Preparation of Hybrid Alginate-Chitosan Aerogel as Potential Carriers for Pulmonary Drug Delivery
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
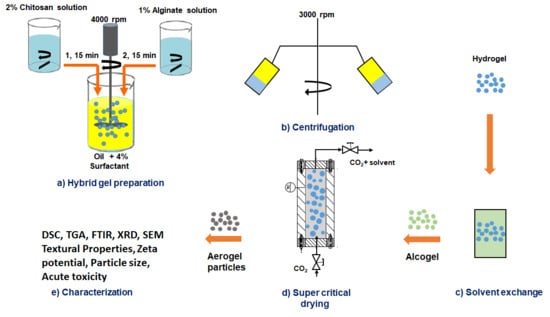
2.2. Preparation of Composite (Alginate-Chitosan) Nanoporous Particles
2.3. Physicochemical Characterizations of the Prepared Particles
2.3.1. Measurement of Particle Size
2.3.2. Zeta Potential
2.3.3. Surface Area and Porosity Analysis
2.3.4. Tapped Density Measurement
2.3.5. True Skeleton Density Measurement
2.3.6. Aerodynamic Diameter (DA)
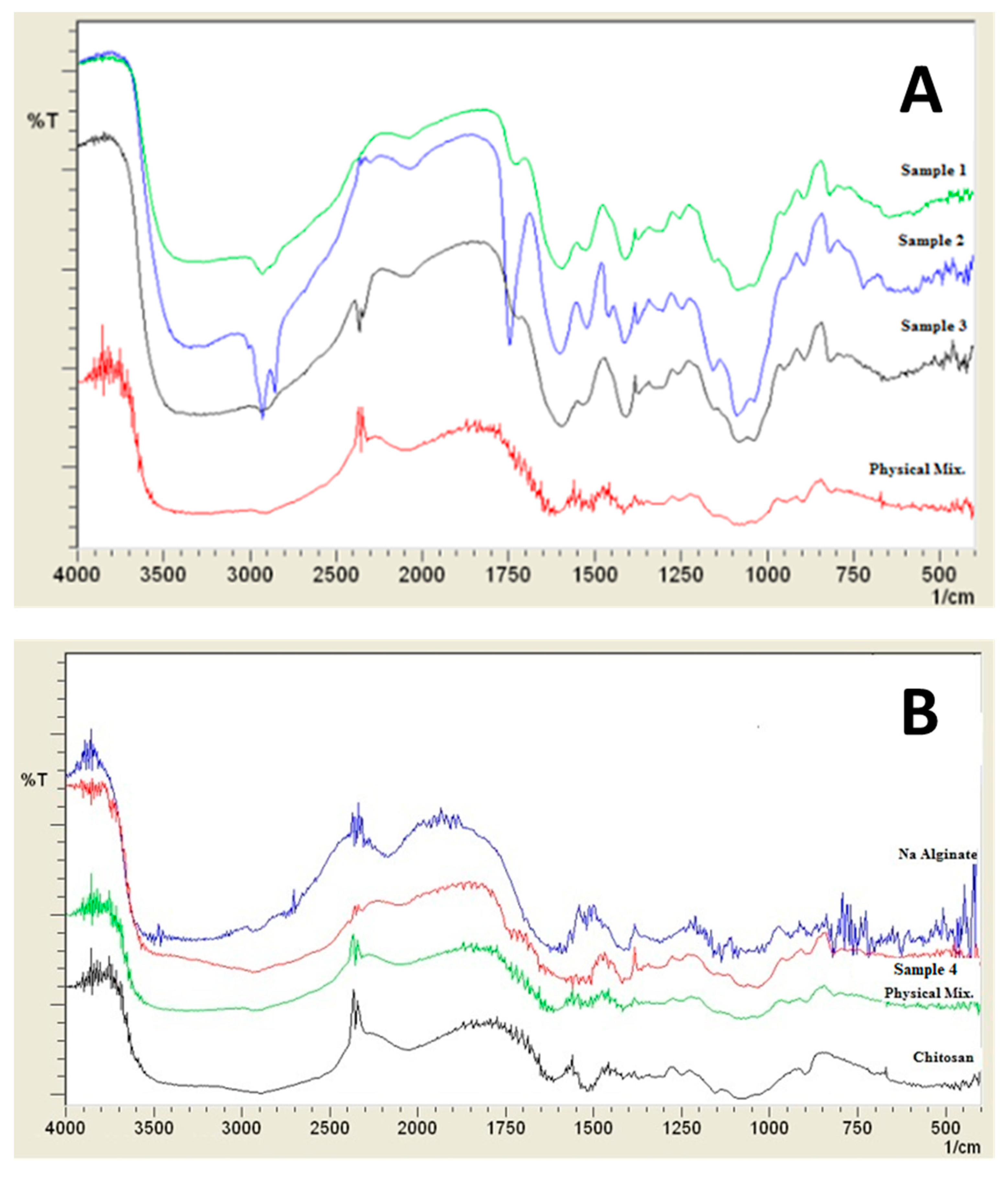
2.3.7. Fourier Transform Infrared Spectroscopy (FTIR)
2.3.8. Powder X-Ray Diffraction (PXRD)
2.3.9. Differential Scanning Calorimetry (DSC)
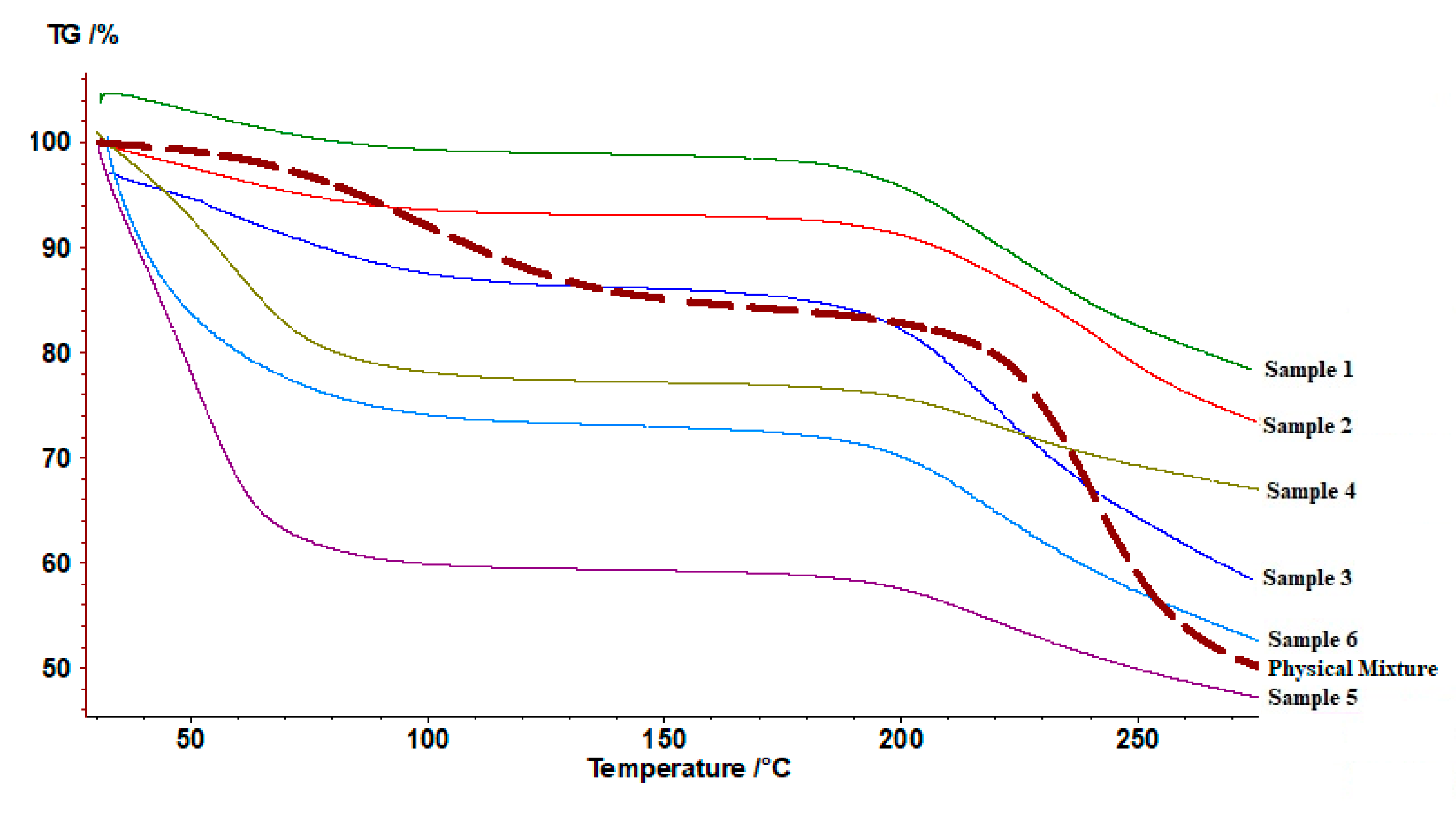
2.3.10. Thermogravimetric Analysis (TGA)
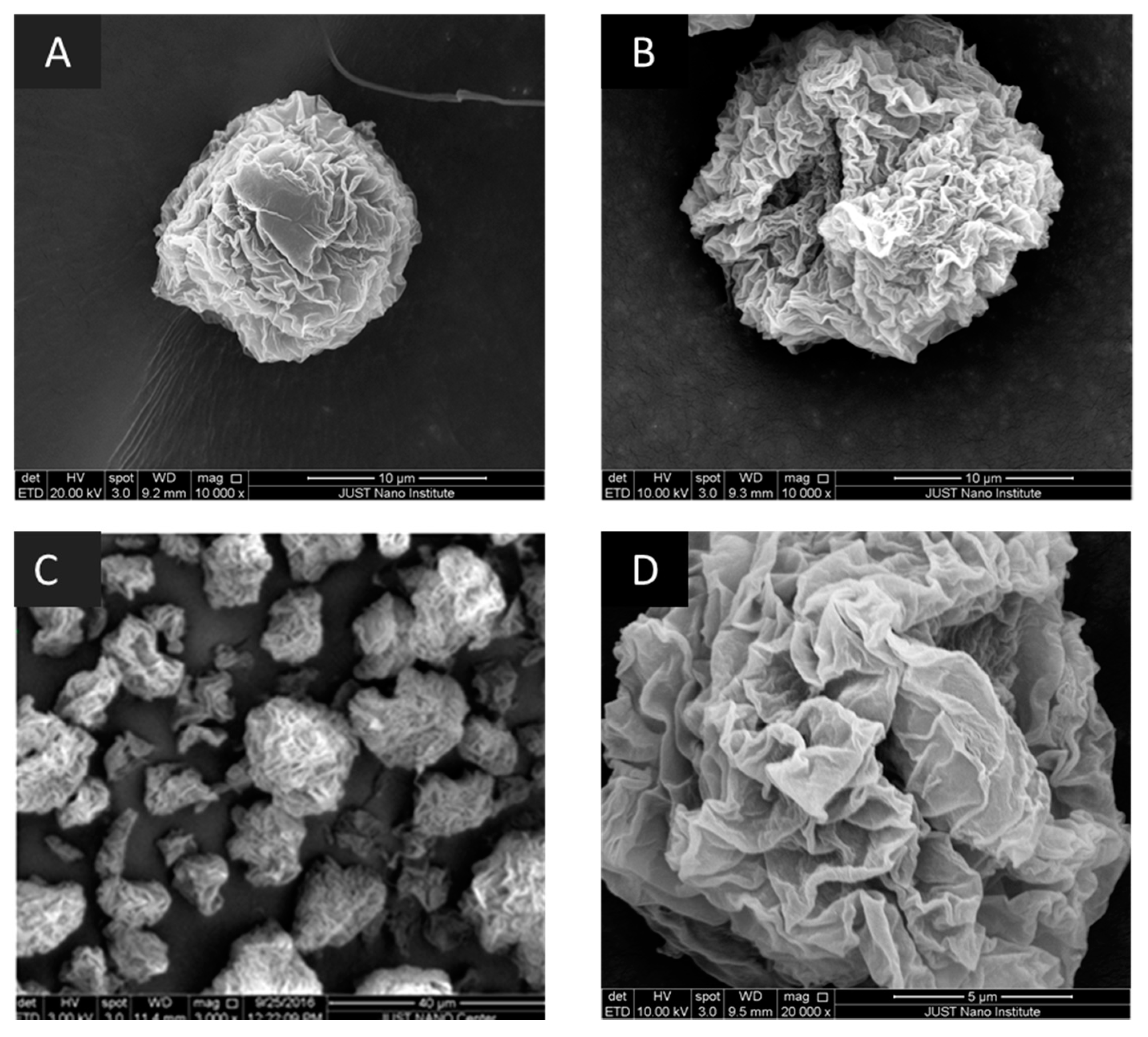
2.3.11. Scanning Electron Microscopy (SEM)
2.3.12. Yield
2.4. Statistics
2.5. In Vivo Toxicity Studies
3. Results
3.1. Structural Properties
3.2. Physicochemical Characterizations of the Prepared Particles
3.2.1. FTIR Analysis
3.2.2. DSC Analysis
3.2.3. TGA Analysis
3.2.4. PXRD Patterns
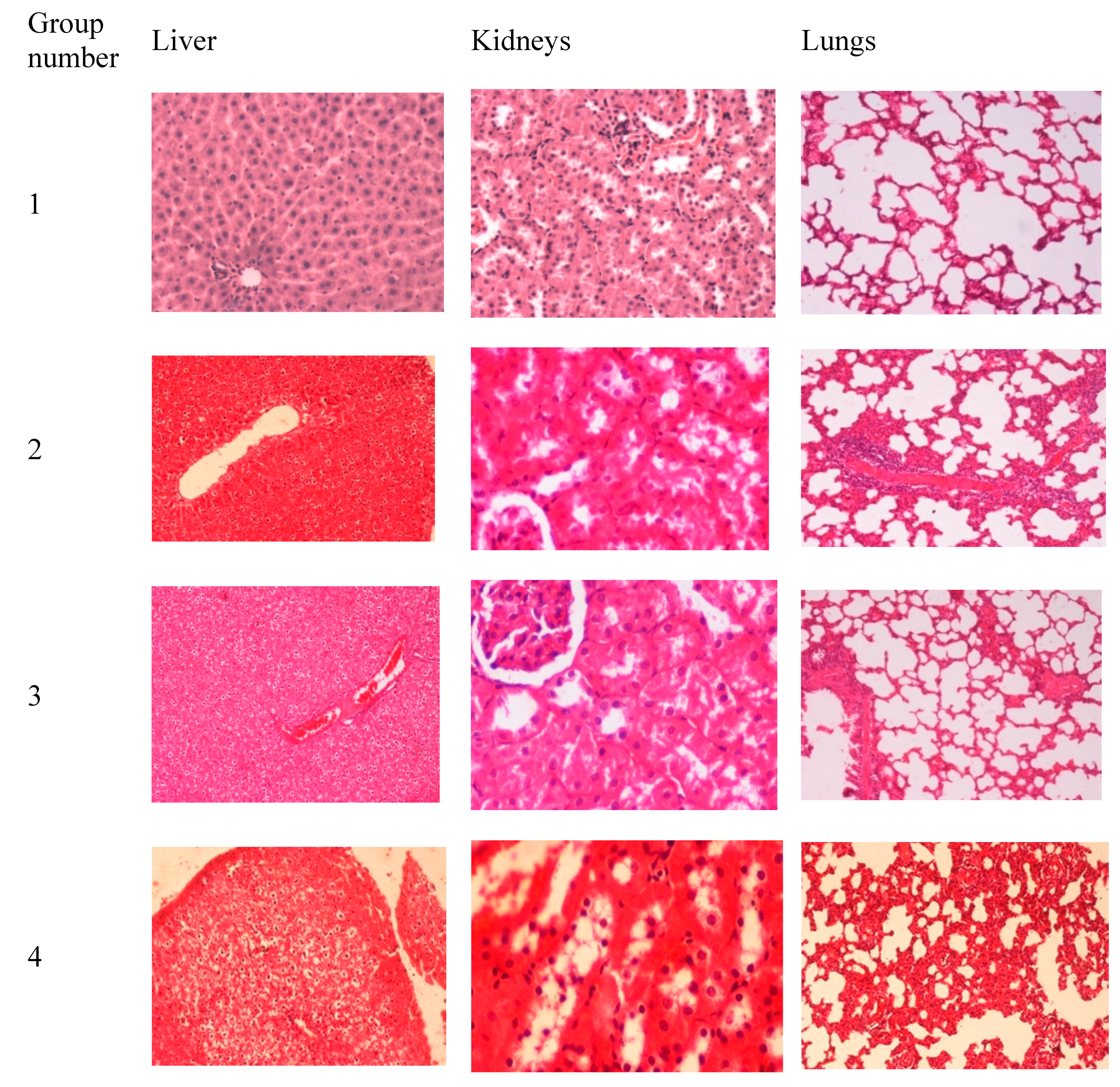
3.3. In Vivo Toxicity Studies
4. Discussion
4.1. Structural Properties
4.2. Effect of Changing the Surfactant Type on Particle Properties
4.3. Effect of the Order of Addition of the Two Polymers on Particle Properties
4.4. Physicochemical Characterizations of Prepared Polyelectrolyte Composite Particles
4.5. In Vivo Toxicity Studies
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sung, J.C.; Pulliam, B.L.; Edwards, D.A. Nanoparticles for drug delivery to the lungs. Trends Biotechnol. 2007, 25, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Sarasija, S.; Patil, J. Pulmonary drug delivery strategies: A concise, systematic review. Lung India 2012, 29, 44. [Google Scholar] [CrossRef] [PubMed]
- Patil, J.; Devi, V.; Devi, K.; Sarasija, S. A novel approach for lung delivery of rifampicin-loaded liposomes in dry powder form for the treatment of tuberculosis. Lung India 2015, 32, 331. [Google Scholar] [CrossRef]
- Ahmed, T.; Aljaeid, B. Preparation, characterization, and potential application of chitosan, chitosan derivatives, and chitosan metal nanoparticles in pharmaceutical drug delivery. Drug Des. Dev. Ther. 2016, 483. [Google Scholar] [CrossRef]
- AboulFotouh, K.; Zhang, Y.; Maniruzzaman, M.; Williams, R.O.; Cui, Z. Amorphous solid dispersion dry powder for pulmonary drug delivery: Advantages and challenges. Int. J. Pharm. 2020, 587, 119711. [Google Scholar] [CrossRef] [PubMed]
- Ho, D.-K.; Nichols, B.L.B.; Edgar, K.J.; Murgia, X.; Loretz, B.; Lehr, C.-M. Challenges and strategies in drug delivery systems for treatment of pulmonary infections. Eur. J. Pharm. Biopharm. 2019, 144, 110–124. [Google Scholar] [CrossRef]
- Deshmukh, R.; Bandyopadhyay, N.; Abed, S.N.; Bandopadhyay, S.; Pal, Y.; Deb, P.K. Chapter 3—Strategies for pulmonary delivery of drugs. In Drug Delivery Systems; Advances in Pharmaceutical Product Development and Research; Tekade, R.K., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 85–129. ISBN 978-0-12-814487-9. [Google Scholar]
- Beck-Broichsitter, M.; Merkel, O.M.; Kissel, T. Controlled pulmonary drug and gene delivery using polymeric nano-carriers. J. Control. Release 2012, 161, 214–224. [Google Scholar] [CrossRef]
- Fromen, C.A.; Rahhal, T.B.; Robbins, G.R.; Kai, M.P.; Shen, T.W.; Luft, J.C.; DeSimone, J.M. Nanoparticle surface charge impacts distribution, uptake and lymph node trafficking by pulmonary antigen-presenting cells. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 677–687. [Google Scholar] [CrossRef]
- Mastrandrea, L.D.; Quattrin, T. Clinical evaluation of inhaled insulin. Adv. Drug Deliv. Rev. 2006, 58, 1061–1075. [Google Scholar] [CrossRef]
- Laube, B.L. The expanding role of aerosols in systemic drug delivery, gene therapy and vaccination: An update. Transl. Respir. Med. 2014, 2, 1. [Google Scholar] [CrossRef][Green Version]
- Poursina, N.; Vatanara, A.; Rouini, M.R.; Gilani, K.; Rouholamini Najafabadi, A. Systemic delivery of parathyroid hormone (1–34) using spray freeze-dried inhalable particles. Pharm. Dev. Technol. 2015, 1–7. [Google Scholar] [CrossRef]
- Taratula, O.; Kuzmov, A.; Shah, M.; Garbuzenko, O.B.; Minko, T. Nanostructured lipid carriers as multifunctional nanomedicine platform for pulmonary co-delivery of anticancer drugs and siRNA. J. Control Release 2013, 171, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Smola, M.; Vandamme, T.; Sokolowski, A. Nanocarriers as pulmonary drug delivery systems to treat and to diagnose respiratory and nonrespiratory diseases. Int. J. Nanomed. 2008, 3, 1. [Google Scholar]
- Peng, H.; Liu, X.; Wang, R.; Jia, F.; Dong, L.; Wang, Q. Emerging nanostructured materials for musculoskeletal tissue engineering. J. Mater. Chem. B 2014, 2, 6435–6461. [Google Scholar] [CrossRef]
- García-González, C.A.; López-Iglesias, C.; Concheiro, A.; Alvarez-Lorenzo, C. Chapter 16 Biomedical Applications of Polysaccharide and Protein Based Aerogels. In Biobased Aerogels: Polysaccharide and Protein-based Materials; The Royal Society of Chemistry: Croydon, UK, 2018; pp. 295–323. ISBN 978-1-78262-765-4. [Google Scholar]
- Thomas, S.; Pothan, L.A.; Mavelil-Sam, R. Biobased Aerogels: Polysaccharide and Protein-based Materials; Royal Society of Chemistry: Croydon, UK, 2018; Volume 58, ISBN 1-78262-765-0. [Google Scholar]
- Kirschning, A.; Dibbert, N.; Dräger, G. Chemical Functionalization of Polysaccharides-Towards Biocompatible Hydrogels for Biomedical Applications. Chem. A Eur. J. 2018, 24, 1231–1240. [Google Scholar] [CrossRef] [PubMed]
- García-González, C.A.; Alnaief, M.; Smirnova, I. Polysaccharide-based aerogels—Promising biodegradable carriers for drug delivery systems. Carbohydr. Polym. 2011, 86, 1425–1438. [Google Scholar] [CrossRef]
- Menon, J.U.; Ravikumar, P.; Pise, A.; Gyawali, D.; Hsia, C.C.W.; Nguyen, K.T. Polymeric nanoparticles for pulmonary protein and DNA delivery. Acta Biomater. 2014, 10, 2643–2652. [Google Scholar] [CrossRef]
- Gurikov, P.; Smirnova, I. Amorphization of drugs by adsorptive precipitation from supercritical solutions: A review. J. Supercrit. Fluids 2017. [Google Scholar] [CrossRef]
- Ho, D.-K.; Costa, A.; De Rossi, C.; de Souza Carvalho-Wodarz, C.; Loretz, B.; Lehr, C.-M. Polysaccharide Submicrocarrier for Improved Pulmonary Delivery of Poorly Soluble Anti-infective Ciprofloxacin: Preparation, Characterization, and Influence of Size on Cellular Uptake. Mol. Pharm. 2018, 15, 1081–1096. [Google Scholar] [CrossRef]
- Tahara, K.; Sakai, T.; Yamamoto, H.; Takeuchi, H.; Hirashima, N.; Kawashima, Y. Improved cellular uptake of chitosan-modified PLGA nanospheres by A549 cells. Int. J. Pharm. 2009, 382, 198–204. [Google Scholar] [CrossRef]
- Martins, M.; Barros, A.A.; Quraishi, S.; Gurikov, P.; Raman, S.P.; Smirnova, I.; Duarte, A.R.C.; Reis, R.L. Preparation of macroporous alginate-based aerogels for biomedical applications. J. Supercrit. Fluids 2015, 106, 152–159. [Google Scholar] [CrossRef]
- Barros, A.; Quraishi, S.; Martins, M.; Gurikov, P.; Subrahmanyam, R.; Smirnova, I.; Duarte, A.R.C.; Reis, R.L. Hybrid Alginate-Based Cryogels for Life Science Applications. Chem. Ing. Tech. 2016, 88, 1770–1778. [Google Scholar] [CrossRef]
- Jia, M.; Li, Z.-B.; Chu, H.-T.; Li, L.; Chen, K.-Y. Alginate-Chitosan Microspheres for Controlled Drug Delivery of Diltiazem Hydrochloride in Cardiac Diseases. J. Biomater. Tissue Eng. 2015, 5, 246–251. [Google Scholar] [CrossRef]
- Takka, S.; Gürel, A. Evaluation of Chitosan/Alginate Beads Using Experimental Design: Formulation and In Vitro Characterization. AAPS Pharmscitech 2010, 11, 460–466. [Google Scholar] [CrossRef]
- Ahmad, Z.; Pandey, R.; Sharma, S.; Khuller, G.K. Alginate nanoparticles as antituberculosis drug carriers: Formulation development, pharmacokinetics and therapeutic potential. Indian J. Chest Dis. Allied Sci. 2006, 48, 171. [Google Scholar] [PubMed]
- Coppi, G.; Iannuccelli, V.; Leo, E.; Bernabei, M.T.; Cameroni, R. Chitosan-Alginate Microparticles as a Protein Carrier. Drug Dev. Ind. Pharm. 2001, 27, 393–400. [Google Scholar] [CrossRef]
- Li, P.; Dai, Y.-N.; Zhang, J.-P.; Wang, A.-Q.; Wei, Q. Chitosan-alginate nanoparticles as a novel drug delivery system for nifedipine. Int. J. Biomed. Sci. 2008, 4, 221–228. [Google Scholar] [PubMed]
- Malesu, V.K.; Sahoo, D.; Nayak, P.L. Chitosan–sodium alginate nanocomposites blended with cloisite 30B as a novel drug delivery system for anticancer drug curcumin. IJABPT 2011, 2, 402–4011. [Google Scholar]
- Chan, G.; Mooney, D.J. Ca2+ released from calcium alginate gels can promote inflammatory responses in vitro and in vivo. Acta Biomater. 2013, 9, 9281–9291. [Google Scholar] [CrossRef]
- Penhasi, A. Preparation and characterization of in-situ ionic cross-linked pectin films: II. Biodegradation and drug diffusion. Carbohydr. Polym. 2017, 157, 651–659. [Google Scholar] [CrossRef]
- Florczyk, S.J.; Kim, D.-J.; Wood, D.L.; Zhang, M. Influence of processing parameters on pore structure of 3D porous chitosan-alginate polyelectrolyte complex scaffolds. J. Biomed. Mater. Res. Part A 2011, 98A, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Sankalia, M.G.; Mashru, R.C.; Sankalia, J.M.; Sutariya, V.B. Reversed chitosan–alginate polyelectrolyte complex for stability improvement of alpha-amylase: Optimization and physicochemical characterization. Eur. J. Pharm. Biopharm. 2007, 65, 215–232. [Google Scholar] [CrossRef] [PubMed]
- Kulig, D.; Zimoch-Korzycka, A.; Jarmoluk, A. Cross-linked alginate/chitosan polyelectrolytes as carrier of active compound and beef color stabilizer. Meat Sci. 2017, 123, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Bilbao-Sainz, C.; Chiou, B.; Punotai, K.; Olson, D.; Williams, T.; Wood, D.; Rodov, V.; Poverenov, E.; McHugh, T. Layer-by-Layer Alginate and Fungal Chitosan Based Edible Coatings Applied to Fruit Bars. J. Food Sci. 2018, 83, 1880–1887. [Google Scholar] [CrossRef]
- Caridade, S.G.; Monge, C.; Gilde, F.; Boudou, T.; Mano, J.F.; Picart, C. Free-Standing Polyelectrolyte Membranes Made of Chitosan and Alginate. Biomacromolecules 2013, 14, 1653–1660. [Google Scholar] [CrossRef]
- Sun, W.; Chen, G.; Wang, F.; Qin, Y.; Wang, Z.; Nie, J.; Ma, G. Polyelectrolyte-complex multilayer membrane with gradient porous structure based on natural polymers for wound care. Carbohydr. Polym. 2018, 181, 183–190. [Google Scholar] [CrossRef]
- Lefnaoui, S.; Moulai-Mostefa, N.; Yahoum, M.M.; Gasmi, S.N. Design of antihistaminic transdermal films based on alginate–chitosan polyelectrolyte complexes: Characterization and permeation studies. Drug Dev. Ind. Pharm. 2018, 44, 432–443. [Google Scholar] [CrossRef]
- Obaidat, R.; Al-Jbour, N.; Al-Sou’d, K.; Sweidan, K.; Al-Remawi, M.; Badwan, A. Some Physico-Chemical Properties of Low Molecular Weight Chitosans and their Relationship to Conformation in Aqueous Solution. J. Solut. Chem. 2010, 39, 575–588. [Google Scholar] [CrossRef]
- Kasaai, M.R. Calculation of Mark–Houwink–Sakurada (MHS) equation viscometric constants for chitosan in any solvent–temperature system using experimental reported viscometric constants data. Carbohydr. Polym. 2007, 68, 477–488. [Google Scholar] [CrossRef]
- Alnaief, M.; Obaidat, R.; Mashaqbeh, H. Loading and evaluation of meloxicam and atorvastatin in carrageenan microspherical aerogels particles. J. Appl. Pharm. Sci. 2019, 9, 83–88. [Google Scholar] [CrossRef]
- Alnaief, M.; Obaidat, R.; Mashaqbeh, H. Effect of processing parameters on preparation of carrageenan aerogel microparticles. Carbohydr. Polym. 2018, 180, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Alsmadi, M.M.; Obaidat, R.M.; Alnaief, M.; Albiss, B.A.; Hailat, N. Development, In Vitro Characterization, and In Vivo Toxicity Evaluation of Chitosan-Alginate Nanoporous Carriers Loaded with Cisplatin for Lung Cancer Treatment. AAPS PharmSciTech 2020, 21, 191. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.S.; Lau, R.W.M. Effect of Particle Shape on Dry Particle Inhalation: Study of Flowability, Aerosolization, and Deposition Properties. AAPS Pharmscitech 2009, 10, 1252–1262. [Google Scholar] [CrossRef] [PubMed]
- Davies, C.N. Particle-fluid interaction. J. Aerosol Sci. 1979, 10, 477–513. [Google Scholar] [CrossRef]
- Edwards, D.A. Delivery of biological agents by aerosols. AICHE J. 2002, 48, 2–6. [Google Scholar] [CrossRef]
- Zelenyuk, A.; Cai, Y.; Imre, D. From Agglomerates of Spheres to Irregularly Shaped Particles: Determination of Dynamic Shape Factors from Measurements of Mobility and Vacuum Aerodynamic Diameters. Aerosol Sci. Technol. 2006, 40, 197–217. [Google Scholar] [CrossRef]
- Mohy Eldin, M.S.; Hashem, A.E.; Tamer, T.M.; Omer, A.M.; Yossuf, M.E.; Sabet, M. Development of Cross linked Chitosan/Alginate Polyelectrolyte Proton Exchanger Membranes for Fuel Cell Applications. Int. J. Electrochem. Sci. 2017, 3840–3858. [Google Scholar] [CrossRef]
- Arianto, A.; Bangun, H.; Harahap, U.; Ilyas, S. Effect of alginate chitosan ratio on the swelling, mucoadhesive, and release of ranitidine from spherical matrices of alginate-chitosan. Int. J. Pharmtech. Res. 2015, 8, 653–665. [Google Scholar]
- Delaney, K.T.; Fredrickson, G.H. Theory of polyelectrolyte complexation—Complex coacervates are self-coacervates. J. Chem. Phys. 2017, 146, 224902. [Google Scholar] [CrossRef]
- Vanbever, R.; Mintzes, J.D.; Wang, J.; Nice, J.; Chen, D.; Batycky, R.; Langer, R.; Edwards, D.A. Formulation and Physical Characterization of Large Porous Particles for Inhalation. Pharm. Res. 1999, 16, 1735–1742. [Google Scholar] [CrossRef]
- Klinkesorn, U. The Role of Chitosan in Emulsion Formation and Stabilization. Food Rev. Int. 2013, 29, 371–393. [Google Scholar] [CrossRef]
- Wang, X. Emulsifying Properties of Chitosan and Chitosan/Gelatin Complexes. Ph.D. Thesis, École Polytechnique de Montréal, Montreal, QC, Canada, 2016. [Google Scholar]
- Xie, Y.; Aillon, K.L.; Cai, S.; Christian, J.M.; Davies, N.M.; Berkland, C.J.; Forrest, M.L. Pulmonary delivery of cisplatin-hyaluronan conjugates via endotracheal instillation for the treatment of lung cancer. Int. J. Pharm. 2010, 392, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.; Khuller, G.K. Chemotherapeutic potential of alginate–chitosan microspheres as anti-tubercular drug carriers. J. Antimicrob. Chemother. 2004, 53, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Li, P.; Zang, J.; Liu, J. Enhanced Hemostatic Performance of Tranexamic Acid-Loaded Chitosan/Alginate Composite Microparticles. J. Biomed. Biotechnol. 2012, 2012, 981321. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mladenovska, K.; Cruaud, O.; Richomme, P.; Belamie, E.; Raicki, R.; Venier-Julienne, M.-C.; Popovski, E.; Benoit, J.; Goracinova, K. 5-ASA loaded chitosan-Ca-alginate microparticles: Preparation and physicochemical characterization. Int. J. Pharm. 2007, 345, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Vert, M.; Doi, Y.; Hellwich, K.-H.; Hess, M.; Hodge, P.; Kubisa, P.; Rinaudo, M.; Schué, F. Terminology for biorelated polymers and applications (IUPAC Recommendations 2012). Pure Appl. Chem. 2012, 84, 377–410. [Google Scholar] [CrossRef]

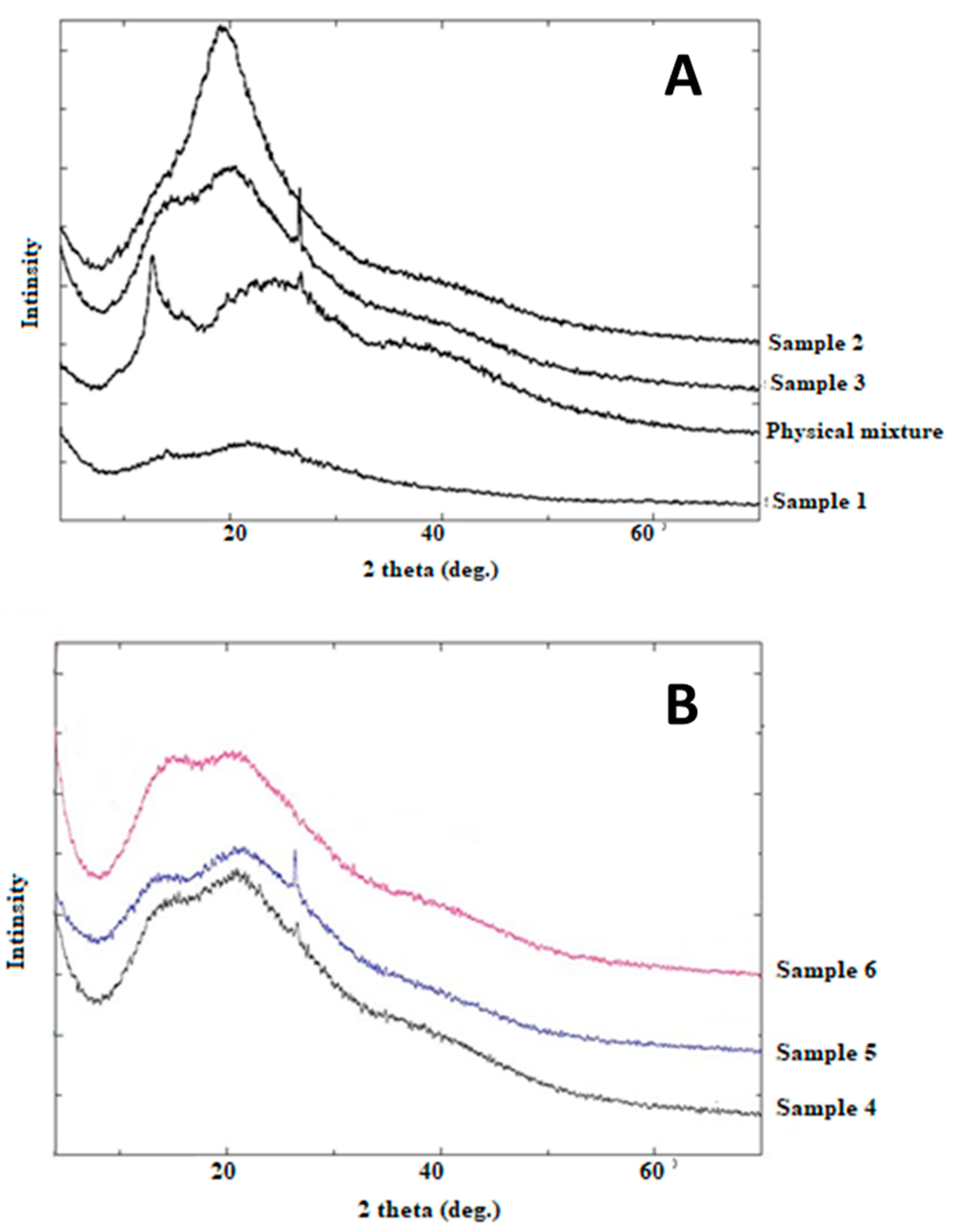
| Samples | Surfactant Type | Order of Polymer Addition to the Oil Phase | Yield (%) |
|---|---|---|---|
| S1 | Span 85 | Chitosan added to Alginate | 73 ± 5 |
| S2 | Span 85 | Alginate added to Chitosan | 78 ± 6 |
| S3 | Span 85 | Both polymers are added simultaneously | 72 ± 8 |
| S4 | Span 80 | Chitosan added to Alginate | 44 ± 3 |
| S5 | Span 80 | Alginate added to Chitosan | 39 ± 5 |
| S6 | Span 80 | Both polymers are added simultaneously | 59 ± 10 |
| Sample | Size (µm) | Zeta Potential (mV) | Specific Surface Area (m2/g) | Porosity (cm3/g) | Pore Diameter (nm) | Bulk Density (g/ cm3) | Tapped Density (g/ cm3) | Calculated Aerodynamic Diameter (µm) |
|---|---|---|---|---|---|---|---|---|
| Span 85 | ||||||||
| S1 | 0.433 ± 0.091 | 45.3 ± 3.44 | 86.2 | 0.288 | 13.38 | 0.113 | 0.16 | 2.29 |
| S2 | 4.170 ± 0.480 | 35.4 ± 5.37 | 79.89 | 0.252 | 12.61 | 0.048 | 0.08 | 0.96 |
| S3 | 2.970 ± 0.420 | 2.2 ± 12.6 | 0.5 | 0 | - | 0.071 | 0.09 | 0.72 |
| Span 80 | ||||||||
| S4 | 0.070 ± 0.043 | −5.98 ± 3.45 | 53.77 | 0.17 | 12.79 | 0.19 | 1.2 | 0.19 |
| S5 | 0.084 ± 0.062 | −2.15 ± 3.70 | 29.36 | 0.094 | 12.78 | 0.17 | 1.14 | 0.17 |
| S6 | 0.081 ± 0.044 | −2.28± 3.61 | 58.84 | 0.184 | 12.48 | 0.19 | 1.2 | 0.19 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alnaief, M.; Obaidat, R.M.; Alsmadi, M.M. Preparation of Hybrid Alginate-Chitosan Aerogel as Potential Carriers for Pulmonary Drug Delivery. Polymers 2020, 12, 2223. https://doi.org/10.3390/polym12102223
Alnaief M, Obaidat RM, Alsmadi MM. Preparation of Hybrid Alginate-Chitosan Aerogel as Potential Carriers for Pulmonary Drug Delivery. Polymers. 2020; 12(10):2223. https://doi.org/10.3390/polym12102223
Chicago/Turabian StyleAlnaief, Mohammad, Rana M. Obaidat, and Mo’tasem M. Alsmadi. 2020. "Preparation of Hybrid Alginate-Chitosan Aerogel as Potential Carriers for Pulmonary Drug Delivery" Polymers 12, no. 10: 2223. https://doi.org/10.3390/polym12102223
APA StyleAlnaief, M., Obaidat, R. M., & Alsmadi, M. M. (2020). Preparation of Hybrid Alginate-Chitosan Aerogel as Potential Carriers for Pulmonary Drug Delivery. Polymers, 12(10), 2223. https://doi.org/10.3390/polym12102223