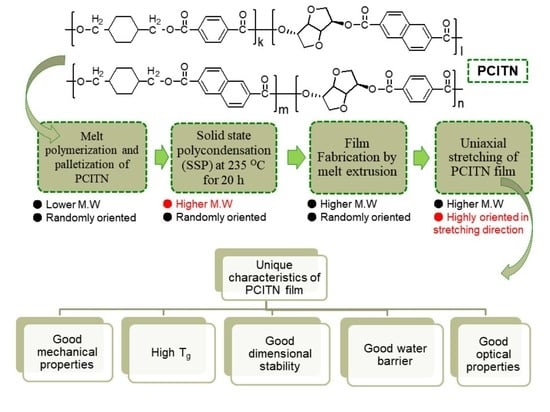
High Thermal Stability, High Tensile Strength, and Good Water Barrier Property of Terpolyester Containing Biobased Monomer for Next-Generation Smart Film Application: Synthesis and Characterization
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Copolyester Followed by Solid-State Polycondensation (SSP)
2.2.1. Copolyester Synthesis
2.2.2. Film Fabrication and Uniaxial Stretching
2.3. Characterization
2.4. Characterization of Film Properties
3. Results and Discussion
3.1. Actual Composition of PCITN Copolyester
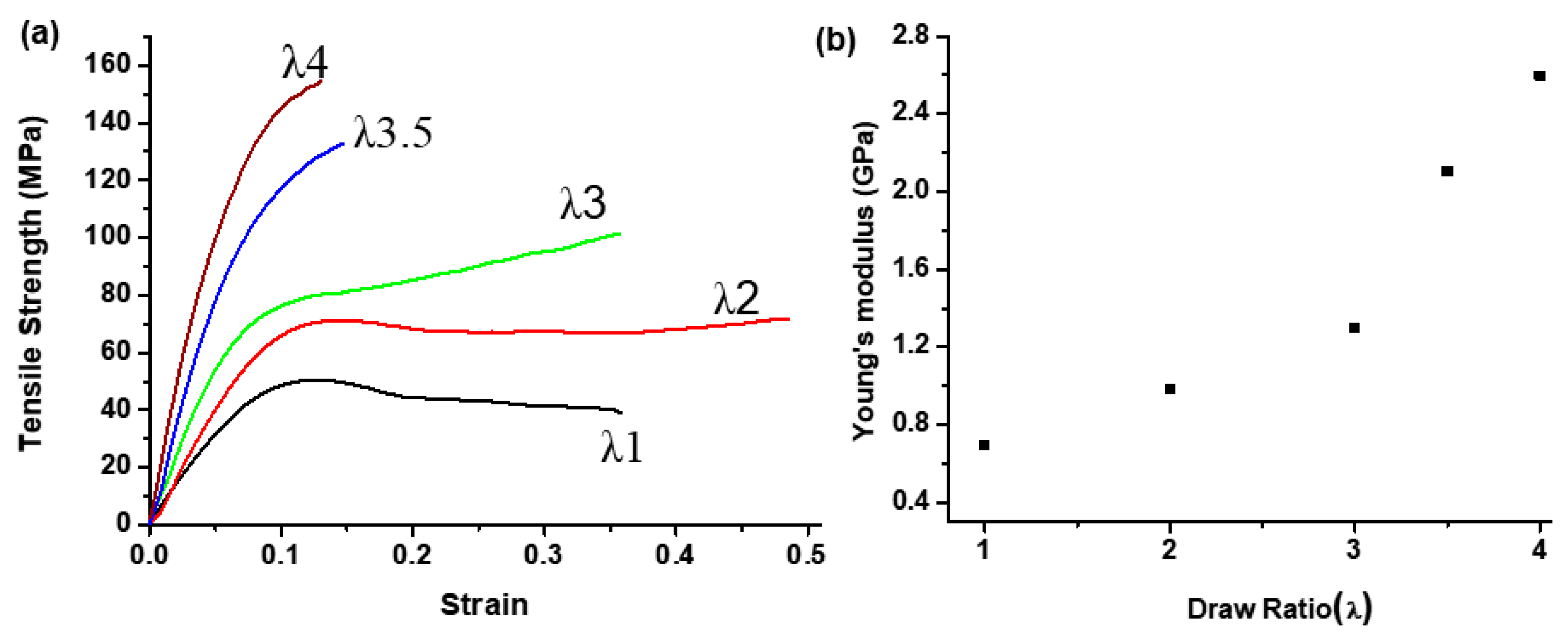
3.2. Mechanical Behavior of PCITN Films
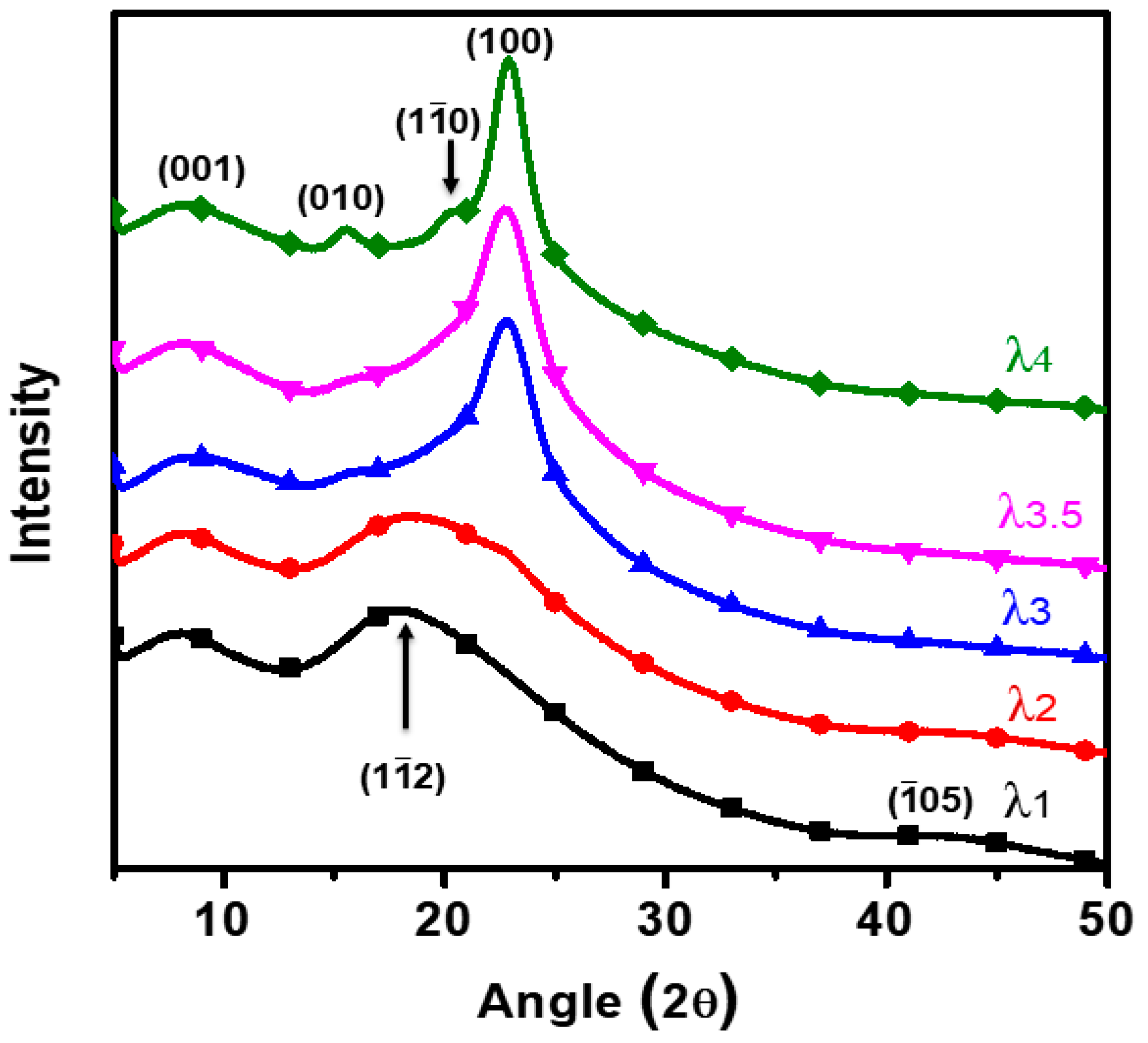
3.3. Direct Evidence of SIC by High Power X-ray Diffraction (XRD) Analysis
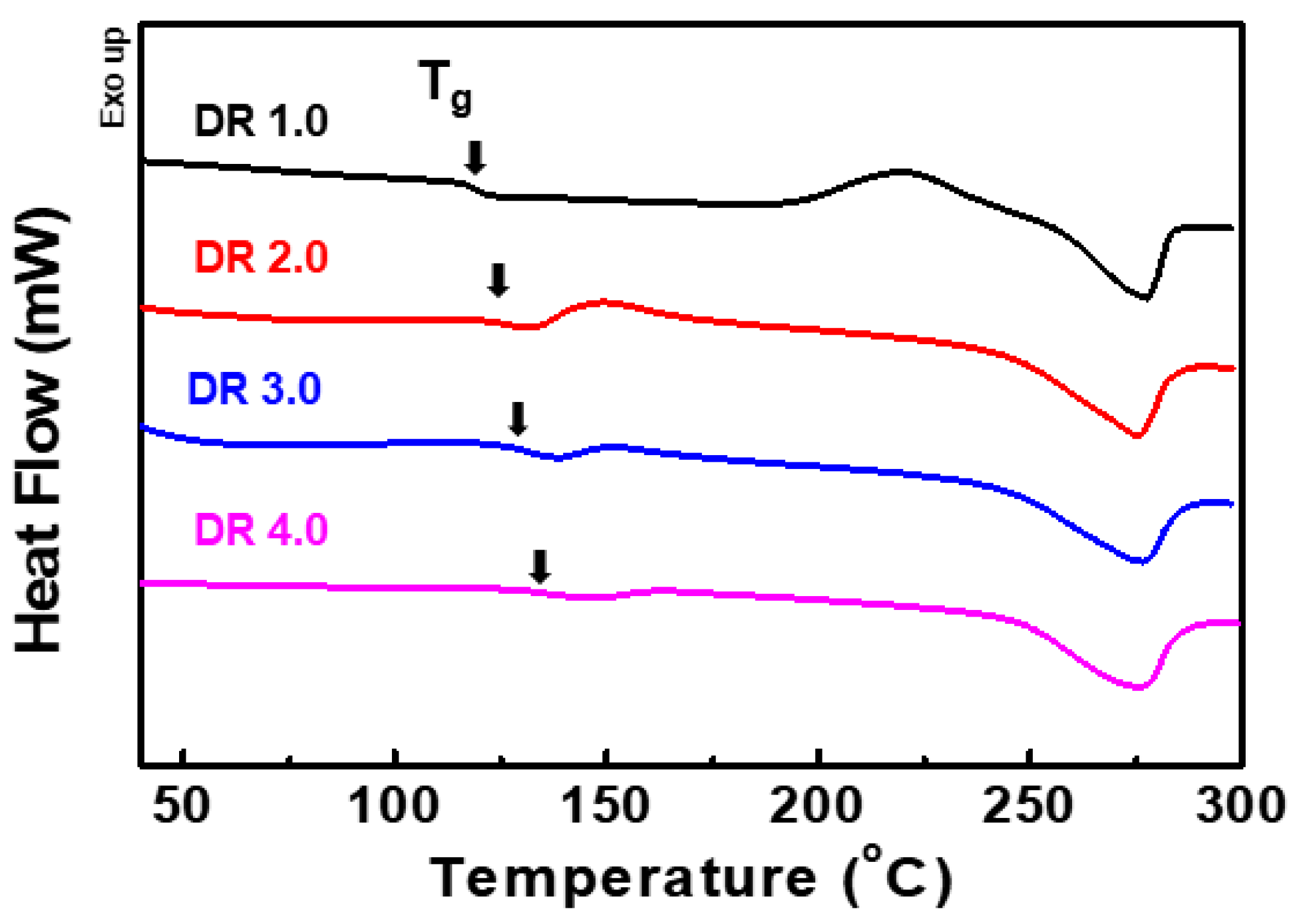
3.4. Thermal Properties and SIC Analysis of PCITN Film
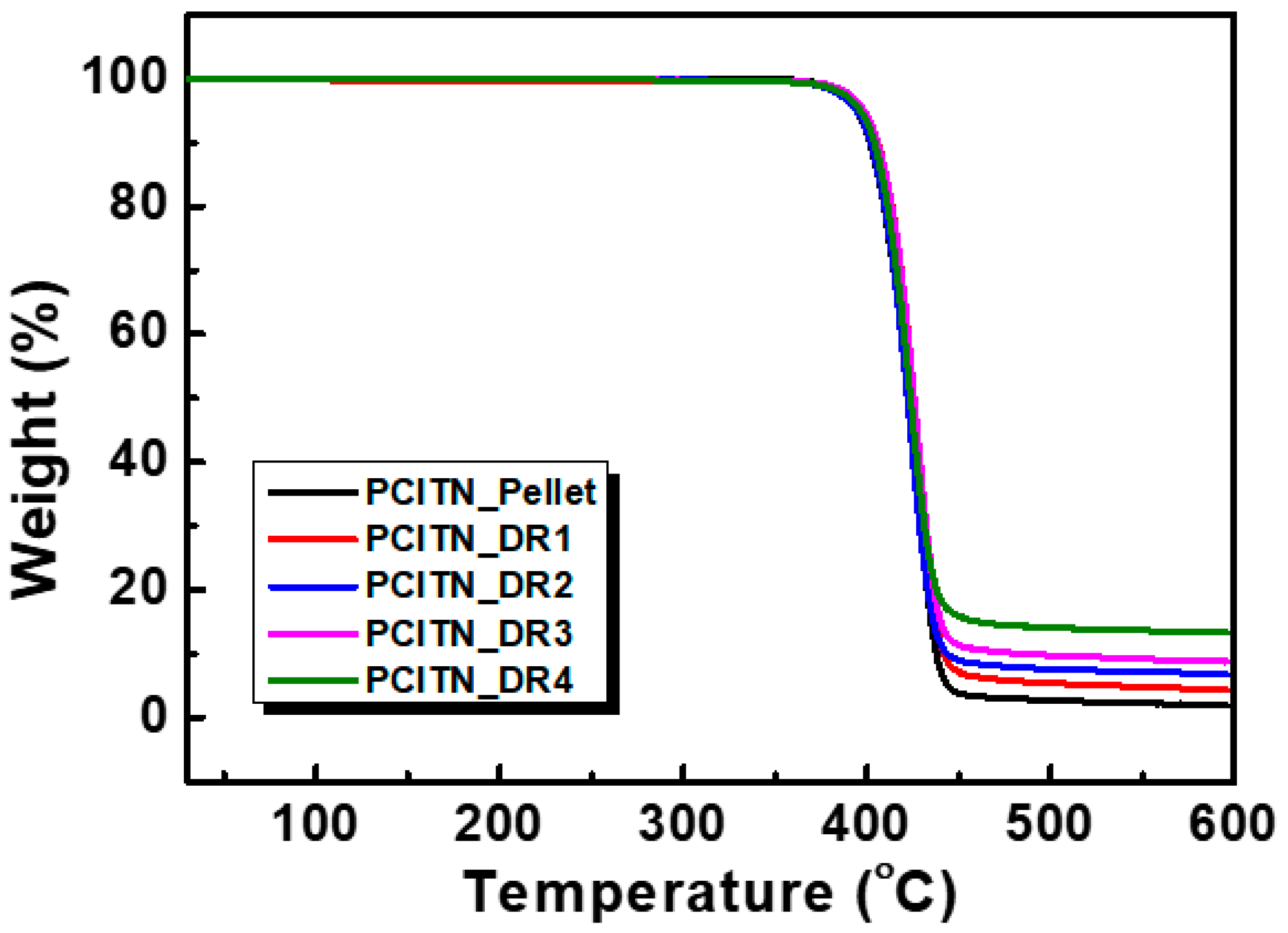
3.5. Thermal Stability of the Fabricated PCITN Films
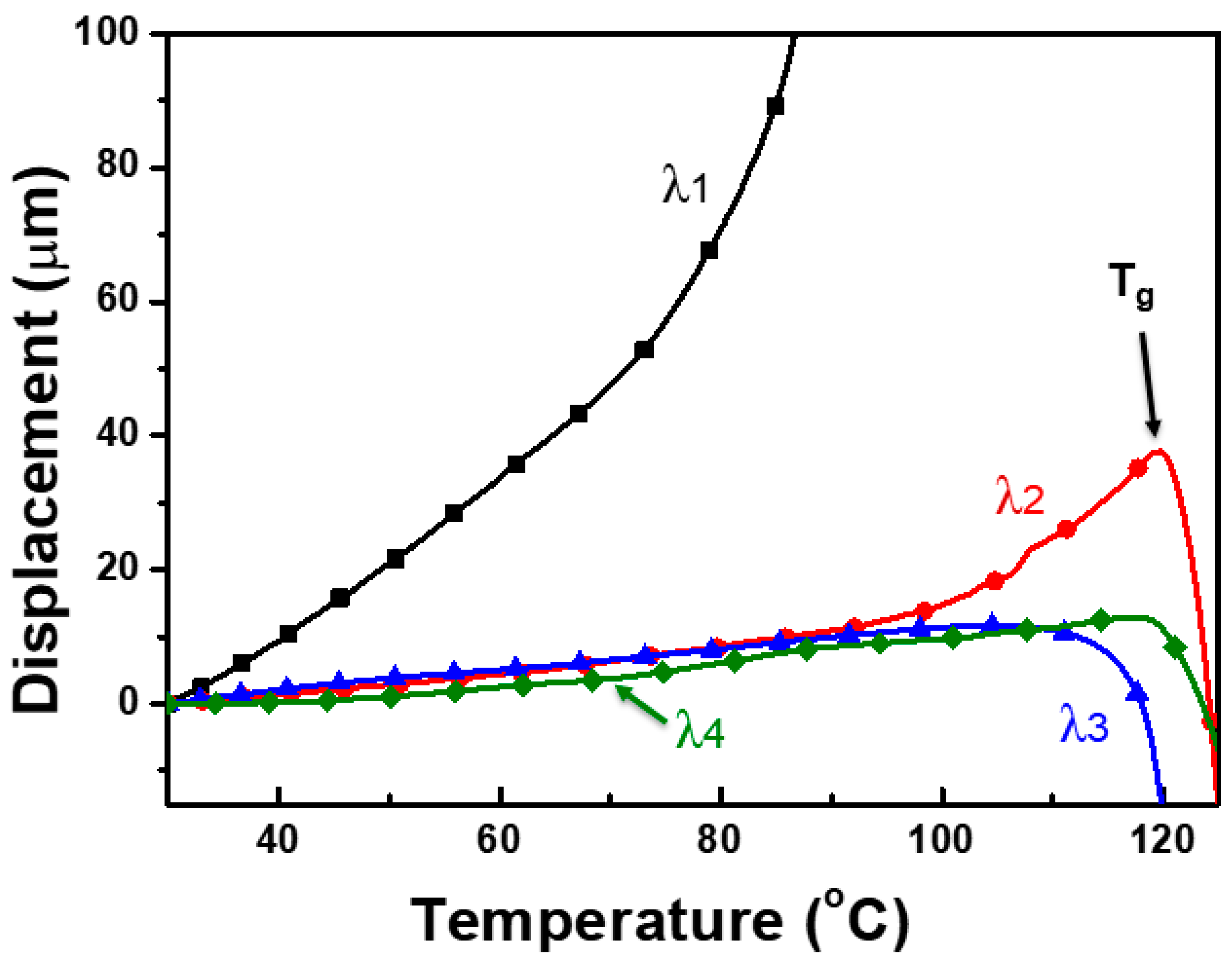
3.6. Improving the Dimensional Stability of Uniaxially Oriented Films
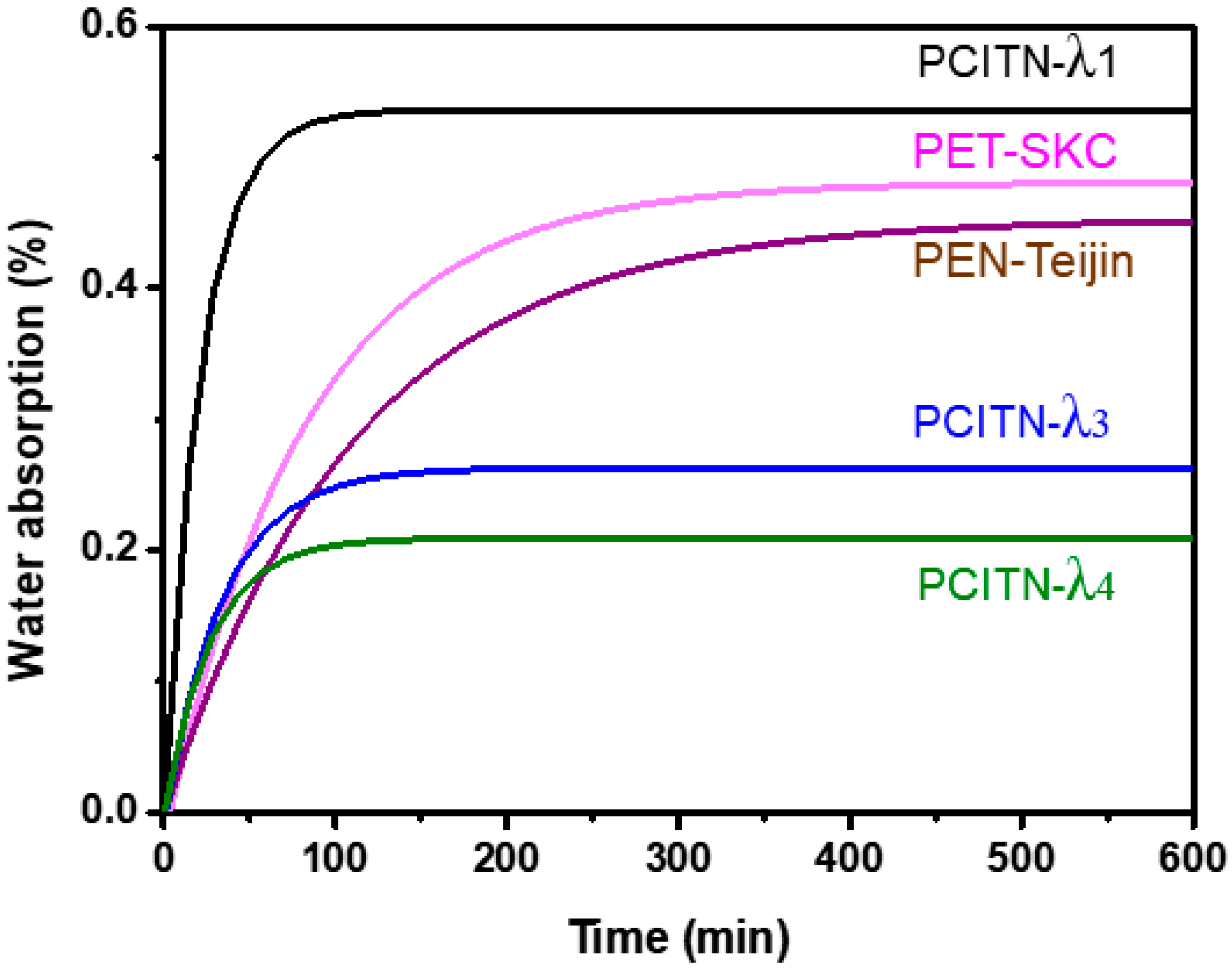
3.7. Water Barrier Property
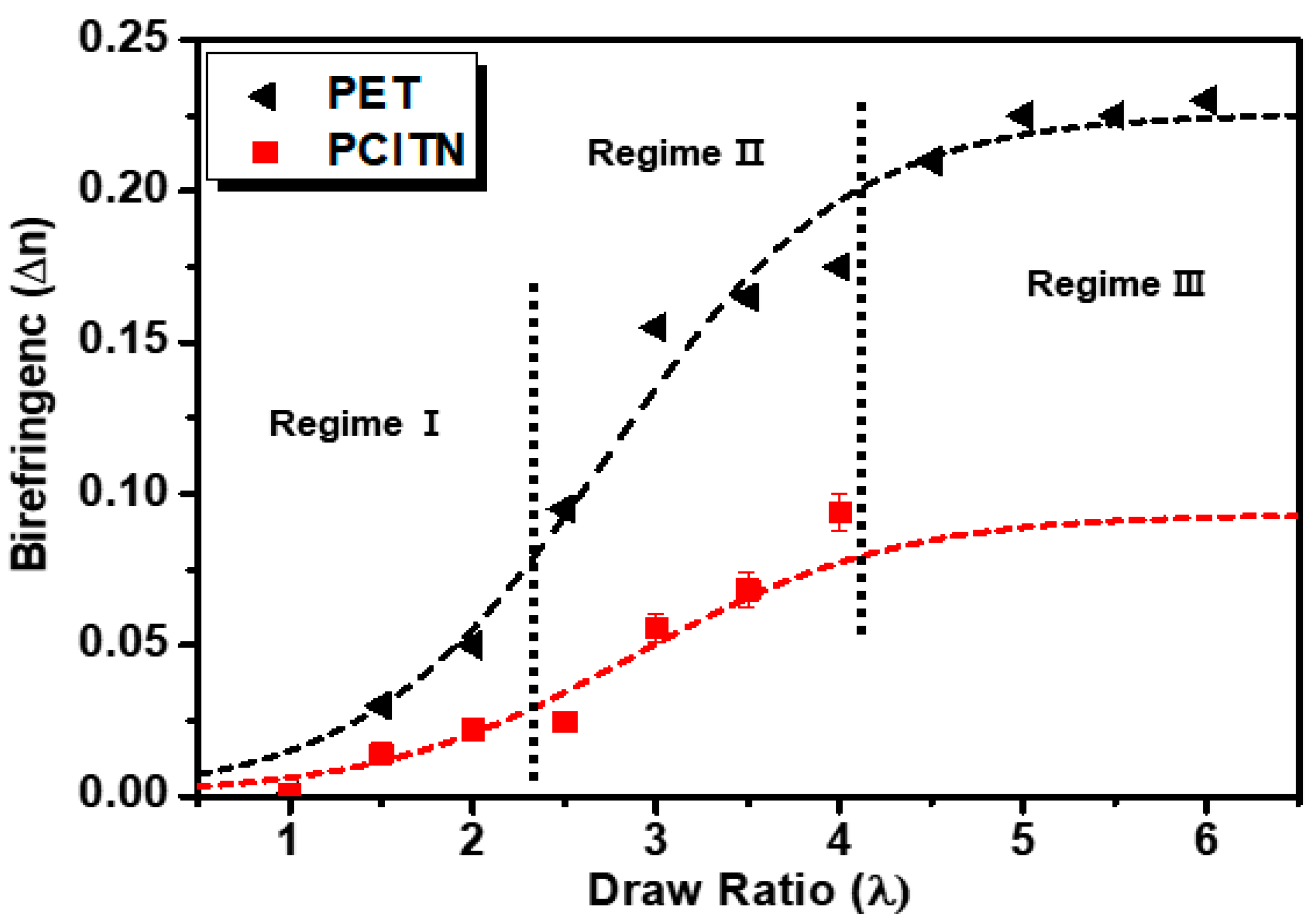
3.8. Birefringence of As-Synthesized and Uniaxially Stretched PCITN Films
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Behera, K.; Chang, Y.H.; Chiu, F.C.; Yang, J.C. Characterization of poly(lactic acid)s with reduced molecular weight fabricated through an autoclave process. Polym. Test. 2017, 60, 132–139. [Google Scholar] [CrossRef]
- Iwata, T. Biodegradable and bio-based polymers: Future prospects of eco-friendly plastics. Angew. Chem. Int. Ed. 2015, 54, 3210–3215. [Google Scholar] [CrossRef] [PubMed]
- Bond, J.Q.; Alonso, D.M.; Wang, D.; West, R.M.; Dumesic, J.A. Integrated Catalytic Conversion of -Valerolactone to Liquid Alkenes for Transportation Fuels. Science 2010, 327, 1110–1114. [Google Scholar] [CrossRef] [PubMed]
- Hussain, F.; Khurshid, M.F.; Masood, R.; Ibrahim, W. Developing antimicrobial calcium alginate fibres from neem and papaya leaves extract. J. Wound Care 2017, 26, 778–783. [Google Scholar] [CrossRef] [PubMed]
- Aricò, F. Isosorbide as biobased platform chemical: Recent advances. Curr. Opin. Green Sustain. Chem. 2020, 21, 82–88. [Google Scholar] [CrossRef]
- Hamad, K.; Kaseem, M.; Ayyoob, M.; Joo, J.; Deri, F. Polylactic acid blends: The future of green, light and tough. Prog. Polym. Sci. 2018, 85, 83–127. [Google Scholar] [CrossRef]
- Girard, M.; Bee, G. Invited review: Tannins as a potential alternative to antibiotics to prevent coliform diarrhea in weaned pigs. Animal 2020, 14, 95–107. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Jia, Z.; Sun, L.; Zhang, Y.; Zhu, J. Modification of poly(ethylene 2,5-furandicarboxylate) (PEF) with 1, 4-cyclohexanedimethanol: Influence of stereochemistry of 1,4-cyclohexylene units. Polymer 2018, 137, 173–185. [Google Scholar] [CrossRef]
- Amulya, K.; Kopperi, H.; Venkata Mohan, S. Tunable production of succinic acid at elevated pressures of CO2 in a high pressure gas fermentation reactor. Bioresour. Technol. 2020, 309, 123327–123335. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Q.; Liu, S.; Sun, T.; Wang, G. Synthesis and properties of poly(isosorbide 2,5-furandicarboxylate-co-ε-caprolactone) copolyesters. Polym. Test. 2020, 81, 106284–106290. [Google Scholar] [CrossRef]
- Musa, C.; Kervoëlen, A.; Danjou, P.E.; Bourmaud, A.; Delattre, F. Bio-based unidirectional composite made of flax fibre and isosorbide-based epoxy resin. Mater. Lett. 2020, 258, 126818–126821. [Google Scholar] [CrossRef]
- Zenner, M.D.; Xia, Y.; Chen, J.S.; Kessler, M.R. Polyurethanes from isosorbide-based diisocyanates. ChemSusChem 2013, 6, 1182–1185. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Lyu, M.Y. Comparison of Rheological Characteristics and Mechanical Properties of Fossil-Based and Bio-Based Polycarbonate. Macromol. Res. 2020, 28, 299–309. [Google Scholar] [CrossRef]
- Eo, Y.S.; Rhee, H.W.; Shin, S. Catalyst screening for the melt polymerization of isosorbide-based polycarbonate. J. Ind. Eng. Chem. 2016, 37, 42–46. [Google Scholar] [CrossRef]
- Behera, K.; Chang, Y.H.; Yadav, M.; Chiu, F.C. Enhanced thermal stability, toughness, and electrical conductivity of carbon nanotube-reinforced biodegradable poly(lactic acid)/poly(ethylene oxide) blend-based nanocomposites. Polymer 2020, 186, 122002–122014. [Google Scholar] [CrossRef]
- Hussain, F.; Jeong, J.; Park, S.; Jeong, E.; Kang, S.-J.; Yoon, K.; Kim, J. Fabrication and characterization of a novel terpolyester film: An alternative substrate polymer for flexible electronic devices. Polymer 2020, 210, 123019–123026. [Google Scholar] [CrossRef]
- Kricheldorf, H.R.; Gomourachvili, Z. Polyanhydrides 10: Aliphatic polyesters and poly(ester-anhydride)s by polycondensation of silylated aliphatic diols. Macromol. Chem. Phys. 1997, 198, 3149–3160. [Google Scholar] [CrossRef]
- Bersot, J.C.; Jacquel, N.; Saint-Loup, R.; Fuertes, P.; Rousseau, A.; Pascault, J.P.; Spitz, R.; Fenouillot, F.; Monteil, V. Efficiency increase of poly(ethylene terephthalate-co-isosorbide terephthalate) synthesis using bimetallic catalytic systems. Macromol. Chem. Phys. 2011, 212, 2114–2120. [Google Scholar] [CrossRef]
- Yoon, W.J.; Hwang, S.Y.; Koo, J.M.; Lee, Y.J.; Lee, S.U.; Im, S.S. Synthesis and characteristics of a biobased high-Tg terpolyester of isosorbide, ethylene glycol, and 1,4-cyclohexane dimethanol: Effect of ethylene glycol as a chain linker on polymerization. Macromolecules 2013, 46, 7219–7231. [Google Scholar] [CrossRef]
- MacDonald, W.A. Latest advances in substrates for flexible electronics. In Large Area and Flexible Electronics; Caironi, M., Noh, Y., Eds.; Wiley: Weinhim, Germany, 2015; pp. 306–307. ISBN 9783527329786. [Google Scholar]
- Lewis, J.S.; Weaver, M.S. Thin-film permeation-barrier technology for flexible organic light-emitting devices. IEEE J. Sel. Top. Quantum Electron. 2004, 10, 45–57. [Google Scholar] [CrossRef]
- Hussain, F.; Park, S.; Jeong, J.; Kang, S.J.; Kim, J. Structure–property relationship of poly(cyclohexane 1,4-dimethylene terephthalate) modified with high trans-1,4-cyclohexanedimethanol and 2,6-naphthalene dicarboxylicacid. J. Appl. Polym. Sci. 2020, 137, 48350–48959. [Google Scholar] [CrossRef]
- Steinborn-Rogulska, I.; Rokicki, G. Solid-state polycondensation (SSP) as a method to obtain high molecular weight polymers: Part II. Synthesis of polylactide and polyglycolide via SSP. Polimery 2013, 58, 85–92. [Google Scholar] [CrossRef]
- Kasmi, N.; Papageorgiou, G.Z.; Achilias, D.S.; Bikiaris, D.N. Solid-State polymerization of poly(Ethylene Furanoate) biobased Polyester, II: An efficient and facile method to synthesize high molecular weight polyester appropriate for food packaging applications. Polymers 2018, 10, 471. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Hussain, F.; Kang, S.; Jeong, J.; Kim, J. Synthesis and properties of copolyesters derived from 1,4-cyclohexanedimethanol, terephthalic acid, and 2,6-naphthalenedicarboxylic acid with enhanced thermal and barrier properties. Polymers 2018, 42, 662–669. [Google Scholar] [CrossRef]
- ASTM (D882-02) Standard Test Method for Tensile Properties of Thin Plastic Sheeting; ASTM International: West Conshohocken, PA, USA, 2002; Volume 14, pp. 1–11.
- ASTM D570-98 Standard Test Method for Water Absorption of Plastics; ASTM International: West Conshohocken, PA, USA, 1998; Volume 16, pp. 1–4.
- Hussain, F.; Park, S.; Jeong, J.; Jeong, E.; Kang, S.; Yoon, K.; Kim, J. Fabrication and characterization of poly(1,4-cyclohexanedimthylene terephthalate-co-1,4-cyclohxylenedimethylene 2,6-naphthalenedicarboxylate) (PCTN) copolyester film: A novel copolyester film with exceptional performances for next generation flexible elec. J. Appl. Polym. Sci. 2020, 138, 49840–49850. [Google Scholar] [CrossRef]
- Hussain, F.; Jeong, J.; Park, S.; Kang, S.-J.; Kim, J. Single-step solution polymerization and thermal properties of copolyesters based on high trans-1,4-cyclohexanedimethanol, terephthaloyl dichloride, and 2,6-naphthalene dicarboxylic chloride. Polymer 2019, 43, 475–484. [Google Scholar] [CrossRef]
- Koo, J.M.; Hwang, S.Y.; Yoon, W.J.; Lee, Y.G.; Kim, S.H.; Im, S.S. Structural and thermal properties of poly(1,4-cyclohexane dimethylene terephthalate) containing isosorbide. Polym. Chem. 2015, 6, 6973–6986. [Google Scholar] [CrossRef]
- Lee, H.; Koo, J.M.; Sohn, D.; Kim, I.S.; Im, S.S. High thermal stability and high tensile strength terpolyester nanofibers containing biobased monomer: Fabrication and characterization. RSC Adv. 2016, 6, 40383–40388. [Google Scholar] [CrossRef]
- Hwang, K.Y.; Kim, S.D.; Kim, Y.W.; Yu, W.R. Mechanical characterization of nanofibers using a nanomanipulator and atomic force microscope cantilever in a scanning electron microscope. Polym. Test. 2010, 29, 375–380. [Google Scholar] [CrossRef]
- Yoon, W.J.; Oh, K.S.; Koo, J.M.; Kim, J.R.; Lee, K.J.; Im, S.S. Advanced polymerization and properties of biobased high Tg polyester of isosorbide and 1,4-cyclohexanedicarboxylic acid through in situ acetylation. Macromolecules 2013, 46, 2930–2940. [Google Scholar] [CrossRef]
- Jeong, Y.G.; Jo, W.H.; Lee, S.C. Synthesis and isodimorphic cocrystallization behavior of poly(1,4-cyclohexylenedimethylene terephthalate-co-1,4-cyclohexylenedimethylene 2,6-naphthalate) copolymers. J. Polym. Sci. Part B 2004, 42, 177–187. [Google Scholar] [CrossRef]
- Jeong, Y.G.; Jo, W.H.; Lee, S.C. Crystal structure determination of poly(1,4-trans-cylcohexylenedimethylene 2,6-naphthalate) by X-ray diffraction and molecular modeling. Macromolecules 2003, 36, 5201–5207. [Google Scholar] [CrossRef]
- Özen, I.; Bozoklu, G.; Dalgiçdir, C.; Yücel, O.; Ünsal, E.; Çakmak, M.; Menceloǧlu, Y.Z. Improvement in gas permeability of biaxially stretched PET films blended with high barrier polymers: The role of chemistry and processing conditions. Eur. Polym. J. 2010, 46, 226–237. [Google Scholar] [CrossRef]
- Ben Doudou, B.; Dargent, E.; Grenet, J. Relationship between draw ratio and strain-induced crystallinity in uniaxially hot-drawn PET-MXD6 films. J. Plast. Film Sheeting 2005, 21, 233–251. [Google Scholar] [CrossRef]
- Ajji, A.; Guèvremont, J.; Cole, K.C.; Dumoulin, M.M. Orientation and structure of drawn poly(ethylene terephthalate). Polymer 1996, 37, 3707–3714. [Google Scholar] [CrossRef]
- Um, I.C.; Ki, C.S.; Kweon, H.Y.; Lee, K.G.; Ihm, D.W.; Park, Y.H. Wet spinning of silk polymer: II. Effect of drawing on the structural characteristics and properties of filament. Int. J. Biol. Macromol. 2004, 34, 107–119. [Google Scholar] [CrossRef]
- MacDonald, B.A.; Rollins, K.; Eveson, R.; Rakos, K.; Rustin, B.A.; Handa, M. New developments in polyester film for flexible electronics. MRS Proc. 2003, 769, 1–8. [Google Scholar] [CrossRef]
- Blumentritt, B.F. Anisotropy and dimensional stability of biaxially oriented poly(ethylene terephthalate) films. J. Appl. Polym. Sci. 1979, 23, 3205–3217. [Google Scholar] [CrossRef]
- Nobukawa, S.; Nakao, A.; Songsurang, K.; Pulkerd, P.; Shimada, H.; Kondo, M.; Yamaguchi, M. Birefringence and strain-induced crystallization of stretched cellulose acetate propionate films. Polymer 2017, 111, 53–60. [Google Scholar] [CrossRef]
- Huijts, R.A.; Peterst, S.M. The relation between molecular orientation and birefringence in PET and PEN fibres. Polymer 1994, 35, 3119–3121. [Google Scholar] [CrossRef]
- MacDonald, W.A. Engineered films for display technologies. J. Mater. Chem. 2004, 14, 4–10. [Google Scholar] [CrossRef]
- MacDonald, W.A.; Looney, M.K.; MacKerron, D.; Eveson, R.; Adam, R.; Hashimoto, K.; Rakos, K. Latest advances in substrates for flexible electronics. J. Soc. Inf. Disp. 2007, 15, 1075–1081. [Google Scholar] [CrossRef]
- Cakmak, M.; Wang, Y.D.; Simhambhatla, M. Processing characteristics, structure development, and properties of uni and biaxially stretched poly(ethylene 2,6 naphthalate) (PEN) films. Polym. Eng. Sci. 1990, 30, 721–733. [Google Scholar] [CrossRef]
- Cheng, I.-C.; Wagner, S. Overview of flexible electronics. In Flexible Electronics: Materials and Applications Electronic Materials: Science and Technology; Wong, W.S., Salleo, A., Eds.; Springer: New York, NY, USA, 2009; pp. 1–28. [Google Scholar]

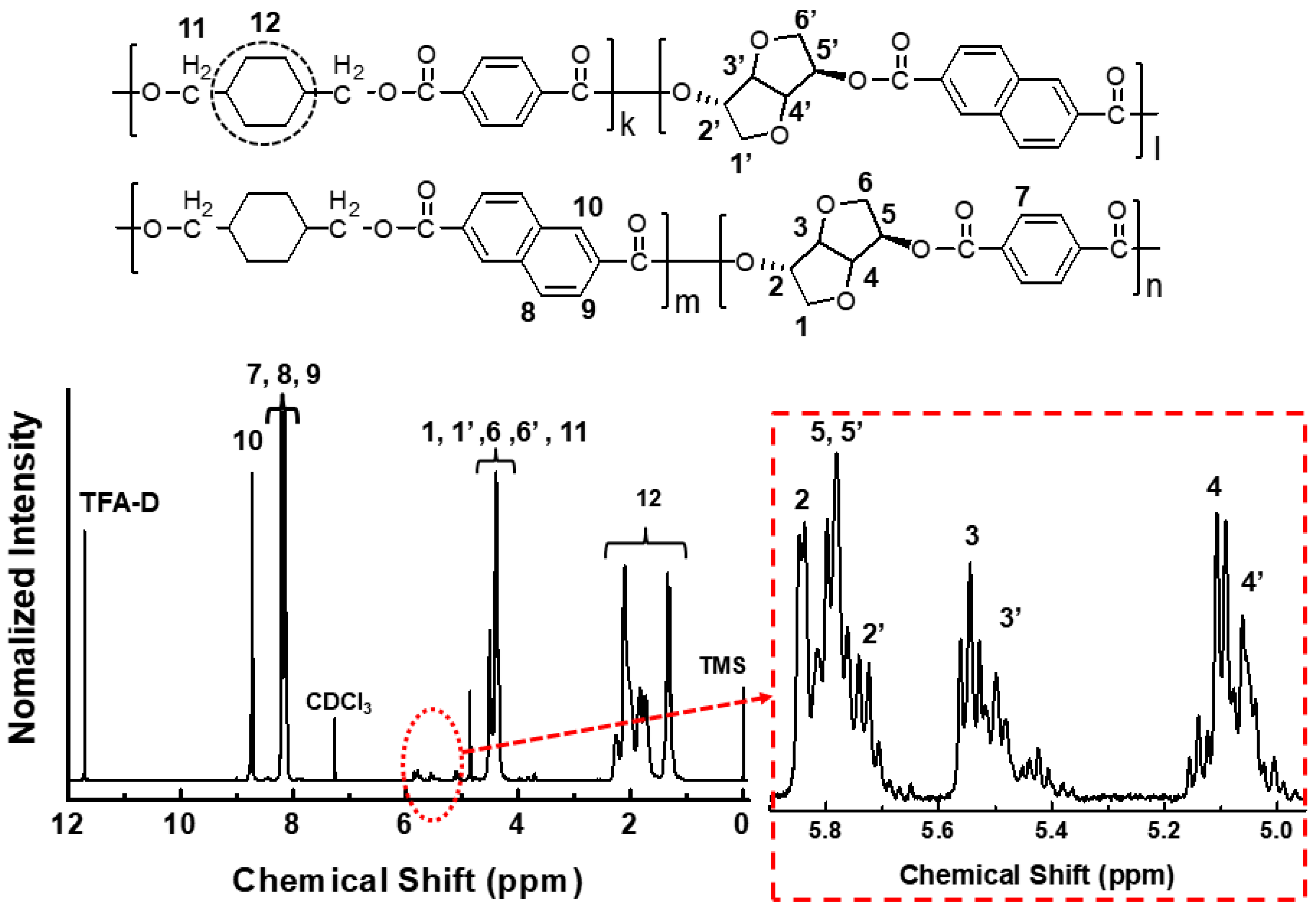
| Tg (°C) a | 123.3 | Mnb | 25,400 |
|---|---|---|---|
| Tm (°C) a | 287.4 | Mwb | 68,900 |
| Tcc (°C) a | 222.9 | PDI (Mn/Mw) b | 2.70 |
| ΔHm (J/g) a | 46.99 | IV c (dL/g) | 0.77 |
| CHDM/ISB Feed Ratio | 75/25 | CHDM/ISB Composition Ratio d | 93.92/6.08 |
| TPA/NDA Feed Ratio | 25/75 | TPA/NDA Composition Ratio d | 30.95/69.05 |
| Draw Ratio | Tg (°C) | Tcc (°C) | ΔHcc (J/g) | Tm (°C) | ΔHm (J/g) | Xc (%) | Tid5% (°C) | Residue at 600 °C (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | 123.9 | 220.5 | 18.20 | 277.5 | 20.49 | 8.65 | 394.7 | 1.9 |
| 2 | 125.1 | 149.2 | 10.61 | 275.1 | 21.86 | 43.43 | 398.3 | 4.3 |
| 3 | 131.9 | - | - | 276.1 | 25.32 | 75.92 | 398.4 | 8.7 |
| 4 | 134.9 | - | - | 275.4 | 26.66 | 97.65 | 396.4 | 13.4 |
| Property | PET (Melinex) | PEN (Teonex) | PI (Kapton) | PCITN (Uniaxially Stretched) |
|---|---|---|---|---|
| Tg (°C) | 78 | 120 | 360 | 140 |
| Tm (°C) | 260 | 268 | - | 275 |
| Commercial Availability | Yes | Yes | Yes | No |
| Transmission (400–700 nm), % | 89 | 87 | Yellow | 86 |
| CTE (−55 to 85 °C) (ppm °C−1) | 15 | 13 | 30–60 | 5.8 |
| Young’s modulus (GPa) | 5.3 | 6.1 | 2.5 | 2.6 |
| Birefringence (△n) (Uniaxially Oriented) | 0.24 | 0.48 | - | 0.09 |
| Water absorption (%) (Randomly Oriented) | 1.08 | 0.98 | - | 0.54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, J.; Hussain, F.; Park, S.; Kang, S.-J.; Kim, J. High Thermal Stability, High Tensile Strength, and Good Water Barrier Property of Terpolyester Containing Biobased Monomer for Next-Generation Smart Film Application: Synthesis and Characterization. Polymers 2020, 12, 2458. https://doi.org/10.3390/polym12112458
Jeong J, Hussain F, Park S, Kang S-J, Kim J. High Thermal Stability, High Tensile Strength, and Good Water Barrier Property of Terpolyester Containing Biobased Monomer for Next-Generation Smart Film Application: Synthesis and Characterization. Polymers. 2020; 12(11):2458. https://doi.org/10.3390/polym12112458
Chicago/Turabian StyleJeong, Jaemin, Fiaz Hussain, Sangwon Park, Soo-Jung Kang, and Jinhwan Kim. 2020. "High Thermal Stability, High Tensile Strength, and Good Water Barrier Property of Terpolyester Containing Biobased Monomer for Next-Generation Smart Film Application: Synthesis and Characterization" Polymers 12, no. 11: 2458. https://doi.org/10.3390/polym12112458
APA StyleJeong, J., Hussain, F., Park, S., Kang, S.-J., & Kim, J. (2020). High Thermal Stability, High Tensile Strength, and Good Water Barrier Property of Terpolyester Containing Biobased Monomer for Next-Generation Smart Film Application: Synthesis and Characterization. Polymers, 12(11), 2458. https://doi.org/10.3390/polym12112458