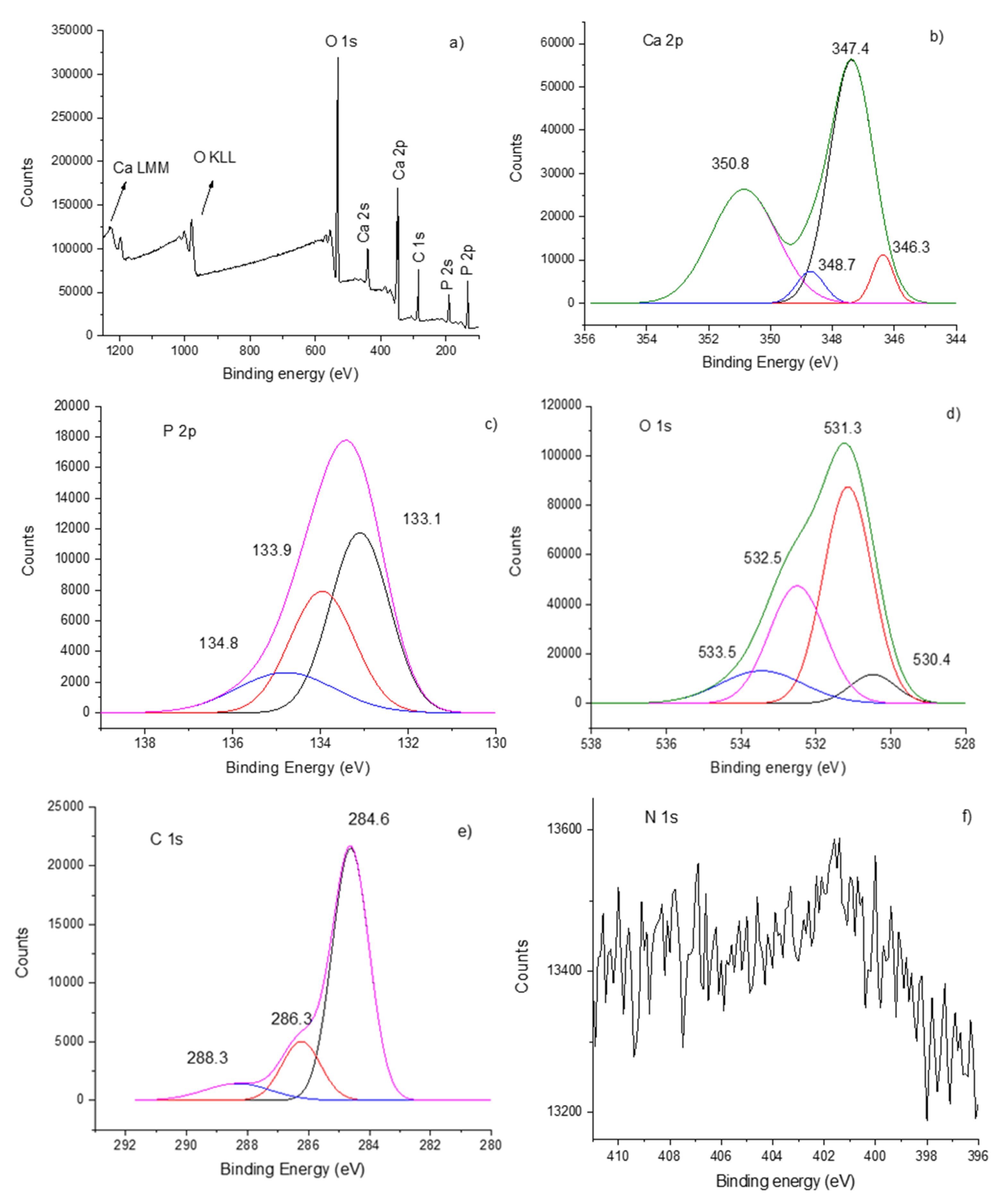
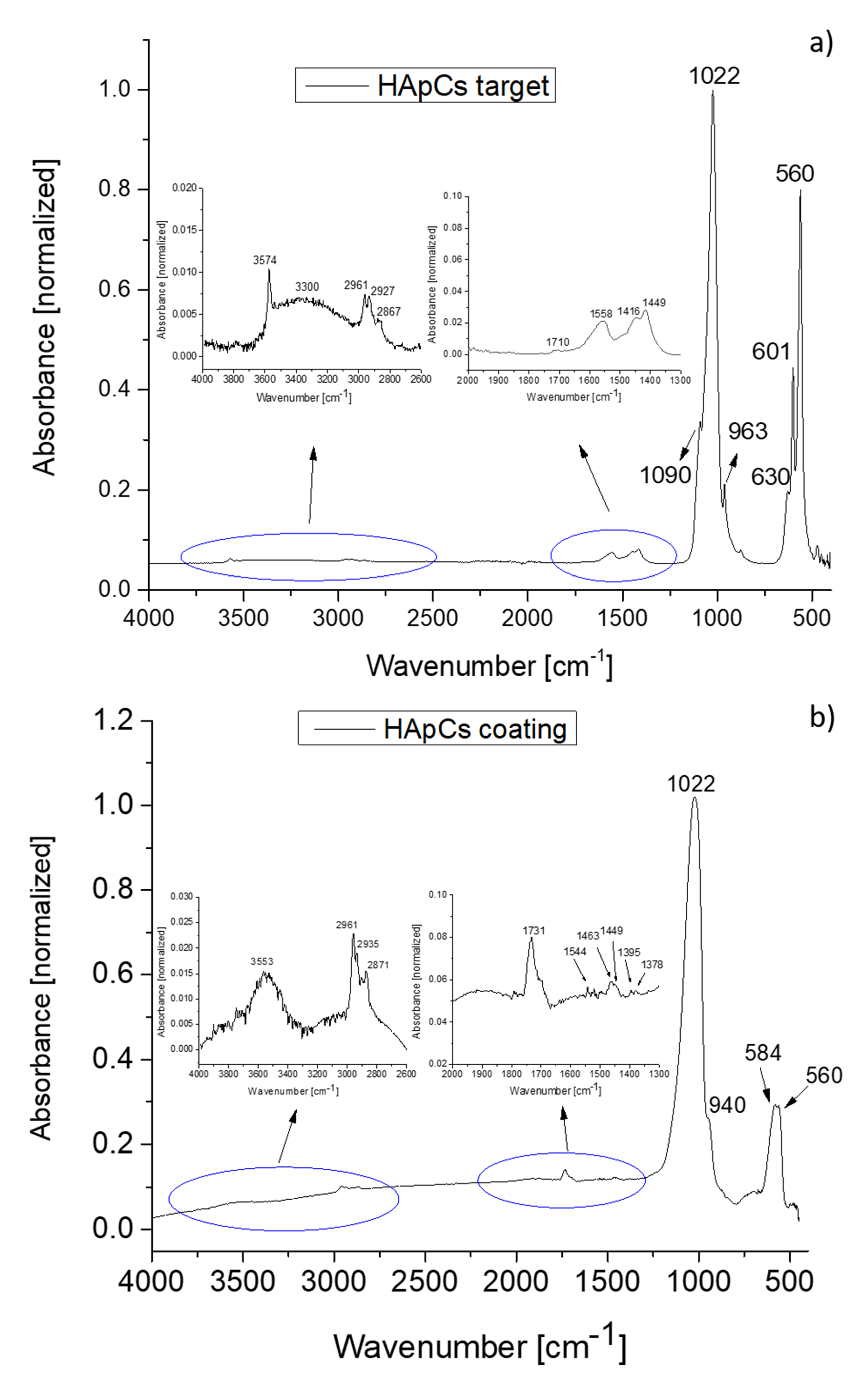


| Ar gas Working Pressure (mbarr) | Ar Gas Flow (sln/min) | Rf Power | Te (eV) HApCh (1 eV = 11,600 K) | ne (m−3) HApCh |
|---|---|---|---|---|
| 2 × 10−3 | 0.8 | 30 W | 3.2 | 7.8 × 1014 |
| 5 × 10−3 | 2.4 | 30 W | 2.5 | 9 × 1014 |
| 1.2 × 10−2 | 4.5 | 30 W | 1.9 | 1 × 1015 |
| 2 × 10−3 | 0.8 | 50W | 2.5 | 1.2 × 1015 |
| 5 × 10−3 | 2.4 | 50W | 2.2 | 1.3 × 1015 |
| 1.2 × 10−2 | 4.5 | 50W | 1.8 | 1.6 × 1015 |
| 2 × 10−3 | 0.8 | 70 W | 2.9 | 1.5 × 1015 |
| 5 × 10−3 | 2.4 | 70 W | 2.1 | 1.8 × 1015 |
| 1.2 × 10−2 | 4.5 | 70 W | 1.7 | 1.9 × 1015 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dreghici, D.B.; Butoi, B.; Predoi, D.; Iconaru, S.L.; Stoican, O.; Groza, A. Chitosan–Hydroxyapatite Composite Layers Generated in Radio Frequency Magnetron Sputtering Discharge: From Plasma to Structural and Morphological Analysis of Layers. Polymers 2020, 12, 3065. https://doi.org/10.3390/polym12123065
Dreghici DB, Butoi B, Predoi D, Iconaru SL, Stoican O, Groza A. Chitosan–Hydroxyapatite Composite Layers Generated in Radio Frequency Magnetron Sputtering Discharge: From Plasma to Structural and Morphological Analysis of Layers. Polymers. 2020; 12(12):3065. https://doi.org/10.3390/polym12123065
Chicago/Turabian StyleDreghici, Dragana Biliana, Bogdan Butoi, Daniela Predoi, Simona Liliana Iconaru, Ovidiu Stoican, and Andreea Groza. 2020. "Chitosan–Hydroxyapatite Composite Layers Generated in Radio Frequency Magnetron Sputtering Discharge: From Plasma to Structural and Morphological Analysis of Layers" Polymers 12, no. 12: 3065. https://doi.org/10.3390/polym12123065
APA StyleDreghici, D. B., Butoi, B., Predoi, D., Iconaru, S. L., Stoican, O., & Groza, A. (2020). Chitosan–Hydroxyapatite Composite Layers Generated in Radio Frequency Magnetron Sputtering Discharge: From Plasma to Structural and Morphological Analysis of Layers. Polymers, 12(12), 3065. https://doi.org/10.3390/polym12123065







