Physico-Chemical and Antiadhesive Properties of Poly(Lactic Acid)/Grapevine Cane Extract Films against Food Pathogenic Microorganisms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Films Preparation
2.3. Films Characterization
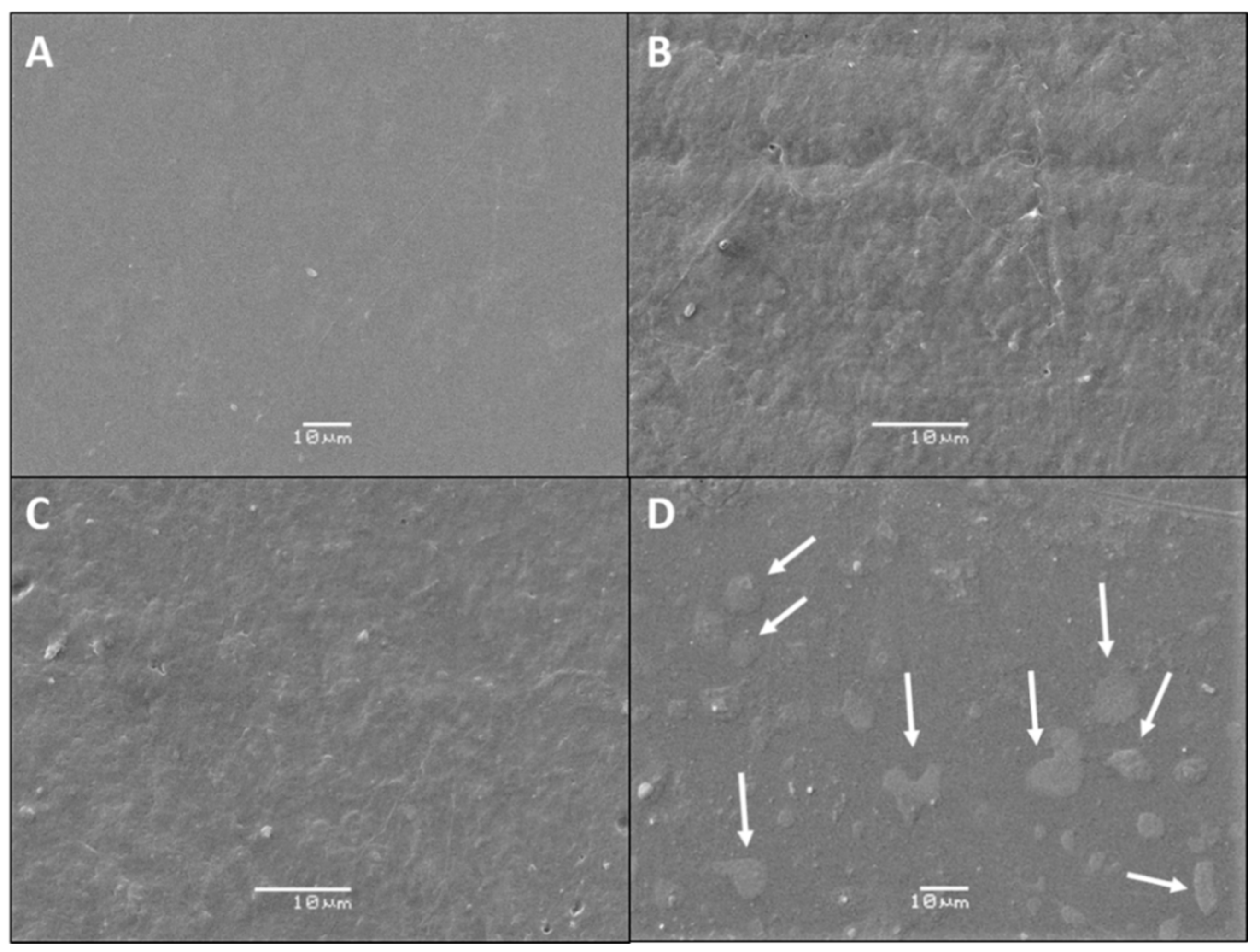
2.3.1. SEM
2.3.2. Mechanical Analysis
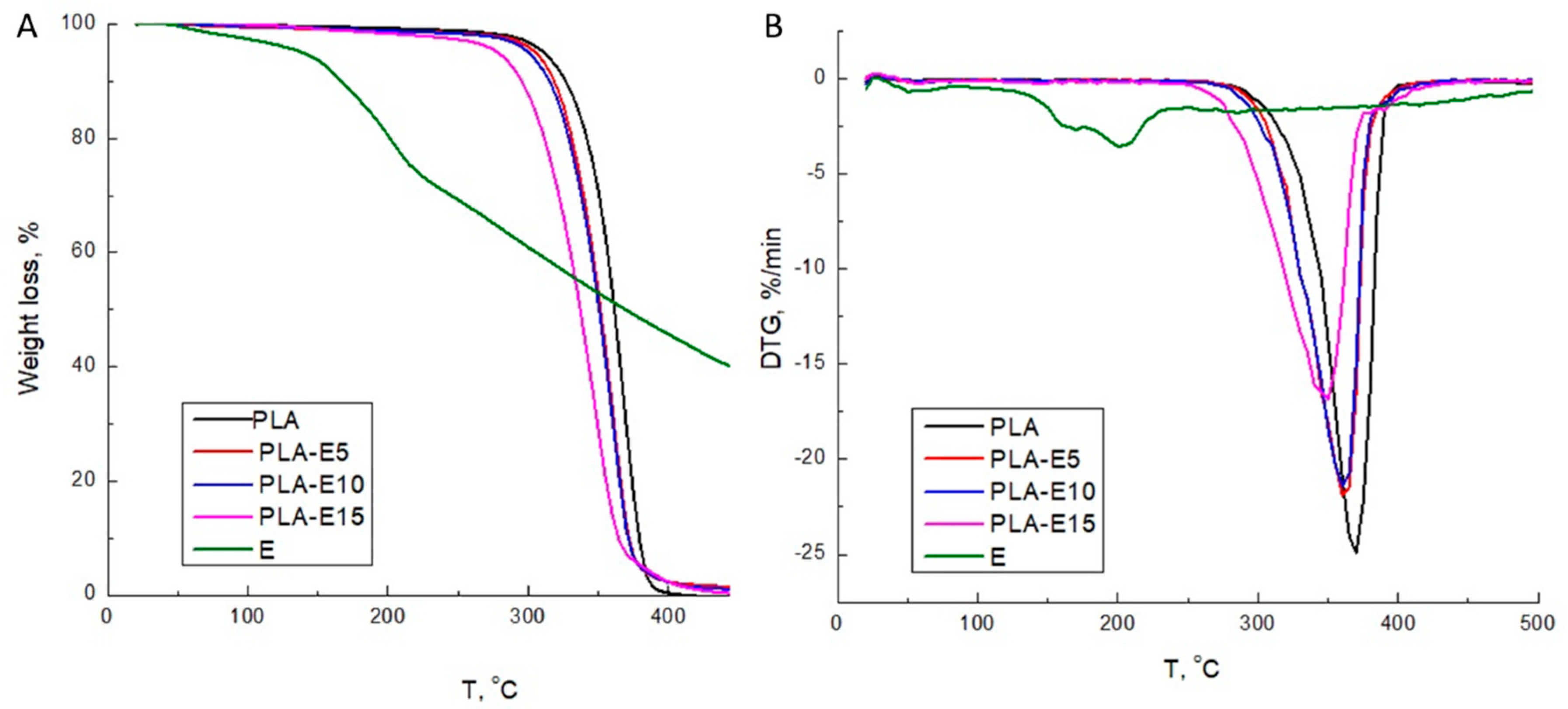
2.3.3. TGA
2.3.4. Water Vapor Permeability
2.3.5. Release of Grapevine Cane Extract from Films and Biological Activity of Films
2.3.6. Bacterial Adhesion on Polyethylene Terephthalate (PET), Polystyrene (PS), and PLA-E Surfaces
2.3.7. Statistical Analysis
3. Results and Discussion
3.1. SEM Analysis
3.2. Mechanical Analysis
3.3. TGA
3.4. Water Vapor Barrier Properties
3.5. Release of Extract from PLA Films and Biological Activity
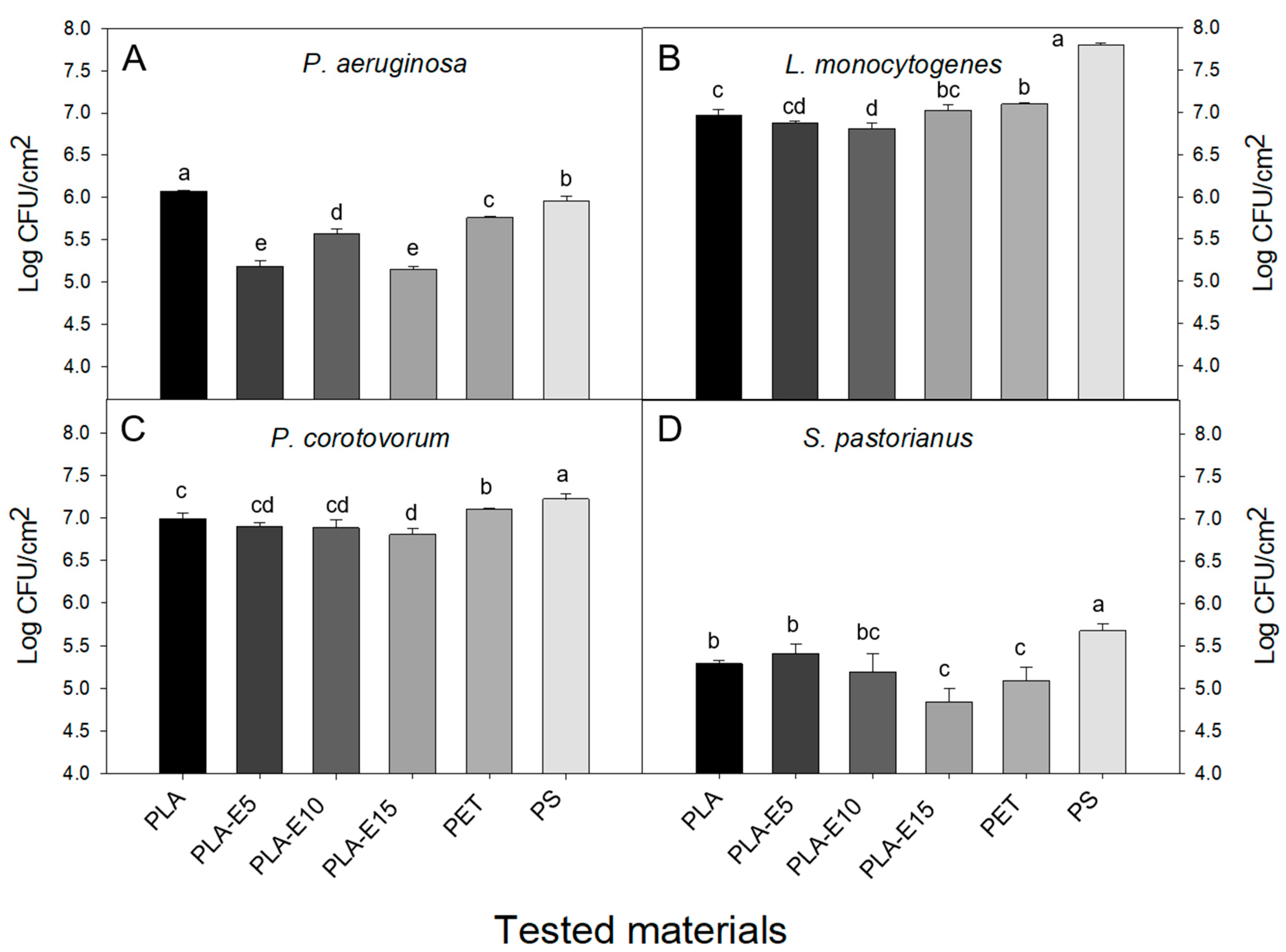
3.6. Adhesion on Polyethylene Terephthalate (PET), Polystyrene (PS), and PLA-E Surfaces
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Plastics-the Facts 2019: An Analysis of European Plastic Production, Demand and Waste Data. 2019. Available online: https://www.plasticseurope.org/es/resources/publications/1804-plastics-facts-2019 (accessed on 11 December 2020).
- Groh, K.J.; Backhaus, T.; Carney-Almroth, B.; Geueke, B.; Inostroza, P.A.; Lennquist, A.; Leslie, H.A.; Maffini, M.; Slunge, D.; Trasande, L.; et al. Overview of known plastic packaging-associated chemicals and their hazards. Sci. Total Environ. 2019, 651, 3253–3268. [Google Scholar] [CrossRef]
- Cacciotti, I.; Mori, S.; Cherubini, V.; Nanni, F. Eco-sustainable systems based on poly(lactic acid), diatomite and coffee grounds extract for food packaging. Int. J. Biol. Macromol. 2018, 112, 567–575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nilsuwan, K.; Benjakul, S.; Prodpran, T. Physical/thermal properties and heat seal ability of bilayer films based on fish gelatin and poly(lactic acid). Food Hydrocoll. 2018, 77, 248–256. [Google Scholar] [CrossRef]
- Ashter, S. Commercial Applications of Bioplastics. In Introduction to Bioplastics Engineering; Ashter, S., William, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 227–249. [Google Scholar]
- Muller, J.; González-Martínez, C.; Chiralt, A. Combination of Poly(lactic) Acid and Starch for Biodegradable Food Packaging. Materials 2017, 10, 952. [Google Scholar] [CrossRef] [PubMed]
- Singha, S.; Hedenqvist, M.S. A Review on Barrier Properties of Poly(Lactic Acid)/Clay Nanocomposites. Polymers 2020, 12, 1095. [Google Scholar] [CrossRef] [PubMed]
- Colomines, G.; Ducruet, V.; Courgneau, C.; Guinault, A.; Domenek, S. Barrier properties of poly(lactic acid) and its morphological changes induced by aroma compound sorption. Polym. Int. 2010, 59, 818–826. [Google Scholar] [CrossRef] [Green Version]
- Requena, R.; Vargas, M.; Chiralt, A. Obtaining antimicrobial bilayer starch and polyester-blend films with carvacrol. Food Hydrocoll. 2018, 83, 118–133. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Peponi, L.; López, D.; Fernández-García, M. Recovery of yerba mate (Ilex paraguariensis) residue for the development of PLA-based bionanocomposite films. Ind. Crops Prod. 2018, 111, 317–328. [Google Scholar] [CrossRef]
- Lukic, I.; Vulic, J.; Ivanovic, J. Antioxidant activity of PLA/PCL films loaded with thymol and/or carvacrol using scCO2 for active food packaging. Food Packag. Shelf Life 2020, 26, 100578. [Google Scholar] [CrossRef]
- Radusin, T.; Torres-Giner, S.; Stupar, A.; Ristic, I.; Miletic, A.; Novakovic, A.; Lagaron, J.M. Preparation, characterization and antimicrobial properties of electrospun polylactide films containing Allium ursinum L. extract. Food Packag. Shelf Life 2019, 21, 100357. [Google Scholar] [CrossRef]
- Agustin-Salazar, S.; Cerruti, P.; Medina-Juárez, L.Á.; Scarinzi, G.; Malinconico, M.; Soto-Valdez, H.; Gamez-Meza, N. Lignin and holocellulose from pecan nutshell as reinforcing fillers in poly (lactic acid) biocomposites. Int. J. Biol. Macromol. 2018, 115, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Risyon, N.P.; Othman, S.H.; Basha, R.K.; Talib, R.A. Characterization of polylactic acid/halloysite nanotubes bionanocomposite films for food packaging. Food Packag. Shelf Life 2020, 23, 100450. [Google Scholar] [CrossRef]
- Chi, H.; Song, S.; Luo, M.; Zhang, C.; Li, W.; Li, L.; Qin, Y. Effect of PLA nanocomposite films containing bergamot essential oil, TiO2 nanoparticles, and Ag nanoparticles on shelf life of mangoes. Sci. Hortic. (Amsterdam) 2019, 249, 192–198. [Google Scholar] [CrossRef]
- Zhang, H.; Hortal, M.; Jordá-Beneyto, M.; Rosa, E.; Lara-Lledo, M.; Lorente, I. ZnO-PLA nanocomposite coated paper for antimicrobial packaging application. LWT 2017, 78, 250–257. [Google Scholar] [CrossRef]
- Baek, N.; Kim, Y.T.; Marcy, J.E.; Duncan, S.E.; O’Keefe, S.F. Physical properties of nanocomposite polylactic acid films prepared with oleic acid modified titanium dioxide. Food Packag. Shelf Life 2018, 17, 30–38. [Google Scholar] [CrossRef]
- Shankar, S.; Rhim, J.-W. Effect of types of zinc oxide nanoparticles on structural, mechanical and antibacterial properties of poly(lactide)/poly(butylene adipate-co-terephthalate) composite films. Food Packag. Shelf Life 2019, 21, 100327. [Google Scholar] [CrossRef]
- Kim, I.; Viswanathan, K.; Kasi, G.; Sadeghi, K.; Thanakkasaranee, S.; Seo, J. Poly(Lactic Acid)/ZnO Bionanocomposite Films with Positively Charged ZnO as Potential Antimicrobial Food Packaging Materials. Polymers 2019, 11, 1427. [Google Scholar] [CrossRef] [Green Version]
- Tang, Z.; Fan, F.; Fan, C.; Jiang, K.; Qin, Y. The Performance Changes and Migration Behavior of PLA/Nano-TiO2 Composite Film by High-Pressure Treatment in Ethanol Solution. Polymers 2020, 12, 471. [Google Scholar] [CrossRef] [Green Version]
- Shavisi, N.; Khanjari, A.; Basti, A.A.; Misaghi, A.; Shahbazi, Y. Effect of PLA films containing propolis ethanolic extract, cellulose nanoparticle and Ziziphora clinopodioides essential oil on chemical, microbial and sensory properties of minced beef. Meat Sci. 2017, 124, 95–104. [Google Scholar] [CrossRef]
- Martins, C.; Vilarinho, F.; Sanches Silva, A.; Andrade, M.; Machado, A.V.; Castilho, M.C.; Sá, A.; Cunha, A.; Vaz, M.F.; Ramos, F. Active polylactic acid film incorporated with green tea extract: Development, characterization and effectiveness. Ind. Crops Prod. 2018, 123, 100–110. [Google Scholar] [CrossRef] [Green Version]
- Arrieta, M.P.; López, J.; Ferrándiz, S.; Peltzer, M.A. Characterization of PLA-limonene blends for food packaging applications. Polym. Test. 2013, 32, 760–768. [Google Scholar] [CrossRef]
- Soto-Valdez, H.; Auras, R.; Peralta, E. Fabrication of poly(lactic acid) films with resveratrol and the diffusion of resveratrol into ethanol. J. Appl. Polym. Sci. 2011, 121, 970–978. [Google Scholar] [CrossRef]
- Vahedikia, N.; Garavand, F.; Tajeddin, B.; Cacciotti, I.; Jafari, S.M.; Omidi, T.; Zahedi, Z. Biodegradable zein film composites reinforced with chitosan nanoparticles and cinnamon essential oil: Physical, mechanical, structural and antimicrobial attributes. Colloids Surf. B Biointerfaces 2019, 177, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Makris, D.P. Green extraction processes for the efficient recovery of bioactive polyphenols from wine industry solid wastes – Recent progress. Curr. Opin. Green Sustain. Chem. 2018, 13, 50–55. [Google Scholar] [CrossRef]
- Aizpurua-Olaizola, O.; Navarro, P.; Vallejo, A.; Olivares, M.; Etxebarria, N.; Usobiaga, A. Microencapsulation and storage stability of polyphenols from Vitis vinifera grape wastes. Food Chem. 2016, 190, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, R.F.; Biais, B.; Richard, T.; Puertas, B.; Waffo-Teguo, P.; Merillon, J.M.; Cantos-Villar, E. Grapevine cane’s waste is a source of bioactive stilbenes. Ind. Crops Prod. 2016, 94, 884–892. [Google Scholar] [CrossRef]
- Vergara, C.; von Baer, D.; Mardones, C.; Wilkens, A.; Wernekinck, K.; Damm, A.; Macke, S.; Gorena, T.; Winterhalter, P. Stilbene Levels in Grape Cane of Different Cultivars in Southern Chile: Determination by HPLC-DAD-MS/MS Method. J. Agric. Food Chem. 2012, 60, 929–933. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Fang, Y.; Wang, H.; Li, H.; Zhang, Z. Free-Radical Scavenging Properties and Reducing Power of Grape Cane Extracts from 11 Selected Grape Cultivars Widely Grown in China. Molecules 2011, 16, 10104–10122. [Google Scholar] [CrossRef]
- Billet, K.; Houillé, B.; Besseau, S.; Mélin, C.; Oudin, A.; Papon, N.; Courdavault, V.; Clastre, M.; Giglioli-Guivarc’h, N.; Lanoue, A. Mechanical stress rapidly induces E-resveratrol and E-piceatannol biosynthesis in grape canes stored as a freshly-pruned byproduct. Food Chem. 2018, 240, 1022–1027. [Google Scholar] [CrossRef]
- Zwingelstein, M.; Draye, M.; Besombes, J.L.; Piot, C.; Chatel, G. Viticultural wood waste as a source of polyphenols of interest: Opportunities and perspectives through conventional and emerging extraction methods. Waste Manag. 2020, 102, 782–794. [Google Scholar] [CrossRef]
- Piñeiro, Z.; Marrufo-Curtido, A.; Serrano, M.J.; Palma, M. Ultrasound-assisted extraction of stilbenes from grape canes. Molecules 2016, 21, 784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Locilento, D.A.; Mercante, L.A.; Andre, R.S.; Mattoso, L.H.C.; Luna, G.L.F.; Brassolatti, P.; Anibal, F.D.F.; Correa, D.S. Biocompatible and Biodegradable Electrospun Nanofibrous Membranes Loaded with Grape Seed Extract for Wound Dressing Application. J. Nanomater. 2019, 2019, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Nikvarz, N.; Khayati, G.R.; Sharafi, S. Preparation of UV absorbent films using polylactic acid and grape syrup for food packaging application. Mater. Lett. 2020, 276, 128187. [Google Scholar] [CrossRef]
- Díaz-Galindo, E.P.; Nesic, A.; Cabrera-Barjas, G.; Mardones, C.; von Baer, D.; Bautista-Baños, S.; Dublan Garcia, O. Physical-Chemical Evaluation of Active Food Packaging Material Based on Thermoplastic Starch Loaded with Grape cane Extract. Molecules 2020, 25, 1306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sáez, V.; Pastene, E.; Vergara, C.; Mardones, C.; Hermosín-Gutiérrez, I.; Gómez-Alonso, S.; Gómez, M.V.; Theoduloz, C.; Riquelme, S.; von Baer, D. Oligostilbenoids in Vitis vinifera L. Pinot Noir grape cane extract: Isolation, characterization, in vitro antioxidant capacity and anti-proliferative effect on cancer cells. Food Chem. 2018, 265, 101–110. [Google Scholar] [CrossRef]
- Kuorwel, K.K.; Cran, M.J.; Sonneveld, K.; Miltz, J.; Bigger, S.W. Evaluation of Antifungal Activity of Antimicrobial Agents on Cheddar Cheese. Packag. Technol. Sci. 2014, 27, 49–58. [Google Scholar] [CrossRef]
- Vazquez-Armenta, F.J.; Bernal-Mercado, A.T.; Lizardi-Mendoza, J.; Silva-Espinoza, B.A.; Cruz-Valenzuela, M.R.; Gonzalez-Aguilar, G.A.; Nazzaro, F.; Fratianni, F.; Ayala-Zavala, J.F. Phenolic extracts from grape stems inhibit Listeria monocytogenes motility and adhesion to food contact surfaces. J. Adhes. Sci. Technol. 2018, 32, 889–907. [Google Scholar] [CrossRef]
- Bonadies, I.; Di Cristo, F.; Valentino, A.; Peluso, G.; Calarco, A.; Di Salle, A. pH-Responsive Resveratrol-Loaded Electrospun Membranes for the Prevention of Implant-Associated Infections. Nanomaterials 2020, 10, 1175. [Google Scholar] [CrossRef]
- Shankar, S.; Wang, L.-F.; Rhim, J.-W. Incorporation of zinc oxide nanoparticles improved the mechanical, water vapor barrier, UV-light barrier, and antibacterial properties of PLA-based nanocomposite films. Mater. Sci. Eng. C 2018, 93, 289–298. [Google Scholar] [CrossRef]
- Qin, Y.; Li, W.; Liu, D.; Yuan, M.; Li, L. Development of active packaging film made from poly (lactic acid) incorporated essential oil. Prog. Org. Coat. 2017, 103, 76–82. [Google Scholar] [CrossRef]
- Hwang, S.W.; Shim, J.K.; Selke, S.E.; Soto-Valdez, H.; Matuana, L.; Rubino, M.; Auras, R. Poly(L-lactic acid) with added α-tocopherol and resveratrol: Optical, physical, thermal and mechanical properties. Polym. Int. 2012, 61, 418–425. [Google Scholar] [CrossRef]
- Agustin-Salazar, S.; Gamez-Meza, N.; Medina-Juàrez, L.À.; Soto-Valdez, H.; Cerruti, P. From Nutraceutics to Materials: Effect of Resveratrol on the Stability of Polylactide. ACS Sustain. Chem. Eng. 2014, 2, 1534–1542. [Google Scholar] [CrossRef]
- Šešlija, S.; Nešić, A.; Ružić, J.; Kalagasidis Krušić, M.; Veličković, S.; Avolio, R.; Santagata, G.; Malinconico, M. Edible blend films of pectin and poly(ethylene glycol): Preparation and physico-chemical evaluation. Food Hydrocoll. 2017, 77, 494–501. [Google Scholar] [CrossRef]
- Ortiz-Vazquez, H.; Shin, J.; Soto-Valdez, H.; Auras, R. Release of butylated hydroxytoluene (BHT) from Poly(lactic acid) films. Polym. Test. 2011, 30, 463–471. [Google Scholar] [CrossRef]
- Jamshidian, M.; Tehrany, E.A.; Imran, M.; Akhtar, M.J.; Cleymand, F.; Desobry, S. Structural, mechanical and barrier properties of active PLA-antioxidant films. J. Food Eng. 2012, 110, 380–389. [Google Scholar] [CrossRef]
- Talebi, F.; Misaghi, A.; Khanjari, A.; Kamkar, A.; Gandomi, H.; Rezaeigolestani, M. Incorporation of spice essential oils into poly-lactic acid film matrix with the aim of extending microbiological and sensorial shelf life of ground beef. LWT 2018, 96, 482–490. [Google Scholar] [CrossRef]
- Albert, S.; Horbach, R.; Deising, H.B.; Siewert, B.; Csuk, R. Synthesis and antimicrobial activity of (E) stilbene derivatives. Bioorg. Med. Chem. 2011, 19, 5155–5166. [Google Scholar] [CrossRef]
- Adrian, M.; Jeandet, P. Effects of resveratrol on the ultrastructure of Botrytis cinerea conidia and biological significance in plant/pathogen interactions. Fitoterapia 2012, 83, 1345–1350. [Google Scholar] [CrossRef]
- Xu, D.; Deng, Y.; Han, T.; Jiang, L.; Xi, P.; Wang, Q.; Jiang, Z.; Gao, L. In vitro and in vivo effectiveness of phenolic compounds for the control of postharvest gray mold of table grapes. Postharvest Biol. Technol. 2018, 139, 106–114. [Google Scholar] [CrossRef]
- Atanacković, M.T.; Gojković-Bukarica, L.C.; Cvejić, J.M. Improving the low solubility of resveratrol. BMC Pharmacol. Toxicol. 2012, 13, A25. [Google Scholar] [CrossRef] [Green Version]
- Pastor, C.; Sánchez-González, L.; Chiralt, A.; Cháfer, M.; González-Martínez, C. Physical and antioxidant properties of chitosan and methylcellulose based films containing resveratrol. Food Hydrocoll. 2013, 30, 272–280. [Google Scholar] [CrossRef] [Green Version]
- Kuruwita, D.P.; Jiang, X.; Darby, D.; Sharp, J.L.; Fraser, A.M. Persistence of Escherichia coli O157: H7 and Listeria monocytogenes on the exterior of three common food packaging materials. Food Control 2020, 112, 107153. [Google Scholar] [CrossRef]
- Kirstein, I.V.; Wichels, A.; Krohne, G.; Gerdts, G. Mature biofilm communities on synthetic polymers in seawater Specific or general? Mar. Environ. Res. 2018, 142, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Zhu, Y.; Zhang, Y.; Zeng, G.; Wen, X.; Yi, H.; Ye, S.; Ren, X.; Song, B. Micro(nano)plastics: Unignorable vectors for organisms. Mar. Pollut. Bull. 2019, 139, 328–331. [Google Scholar] [CrossRef] [PubMed]
- Gouvinhas, I.; Santos, R.A.; Queiroz, M.; Leal, C.; Saavedra, M.J.; Domínguez-Perles, R.; Rodrigues, M.; Barros, A.I.R.N.A. Monitoring the antioxidant and antimicrobial power of grape (Vitis vinifera L.) stems phenolics over long-term storage. Ind. Crops Prod. 2018, 126, 83–91. [Google Scholar] [CrossRef]
- Chalal, M.; Klinguer, A.; Echairi, A.; Meunier, P.; Vervandier-Fasseur, D.; Adrian, M. Antimicrobial activity of resveratrol analogues. Molecules 2014, 19, 7679–7688. [Google Scholar] [CrossRef] [Green Version]

| Sample | PLA (wt%) | PEG1500 (wt%) | Grapevine Cane Extract (wt%) |
|---|---|---|---|
| PLA | 95 | 5 | 0 |
| PLA-E5 | 90 | 5 | 5 |
| PLA-E10 | 85 | 5 | 10 |
| PLA-E15 | 80 | 5 | 15 |
| Strain | Type of Microorganism | Cultivation Temperature (°C) |
|---|---|---|
| Listeria monocytogenes ATCC 7644 | Gram positive (+) | 37 ± 2 |
| Saccharomyces pastorianus ATCC 2345 | Yeast | 30 |
| Pseudomonas aeruginosa ATCC 10145 | Gram-negative (−) | 37 ± 2 |
| Pectobacterium carotovorum ATCC 15713 | Gram-negative (−) | 25 ± 2 |
| Sample | E (MPa) | TS (MPa) | e (%) | Tonset (°C) | Tdeg (°C) | WVP × 1011 (g/m s Pa) |
|---|---|---|---|---|---|---|
| PLA | 735 ± 17 a | 33.1 ± 0.08 a | 27.4 ± 1.0 c | 304 | 369 | 4.74 ± 0.31 |
| PLA-E5 | 512 ± 19 b | 23.8 ± 1.3 b | 34.5 ± 1.5 b | 302 | 364 | 2.37 ± 0.12 |
| PLA-E10 | 342 ± 12 c | 19.6 ± 0.09 c | 37.6 ± 1.2 a | 297 | 363 | 2.14 ± 0.11 |
| PLA-E15 | 325 ± 45 c | 16.3 ± 2.1 c | 35.6 ± 3.5 ab | 297 | 351 | 3.40 ± 0.47 |
| Sample | Release of Extract (mg/L) | Inhibition of Mycelial Growth (%) | ||
|---|---|---|---|---|
| 2 Day | 7 Day | 2 Day | 7 Day | |
| PLA-E5 | 1.66 ± 0.12 b | 1.90 ± 0.18 b | 16.2 ± 2.1 c | 4.9 ± 2.7 c |
| PLA-E10 | 1.73 ± 0.15 ab | 2.23 ± 0.21 b | 22.5 ± 1.7 b | 25.0 ± 3.9 b |
| PLA-E15 | 2.03 ± 0.18 a | 4.39 ± 0.46 a | 34.7 ± 0.4 a | 35.8 ± 1.1 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-Galindo, E.P.; Nesic, A.; Cabrera-Barjas, G.; Dublan-García, O.; Ventura-Aguilar, R.I.; Vázquez-Armenta, F.J.; Aguilar-Montes de Oca, S.; Mardones, C.; Ayala-Zavala, J.F. Physico-Chemical and Antiadhesive Properties of Poly(Lactic Acid)/Grapevine Cane Extract Films against Food Pathogenic Microorganisms. Polymers 2020, 12, 2967. https://doi.org/10.3390/polym12122967
Díaz-Galindo EP, Nesic A, Cabrera-Barjas G, Dublan-García O, Ventura-Aguilar RI, Vázquez-Armenta FJ, Aguilar-Montes de Oca S, Mardones C, Ayala-Zavala JF. Physico-Chemical and Antiadhesive Properties of Poly(Lactic Acid)/Grapevine Cane Extract Films against Food Pathogenic Microorganisms. Polymers. 2020; 12(12):2967. https://doi.org/10.3390/polym12122967
Chicago/Turabian StyleDíaz-Galindo, Edaena Pamela, Aleksandra Nesic, Gustavo Cabrera-Barjas, Octavio Dublan-García, Rosa Isela Ventura-Aguilar, Francisco Javier Vázquez-Armenta, Saúl Aguilar-Montes de Oca, Claudia Mardones, and Jesús Fernando Ayala-Zavala. 2020. "Physico-Chemical and Antiadhesive Properties of Poly(Lactic Acid)/Grapevine Cane Extract Films against Food Pathogenic Microorganisms" Polymers 12, no. 12: 2967. https://doi.org/10.3390/polym12122967
APA StyleDíaz-Galindo, E. P., Nesic, A., Cabrera-Barjas, G., Dublan-García, O., Ventura-Aguilar, R. I., Vázquez-Armenta, F. J., Aguilar-Montes de Oca, S., Mardones, C., & Ayala-Zavala, J. F. (2020). Physico-Chemical and Antiadhesive Properties of Poly(Lactic Acid)/Grapevine Cane Extract Films against Food Pathogenic Microorganisms. Polymers, 12(12), 2967. https://doi.org/10.3390/polym12122967










