New Functionalized Polymeric Sensor Based NiO/MgO Nanocomposite for Potentiometric Determination of Doxorubicin Hydrochloride in Commercial Injections and Human Plasma
Abstract
1. Introduction
2. Experimental
2.1. Chemicals and Reagents
2.2. Instruments
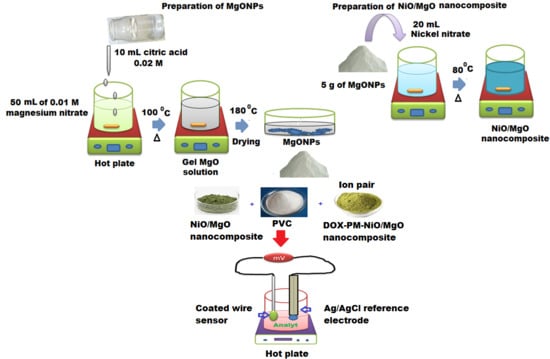
2.3. Synthesis of Magnesium Oxide Nanoparticles
2.4. Synthesis of Nickel Oxide/Magnesium Oxide Nanocomposite
2.5. Characterization of Nanoparticles
2.6. Preparation of Stock Drug Solution
2.7. Preparation of Electroactive Complex
2.8. Membrane Composition and Sensor Fabrication
2.9. Calibration Graphs
2.10. Optimization of Potential Readings Conditions
2.11. Analysis of DOX in Adriamycin® Injections
2.12. Analysis of DOX in Real Human Plasma Samples
3. Results and Discussion
3.1. Characterization of NiO/MgO Nanocomposite
3.2. The Nature of the Fabricated Sensors
3.3. Quantification of DOX in Its Bulk Powder
3.4. Method Validation
3.5. Quantification of DOX in Adriamycin® Injections
| Statistical Analysis | Conventional DOX-PM Coated Wire Sensor | Modified DOX-PM NiO/MgO Sensor | Reported Method [41] | ||
| * Test Solution | % Recovery | * Test Solution | % Recovery | ||
| 6 | 99.0 | 10 | 99.5 | 99.5 ± 0.4 | |
| 5.3 | 99.3 | 8 | 99.8 | ||
| 5 | 99.5 | 6 | 99.5 | ||
| 4 | 99.8 | 4 | 99.8 | ||
| 3 | 99.2 | 3 | 100.2 | ||
| 2 | 98.8 | 2 | 99.9 | ||
| Mean ± SD | 99.3 ± 0.3 | 99.8 ± 0.3 | |||
| n | 6 | 6 | |||
| Variance | 0.09 | 0.09 | |||
| %SE ** | 0.12 | 0.12 | |||
| %RSD | 0.30 | 0.30 | |||
| t-test | 1.000 (2.228) *** | 1.500 (2.228) *** | |||
| F-test | 1.78 (5.05) *** | 1.78 (5.05) *** | |||
3.6. Quantification of DOX in Human Plasma Samples
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anuar, N.S.; Basirun, W.J.; Shalauddin, M.; Akhter, S. A dopamine electrochemical sensor based on a platinum–silver graphene nanocomposite modified electrode. RSC Adv. 2020, 10, 17336–17344. [Google Scholar] [CrossRef]
- Bera, B.; Banerjee, D. Synthesis, Characterization of MoS2 nanosheets, amorphous carbon nanotube (ACNT) and ACNT-MoS2 nanohybrids: A Comparative research analysis. J. Nanosci. Nanoeng. Appl. 2020, 9, 27–36. [Google Scholar]
- Bayda, S.; Adeel, M.; Tuccinardi, T.; Cordani, M.; Rizzolio, F. The history of nanoscience and nanotechnology: From chemical–physical applications to nanomedicine. Molecules 2020, 25, 112. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Lyu, H.; Ji, Z.; Zhu, C.; Yaghi, O.M. Ester-Linked Crystalline Covalent Organic Frameworks. J. Am. Chem. Soc. 2020, 142, 14450–14454. [Google Scholar] [CrossRef] [PubMed]
- İpek, Z.; Atik, A.D.; Tan, S.; Erkoc, F. Opinions of biology teachers about nanoscience and nanotechnology education in Turkey. Int. J. Prog. Educ. 2020, 16, 205–222. [Google Scholar]
- Mousavi, S.M.; Hashemi, S.A.; Zarei, M.; Amani, A.M.; Babapoor, A. Nanosensors for chemical and biological and medical applications. Med. Chem. 2018, 8, 205–217. [Google Scholar] [CrossRef]
- Wei, H.; Wang, H.; Li, A.; Cui, D.; Zhao, Z.; Chu, L.; Wei, X.; Wang, L.; Pan, D.; Fan, J.; et al. Multifunctions of polymer nanocomposites: Environmental remediation, electromagnetic interference shielding, and sensing applications. Chem. Nano Mat. 2020, 6, 174–184. [Google Scholar] [CrossRef]
- Li, S.; Huang, L.; Zhang, B.; Chen, C.; Fu, X.; Huang, Q. Fabrication and characterization of starch/zein nanocomposites with pH-responsive emulsion behavior. Food Hydrocoll. 2020, 112, 106341. [Google Scholar] [CrossRef]
- Musarat, M.; Al Musayeib, N.M.; Alarfaj, N.A.; El-Tohamy, M.F.; Oraby, H.F.; Al Hamoud, G.A.; Bukhari, S.I.; Moubayed, N.M.S. Biogenic green synthesis of MgO nanoparticles using Saussurea costus biomasses for a comprehensive detection of their antimicrobial, cytotoxicity against MCF-7 breast cancer cells and photocatalysis potentials. PLoS ONE 2020, 15, e0237567. [Google Scholar] [CrossRef]
- Alarfaj, N.A.; El-Tohamy, M.F.; Oraby, H.F. New Immunosensing-Fluorescence Detection of Tumor Marker Cytokeratin-19 Fragment (CYFRA 21-1) Via Carbon Quantum Dots/Zinc Oxide Nanocomposite. Nanoscale Res. Lett. 2020, 15, 12. [Google Scholar] [CrossRef]
- Amina, M.; Al Musayeib, N.M.; Alarfaj, N.A.; El-Tohamy, M.F.; Orabi, H.E.; Bukhari, S.I.; Mahmoud, A.Z. Exploiting the Potential of Moringa oleifera Oil/Polyvinyl Chloride Polymeric Bionanocomposite Film Enriched with Silver Nanoparticles for Antimicrobial Activity. Int. J. Polym. Sci. 2019, 2019. [Google Scholar] [CrossRef]
- Gholamali, I.; Asnaashariisfahani, M.; Alipour, E. Silver nanoparticles incorporated in pH-sensitive nanocomposite hydrogels based on carboxymethyl chitosan-poly (vinyl alcohol) for use in a drug delivery system. Regen. Eng. Transl. Med. 2020, 6, 138–153. [Google Scholar] [CrossRef]
- Al-Mohaimeed, A.M.; Alarfaj, N.A.; El-Tohamy, M.F.; Al-Harbi, H. Prospective of Ultrasenstive Nanometal Oxides Electrochemical Sensors for Pharmaceutical Analysis of Antihistamine Drug Fexofenadine Hydrochloride. Int. J. Electrochem. Sci. 2020, 15, 4774–4788. [Google Scholar] [CrossRef]
- Ren, H.; Gu, C.; Joo, S.W.; Zhao, J.; Sun, Y.; Huang, J. Effective hydrogen gas sensor based on NiO@ rGO nanocomposite. Sens. Actuators B Chem. 2018, 266, 506–513. [Google Scholar] [CrossRef]
- Bharathy, G.; Raji, P. Room temperature ferromagnetic behavior of Mn doped NiO nanoparticles: A suitable electrode material for supercapacitors. J. Mater. Sci. Mater. Electron. 2017, 28, 17889–17895. [Google Scholar] [CrossRef]
- Suvith, V.S.; Devu, V.S.; Philip, D. Tannic acid mediated synthesis of nanostructured NiO and SnO2 for catalytic degradation of methylene blue. Opt. Quantum Electron. 2020, 52, 12. [Google Scholar] [CrossRef]
- Chen, Z.J.; Cao, G.X.; Gan, L.Y.; Dai, H.; Xu, N.; Zang, M.J.; Dai, H.B.; Wu, H.; Wang, P. Highly dispersed platinum on honeycomb-like NiO@ Ni film as a synergistic electrocatalyst for the hydrogen evolution reaction. ACS Catal. 2018, 8, 8866–8872. [Google Scholar] [CrossRef]
- Bindhu, M.R.; Umadevi, M.; Micheal, M.K.; Arasu, M.V.; Al-Dhabi, N.A. Structural, morphological and optical properties of MgO nanoparticles for antibacterial applications. Mater. Lett. 2016, 166, 19–22. [Google Scholar] [CrossRef]
- Chang, C.W.; Gong, Z.J.; Huang, N.C.; Wang, C.Y.; Yu, W.Y. MgO nanoparticles confined in ZIF-8 as acid-base bifunctional catalysts for enhanced glycerol carbonate production from transesterification of glycerol and dimethyl carbonate. Catal. Today 2020, 351, 21–29. [Google Scholar] [CrossRef]
- Sahmani, S.; Saber-Samandari, S.; Khandan, A.; Aghdam, M.M. Influence of MgO nanoparticles on the mechanical properties of coated hydroxyapatite nanocomposite scaffolds produced via space holder technique: Fabrication, characterization and simulation. J. Mech. Behav. Biomed. Mater. 2019, 95, 76–88. [Google Scholar] [CrossRef]
- Verma, S.K.; Nisha, K.; Panda, P.K.; Patel, P.; Kumari, P.; Mallick, M.A.; Sarkar, B.; Das, B. Green synthesized MgO nanoparticles infer biocompatibility by reducing in vivo molecular nanotoxicity in embryonic zebrafish through arginine interaction elicited apoptosis. Sci. Total Environ. 2020, 713, 136521. [Google Scholar] [CrossRef] [PubMed]
- Mahendiran, C.; Maiyalagan, T.; Scott, K.; Gedanken, A. Synthesis of a carbon-coated NiO/MgO core/shell nanocomposite as a Pd electro-catalyst support for ethanol oxidation. Mater. Chem. Phys. 2011, 128, 341–347. [Google Scholar] [CrossRef]
- Fuku, X.; Matinise, N.; Masikini, M.; Kasinathan, K.; Maaza, M. An electrochemically active green synthesized polycrystalline NiO/MgO catalyst: Use in photo-catalytic applications. Mater. Res. Bull. 2018, 97, 457–465. [Google Scholar] [CrossRef]
- Ghazal, S.; Akbari, A.; Hosseini, H.A.; Sabouri, Z.; Forouzanfar, F.; Khatami, M.; Darroudi, M. Sol-gel biosynthesis of nickel oxide nanoparticles using Cydonia oblonga extract and evaluation of their cytotoxicity and photocatalytic activities. J. Mol. Str. 2020, 128378. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Chan, Y.S.; Danquah, M.K. Calcination dependent morphology transformation of sol-gel-synthesized MgO nanoparticles. Chem. Select 2017, 2, 10393–10404. [Google Scholar] [CrossRef]
- Gondal, M.A.; Saleh, T.A.; Drmosh, Q.A. Synthesis of nickel oxide nanoparticles using pulsed laser ablation in liquids and their optical characterization. Appl. Surf. Sci. 2012, 258, 6982–6986. [Google Scholar] [CrossRef]
- Nouh, S.A.; Alsobhi, B.O.; Abou Elfadl, A.; Benthami, K.; Khalil, K.D.; Riyadh, S.M. Structure and thermal investigation of the effect of laser radiation in Chitosan-MgO nanocomposite film. Radiat. Eff. Defects Solids 2020, 175, 422–432. [Google Scholar] [CrossRef]
- Brewster, D.A.; Bian, Y.; Knowles, K.E. Direct Solvothermal Synthesis of Phase-Pure Colloidal NiO Nanocrystals. Chem. Mater. 2020, 32, 2004–2013. [Google Scholar] [CrossRef]
- Ling, Z.; Zheng, M.; Du, Q.; Wang, Y.; Song, J.; Dai, W.; Zhang, L.; Ji, G.; Cao, J. Synthesis of mesoporous MgO nanoplate by an easy solvothermal- annealing method. Solid State Sci. 2011, 13, 2073–2079. [Google Scholar] [CrossRef]
- Israr-Qadir, M.; Jamil-Rana, S.; Nur, O.; Willander, M. Zinc oxide-based self-powered potentiometric chemical sensors for biomolecules and metal ions. Sensors 2017, 17, 1645. [Google Scholar] [CrossRef]
- Pechenkina, I.A.; Mikhelson, K.N. 2015. Materials for the ionophore-based membranes for ion-selective electrodes: Problems and achievements. Russ. J. Electrochem. 2015, 51, 93–102. [Google Scholar] [CrossRef]
- Tumur, M.; Kanberoglu, G.S.; Coldur, F. A novel potentiometric PVC-membrane cysteamine-selective electrode based on cysteamine-phosphomolybdate ion-pair. Curr. Pharm. Anal. 2020, 16, 168–175. [Google Scholar] [CrossRef]
- Soleymanpour, A.; Shafaatian, B.; Kor, K.; Hasaninejad, A.R. Coated wire lead (II)-selective electrode based on a Schiff base ionophore for low concentration measurements. Mon. Chem. Chem. Mon. 2012, 143, 181–188. [Google Scholar] [CrossRef]
- Elashery, S.E.; Frag, E.Y.; Mousa, M.G. A comparative study of tetra-n-butylammonium bromide potentiometric selective screen printed, carbon paste and carbon nanotube modified graphite sensors. J. Iran. Chem. Soc. 2020, 17, 911–921. [Google Scholar] [CrossRef]
- Licata, S.; Saponiero, A.; Mordente, A. Doxorubicin metabolism and toxicity in human myocardium: Role of cytoplasmic deglycosidation and carbonyl reduction. Chem. Res. Toxicol. 2000, 13, 414–420. [Google Scholar] [CrossRef]
- Dhanalingam, S.R.; Ramamurthy, S.; Chidambaram, K.; Nadaraju, S.A. Simple HPLC bioanalytical method for the determination of doxorubicin hydrochloride in rat plasma: Application to pharmacokinetic studies. Trop. J. Pharm. Res. 2014, 13, 409–415. [Google Scholar] [CrossRef]
- Choi, W.G.; Kim, D.K.; Shin, Y.; Park, R.; Cho, Y.Y.; Lee, J.Y.; Kang, H.C.; Lee, H.S. Liquid Chromatography–Tandem Mass Spectrometry for the Simultaneous Determination of Doxorubicin and its Metabolites Doxorubicinol, Doxorubicinone, Doxorubicinolone, and 7-Deoxydoxorubicinone in Mouse Plasma. Molecules 2020, 25, 1254. [Google Scholar] [CrossRef]
- Kim, H.S.; Wainer, I.W. Simultaneous analysis of liposomal doxorubicin and doxorubicin using capillary electrophoresis and laser induced fluorescence. J. Pharm. Biomed. Anal. 2010, 52, 372–376. [Google Scholar] [CrossRef]
- Yan-Fang, W.J.M.T.; Ying-Yue, Q.I.N. Determination of adriamycin in polylactic acid microballoon by ultraviolet spectrophotometry. Chin. J. Spectrosc. Lab. 2011, 4, 91. [Google Scholar]
- Skalova, S.; Langmaier, J.; Barek, J.; Vyskocil, V.; Navrátil, T. Doxorubicin determination using two novel voltammetric approaches: A comparative study. Electrochim. Acta 2020, 330, 135180. [Google Scholar] [CrossRef]
- Patel, D.; Patel, S.; Parmar, Y.; Chauhan, K.; Sannigrahi, P.; Rawat, A.S.; Vardhan, A. A novel potentiometric titration method for quantitative determination of bromide content in doxorubicin hydrochloride. Int. J. Pharma Res. Rev. 2013, 2, 10–15. [Google Scholar]
- Chen, L.; Sun, X.; Liu, Y.; Li, Y. Preparation and characterization of porous MgO and NiO/MgO nanocomposites. Appl. Catal. A Gen. 2004, 265, 123–128. [Google Scholar] [CrossRef]
- Egorov, V.V.; Zdrachek, E.A.; Nazarov, V.A. Improved separate solution method for determination of low selectivity coefficients. Anal. Chem. 2014, 86, 3693–3696. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.V.; Smakula, A. Dielectric properties of cobalt oxide, nickel oxide, and their mixed crystals. J. Appl. Phys. 1965, 36, 2031–2038. [Google Scholar] [CrossRef]
- Mageshwari, K.; Mali, S.S.; Sathyamoorthy, R.; Patil, P.S. Template-free synthesis of MgO nanoparticles for effective photocatalytic applications. Powder Technol. 2013, 249, 456–462. [Google Scholar] [CrossRef]
- International Union of Pure and Applied Chemistry Analytical Chemistry Division Commission on Analytical Nomenclature. Pure Appl. Chem.; Pergamon Press: New York, NY, USA, 1976; Volume 48, pp. 127–132.
- Kakhkiz, R.M. Application of nanoparticles in the potentiometric ion selective electrodes. Russ. J. Electrochem. 2013, 49, 458–465. [Google Scholar] [CrossRef]
- Isa, I.M.; Sohaimi, N.M.; Hashim, N.; Kamari, A.; Mohamed, A.; Ahmad, M.; Ghani, S.A. Determination of salicylate ion by potentiometric membrane electrode based on zinc aluminium layered double hydroxides-4 (2,4-dichlorophenoxy) butyrate nanocomposites. Int. J. Electrochem. Sci. 2013, 8, 2112–2121. [Google Scholar]
- Ma, T.S.; Hassan, S.S. Organic Analysis Using Ion Selective Electrodes; Academic Press: London, UK, 1982; Volume I–II. [Google Scholar]
- FDA. ICH-Q2 (R1) Validation and Analytical Procedures: Text and Methodology. In Proceedings of the International Conference on Harmonization Guidelines, Geneva, Switzerland, 17 November 2005. [Google Scholar]
- Miller, J.C.; Miller, J.N. Statistics for Analytical Chemistry, 3rd ed.; Ellis Horwood PTR Prentice Hall: New York, NY, USA, 1993. [Google Scholar]
- Harahap, Y.; Ardiningsih, P.; Winarti, A.C.; Purwanto, D.J. Analysis of the Doxorubicin and Doxorubicinol in the Plasma of Breast Cancer Patients for Monitoring the Toxicity of Doxorubicin. Drug Des. Devel. Ther. 2020, 14, 3469. [Google Scholar] [CrossRef]









| Parameter | Conventional Coated Wire DOX-PM Sensor | Modified DOX-PM-NiO/MgO Nanocomposite Sensor |
|---|---|---|
| Slope (mV. Decade−1) | 52.9 ± 0.5 | 57.9 ± 0.3 |
| Intercept | 453.4 | 723.2 |
| Regression equation | EmV = (52.9 ± 0.5) log [DOX] + 453.4 | EmV = (57.9 ± 0.3) log [DOX] + 723.2 |
| Correlation coefficient, r | 0.9989 | 0.9999 |
| Linear range (mol L−1) | 10 × 10−6–1.0 × 10−2 | 1.0 × 10−11–1.0 × 10−2 |
| LOD | 5.0 × 10−7 | 5.4 × 10−12 |
| Response time/s | 75 | 40 |
| Working pH range | 2–5 | 2–5 |
| Lifetime/day | 30 | 75 |
| Temperature (°C) | 25 | 25 |
| Accuracy (%) | 99.2 ± 0.6 | 99.8 ± 0.2 |
| Interferences | Conventional Coated Wire DOX-PM Sensor | Modified DOX-PM-NiO/MgO Nanocomposite Sensor |
|---|---|---|
| Na+ | 4.4 × 10−3 | 9.5 × 10−5 |
| K+ | 1.4 × 10−3 | 5.8 × 10−4 |
| Ca2+ | 2.9 × 10−3 | 6.4 × 10−4 |
| Mg2+ | 6.6 × 10−3 | 2.5 × 10−5 |
| Cu2+ | 4.8 × 10−3 | 8.9 × 10−4 |
| Zn2+ | 7.6 × 10−3 | 2.8 × 10−5 |
| Ag+ | 6.9 × 10−3 | 5.9 × 10−4 |
| Glucose | 4.6 × 10−3 | 4.4 × 10−5 |
| Lactose | 5.9 × 10−3 | 5.7 × 10−4 |
| Starch | 4.4 × 10−3 | 2.3 × 10−5 |
| Valine | 1.3 × 10−3 | 9.9 × 10−4 |
| Lysine | 4.6 × 10−3 | 3.4 × 10−5 |
| Tryptophan | 9.5 × 10−3 | 5.9 × 10−5 |
| Glycine | 5.4 × 10−3 | 6.7 × 10−5 |
| Leucine | 1.6 × 10−3 | 2.6 × 10−4 |
| L-histidine | 4.7 × 10−3 | 2.8 × 10−5 |
| Statistical analysis | Conventional DOX-PM Coated Wire Sensor | Modified DOX-PM-NiO/MgO Nanocomposite | ||
| * Test solution | % Recovery | * Test Solution | % Recovery | |
| 6 5.3 5 4 3 2 | 98.5 98.2 99.4 99.3 98.8 98.6 | 11 10 9 8 7 6 5 4 3 2 | 100.0 99.5 99.7 100.0 98.9 99.2 99.6 100.0 99.6 99.7 | |
| Mean ± SD | 98.8 ± 0.5 | 99.6 ± 0.4 | ||
| n | 6 | 10 | ||
| Variance | 0.25 | 0.16 | ||
| %SE | 0.20 | 0.13 | ||
| %RSD | 0.51 | 0.40 | ||
| Statistical analysis | Modified DOX-PM-NiO@MgO Nanocomposite Coated Wire Sensor | |||||
| Intra-day assay | Inter-day assay | |||||
| * Test solution | * Found | % Recovery | * Test solution | * Found | % Recovery | |
| 11.0 | 11.0 | 100.0 | 11.0 | 10.99 | 99.9 | |
| 8.0 | 7.99 | 99.9 | 8.0 | 7.98 | 99.8 | |
| 4.0 | 4.01 | 100.3 | 4.0 | 3.98 | 99.5 | |
| Mean ± SD | 100.06 ± 0.2 | 99.7 ± 0.2 | ||||
| n | 3 | 3 | ||||
| Variance | 0.04 | 0.04 | ||||
| %SE ** | 0.11 | 0.11 | ||||
| %RSD | 0.20 | 0.20 | ||||
| Initial [DOX] −log Conc. mol L−1 | Added [DOX] −log Conc. mol L−1 | DOX-PM- NiO/MgONPs Sensor | Reported Method [52] |
|---|---|---|---|
| % Recovery ± %RSD | % Recovery ± %RSD | ||
| 8.3 | 0.5 | 98.4 ± 0.6 | 96.4 ± 0.5 |
| 6.5 | 0.5 | 99.0 ± 0.4 | 96.5 ± 0.7 |
| 8.8 | 0.5 | 98.2 ± 0.3 | 97.3 ± 0.8 |
| 7.6 | 0.5 | 99.5 ± 0.2 | 96.9 ± 0.4 |
| 8.2 | 0.5 | 98.9 ± 0.7 | 97.6 ± 0.2 |
| 5.9 | 0.5 | 98.4 ± 0.9 | 98.6 ± 1.4 |
| 7.7 | 0.5 | 99.2 ± 0.3 | 96.4 ± 1.2 |
| 9.7 | 0.5 | 99.6 ± 0.4 | 97.8 ± 0.7 |
| 6.2 | 0.5 | 98.5 ± 1.1 | 98.5 ± 0.4 |
| 8.6 | 0.5 | 98.8 ± 0.9 | 96.6 ± 1.2 |
| 7.2 | 0.5 | 98.6 ± 1.2 | 97.4 ± 0.3 |
| 6.1 | 0.5 | 98.3 ± 0.5 | 98.8 ± 14 |
| 8.7 | 0.5 | 98.2 ± 0.3 | 96.9 ± 0.5 |
| 7.8 | 0.5 | 99.3 ± 0.9 | 97.4 ± 0.6 |
| 7.4 | 0.5 | 98.6 ± 1.0 | 98.3 ± 0.3 |
| 8.6 | 0.5 | 99.8 ± 0.8 | 97.9 ± 1.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alarfaj, N.A.; El-Tohamy, M.F. New Functionalized Polymeric Sensor Based NiO/MgO Nanocomposite for Potentiometric Determination of Doxorubicin Hydrochloride in Commercial Injections and Human Plasma. Polymers 2020, 12, 3066. https://doi.org/10.3390/polym12123066
Alarfaj NA, El-Tohamy MF. New Functionalized Polymeric Sensor Based NiO/MgO Nanocomposite for Potentiometric Determination of Doxorubicin Hydrochloride in Commercial Injections and Human Plasma. Polymers. 2020; 12(12):3066. https://doi.org/10.3390/polym12123066
Chicago/Turabian StyleAlarfaj, Nawal A., and Maha F. El-Tohamy. 2020. "New Functionalized Polymeric Sensor Based NiO/MgO Nanocomposite for Potentiometric Determination of Doxorubicin Hydrochloride in Commercial Injections and Human Plasma" Polymers 12, no. 12: 3066. https://doi.org/10.3390/polym12123066
APA StyleAlarfaj, N. A., & El-Tohamy, M. F. (2020). New Functionalized Polymeric Sensor Based NiO/MgO Nanocomposite for Potentiometric Determination of Doxorubicin Hydrochloride in Commercial Injections and Human Plasma. Polymers, 12(12), 3066. https://doi.org/10.3390/polym12123066