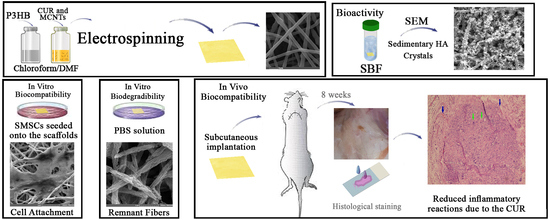
Poly(3-Hydroxybutyrate)-Multiwalled Carbon Nanotubes Electrospun Scaffolds Modified with Curcumin
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
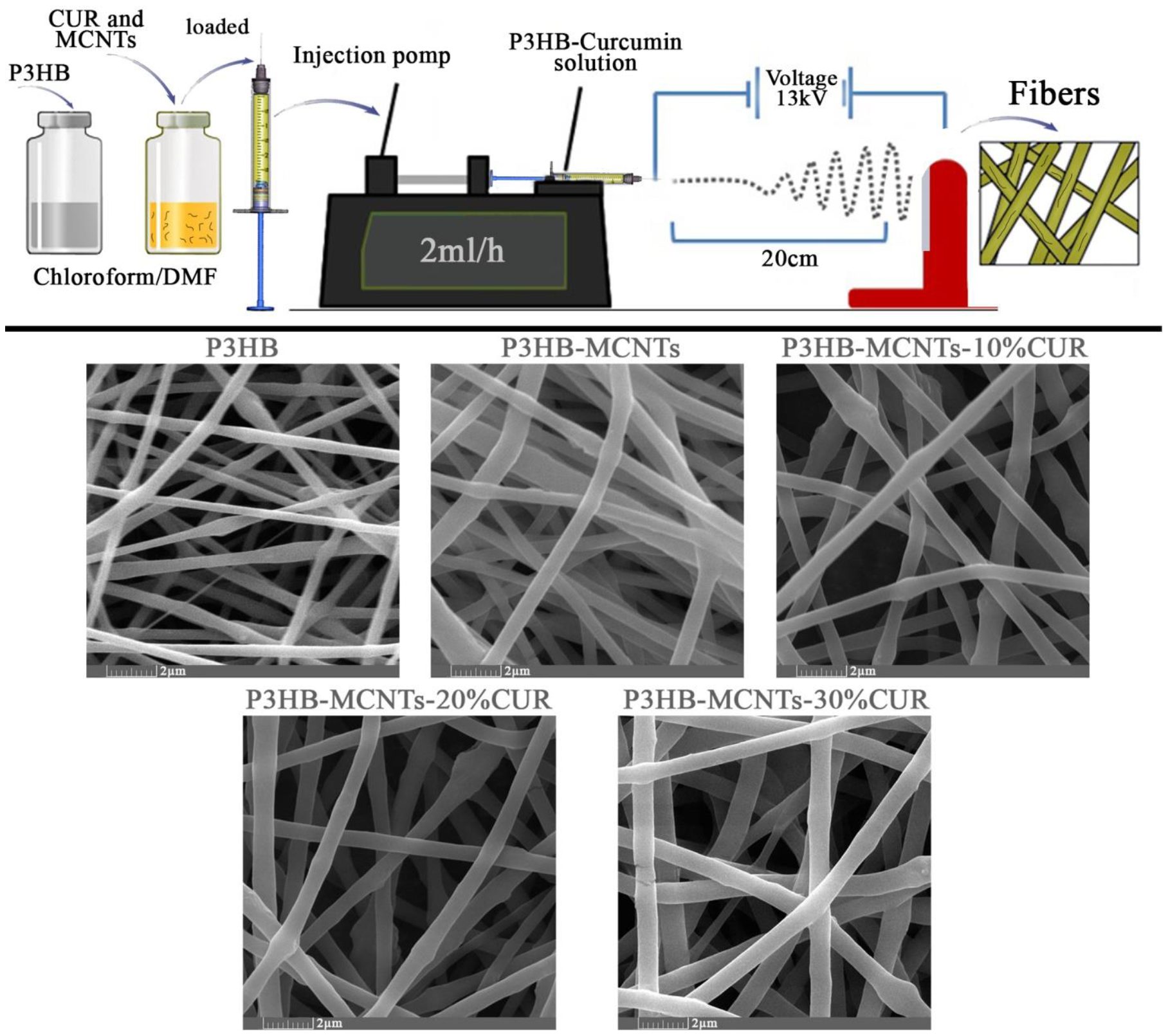
2.2. Electrospinning of Fibrous Scaffolds
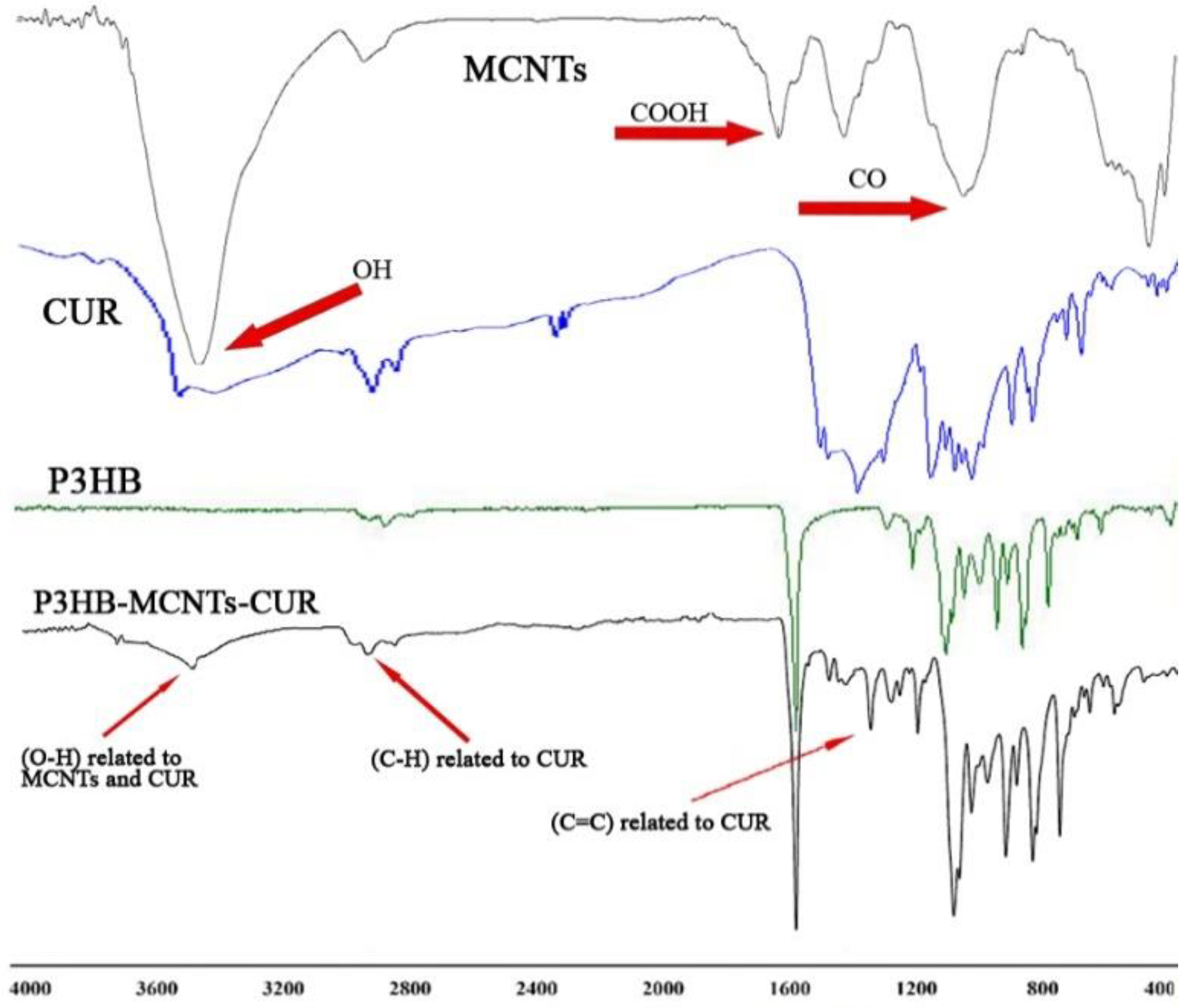
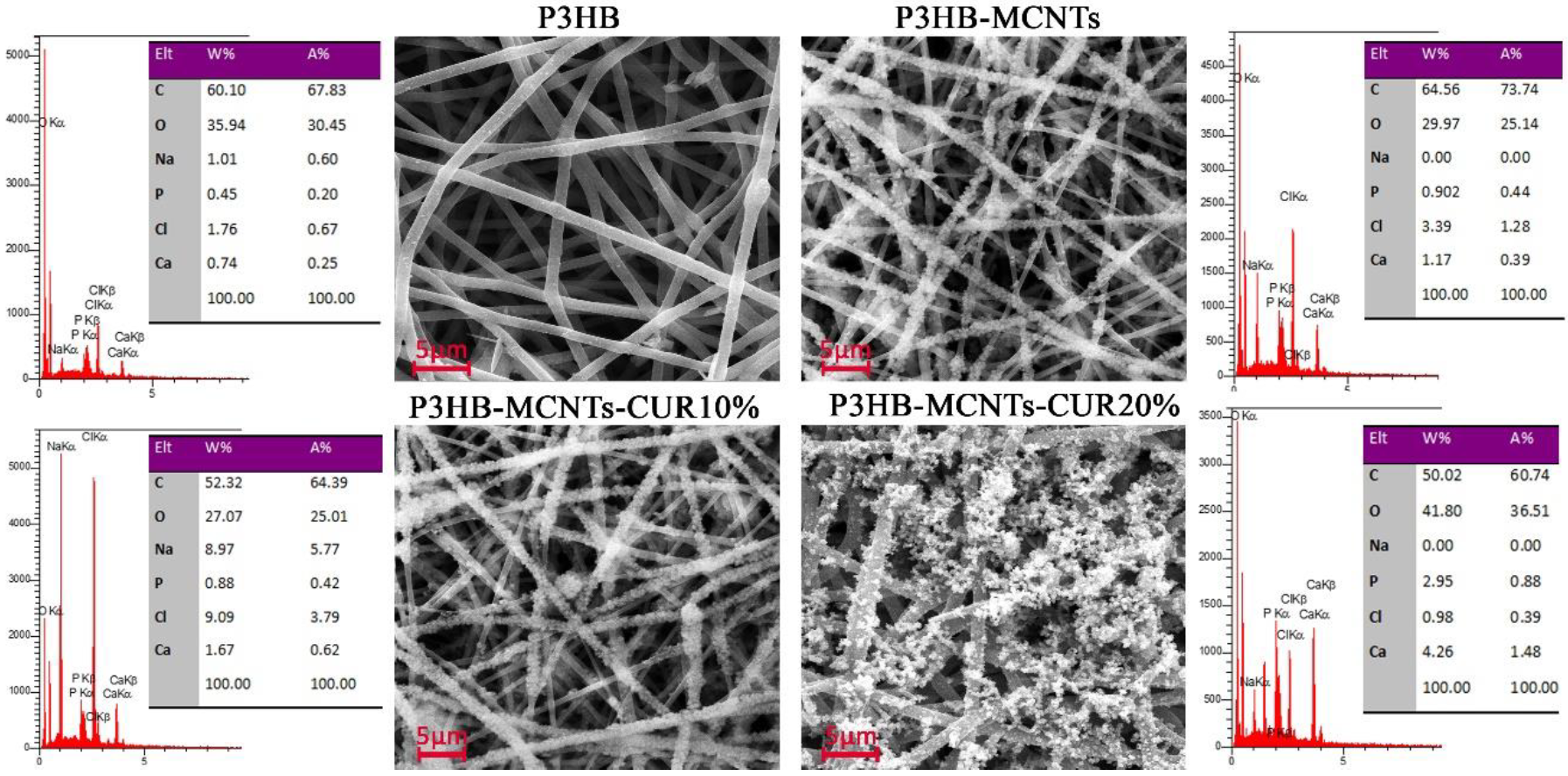
2.3. Chemical and Structural Characterization
2.4. Mechanical Properties
2.5. Hydrolytic Degradation
2.6. In Vitro Bioactivity
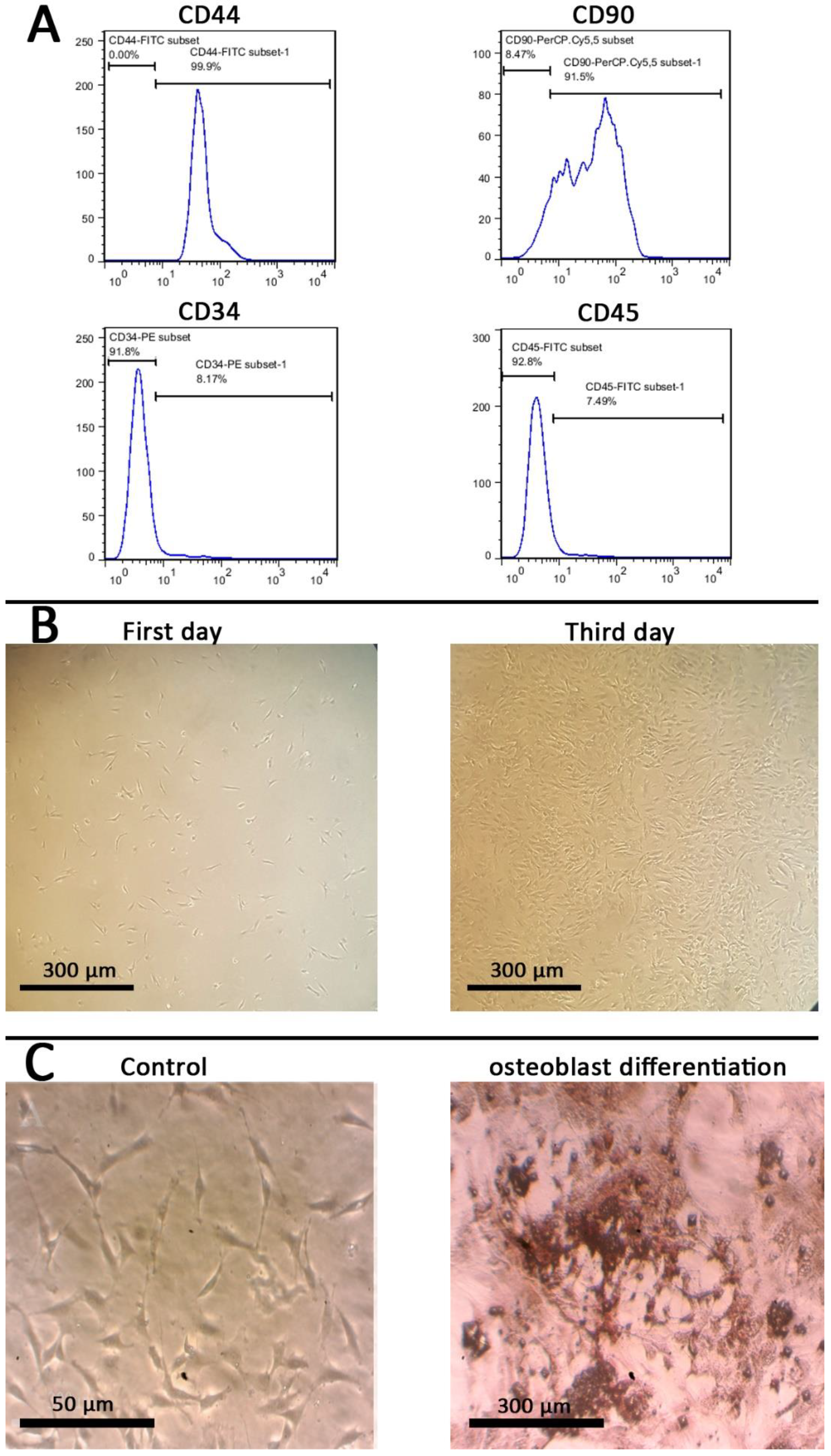
2.7. Stem Cells Isolation and Identification
2.8. In Vitro Biocompatibility
2.9. In Vivo Biocompatibility
2.10. Statistical Analysis
3. Results and Discussion
3.1. Chemical Structure
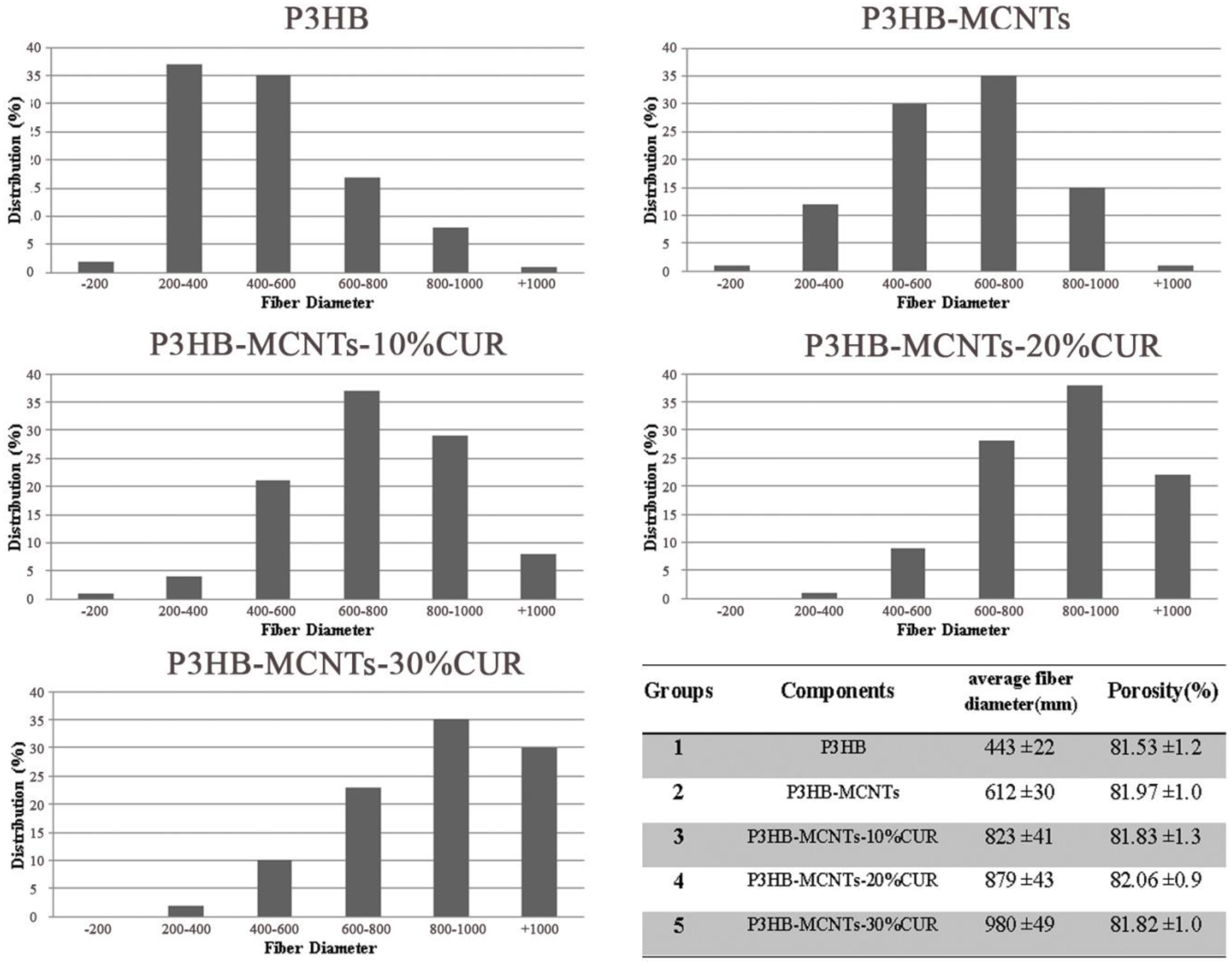
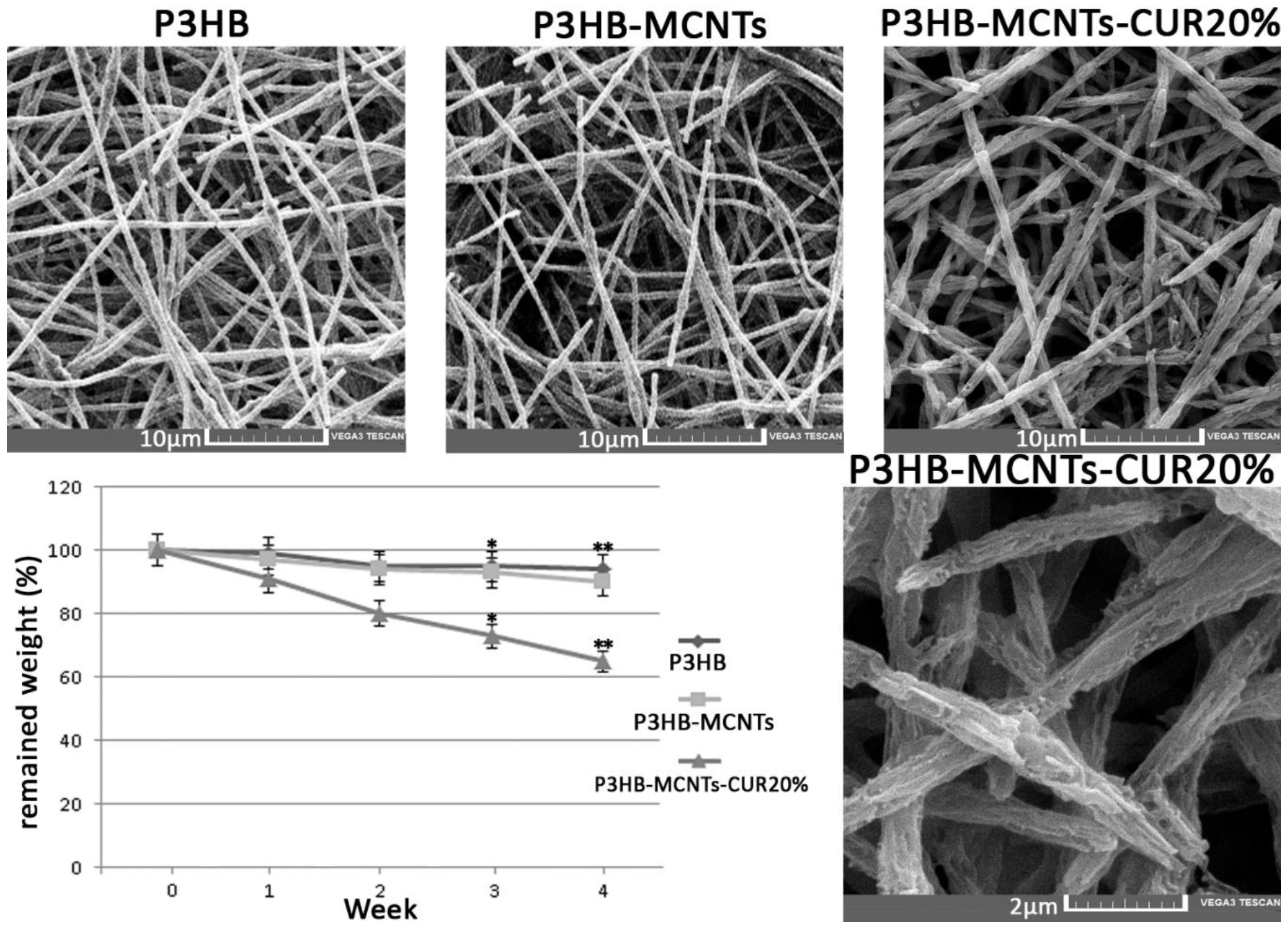
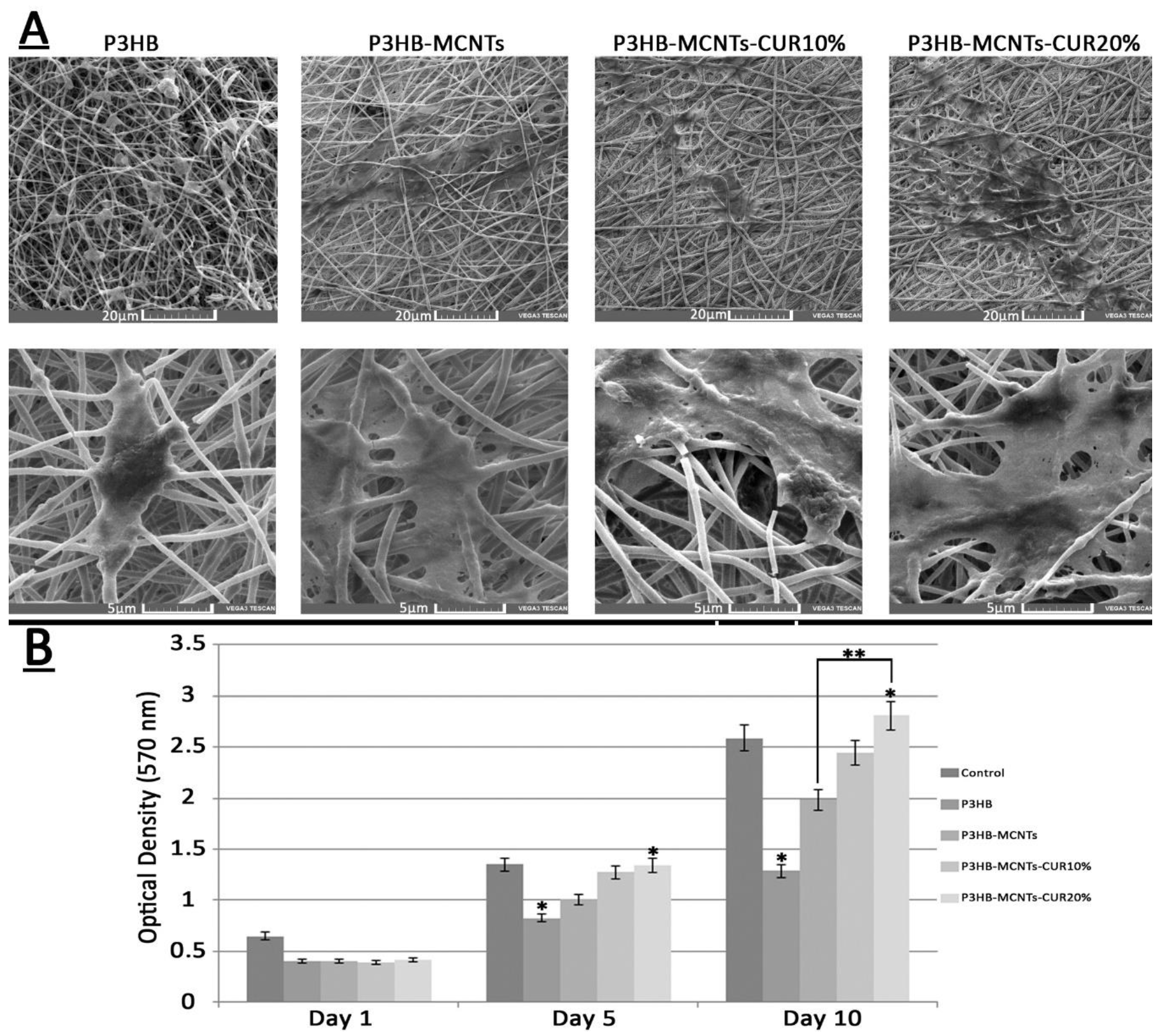
3.2. Characterization of Fibrous Scaffolds
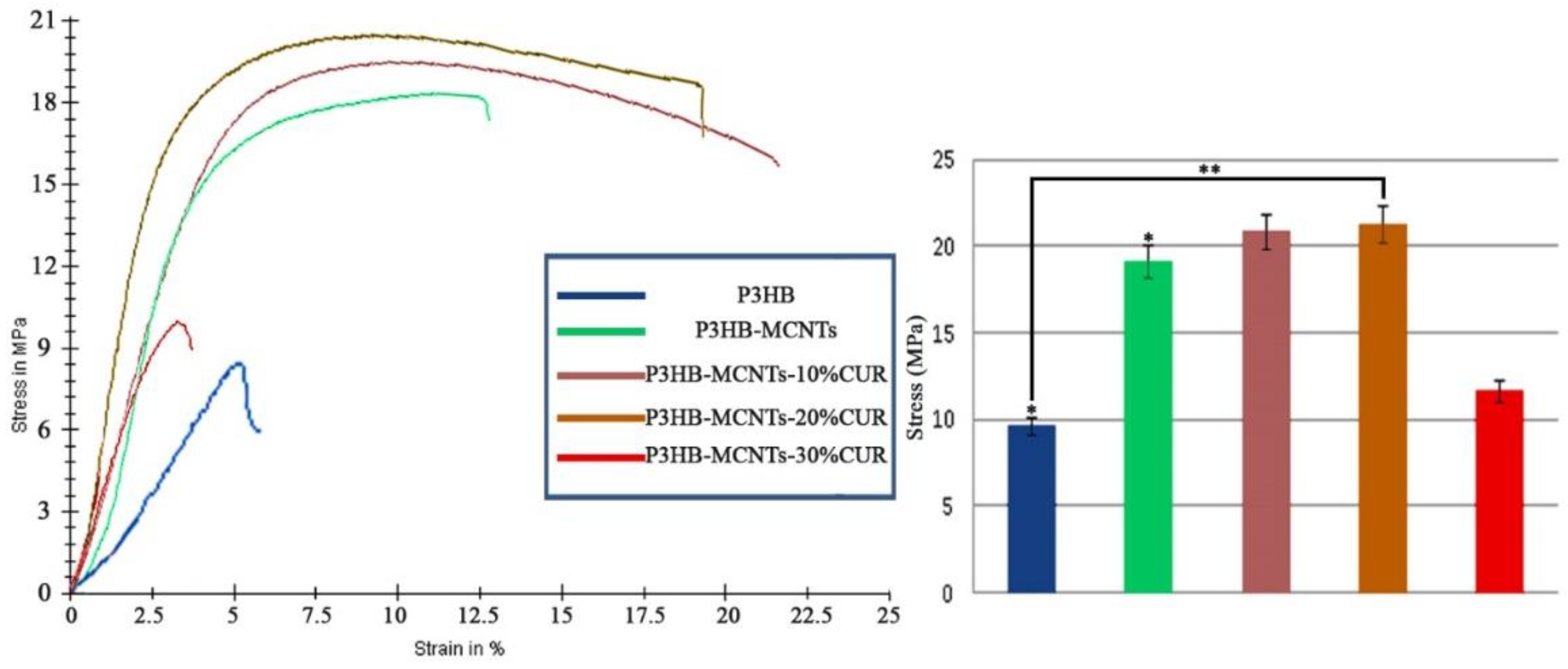
3.3. Mechanical Properties
3.4. In Vitro Degradation
3.5. Scaffolds Bioactivity in SBF
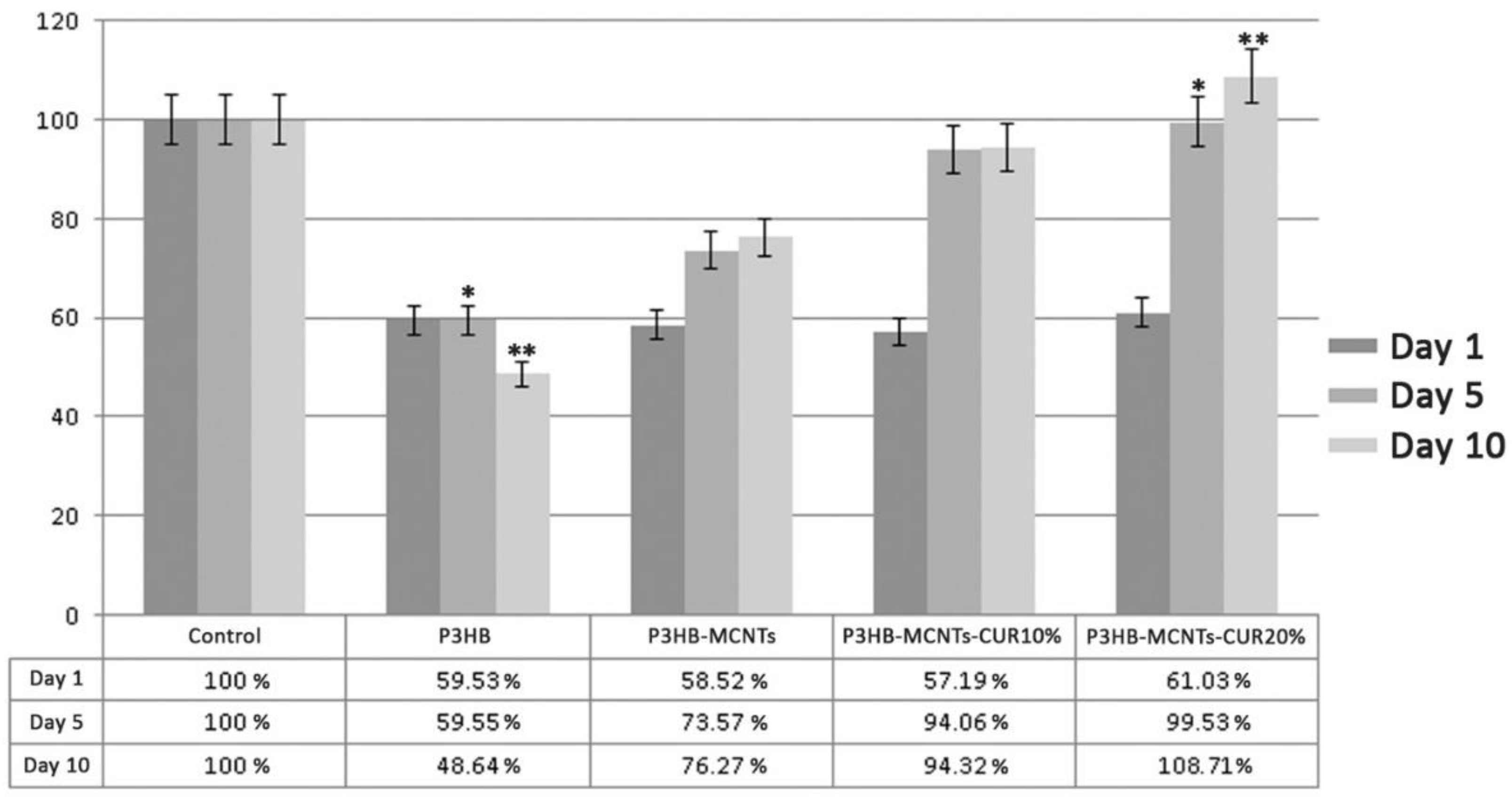
3.6. In Vitro Cytotoxicity
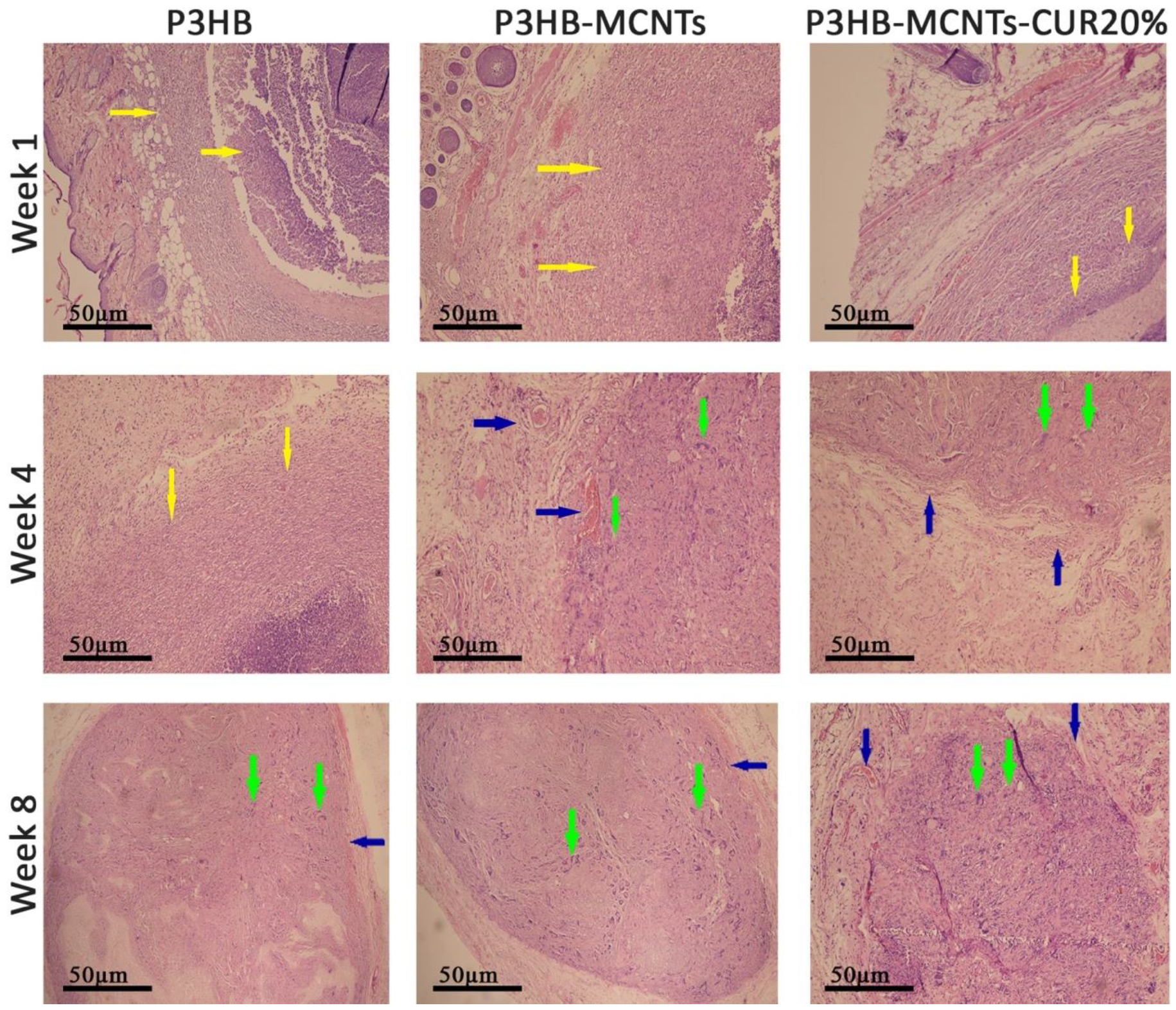
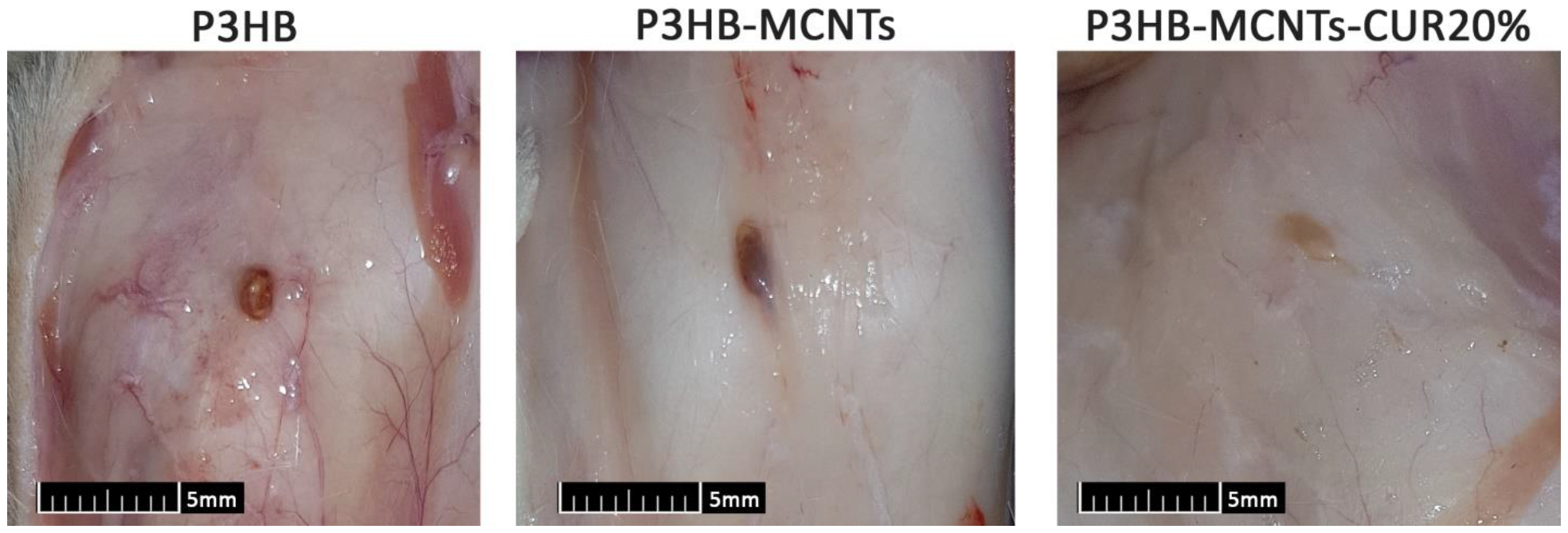
3.7. In Vivo Biocompatibility
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Naderi, P.; Zarei, M.; Karbasi, S.; Salehi, H. Evaluation of the effects of keratin on physical, mechanical and biological properties of poly(3-hydroxybutyrate) electrospun scaffold: Potential application in bone tissue engineering. Eur. Polym. J. 2020, 124, 109502. [Google Scholar] [CrossRef]
- Lanza, R.; Langer, R.; Vacanti, J.P. Principles of Tissue Engineering, 4th ed.; Academic Press: Cambridge, MA, USA, 2013; ISBN 9780123983589. [Google Scholar]
- Khademhosseini, A.; Langer, R. A decade of progress in tissue engineering. Nat. Protoc. 2016, 11, 1775–1781. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Hollister, S.J. Porous scaffold design for tissue engineering. Nat. Mater. 2005, 4, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Gouma, P.I. Electrospun bioscaffolds that mimic the topology of extracellular matrix. Nanomed. Nanotechnol. Biol. Med. 2006, 2, 37–41. [Google Scholar] [CrossRef]
- Wang, K.; Wang, P.; Wang, M.; Yu, D.G.; Wan, F.; Bligh, S.W.A. Comparative study of electrospun crystal-based and composite-based drug nano depots. Mater. Sci. Eng. C 2020, 113. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Dou, C.; Chang, S.; Xie, Z.; Yu, D.G.; Liu, Y.; Shao, J. Core-shell eudragit S100 nanofibers preparedvia triaxial electrospinning to providea colon-targeted extended drug release. Polymers 2020, 12, 2034. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, X.; Liu, P.; Chen, X.; Yu, D.-G. Electrospun multiple-chamber nanostructure and its potential self-healing applications. Polymers 2020, 12, 2413. [Google Scholar] [CrossRef] [PubMed]
- Ogueri, K.S.; Jafari, T.; Escobar Ivirico, J.L.; Laurencin, C.T. Polymeric Biomaterials for Scaffold-Based Bone Regenerative Engineering. Regen. Eng. Transl. Med. 2019, 5, 128–154. [Google Scholar] [CrossRef]
- Roseti, L.; Parisi, V.; Petretta, M.; Cavallo, C.; Desando, G.; Bartolotti, I.; Grigolo, B. Scaffolds for Bone Tissue Engineering: State of the art and new perspectives. Mater. Sci. Eng. C 2017, 78, 1246–1262. [Google Scholar] [CrossRef]
- Nair, L.S.; Laurencin, C.T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Chen, G.Q.; Wu, Q. The application of polyhydroxyalkanoates as tissue engineering materials. Biomaterials 2005, 26, 6565–6578. [Google Scholar] [CrossRef] [PubMed]
- Niaounakis, M. Biopolymers: Applications and Trends; william Andrew: Norwich, NY, USA, 2015; ISBN 9780323353991. [Google Scholar]
- Tehrani, A.H.; Zadhoush, A.; Karbasi, S.; Khorasani, S.N. Experimental investigation of the governing parameters in the electrospinning of poly(3-hydroxybutyrate) scaffolds: Structural characteristics of the pores. J. Appl. Polym. Sci. 2010, 118, 2682–2689. [Google Scholar] [CrossRef]
- Tehrani, A.H.; Zadhoush, A.; Karbasi, S. Preparing nanocomposite fibrous scaffolds of P3HB/nHA for bone tissue engineering. In Proceedings of the 2010 17th Iranian Conference of Biomedical Engineering, ICBME 2010-Proceedings, Isfahan, Iran, 3–4 November 2010. [Google Scholar]
- Karbasi, S.; Zarei, M.; Foroughi, M.R. Effects of Multi-Wall carbon Nano-Tubes (MWNTs) on structural and mechanical properties of electrospun poly(3-hydroxybutyrate) scaffold for tissue engineering applications. Sci. Iran. 2016, 23, 3145–3152. [Google Scholar] [CrossRef]
- Haddadi, M.H.; Asadolahi, R.; Negahdari, B. The bioextraction of bioplastics with focus on polyhydroxybutyrate: A review. Int. J. Environ. Sci. Technol. 2019, 16, 3935–3948. [Google Scholar] [CrossRef]
- Mirmusavi, M.H.; Karbasi, S.; Semnani, D.; Kharazi, A.Z. Characterization of Silk/Poly 3-Hydroxybutyrate-chitosan-multi-walled Carbon Nanotube Micro-nano Scaffold: A New Hybrid Scaffold for Tissue Engineering Applications. J. Med. Signals Sens. 2018, 8, 46–52. [Google Scholar] [CrossRef]
- Sadeghi, D.; Karbasi, S.; Razavi, S.; Mohammadi, S.; Shokrgozar, M.A.; Bonakdar, S. Electrospun poly(hydroxybutyrate)/chitosan blend fibrous scaffolds for cartilage tissue engineering. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Zarei, M.; Karbasi, S. Evaluation of the effects of multiwalled carbon nanotubes on electrospun poly(3-hydroxybutirate) scaffold for tissue engineering applications. J. Porous Mater. 2018, 25, 259–272. [Google Scholar] [CrossRef]
- Zarei, M.; Tanideh, N.; Zare, S.; Aslani, F.S.; Koohi-Hosseinabadi, O.; Rowshanghias, A.; Pourjavaheri, F.; Mehryar, P.; Muthuraj, R. Electrospun poly(3-hydroxybutyrate)/chicken feather-derived keratin scaffolds: Fabrication, in vitro and in vivo biocompatibility evaluation. J. Biomater. Appl. 2020, 34, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Zarei, M.; Tanideh, N.; Zare, S.; Sari Aslani, F.; Koohi-Hosseinabadi, O.; Muthuraj, R.; Jamhiri, I.; Rowshanghias, A.; Mehryar, P. Preparation and performance evaluation of electrospun poly(3-hydroxybutyrate) composite scaffolds as a potential hard tissue engineering application. J. Bioact. Compat. Polym. 2019, 34, 386–400. [Google Scholar] [CrossRef]
- Tran, P.A.; Zhang, L.; Webster, T.J. Carbon nanofibers and carbon nanotubes in regenerative medicine. Adv. Drug Deliv. Rev. 2009, 61, 1097–1114. [Google Scholar] [CrossRef]
- Harrison, B.S.; Atala, A. Carbon nanotube applications for tissue engineering. Biomaterials 2007, 28, 344–353. [Google Scholar] [CrossRef]
- Mikael, P.E.; Amini, A.R.; Basu, J.; Arellano-Jimenez, M.J.; Laurencin, C.T.; Sanders, M.M.; Carter, C.B.; Nukavarapu, S.P. Functionalized carbon nanotube reinforced scaffolds for bone regenerative engineering: Fabrication, in vitro and in vivo evaluation. Biomed. Mater. 2014, 9. [Google Scholar] [CrossRef]
- Zarei, M.; Karbasi, S.; Sari Aslani, F.; Zare, S.; Koohi-Hosseinabad, O.; Tanideh, N. In Vitro and In Vivo Evaluation of Poly(3-hydroxybutyrate)/Carbon Nanotubes Electrospun Scaffolds for Periodontal Ligament Tissue Engineering. J. Dent. 2020, 21, 18–30. [Google Scholar] [CrossRef]
- Zhijiang, C.; Cong, Z.; Jie, G.; Qing, Z.; Kongyin, Z. Electrospun carboxyl multi-walled carbon nanotubes grafted polyhydroxybutyrate composite nanofibers membrane scaffolds: Preparation, characterization and cytocompatibility. Mater. Sci. Eng. C 2018, 82, 29–40. [Google Scholar] [CrossRef]
- Mesgar, A.S.; Mohammadi, Z.; Khosrovan, S. Improvement of mechanical properties and in vitro bioactivity of freeze-dried gelatin/chitosan scaffolds by functionalized carbon nanotubes. Int. J. Polym. Mater. Polym. Biomater. 2018, 67, 267–276. [Google Scholar] [CrossRef]
- Chen, Y.C.; Shie, M.Y.; Wu, Y.H.A.; Lee, K.X.A.; Wei, L.J.; Shen, Y.F. Anti-inflammation performance of curcumin-loaded mesoporous calcium silicate cement. J. Formos. Med. Assoc. 2017, 116, 679–688. [Google Scholar] [CrossRef]
- Hocking, A.; Tommasi, S.; Sordillo, P.; Klebe, S. The safety and exploration of the pharmacokinetics of intrapleural liposomal curcumin. Int. J. Nanomed. 2020, 15, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Ahangari, N.; Kargozar, S.; Ghayour-Mobarhan, M.; Baino, F.; Pasdar, A.; Sahebkar, A.; Ferns, G.A.A.; Kim, H.W.; Mozafari, M. Curcumin in tissue engineering: A traditional remedy for modern medicine. BioFactors 2019, 45, 135–151. [Google Scholar] [CrossRef]
- Mardani, M.; Sadeghzadeh, A.; Tanideh, N.; Andisheh Tadbir, A.; Lavaee, F.; Zarei, M.; Moayedi, J. The effects of adipose tissue-derived stem cells seeded onto the curcumin-loaded collagen scaffold in healing of experimentally induced oral mucosal ulcers in rat. Iran. J. Basic Med. Sci. 2020, 23, 1618–1627. [Google Scholar] [CrossRef]
- Kasoju, N.; Bora, U. Fabrication and characterization of curcumin-releasing silk fibroin scaffold. J. Biomed. Mater. Res. Part B 2012, 100 B, 1854–1866. [Google Scholar] [CrossRef]
- Sarkar, N.; Bose, S. Liposome-Encapsulated Curcumin-Loaded 3D Printed Scaffold for Bone Tissue Engineering. ACS Appl. Mater. Interfaces 2019, 11, 17184–17192. [Google Scholar] [CrossRef]
- Rezaii, M.; Oryan, S.; Javeri, A. Curcumin nanoparticles incorporated collagen-chitosan scaffold promotes cutaneous wound healing through regulation of TGF-β1/Smad7 gene expression. Mater. Sci. Eng. C 2019, 98, 347–357. [Google Scholar] [CrossRef]
- Mirtaghavi, A.; Baldwin, A.; Tanideh, N.; Zarei, M.; Muthuraj, R.; Cao, Y.; Zhao, G.; Geng, J.; Jin, H.; Luo, J. Crosslinked porous three-dimensional cellulose nanofibers-gelatine biocomposite scaffolds for tissue regeneration. Int. J. Biol. Macromol. 2020, 164, 1949–1959. [Google Scholar] [CrossRef]
- Amiri, S.; Rahimi, A. Poly(ε-caprolactone) electrospun nanofibers containing curcumin nanocontainers: Enhanced solubility, dissolution and physical stability of curcumin via formation of inclusion complex with cyclodextrins. Int. J. Polym. Mater. Polym. Biomater. 2019, 68, 669–679. [Google Scholar] [CrossRef]
- Ranjbar-Mohammadi, M.; Bahrami, S.H. Electrospun curcumin loaded poly(ε-caprolactone)/gum tragacanth nanofibers for biomedical application. Int. J. Biol. Macromol. 2016, 84, 448–456. [Google Scholar] [CrossRef]
- Mohammadi, M.R.; Rabbani, S.; Bahrami, S.H.; Joghataei, M.T.; Moayer, F. Antibacterial performance and in vivo diabetic wound healing of curcumin loaded gum tragacanth/poly(ε-caprolactone) electrospun nanofibers. Mater. Sci. Eng. C 2016, 69, 1183–1191. [Google Scholar] [CrossRef]
- Shababdoust, A.; Ehsani, M.; Shokrollahi, P.; Zandi, M. Fabrication of curcumin-loaded electrospun nanofiberous polyurethanes with anti-bacterial activity. Prog. Biomater. 2018, 7, 23–33. [Google Scholar] [CrossRef]
- Pankongadisak, P.; Sangklin, S.; Chuysinuan, P.; Suwantong, O.; Supaphol, P. The use of electrospun curcumin-loaded poly(L-lactic acid) fiber mats as wound dressing materials. J. Drug Deliv. Sci. Technol. 2019, 53. [Google Scholar] [CrossRef]
- Sampath, M.; Lakra, R.; Korrapati, P.S.; Sengottuvelan, B. Curcumin loaded poly(lactic-co-glycolic) acid nanofiber for the treatment of carcinoma. Colloids Surfaces B 2014, 117, 128–134. [Google Scholar] [CrossRef]
- Moradkhannejhad, L.; Abdouss, M.; Nikfarjam, N.; Mazinani, S.; Sayar, P. Electrospun curcumin loaded poly(lactic acid) nanofiber mat on the flexible crosslinked PVA/PEG membrane film: Characterization and in vitro release kinetic study. Fibers Polym. 2017, 18, 2349–2360. [Google Scholar] [CrossRef]
- Wang, N.; Wang, F.; Gao, Y.; Yin, P.; Pan, C.; Liu, W.; Zhou, Z.; Wang, J. Curcumin protects human adipose-derived mesenchymal stem cells against oxidative stress-induced inhibition of osteogenesis. J. Pharmacol. Sci. 2016, 132, 192–200. [Google Scholar] [CrossRef]
- Ghasemi-Mobarakeh, L.; Semnani, D.; Morshed, M. A novel method for porosity measurement of various surface layers of nanofibers mat using image analysis for tissue engineering applications. J. Appl. Polym. Sci. 2007, 106, 2536–2542. [Google Scholar] [CrossRef]
- Yang, S.; Leong, K.F.; Du, Z.; Chua, C.K. The design of scaffolds for use in tissue engineering. Part I. Traditional factors. Tissue Eng. 2001, 7, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Mouthuy, P.A.; Škoc, M.S.; Gašparović, A.Č.; Milković, L.; Carr, A.J.; Žarković, N. Investigating the use of curcumin-loaded electrospun filaments for soft tissue repair applications. Int. J. Nanomed. 2017, 12, 3977–3991. [Google Scholar] [CrossRef]
- Mohammadalizadeh, Z.; Karbasi, S.; Arasteh, S. Physical, mechanical and biological evaluation of poly(3-hydroxybutyrate)-chitosan/MWNTs as a novel electrospun scaffold for cartilage tissue engineering applications. Polym. Technol. Mater. 2020, 59, 417–429. [Google Scholar] [CrossRef]
- Hiruta, T.; Yabutsuka, T.; Watanabe, S.; Fukushima, K.; Takai, S.; Yao, T. Apatite formation ability of bioactive bearing grade polyetheretherketone fabricated by incorporation of apatite nuclei. Key Eng. Mater. 2017, 758, 69–74. [Google Scholar] [CrossRef]
- Subramanian, A.; Krishnan, U.M.; Sethuraman, S. In vivo biocompatibility of PLGA-polyhexylthiophene nanofiber scaffolds in a rat model. BioMed Res. Int. 2013, 2013. [Google Scholar] [CrossRef]
- Ishak, S.A.; Djuansjah, J.R.P.; Kadir, M.R.A.; Sukmana, I. Angiogenesis in tissue engineering: From concept to the vascularization of scaffold construct. IOP Conf. Ser. Mater. Sci. Eng. 2014, 58, 012015. [Google Scholar] [CrossRef]

| Compound | Moiety | Chemical Shift (ppm) | Integral |
|---|---|---|---|
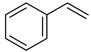
| CUR |  | 6.472–7.501 | 12 |
 | 5.810–5.823 | 2 | |
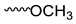 | 3.967 | 6 | |
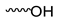 | 7.585–7.637 | 2 | |
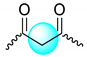
| P3HB | 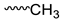 | 1.278–1.299 | 3 |
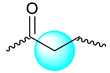 | 2.449–2.662 | 2 | |
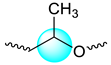 | 5.220–5.326 | 1 | |
| Mixture | 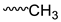 | 1.283–1.303 | 3 |
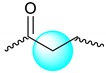 | 2.195–2.667 | 2 | |
 | 3.973 | 0.3 | |
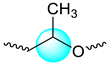 | 5.248–5.309 | 1 | |
 | 5.825–5.890 | 0.13 | |
 | 6.476–7.505 | 1 | |
 | 7.589–7.641 | 0.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanideh, N.; Azarpira, N.; Sarafraz, N.; Zare, S.; Rowshanghiyas, A.; Farshidfar, N.; Iraji, A.; Zarei, M.; El Fray, M. Poly(3-Hydroxybutyrate)-Multiwalled Carbon Nanotubes Electrospun Scaffolds Modified with Curcumin. Polymers 2020, 12, 2588. https://doi.org/10.3390/polym12112588
Tanideh N, Azarpira N, Sarafraz N, Zare S, Rowshanghiyas A, Farshidfar N, Iraji A, Zarei M, El Fray M. Poly(3-Hydroxybutyrate)-Multiwalled Carbon Nanotubes Electrospun Scaffolds Modified with Curcumin. Polymers. 2020; 12(11):2588. https://doi.org/10.3390/polym12112588
Chicago/Turabian StyleTanideh, Nader, Negar Azarpira, Najmeh Sarafraz, Shahrokh Zare, Aida Rowshanghiyas, Nima Farshidfar, Aida Iraji, Moein Zarei, and Miroslawa El Fray. 2020. "Poly(3-Hydroxybutyrate)-Multiwalled Carbon Nanotubes Electrospun Scaffolds Modified with Curcumin" Polymers 12, no. 11: 2588. https://doi.org/10.3390/polym12112588
APA StyleTanideh, N., Azarpira, N., Sarafraz, N., Zare, S., Rowshanghiyas, A., Farshidfar, N., Iraji, A., Zarei, M., & El Fray, M. (2020). Poly(3-Hydroxybutyrate)-Multiwalled Carbon Nanotubes Electrospun Scaffolds Modified with Curcumin. Polymers, 12(11), 2588. https://doi.org/10.3390/polym12112588