Characterization of Porous Structures of Cellulose Nanofibrils Loaded with Salicylic Acid
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Production and Characterization of CNFs
2.3. Characterization of CNFs Loaded with Salicylic Acid
2.4. Quantification of Release of Salicylic Acid
3. Results and Discussion
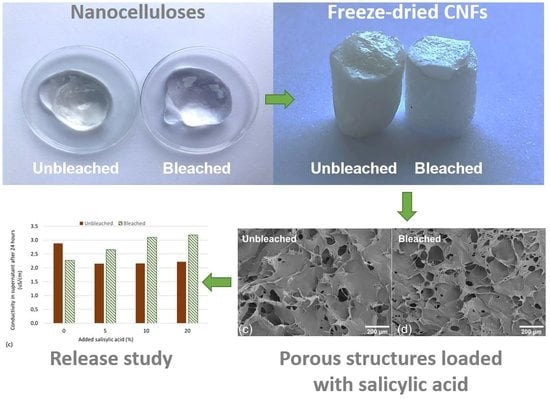
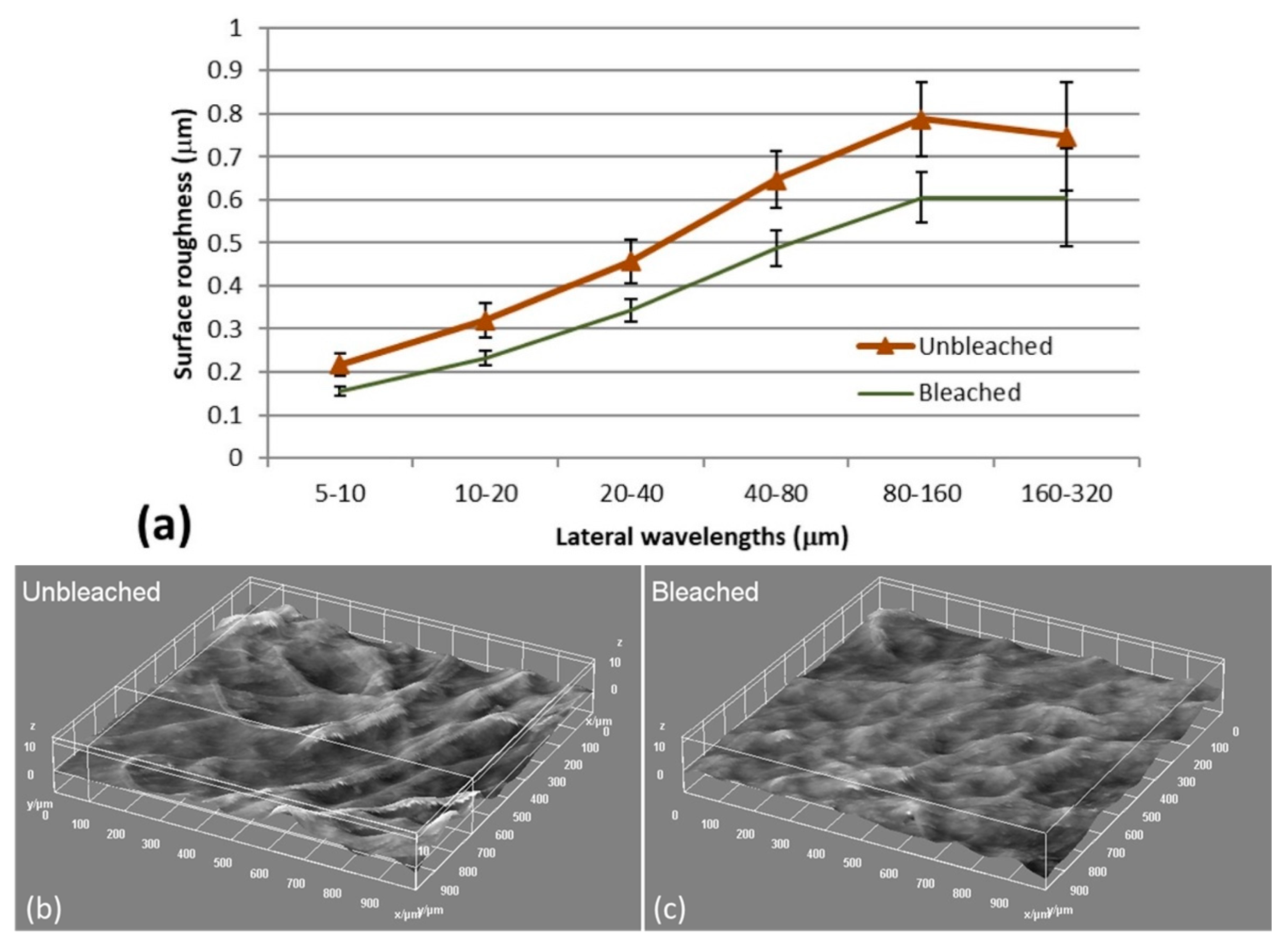
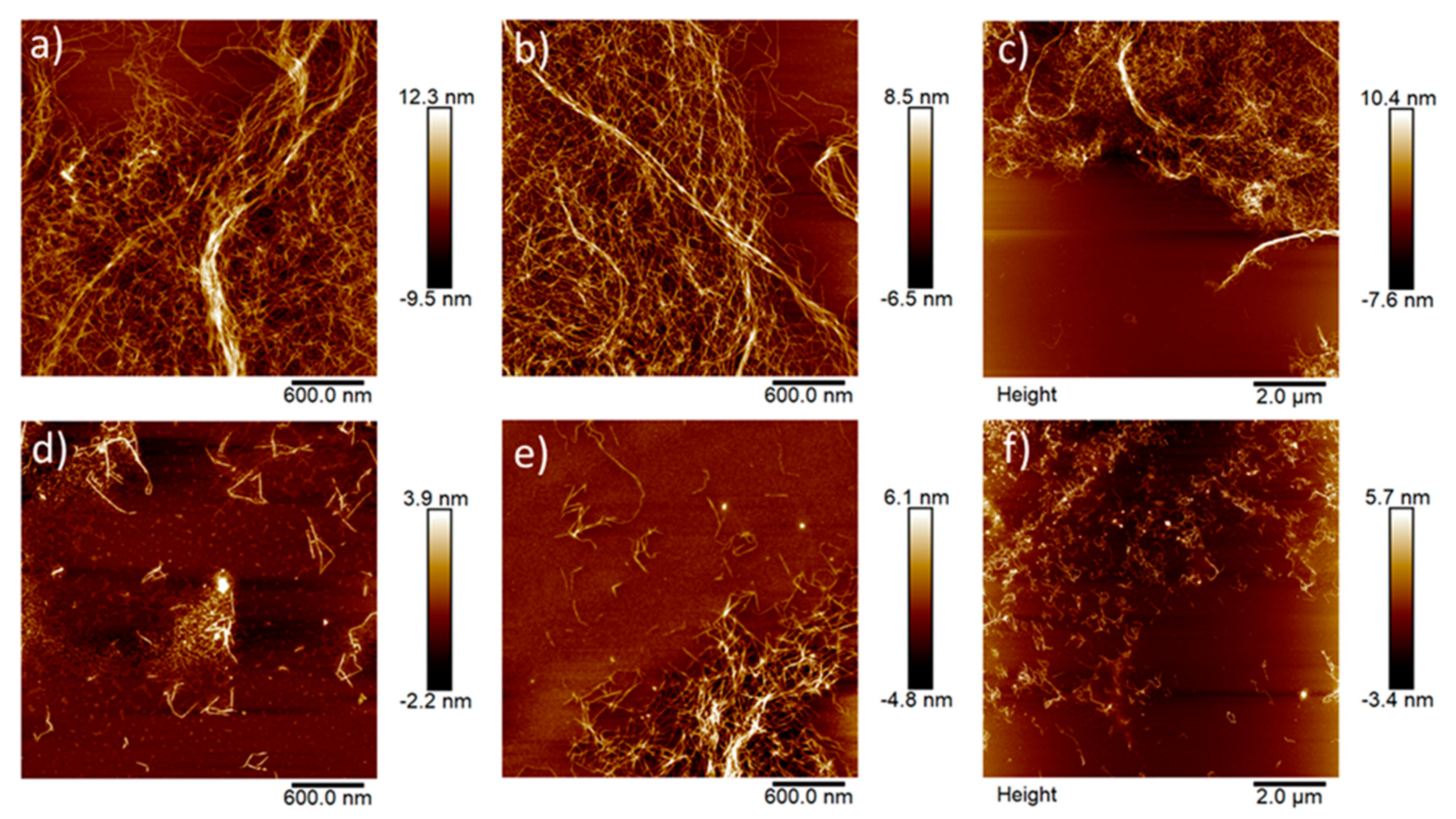
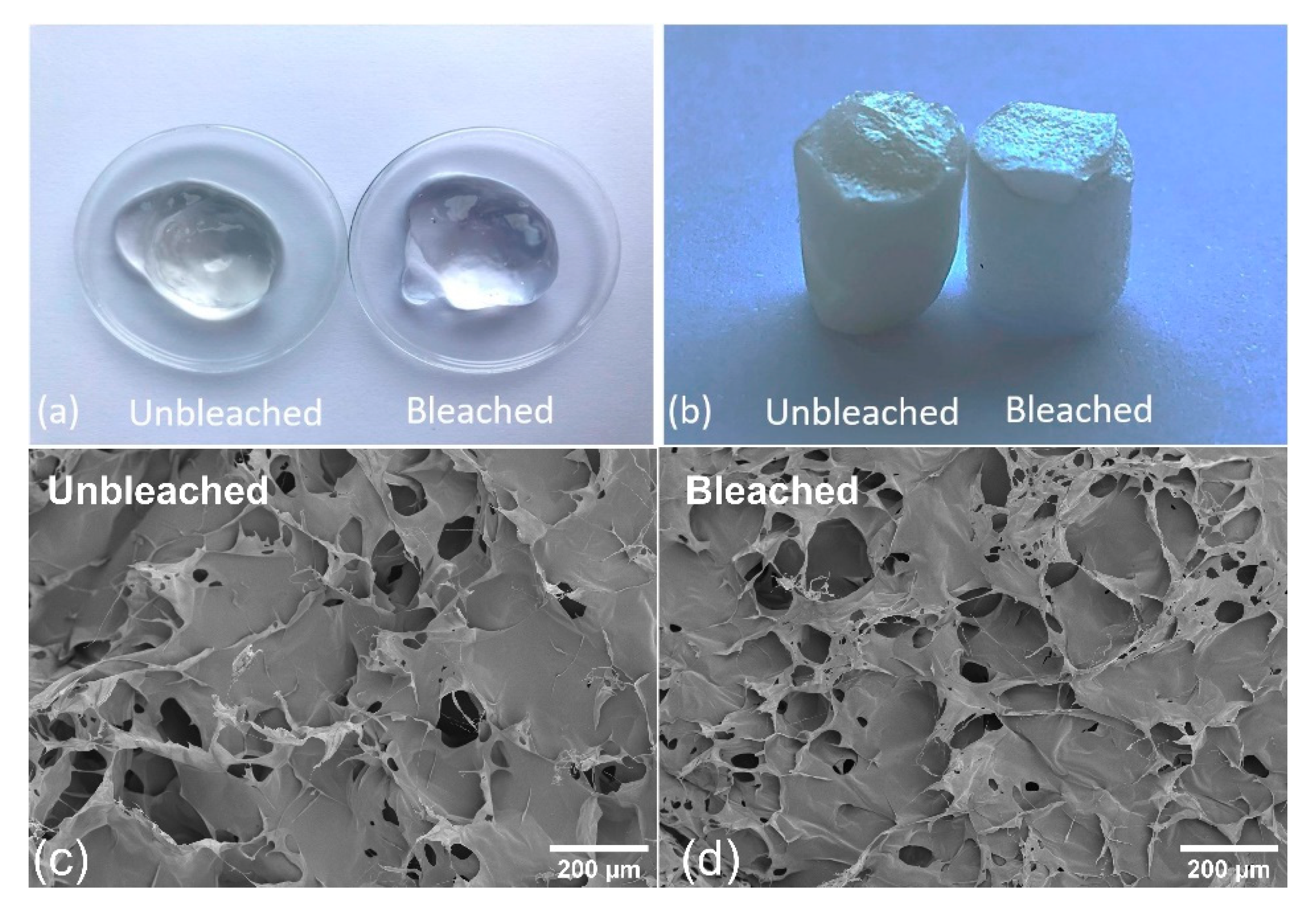
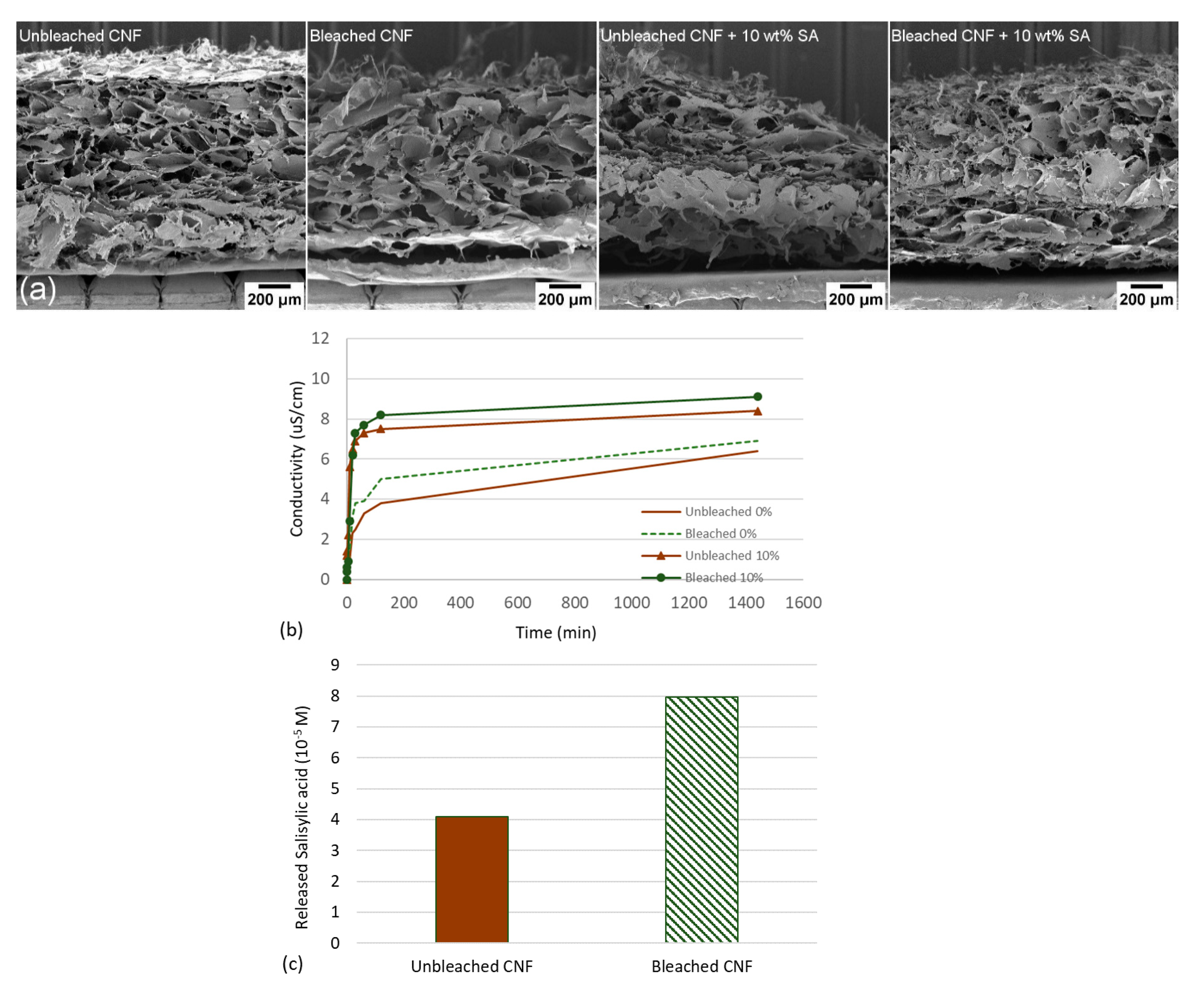
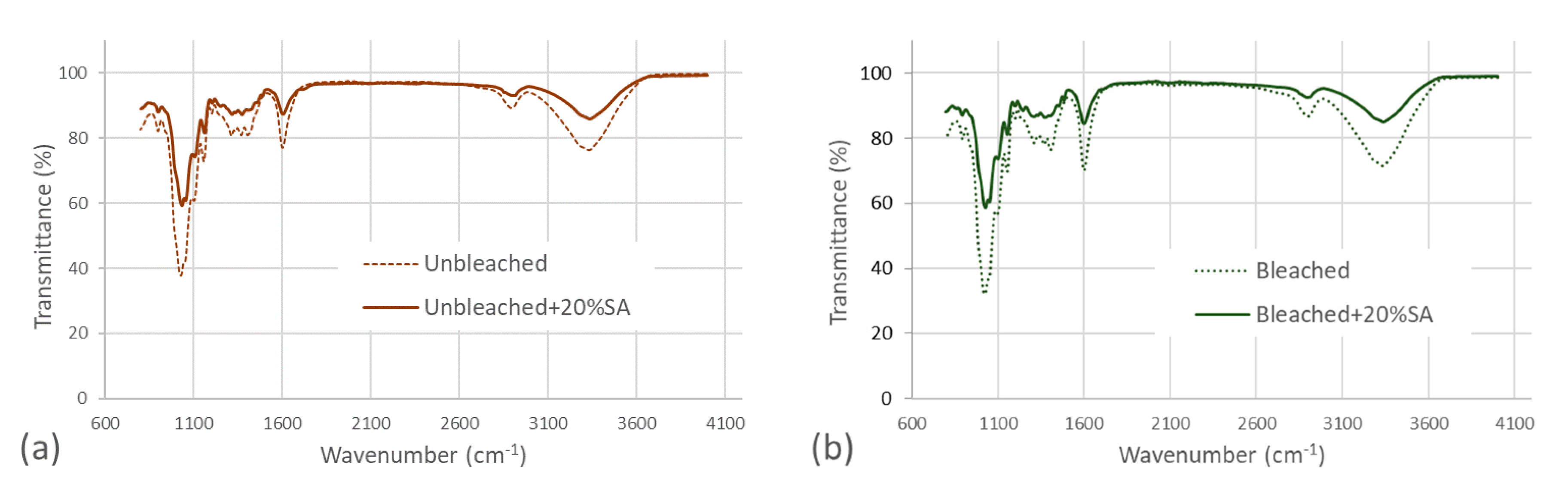
3.1. Characterization of Bleached and Unbleached CNFs
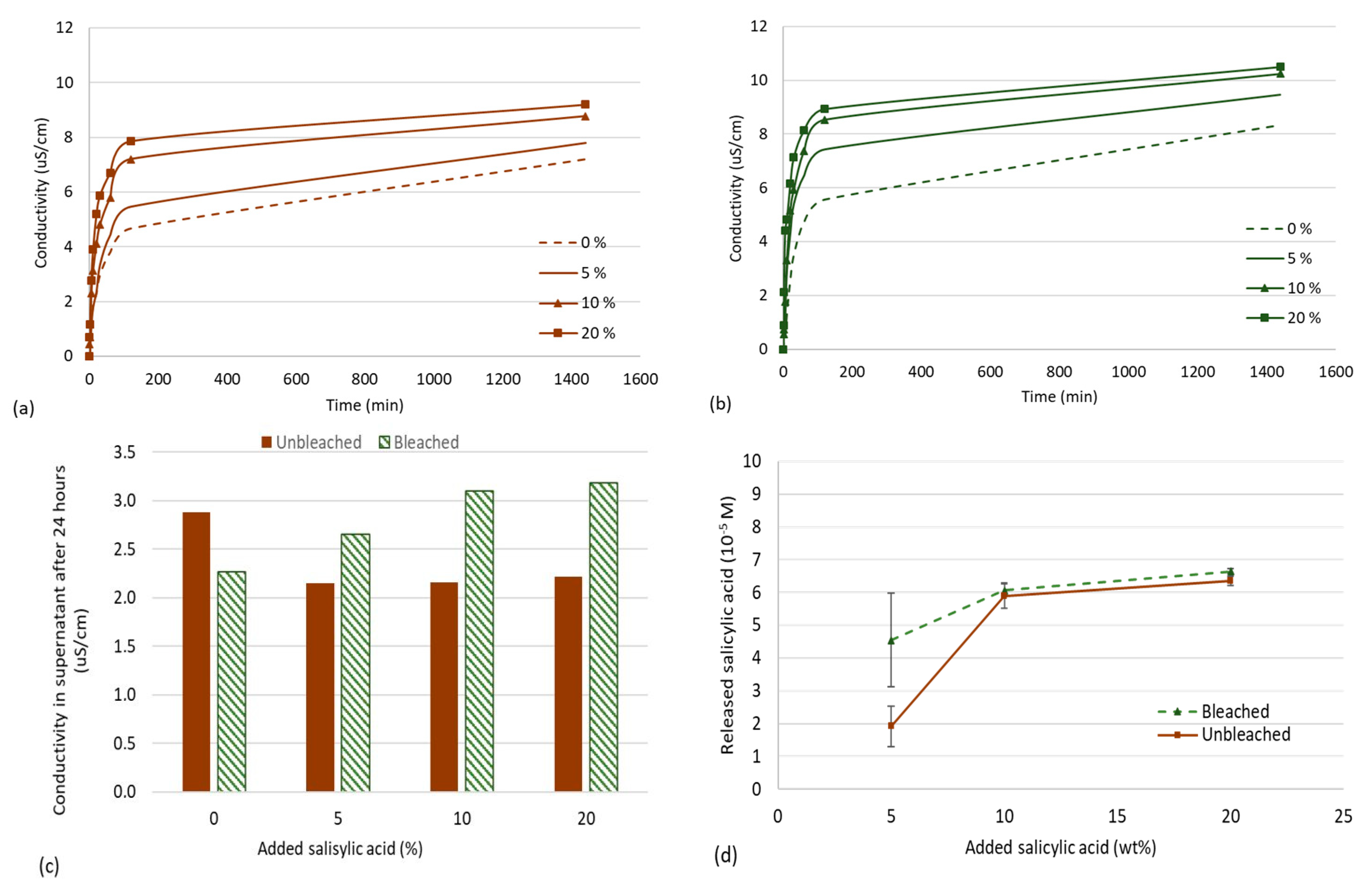
3.2. Release Studies of Salicylic Acid from CNF Aerogels
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cocco, P. Pesticides and Human Health; Oxford University Press: Oxford, UK, 2016. [Google Scholar]
- Di Fiori, E.; Pizarro, H.; dos Santos Afonso, M.; Cataldo, D. Impact of the invasive mussel Limnoperna fortunei on glyphosate concentration in water. Ecotoxicol. Environ. Saf. 2012, 81, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.R.; Fatma, M.; Per, T.S.; Anjum, N.A.; Khan, N.A. Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front. Plant Sci. 2015, 6, 462. [Google Scholar] [CrossRef]
- Bektas, Y.; Eulgem, T. Synthetic plant defense elicitors. Front. Plant Sci. 2015, 5, 804. [Google Scholar] [CrossRef]
- Balakireva, A.V.; Zamyatnin, A.A. Indispensable Role of Proteases in Plant Innate Immunity. Int. J. Mol. Sci. 2018, 19, 629. [Google Scholar] [CrossRef]
- Durrant, W.E.; Dong, X. SYSTEMIC ACQUIRED RESISTANCE. Annu. Rev. Phytopathol. 2004, 42, 185–209. [Google Scholar] [CrossRef]
- Arfan, M.; Athar, H.R.; Ashraf, M. Does exogenous application of salicylic acid through the rooting medium modulate growth and photosynthetic capacity in two differently adapted spring wheat cultivars under salt stress? J. Plant Physiol. 2007, 164, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Nazar, R.; Umar, S.; Khan, N.A. Exogenous salicylic acid improves photosynthesis and growth through increase in ascorbate-glutathione metabolism and S assimilation in mustard under salt stress. Plant Signal. Behav. 2015, 10, e1003751. [Google Scholar] [CrossRef] [PubMed]
- Hayat, Q.; Hayat, S.; Irfan, M.; Ahmad, A. Effect of exogenous salicylic acid under changing environment: A review. Environ. Exp. Bot. 2010, 68, 14–25. [Google Scholar] [CrossRef]
- Raskin, I. Role of Salicylic Acid in Plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1992, 43, 439–463. [Google Scholar] [CrossRef]
- Singh, H.; Singh, N.B.; Singh, A.; Hussain, I. Exogenous Application of Salicylic Acid to Alleviate Glyphosate Stress in Solanum lycopersicum. Int. J. Veg. Sci. 2017, 23, 552–566. [Google Scholar] [CrossRef]
- Agami, R.A.; Mohamed, G.F. Exogenous treatment with indole-3-acetic acid and salicylic acid alleviates cadmium toxicity in wheat seedlings. Ecotoxicol. Environ. Saf. 2013, 94, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.-M.; Wang, J.; Wang, S.-H.; Xu, L.-L. Salicylic acid-induced aluminum tolerance by modulation of citrate efflux from roots of Cassia tora L. Planta 2003, 217, 168–174. [Google Scholar] [CrossRef]
- Kangas, H.; Pitkänen, M.; Vikman, M.; Vartiainen, J.; Tsitko, I. Biodegradability, Compostability and Safety of Cellulose Nanofibrils (CNF) and CNF Based Products. In Proceedings of the TAPPI International Conference on Nanotechnology for Renewable Materials Proceedings, Atlanta, GA, USA, 22–25 June 2015. [Google Scholar]
- Vikman, M.; Vartiainen, J.; Tsitko, I.; Korhonen, P. Biodegradability and Compostability of Nanofibrillar Cellulose-Based Products. J. Polym. Environ. 2015, 23, 206–215. [Google Scholar] [CrossRef]
- Chinga-Carrasco, G.; Yu, Y.D.; Diserud, O. Quantitative Electron Microscopy of Cellulose Nanofibril Structures from Eucalyptus and Pinus radiata Kraft Pulp Fibers. Microsc. Microanal. 2011, 17, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Fukuzumi, H.; Saito, T.; Isogai, A. Influence of TEMPO-oxidized cellulose nanofibril length on film properties. Carbohydr. Polym. 2013, 93, 172–177. [Google Scholar] [CrossRef]
- Gamelas, J.A.F.; Pedrosa, J.; Lourenco, A.F.; Mutje, P.; Gonzalez, I.; Chinga-Carrasco, G.; Singh, G.; Ferreira, P.J.T. On the morphology of cellulose nanofibrils obtained by TEMPO-mediated oxidation and mechanical treatment. Micron 2015, 72, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Mattos, B.D.; Tardy, B.L.; Rojas, O.J. Accounting for Substrate Interactions in the Measurement of the Dimensions of Cellulose Nanofibrils. Biomacromolecules 2019, 20, 2657–2665. [Google Scholar] [CrossRef]
- Shinoda, R.; Saito, T.; Okita, Y.; Isogai, A. Relationship between Length and Degree of Polymerization of TEMPO-Oxidized Cellulose Nanofibrils. Biomacromolecules 2012, 13, 842–849. [Google Scholar] [CrossRef]
- Bhandari, J.; Mishra, H.; Mishra, P.K.; Wimmer, R.; Ahmad, F.J.; Talegaonkar, S. Cellulose nanofiber aerogel as a promising biomaterial for customized oral drug delivery. Int. J. Nanomed. 2017, 12, 2021. [Google Scholar] [CrossRef]
- Zhao, J.Q.; Lu, C.H.; He, X.; Zhang, X.F.; Zhang, W.; Zhang, X.M. Polyethylenimine-Grafted Cellulose Nanofibril Aerogels as Versatile Vehicles for Drug Delivery. ACS Appl. Mater. Interfaces 2015, 7, 2607–2615. [Google Scholar] [CrossRef]
- Chinga-Carrasco, G.; Kuznetsova, N.; Garaeva, M.; Leirset, I.; Galiullina, G.; Kostochko, A.; Syverud, K. Bleached and unbleached MFC nanobarriers: Properties and hydrophobisation with hexamethyldisilazane. J. Nanopart. Res. 2012, 14, 1280. [Google Scholar] [CrossRef]
- Saito, T.; Isogai, A. TEMPO-mediated oxidation of native cellulose. The effect of oxidation conditions on chemical and crystal structures of the water-insoluble fractions. Biomacromolecules 2004, 5, 1983–1989. [Google Scholar] [CrossRef] [PubMed]
- Isogai, A.; Saito, T.; Fukuzumi, H. TEMPO-oxidized cellulose nanofibers. Nanoscale 2011, 3, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Ehman, N.V.; Lourenco, A.F.; McDonagh, B.H.; Vallejos, M.E.; Felissia, F.E.; Ferreira, P.J.T.; Chinga-Carrasco, G.; Area, M.C. Influence of initial chemical composition and characteristics of pulps on the production and properties of lignocellulosic nanofibers. Int. J. Biol. Macromol. 2020, 143, 453–461. [Google Scholar] [CrossRef]
- Heggset, E.B.; Chinga-Carrasco, G.; Syverud, K. Temperature stability of nanocellulose dispersions. Carbohydr. Polym. 2017, 157, 114–121. [Google Scholar] [CrossRef]
- Geng, L.; Mittal, N.; Zhan, C.; Ansari, F.; Sharma, P.R.; Peng, X.; Hsiao, B.S.; Söderberg, L.D. Understanding the Mechanistic Behavior of Highly Charged Cellulose Nanofibers in Aqueous Systems. Macromolecules 2018, 51, 1498–1506. [Google Scholar] [CrossRef]
- Chinga-Carrasco, G.; Averianova, N.; Kondalenko, O.; Garaeva, M.; Petrov, V.; Leinsvang, B.; Karlsen, T. The effect of residual fibres on the micro-topography of cellulose nanopaper. Micron 2014, 56, 80–84. [Google Scholar] [CrossRef]
- Fujisawa, S.; Okita, Y.; Fukuzumi, H.; Saito, T.; Isogai, A. Preparation and characterization of TEMPO-oxidized cellulose nanofibril films with free carboxyl groups. Carbohyd. Polym. 2011, 84, 579–583. [Google Scholar] [CrossRef]
- De France, K.J.; Hoare, T.; Cranston, E.D. Review of Hydrogels and Aerogels Containing Nanocellulose. Chem. Mater. 2017, 29, 4609–4631. [Google Scholar] [CrossRef]
- Jiang, F.; Hsieh, Y.-L. Self-assembling of TEMPO Oxidized Cellulose Nanofibrils As Affected by Protonation of Surface Carboxyls and Drying Methods. ACS Sustain. Chem. Eng. 2016, 4, 1041–1049. [Google Scholar] [CrossRef]
- Syverud, K.; Kirsebom, H.; Hajizadeh, S.; Chinga-Carrasco, G. Cross-linking cellulose nanofibrils for potential elastic cryo-structured gels. Nanoscale Res. Lett. 2011, 6, 1–6. [Google Scholar] [CrossRef]
- Chaikumpollert, O.; Methacanon, P.; Suchiva, K. Structural elucidation of hemicelluloses from Vetiver grass. Carbohyd. Polym. 2004, 57, 191–196. [Google Scholar] [CrossRef]
- Sanchez-Gutierrez, M.; Espinosa, E.; Bascon-Villegas, I.; Perez-Rodriguez, F.; Carrasco, E.; Rodriguez, A. Production of Cellulose Nanofibers from Olive Tree Harvest-A Residue with Wide Applications. Agronomy 2020, 10, 696. [Google Scholar] [CrossRef]
- Chinga-Carrasco, G.; Ehman, N.V.; Pettersson, J.; Vallejos, M.E.; Brodin, M.W.; Felissia, F.E.; Hakansson, J.; Area, M.C. Pulping and Pretreatment Affect the Characteristics of Bagasse Inks for Three-dimensional Printing. ACS Sustain. Chem. Eng. 2018, 6, 4068–4075. [Google Scholar] [CrossRef]
- Rozali, M.L.H.; Ahmad, Z.; Isa, M.I.N. Interaction between Carboxy Methylcellulose and Salicylic Acid Solid Biopolymer Electrolytes. Adv. Mater. Res. 2015, 1107, 223–229. [Google Scholar] [CrossRef]
- Harper, J.R.; Balke, N.E. Characterization of the Inhibition of K+; Absorption in Oat Roots by Salicylic Acid. Plant Physiol. 1981, 68, 1349. [Google Scholar] [CrossRef]
- Fariduddin, Q.; Hayat, S.; Ahmad, A. Salicylic Acid Influences Net Photosynthetic Rate, Carboxylation Efficiency, Nitrate Reductase Activity, and Seed Yield in Brassica juncea. Photosynthetica 2003, 41, 281–284. [Google Scholar] [CrossRef]
| Sample Name | Carboxyl Content (µmol/g) | Standard Deviation (µmol/g) |
|---|---|---|
| Unbleached CNFs | 1057 | 18.4 |
| Bleached CNFs | 1376 | 23.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
McDonagh, B.H.; Chinga-Carrasco, G. Characterization of Porous Structures of Cellulose Nanofibrils Loaded with Salicylic Acid. Polymers 2020, 12, 2538. https://doi.org/10.3390/polym12112538
McDonagh BH, Chinga-Carrasco G. Characterization of Porous Structures of Cellulose Nanofibrils Loaded with Salicylic Acid. Polymers. 2020; 12(11):2538. https://doi.org/10.3390/polym12112538
Chicago/Turabian StyleMcDonagh, Birgitte Hjelmeland, and Gary Chinga-Carrasco. 2020. "Characterization of Porous Structures of Cellulose Nanofibrils Loaded with Salicylic Acid" Polymers 12, no. 11: 2538. https://doi.org/10.3390/polym12112538
APA StyleMcDonagh, B. H., & Chinga-Carrasco, G. (2020). Characterization of Porous Structures of Cellulose Nanofibrils Loaded with Salicylic Acid. Polymers, 12(11), 2538. https://doi.org/10.3390/polym12112538