Cross-Linking Modification of Ammonium Polyphosphate via Ionic Exchange and Self-Assembly for Enhancing the Fire Safety Properties of Polypropylene
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Modification of APP
2.3. Sample Preparation
2.4. Characterization
3. Results and Discussion
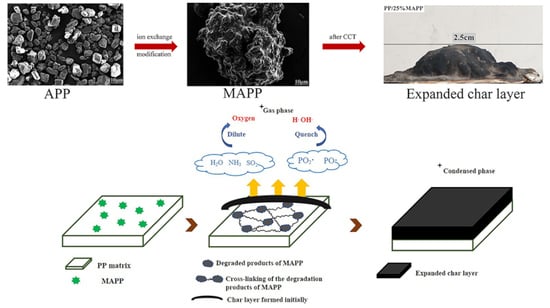
3.1. Characterization of MAPP
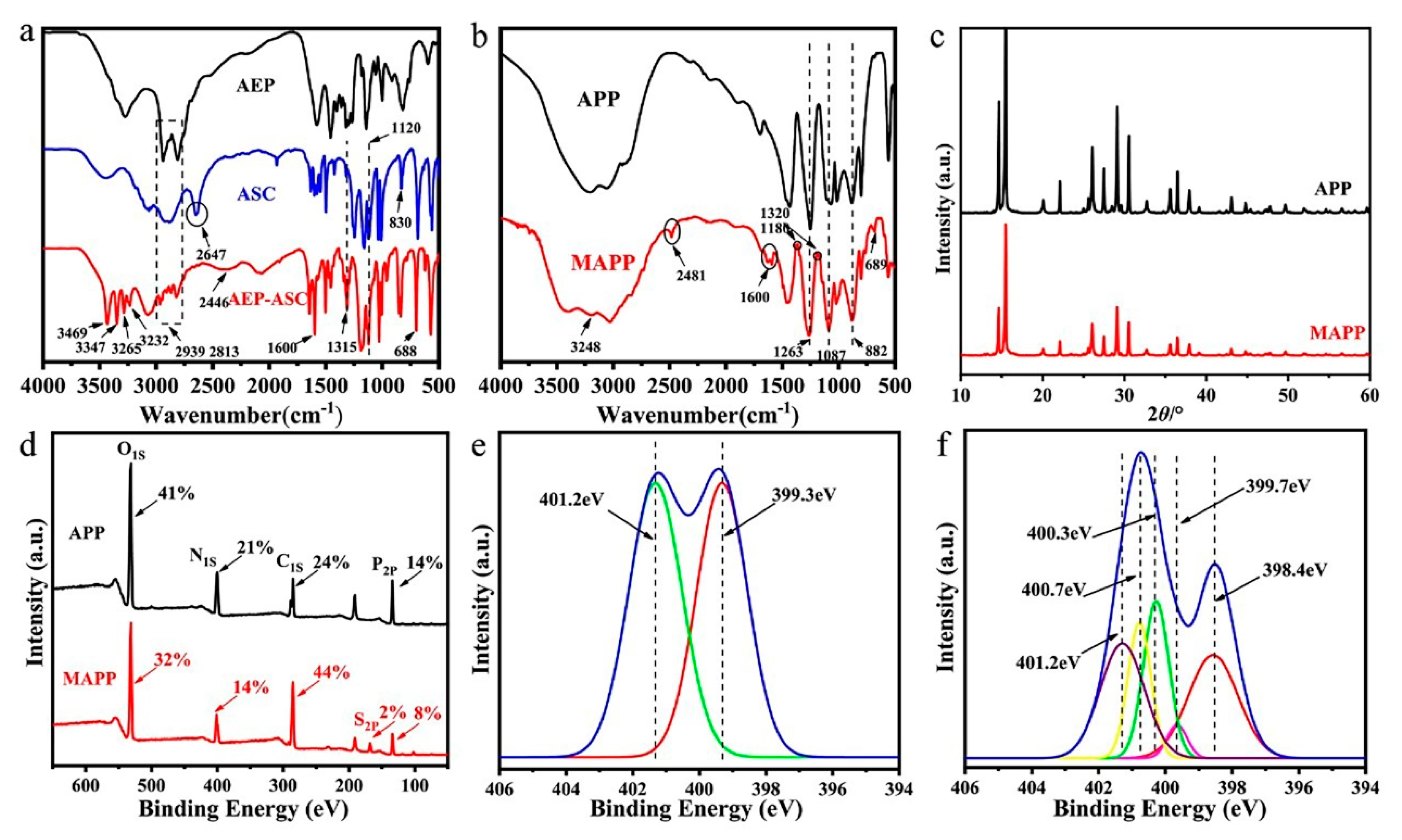
3.1.1. FTIR Analysis
3.1.2. XRD Analysis
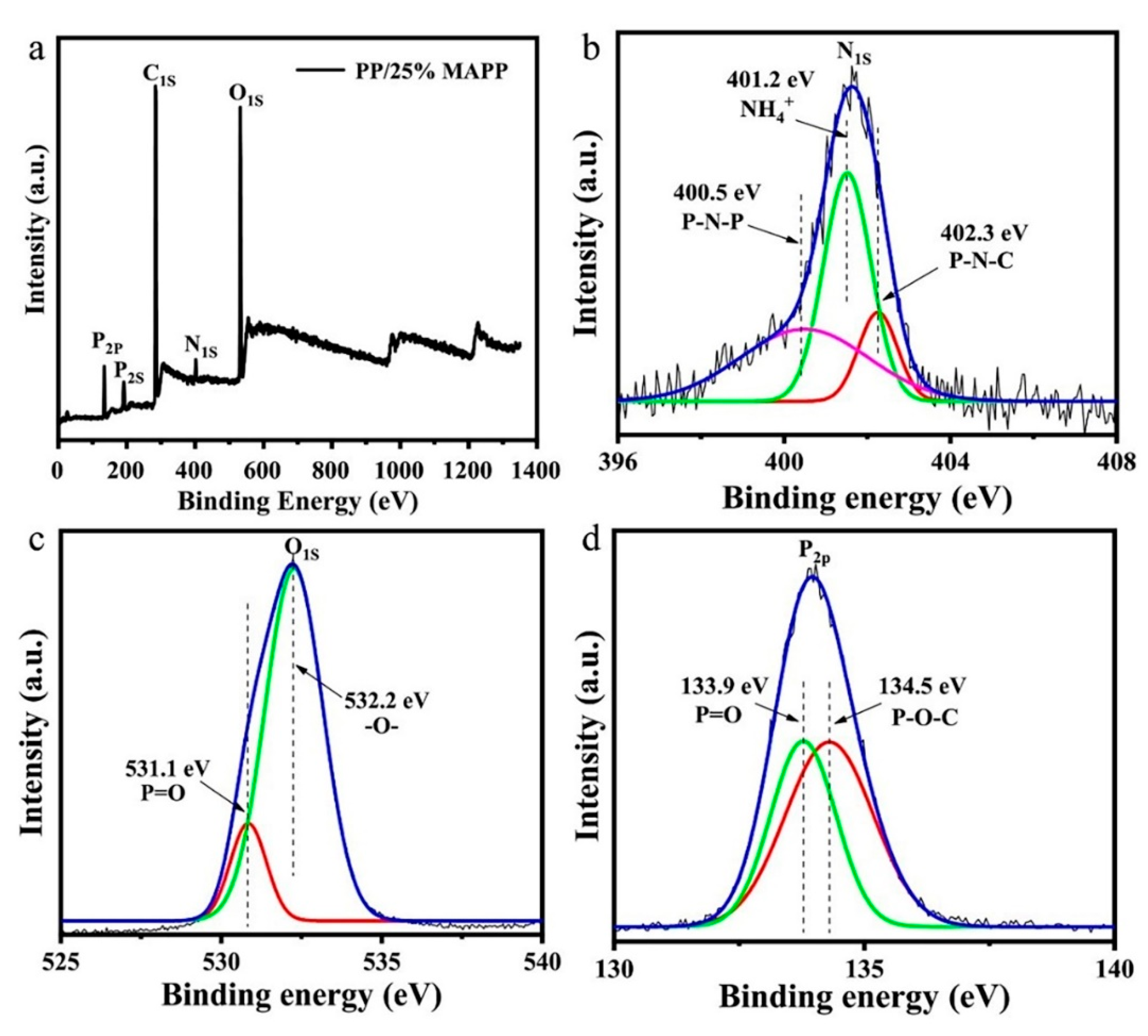
3.1.3. XPS Analysis
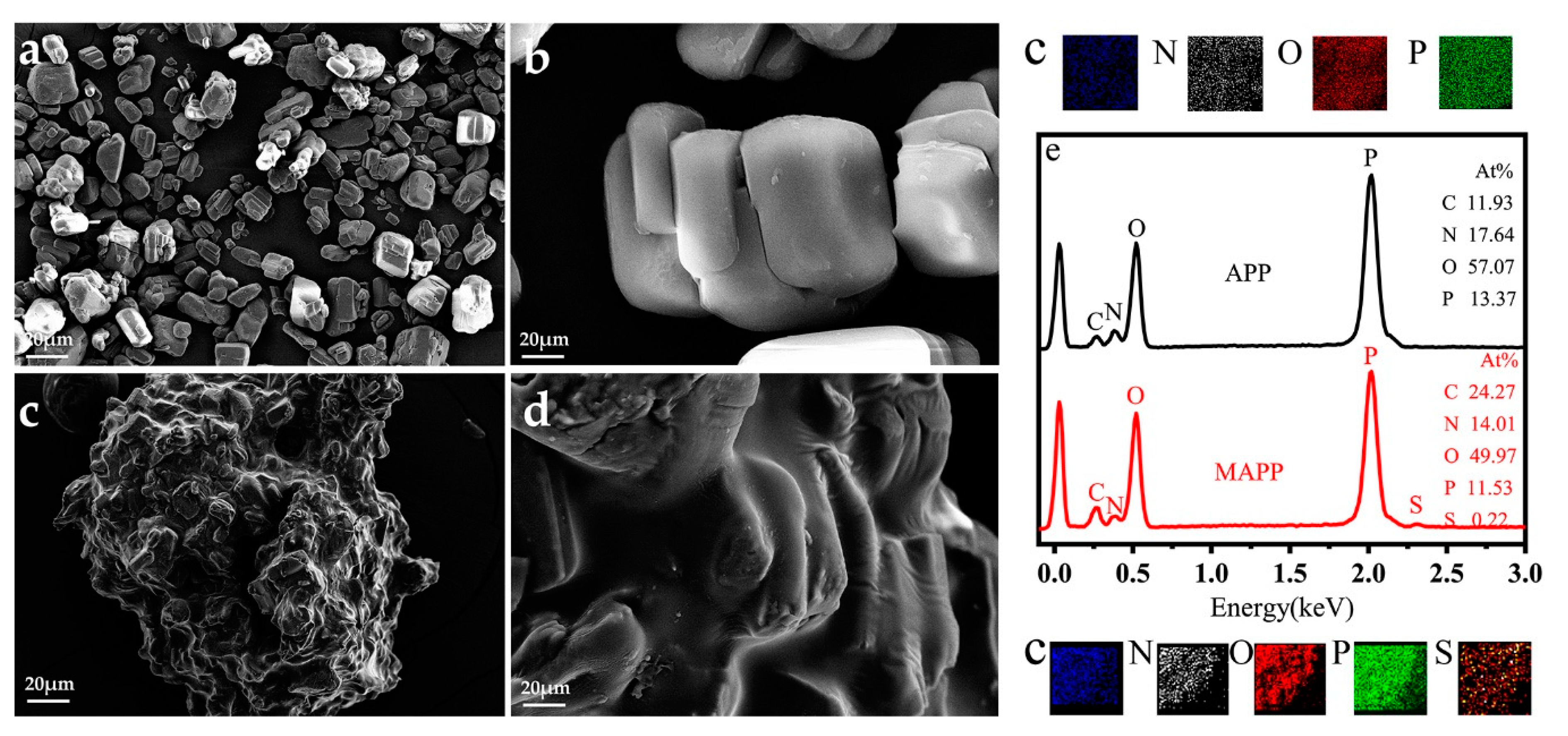
3.1.4. Surface Morphology and EDS test
3.2. Fire Behavior
3.2.1. Reaction to Small Flame (UL 94 Vertical Burning and LOI)
3.2.2. Combustion Behavior Under Forced-Flaming Scenario (Cone Calorimetry Test)
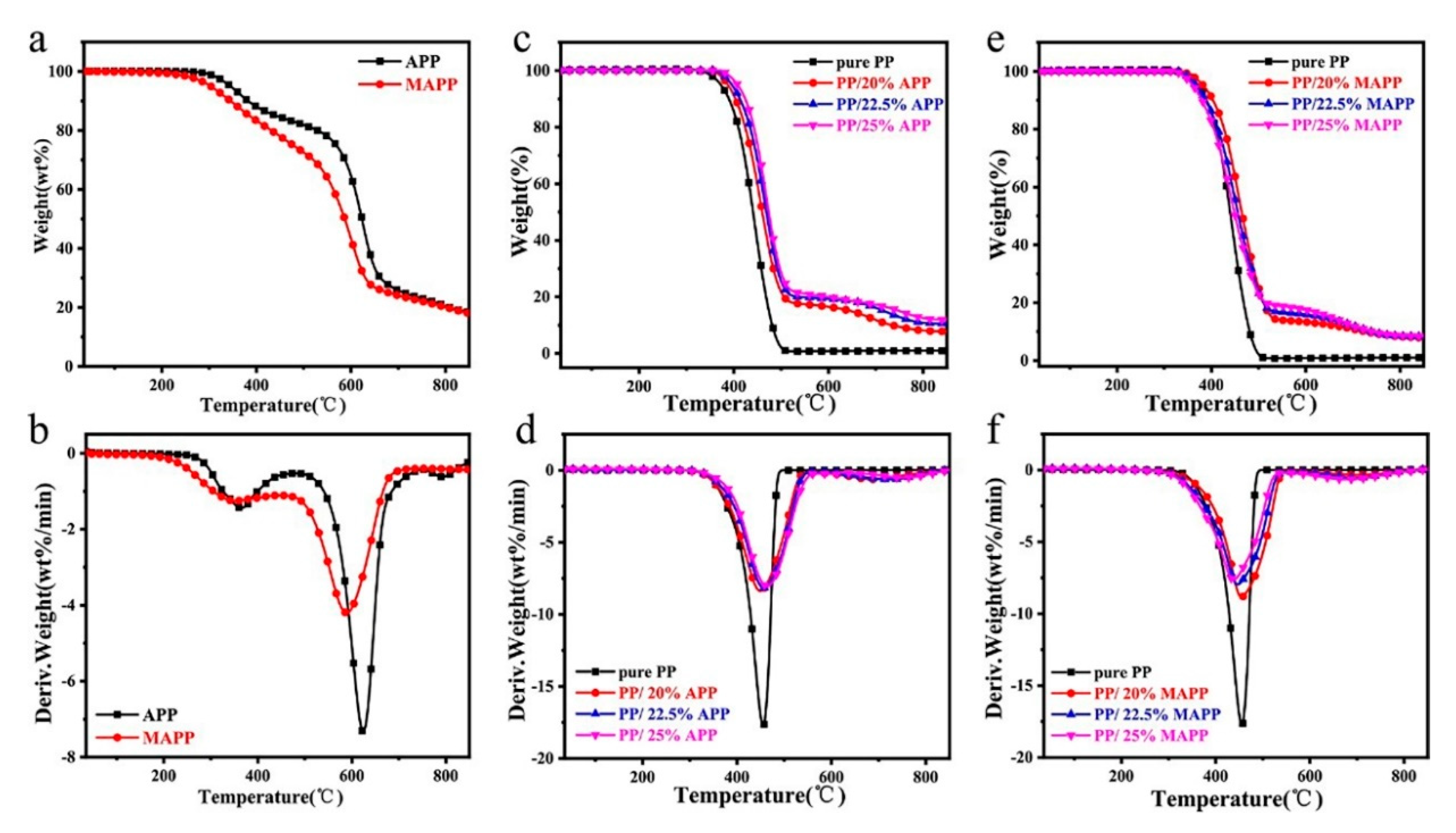
3.3. Thermal Stability Analysis
3.4. Flame-Retardant Mechanism Analysis
3.4.1. Gas Phase Analysis
3.4.2. Analysis of Char Residue
3.4.3. Possible Flame-Retardant Mechanism
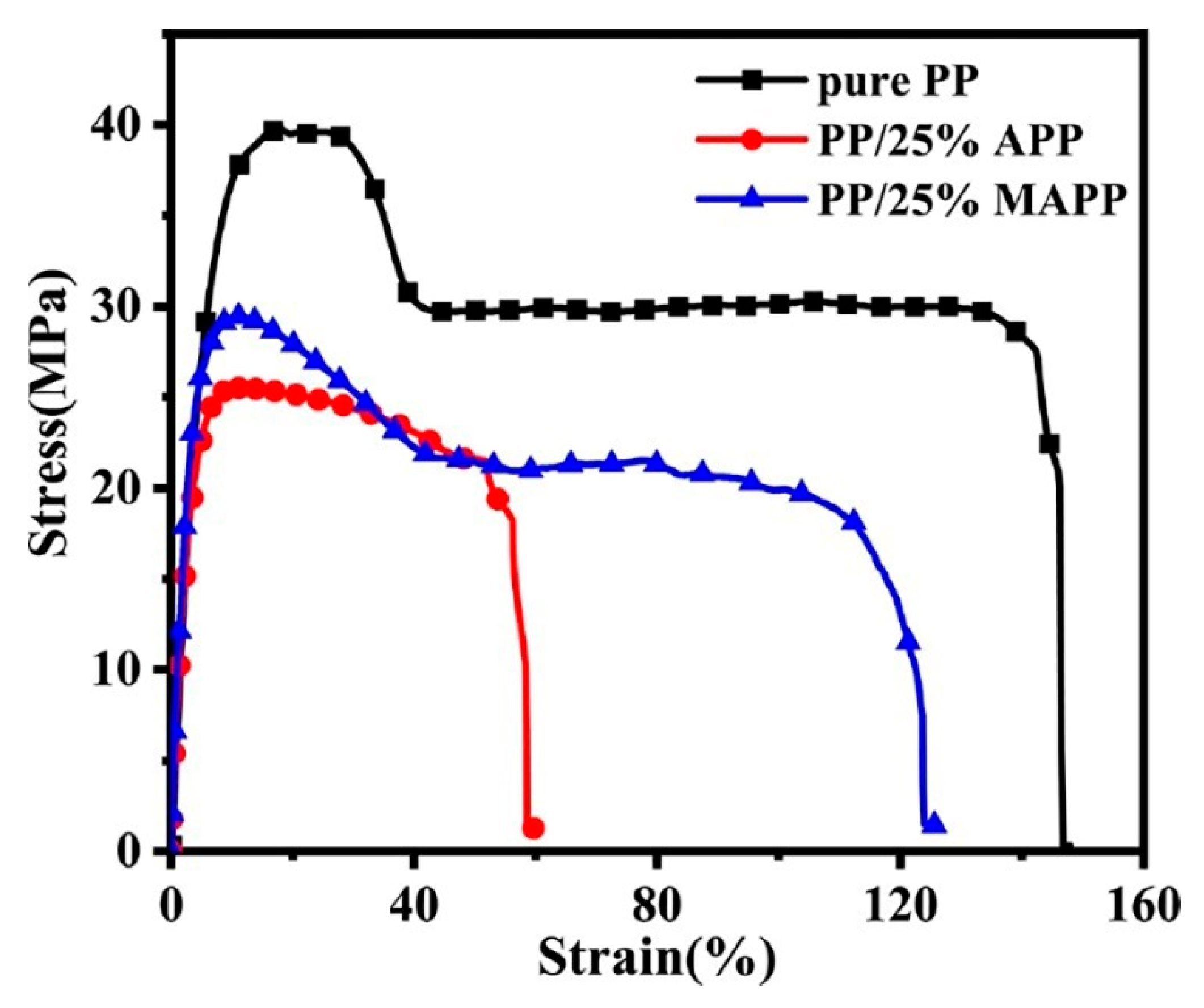
3.5. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhao, Z.; Jin, Q.; Zhang, N.; Guo, X.; Yan, H. Preparation of a novel polysiloxane and its synergistic effect with ammonium polyphosphate on the flame retardancy of polypropylene. Polym. Degrad. Stab. 2018, 150, 73–85. [Google Scholar] [CrossRef]
- Yang, K.; Xu, M.J.; Li, B. Synthesis of N-ethyl triazineepiperazine copolymer and flame retardancy and water resistance of intumescent flame retardant polypropylene. Polym. Degrad. Stab. 2013, 98, 1397–1406. [Google Scholar] [CrossRef]
- Qi, H.S.; Liu, S.W.; Chen, X.L.; Shen, C.H.; Gao, S.J. The flame retardant and thermal performances of polypropylene with a novel intumescent flame retardant. J. Appl. Polym. Sci. 2020, 137, 49047. [Google Scholar] [CrossRef]
- Qi, C.; Yuan, B.; Dong, H.; Li, K.; Shang, S.; Sun, Y.; Chen, G.; Zhan, Y. Supramolecular self-assembly modification of ammonium polyphosphate and its flame retardant application in polypropylene. Polym. Adv. Technol. 2020, 31, 1099–1109. [Google Scholar] [CrossRef]
- Yu, G.; Ma, C.; Li, J. Flame retardant effect of cytosine pyrophosphate and pentaerythritol on polypropylene. Compos. B Eng. 2020, 180, 107520. [Google Scholar] [CrossRef]
- Rault, F.; Giraud, S.; Salaün, F.; Almeras, X. Development of a Halogen Free Flame Retardant Masterbatch for Polypropylene Fibers. Polymers 2015, 7, 220–234. [Google Scholar] [CrossRef]
- Seidi, F.; Movahedifar, E.; Naderi, G.; Akbari, V.; Ducos, F.; Shamsi, R.; Vahabi, H.; Saeb, M.R. Flame Retardant Polypropylenes: A Review. Polymers 2020, 12, 1701. [Google Scholar] [CrossRef]
- Tang, W.; Qian, L.; Chen, Y.; Qiu, Y.; Xu, B. Intumescent flame retardant behavior of charring agents with different aggregation of piperazine/triazine groups in polypropylene. Polym. Degrad. Stab. 2019, 169, 108982. [Google Scholar] [CrossRef]
- Ding, S.; Liu, P.; Zhang, S.; Ding, Y.; Wang, F.; Gao, C.; Yang, M. Preparation and characterization of cyclodextrin microencapsulated ammonium polyphosphate and its application in flame retardant polypropylene. J. Appl. Polym. Sci. 2020, 137, 49001. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, J.; Yan, H.; Guo, X.; Sun, Q.; Guo, R. A novel organic-inorganic hybrid K-HBPE@APP performing excellent flame retardancy and smoke suppression for polypropylene. J. Hazard. Mater. 2019, 373, 856–865. [Google Scholar] [CrossRef]
- Zhang, T.; Tao, Y.; Zhou, F.; Sheng, H.; Qiu, S.; Ma, C.; Hu, Y. Synthesis of a hyperbranched phosphorus-containing polyurethane as char forming agent combined with ammonium polyphosphate for reducing fire hazard of polypropylene. Polym. Degrad. Stab. 2019, 165, 207–219. [Google Scholar] [CrossRef]
- Qiu, S.; Ma, C.; Wang, X.; Zhou, X.; Feng, X.; Yuen, R.K.; Hu, Y. Melamine-containing polyphosphazene wrapped ammonium polyphosphate: A novel multifunctional organic-inorganic hybrid flame retardant. J. Hazard. Mater. 2018, 344, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Liu, G. Microencapsulation of ammonium polyphosphate with melamine-formaldehyde-tris(2-hydroxyethyl)isocyanurate resin and its flame retardancy in polypropylene. RSC Adv. 2015, 5, 88445–88455. [Google Scholar] [CrossRef]
- Shao, Z.-B.; Deng, C.; Tan, Y.; Chen, M.-J.; Chen, L.; Wang, Y.-Z. Flame retardation of polypropylene via a novel intumescent flame retardant: Ethylenediamine-modified ammonium polyphosphate. Polym. Degrad. Stab. 2014, 106, 88–96. [Google Scholar] [CrossRef]
- Shao, Z.-B.; Deng, C.; Tan, Y.; Yu, L.; Chen, M.; Chen, L.; Wang, Y.-Z. Ammonium polyphosphate chemically-modified with ethanolamine as an efficient intumescent flame retardant for polypropylene. J. Mater. Chem. A 2014, 2, 13955–13965. [Google Scholar] [CrossRef]
- Tan, Y.; Shao, Z.-B.; Chen, X.-F.; Long, J.-W.; Chen, L.; Wang, Y.-Z. Novel Multifunctional Organic–Inorganic Hybrid Curing Agent with High Flame-Retardant Efficiency for Epoxy Resin. ACS Appl. Mater. Interfaces 2015, 7, 17919–17928. [Google Scholar] [CrossRef]
- Shao, Z.-B.; Deng, C.; Tan, Y.; Chen, M.-J.; Chen, L.; Wang, Y.-Z. An Efficient Mono-Component Polymeric Intumescent Flame Retardant for Polypropylene: Preparation and Application. ACS Appl. Mater. Interfaces 2014, 6, 7363–7370. [Google Scholar] [CrossRef]
- Shang, S.; Yuan, B.; Sun, Y.; Chen, G.; Huang, C.; Yu, B.; He, S.; Dai, H.; Chen, X. Facile preparation of layered melamine-phytate flame retardant via supramolecular self-assembly technology. J. Colloid Interface Sci. 2019, 553, 364–371. [Google Scholar] [CrossRef]
- Shang, S.; Ma, X.; Yuan, B.; Chen, G.; Sun, Y.; Huang, C.; He, S.; Dai, H.; Chen, X. Modification of halloysite nanotubes with supramolecular self-assembly aggregates for reducing smoke release and fire hazard of polypropylene. Compos. B Eng. 2019, 177, 107371. [Google Scholar] [CrossRef]
- Dong, H.; Yuan, B.; Qi, C.; Li, K.; Shang, S.; Sun, Y.; Chen, G.; Zhang, H.; Chen, X. Preparation of piperazine cyanurate by hydrogen-bonding self-assembly reaction and its application in intumescent flame-retardant polypropylene composites. Polym. Adv. Technol. 2019, 31, 1027–1037. [Google Scholar] [CrossRef]
- Sunab, Y.; Yuana, B.; Shanga, S.; Zhangc, H.; Shid, Y.; Yue, B.; Qia, C.; Donga, H.; Chena, X.; Yangb, X. Surface modification of ammonium polyphosphate by supramolecular assembly for enhancing fire safety properties of polypropylene. Compos. B Eng. 2020, 181, 107588. [Google Scholar] [CrossRef]
- Jin, X.; Sun, J.; Zhang, J.S.; Gu, X.; Bourbigot, S.; Li, H.; Tang, W.; Zhang, S. Preparation of a Novel Intumescent Flame Retardant Based on Supramolecular Interactions and Its Application in Polyamide 11. ACS Appl. Mater. Interfaces 2017, 9, 24964–24975. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Cui, S.-P.; Sun, S.; Gu, X.; Li, H.; Sun, J.; Zhang, S.; Bourbigot, S. The Preparation of an Intumescent Flame Retardant by Ion Exchange and Its Application in Polylactic Acid. ACS Appl. Polym. Mater. 2019, 1, 755–764. [Google Scholar] [CrossRef]
- Duan, L.; Yang, H.; Song, L.; Hou, Y.; Wang, W.; Gui, Z.; Hu, Y. Hyperbranched phosphorus/nitrogen-containing polymer in combination with ammonium polyphosphate as a novel flame retardant system for polypropylene. Polym. Degrad. Stab. 2016, 134, 179–185. [Google Scholar] [CrossRef]
- Ren, Y.; Yuan, D.; Li, W.; Cai, X. Flame retardant efficiency of KH-550 modified urea-formaldehyde resin cooperating with ammonium polyphosphate on polypropylene. Polym. Degrad. Stab. 2018, 151, 160–171. [Google Scholar] [CrossRef]
- Guan, Y.-H.; Huang, J.-Q.; Yang, J.-C.; Shao, Z.-B.; Wang, Y.-Z. An Effective Way to Flame-Retard Biocomposite with Ethanolamine Modified Ammonium Polyphosphate and Its Flame Retardant Mechanisms. Ind. Eng. Chem. Res. 2015, 54, 3524–3531. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Y.; Li, T.; Zhang, S.; Ma, P.; Shi, D.; Chen, M.; Dong, W. A Bio-Based Flame-Retardant Starch Based on Phytic Acid. ACS Sustain. Chem. Eng. 2020, 8, 10265–10274. [Google Scholar] [CrossRef]
- Xiong, Z.; Zhang, Y.; Du, X.; Song, P.; Fang, Z. Green and Scalable Fabrication of Core–Shell Biobased Flame Retardants for Reducing Flammability of Polylactic Acid. ACS Sustain. Chem. Eng. 2019, 7, 8954–8963. [Google Scholar] [CrossRef]
- Tian, N.; Wen, X.; Jiang, Z.; Gong, J.; Wang, Y.; Xue, J.; Tang, T. Synergistic Effect between a Novel Char Forming Agent and Ammonium Polyphosphate on Flame Retardancy and Thermal Properties of Polypropylene. Ind. Eng. Chem. Res. 2013, 52, 10905–10915. [Google Scholar] [CrossRef]
- Jiang, W.; Hao, J.-W.; Han, Z. Study on the thermal degradation of mixtures of ammonium polyphosphate and a novel caged bicyclic phosphate and their flame retardant effect in polypropylene. Polym. Degrad. Stab. 2012, 97, 632–637. [Google Scholar] [CrossRef]
- Zheng, Z.; Liu, Y.; Zhang, L.; Dai, B.; Yang, X.; Wang, H. Fabrication of halogen-free ammonium phosphate with two components via a simple method and its flame retardancy in polypropylene composites. J. Therm. Anal. Calorim. 2016, 127, 2013–2023. [Google Scholar] [CrossRef]
- Ran, J.C.; Qiu, J.D.; Xie, H.L.; Lai, X.J.; Li, H.Q.; Zeng, X.R. Combination effect of zirconium phosphate nanosheet and PU-coated carbon fiber on flame retardancy and thermal behavior of PA46/PPO alloy. Compos. B. Eng. 2019, 166, 621–632. [Google Scholar] [CrossRef]
- Huang, W.; He, W.; Long, L.; Yan, W.; He, M.; Qin, S.; Yu, J. Highly efficient flame-retardant glass-fiber-reinforced polyamide 6T system based on a novel DOPO-based derivative: Flame retardancy, thermal decomposition, and pyrolysis behavior. Polym. Degrad. Stab. 2018, 148, 26–41. [Google Scholar] [CrossRef]
- Qiao, Y.H.; Wang, Y.B.; Zou, M.H.; Xu, D.H.; Pan, Y.T.; Luo, Z.L.; Wang, B.B. One-Step Synthesis of Highly Efficient Oligo (phenyl phosphonic Dihydroxypropyl Silicone Oil) Flame Retardant for Polycarbonate. Polymers 2019, 11, 1977. [Google Scholar] [CrossRef] [PubMed]









| Sample ID | PP wt.% | APP wt.% | MAPP wt.% | Dripping or Not | UL-94 Rating 3.2 mm | LOI % |
|---|---|---|---|---|---|---|
| Pure PP | 100 | 0 | 0 | Yes | No rate | 17 |
| PP/20%APP | 80 | 20 | 0 | Yes | No rate | 17 |
| PP/22.5%APP | 77.5 | 22.5 | 0 | Yes | No rate | 18 |
| PP/25%APP | 75 | 25 | 0 | Yes | V-2 | 19 |
| PP/20%MAPP | 80 | 0 | 20 | Yes | V-2 | 22 |
| PP/22.5%MAPP | 77.5 | 0 | 22.5 | No | V-0 | 30 |
| PP/25%MAPP | 75 | 0 | 25 | No | V-0 | 32 |
| Sample (Units) | Pure PP | PP/25%APP | PP/20%MAPP | PP/22.5%MAPP | PP/25%MAPP |
|---|---|---|---|---|---|
| TTI (s) | 47 | 49 | 40 | 38 | 35 |
| PHRR (kW/m2) | 718.3 | 354.7 | 201.0 | 192.4 | 155.9 |
| TPHRR (s) | 105 | 190 | 255 | 235 | 215 |
| THR (MJ/m2) | 57.3 | 47.9 | 50.7 | 47.2 | 44.9 |
| TSP (m2) | 48.8 | 6.4 | 6.5 | 5.2 | 4.2 |
| Mean COY (kg/kg) | 0.32 | 0.11 | 0.17 | 0.17 | 0.15 |
| PSPR (m2/s) | 0.5 | 0.045 | 0.026 | 0.024 | 0.016 |
| AMLR (g/s) | 0.059 | 0.043 | 0.033 | 0.032 | 0.027 |
| FPI (m2s/kW) | 0.07 | 0.14 | 0.20 | 0.20 | 0.22 |
| FGI (kW/m2s) | 6.84 | 1.87 | 0.79 | 0.82 | 0.72 |
| Sample | T5wt% (°C) | Tmax1 (°C) | Tmax2 (°C) | Rate of Tmax1 (wt.%/min) | 800 °C Char Residues (%) |
|---|---|---|---|---|---|
| APP | 345.1 | 364.4 | 623.9 | 7.3 | 20.5 |
| MAPP | 303.1 | 351.7 | 588.4 | 4.2 | 20.1 |
| pure PP | 373.5 | 455.4 | / | 17.7 | 0 |
| PP/20%APP | 387.4 | 448.8 | 687.9 | 8.4 | 8.1 |
| PP/22.5%APP | 395.9 | 456.6 | 719.1 | 8.2 | 10.9 |
| PP/25%APP | 407.9 | 463.7 | 747.4 | 8.1 | 12.5 |
| PP/20%MAPP | 383.0 | 454.6 | 704.6 | 8.8 | 8.3 |
| PP/22.5%MAPP | 368.0 | 446.1 | 694.8 | 8.0 | 8.5 |
| PP/25%MAPP | 361.2 | 437.6 | 670.9 | 7.6 | 8.6 |
| Sample | T5wt% (°C) | Tmax1 (°C) | Tmax2 (°C) | Rate of Tmax1 (wt.%/min) | 800 °C Char Residues (%) |
|---|---|---|---|---|---|
| APP | 346.6 | 359.5 | 626.8 | 7.4 | 22.9 |
| MAPP | 270.9 | 320.7 | 615.0 | 6.7 | 5.7 |
| pure PP | 246.4 | 334.8 | / | 8.5 | 0 |
| PP/20%APP | 287.9 | 381.1 | 561.3 | 6.0 | 3.7 |
| PP/22.5%APP | 284.2 | 374.1 | 583.7 | 5.7 | 3.9 |
| PP/25%APP | 275.0 | 366.0 | 589.9 | 5.3 | 5.3 |
| PP/20%MAPP | 279.3 | 362.1 | 557.1 | 6.4 | 3.3 |
| PP/22.5%MAPP | 277.3 | 354.1 | 591.0 | 5.9 | 4.1 |
| PP/25%MAPP | 273.8 | 346.0 | 596.6 | 5.6 | 5.4 |
| Sample | Tensile Strength (MPa) | Elongation at Break (%) | Impact Strength (kJ m−2) |
|---|---|---|---|
| Pure PP | 39.1 ± 0.4 | 147.1 ± 3.0 | 4.6 ± 0.5 |
| PP/25%APP | 25.3 ± 2.5 | 59.8 ± 6.8 | 2.9 ± 0.2 |
| PP/25%MAPP | 28.4 ± 1.1 | 125.9 ± 4.8 | 4.0 ± 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, Y.; Luo, Z.; Wang, B. Cross-Linking Modification of Ammonium Polyphosphate via Ionic Exchange and Self-Assembly for Enhancing the Fire Safety Properties of Polypropylene. Polymers 2020, 12, 2761. https://doi.org/10.3390/polym12112761
Pan Y, Luo Z, Wang B. Cross-Linking Modification of Ammonium Polyphosphate via Ionic Exchange and Self-Assembly for Enhancing the Fire Safety Properties of Polypropylene. Polymers. 2020; 12(11):2761. https://doi.org/10.3390/polym12112761
Chicago/Turabian StylePan, Yingtong, Zhonglin Luo, and Biaobing Wang. 2020. "Cross-Linking Modification of Ammonium Polyphosphate via Ionic Exchange and Self-Assembly for Enhancing the Fire Safety Properties of Polypropylene" Polymers 12, no. 11: 2761. https://doi.org/10.3390/polym12112761
APA StylePan, Y., Luo, Z., & Wang, B. (2020). Cross-Linking Modification of Ammonium Polyphosphate via Ionic Exchange and Self-Assembly for Enhancing the Fire Safety Properties of Polypropylene. Polymers, 12(11), 2761. https://doi.org/10.3390/polym12112761