Abstract
A series of linear amphiphilic pentablock terpolymer PAAx-b-PS48-b-PEO46-b-PS48-b-PAAx (AxS48O46S48Ax) with various lengths x of the PAA block (x = 15, 40, 60, and 90) were synthesized via a two-step atom transfer radical polymerization (ATRP) using Br-poly(ethylene oxide)-Br (Br-PEO46-Br) as the macroinitiator, styrene (St) as the first monomer, and tert-butyl acrylate (tBA) as the second monomer, followed with the hydrolysis of PtBA blocks. The AxS48O46S48Ax pentablock terpolymers formed micelles in dilute aqueous solution, of which the morphologies were dependent on the length x of the PAA block. Cryogenic transmission electron microscopy (cryo-TEM), dynamic light scattering (DLS), and zeta potential measurement were employed to investigate the morphologies, chain structures, size, and size distribution of the obtained micelles. The morphology of AxS48O46S48Ax micelles changed from spherical vesicles with ordered porous membranes to long double nanotubes, then to long nanotubes with inner modulated nanotubes or short nanotubes, and finally, to spherical micelles or large compound vesicles with spherical micelles inside when x increased from 15 to 90. The hydrophobic PS blocks formed the walls of vesicles and nanotubes as well as the core of spherical micelles. The hydrophilic PEO and PAA block chains were located on the surfaces of vesicle membranes, nanotubes, and spherical micelles. The PAA block chains were partially ionized, leading to the negative zeta potential of AxS48O46S48Ax micelles in dilute aqueous solutions.
1. Introduction
Amphiphilic block copolymers, with hydrophilic and hydrophobic segments connected by covalent bonds, can self-assemble into micelles with various morphologies and structures in selective solvents [1,2,3,4]. Because of the unique physical and chemical properties, block copolymer micelles have been used in many technical fields, including drug delivery [5,6], templates [7,8,9], hydrogels [10,11], and membranes [12,13,14]. In past decades, the self-assembly of block copolymers in selective solvents has been extensively studied experimentally [15,16] and theoretically [17,18,19,20]. The morphologies and structures of block copolymer micelles are strongly dependent on the monomer type, composition, and molecular weight of block copolymers as well as the use of selective solvents. Spherical and rod-like micelles, vesicles, large compound micelles, micelles with octopi, jellyfish, and bamboo cage shapes as well as spindle worm-like micelles have been reported for AB diblock copolymer and ABC triblock terpolymers in various selective solvents like aqueous solution, DMF, methanol-water mixture, and THF-water mixture [21,22,23,24,25].
Recently, the self-assembly of linear ABCBA pentablock terpolymers in selective solvents has attracted increasing interest. The ABCBA pentablock terpolymers were thought to serve as the simplest model system of multiblock copolymers, which provide more controllable structure parameters like number, sequence, and composition of the blocks. The investigation of self-assembly multiblock copolymers in solutions will shed light on the understanding of solution properties of biomacromolecules [26,27]. How to systematically manipulate chain architectures, assembly behavior, and microstructures of multiblock copolymers in solutions has been becoming an active area [28,29,30]. For instance, Zhang et al. [28] synthesized poly(2-dimethylaminoethyl methacrylate)-block-poly(2,2,2-trifluoroethylmethacrylate)-block-poly(e-caprolactone)-block-poly(2,2,2-trifluoroethylmethacrylate)-block-poly(2-dimethylaminoethyl methacrylate) (PDMAEMA-b-PTFEMA-b-PCL-b-PTFEMA-b-PDMAEMA) pentablock terpolymers via a two-step atom transfer radical polymerization (ATRP) and obtained spherical micelles in aqueous solution with different pH values. They found that the mean diameter of micelles increased with the decrease in pH value. Mallapragada et al. [31,32] reported the syntheses of a series of pentablock terpolymers by using modified poly(ethylene oxide)100-poly(propylene oxide)65-poly(ethylene oxide)100 (PEO100-PPO65-PEO100 or F127) as the macroinitiator and 2-(diethylamino)ethyl methacrylate (DEAEMA), DMAEMA, 2-diisopropylaminoethyl methacrylate (DiPAEMA), or (tert-butylamino)ethyl methacrylate (tBAEMA) as the monomer and their formation of thermo- and pH-responsive micelles in dilute aqueous solutions. Thunemann et al. [29] reported the formation of two-compartment cylindrical micelles for poly(ethylene oxide)-block-poly(γ-benzyl L-glutamate)-block-poly(perfluoro ether)-block-poly(γ-benzyl L-glutamate)-block-poly(ethylene oxide) (PEO-b-PBLG-b-PFPE-b-PBLG-b-PEO) pentablock terpolymers in aqueous solutions. The formation of thermosensitive spherical micelles of poly(N-isopropylacrylamide)-block-poly(ethylene oxide)-block-poly(propylene oxide)-block-poly(ethylene oxide)-block-poly(N-isopropylacrylamide) (PNIPAM-b-PEO-b-PPO-b-PEO-b-PNIPAM) pentablock terpolymers in aqueous solutions have been reported by Du et al. [33,34] and Parekh et al. [30]. Lv et al. [35] reported that poly(N-isopropylacrylamide)x-b-poly(tert-butyl acrylate)90-b-poly(propylene oxide)36-b-poly(tert-butyl acrylate)90-b-poly(N-isopropylacrylamide)x (PNIPAMx-b-PtBA90-b-PPO36-b-PtBA90-b-PNIPAMx, x = 111, 107, 95, 58, 33, and 26) formed core-corona spherical micelles with a homogeneous hydrophobic core, rod-like micelles, core-shell-corona spherical micelles with clear or blurry boundaries, and large compound spherical micelles with embedded hydrophilic chains in the hydrophobic region, respectively, when the length x of PNIPAM blocks decreased from 111 to 26.
However, up to now, most ABCBA pentablock terpolymer systems reported were mainly designed to be hydrophilic–hydrophobic–hydrophilic or hydrophobic–hydrophilic–hydrophobic, where A and B blocks or B and C blocks were both soluble or insoluble in aqueous solution at room temperature. The study of ABCBA pentablock terpolymers with alternated hydrophilicity, where A and C blocks are water soluble with water-insoluble B block or vice versa, is still rare. Furthermore, the most reported self-assembly of ABCBA pentablock terpolymers mainly focused on the stimuli-responsiveness of micelles in aqueous solution instead of finding new micelle morphologies. Therefore, we design a new ABCBA pentablock terpolymer with hydrophobic PS as B block and hydrophilic PAA and PEO as A and C blocks in the present work. Four linear ABCBA-type amphiphilic pentablock terpolymers, poly(acrylic acid)x-b-poly(styrene)48-b-poly(ethylene oxide)46-b-poly(styrene)48-b-poly(acrylic acid)x (PAAx-b-PS48-b-PEO46-b-PS48-b-PAAx or AxS48O46S48Ax) with various lengths x of PAA block (x = 15, 40, 60, and 90) were synthesized by a two-step ATRP process followed with hydrolysis treatment. AxS48O46S48Ax formed micelles in dilute aqueous solutions, which were investigated by cryogenic transmission electron microscopy (cryo-TEM), dynamic light scattering (DLS), and Zeta potential measurement. Interestingly, new micelle morphologies, namely long double nanotubes with different inner nanotube structures, were observed for A40S48O46S48A40 and A60S48O46S48A60. The effects of PAA block length x on the morphologies and chain structures of AxS48O46S48Ax micelles in dilute aqueous solutions were discussed.
2. Materials and Methods
2.1. Materials
Tert-Butyl acrylate (tBA, 99%), 2-bromoisobutyryl bromide (BIBB, 98%), N,N,N′,N″,N″-pentamethyldiethylenetriamine (PMDETA, 98%), copper(I) bromide (CuBr, 98%), trifluoroacetic acid (TFA, 99.5%), 4-dimethylaminopyridine (DMAP, 98%), and triethylamine (TEA, ultra-dry) were purchased from J&K Chemical Ltd., Shanghai, China. Styrene (St, 99%) and poly(ethylene oxide) (HO-PEO46-OH, Mn = 2050, Mw/Mn = 1.06) were purchased from Sinopharm Chemical Reagent Co., Ltd., Shanghai, China, and Sigma-Aldrich Chemical Ltd., St. Louis, MO, USA, respectively. St and tBA were purified by passing through alkaline alumina column (200–300 mesh), respectively. CuBr was purified by sequential washing with glacial acetic acid, absolute ethanol, and anhydrous ether, followed by drying in a vacuum overnight. The purified CuBr was sealed in a brown bottle for further usage. Other chemicals and solvents with analytical grade were used as received.
2.2. Synthesis of Br-PEO46-Br Macroinitiator
Macroinitiator, Br-PEO46-Br, was synthesized by reacting HO-PEO46-OH and BIBB, with DMAP and TEA as catalysts in anhydrous dichloromethane (DCM). Briefly, 20.5 g HO-PEO46-OH, 0.2 g DMAP, and 8.5 mL TEA were completely dissolved in 100 mL DCM under nitrogen atmosphere in an ice/water bath. Then, 10 mL BIBB DCM solutions (4 mol/L) were dropwise added into the mixed solution. After 2 h, the ice/water bath was taken away and the reaction continued for 24 h at room temperature. Afterward, the mixture was filtered and washed with saturated NaHCO3 solution and deionized water. The mixture was further dried with anhydrous Na2SO4 powders overnight. The Na2SO4 powders were filtered and the residue dark-brown solution was concentrated and then, precipitated in cold n-hexane. The obtained light-yellow powders were dried at 40 °C under vacuum overnight to give the macroinitiator, Br-PEO46-Br. A 1H NMR spectrum of Br-PEO46-Br macroinitiator indicated the bromination extent of PEO46 was 100%, as shown in Figure S1.
2.3. Synthesis of PtBAx-PS48-PEO46-PS48-PtBAx Pentablock Terpolymers
The pentablock terpolymers PtBAx-PS48-PEO46-PS48-PtBAx with various PtBA block lengths x and fixed PS block length of 48 were synthesized via a two-step ATRP process using Br-PEO46-Br as the macroinitiator, St as the first monomer, and tBA as the second monomer. Firstly, 0.43 mmol Br-PEO46-Br, 262 mmol St, and 3.4 mmol PMDETA were completely mixed in a 50 mL Schlenk tube under nitrogen atmosphere. The mixture was degassed via freezing–pumping–thawing process three times. Then, 1.7 mmol CuBr was added and the Schlenk tube was sealed and then immersed into an oil bath with reset temperature of 90 °C. After 1 h, the reaction was quenched by putting the Schlenk tube in liquid nitrogen. The reaction mixture was passed through a neutral alumina column (100–200 mesh) by using DCM as the eluent. The purified mixture was concentrated and then precipitated in cold n-hexane. The resultant white precipitates were dried overnight at 40 °C under vacuum to give Br-PS48-PEO46-PS48-Br triblock copolymer.
The obtained Br-PS48-PEO46-PS48-Br triblock copolymer was used as the macroinitiator to further polymerize the second monomer, tBA, via a similar ATRP procedure. Note that the ATRP of tBA was carried out at 75 °C for 1 h. Four pentablock terpolymers, namely PtBA15-PS48-PEO46-PS48-PtBA15, PtBA40-PS48-PEO46-PS48-PtBA40, PtBA60-PS48-PEO46-PS48-PtBA60, and PtBA90-PS48-PEO46-PS48-PtBA90, were synthesized.
2.4. Preparation of PAAx-PS48-PEO46-PS48-PAAx Pentablock Terpolymers
The PAAx-PS48-PEO46-PS48-PAAx pentablock terpolymers were prepared from the corresponding PtBAx-PS48-PEO46-PS48-PtBAx pentablock terpolymers via the hydrolysis of PtBA blocks. Briefly, given amounts of PtBAx-PS48-PEO46-PS48-PtBAx and TFA were dissolved in 2 mL DCM and stirred at room temperature for 24 h. The PtBA blocks were completely hydrolyzed to give PAA blocks. The hydrolysis products were precipitated in cold n-hexane and the precipitates were dried under vacuum overnight to give PAAx-PS48-PEO46-PS48-PAAx pentablock terpolymers.
The PtBAx-PS48-PEO46-PS48-PtBAx and PAAx-PS48-PEO46-PS48-PAAx pentablock terpolymers were then coded as TxS48O46S48Tx and AxS48O46S48Ax, respectively. The capital letters O, S, A, and T were used to represent the PEO, PS, PAA, and PtBA blocks, respectively.
2.5. Preparation of AxS48O46S48Ax Micelles in Dilute Aqueous Solutions
Certain amounts of AxS48O46S48Ax pentablock terpolymers were completely dissolved in tetrahydrofuran (THF) to give a concentration of 1 mg/mL. Deionized water was then dropped into the AxS48O46S48Ax THF solutions at a constant rate (ca. 1 drop per 10 s) under vigorous stirring until the water content reached 35 wt%. Afterward, the mixing solutions were transferred into a dialysis tube (molecular weight cutoff of 3500), which was then dialyzed against deionized water for 3 days. The final micelle solutions were transferred into a clean volumetric glass flask and diluted with deionized water to give a final concentration of 0.3 mg/mL. The pH values of micelle aqueous solutions were measured to be 5.23, 5.25, 5.20, and 5.12 for A15S48O46S48A15, A40S48O46S48A40, A60S48O46S48A60, and A90S48O46S48A90, respectively, by a pH meter (FE28, METTLER TOLEDO, Zurich, Switzerland).
2.6. Measurements
Gel permeation chromatograph (GPC, Waters 1515 system, Waters Corp., Milford, MA, USA) was used to measure the dispersities (Mw/Mns) of TxS48O46S48Tx pentablock terpolymers with polystyrene as the calibration standard and THF as the eluent (1.0 mL/min) at 40 °C. Number-average molecular weights (Mns) of TxS48O46S48Tx and AxS48O46S48Ax were determined from their 1H NMR spectra, which were recorded on a Bruker DMX-400 MHz instrument (Bruker Corp., Karlsruhe, Germany).
Dynamic light scattering (DLS) measurements of AxS48O46S48Ax micelle solutions were performed on a Brookhaven BI-200SM Instrument (Brookhaven Instruments Corp., Holtsville, NY, USA). with a laser wavelength of 657 nm at 25 °C. The scattering angle was fixed at 90°. The Zeta potentials ξ of AxS48O46S48Ax micelles were also measured by electrophoretic light scattering (ELS) using the same BI-200SM Instrument.
The morphologies of AxS48O46S48Ax micelles were observed by cryogenic transmission electron microscopy (cryo-TEM) on a Talos F200C cryogenic electron microscopy (FEI Company, Hillsboro, Oregon) at an acceleration voltage of 200 kV. The micelle solutions were dropped onto bare copper grids, which were immediately submerged into liquid nitrogen for flash freezing. The cryo-grids were then mounted on a cryogenic sample holder. Images were recorded on a Ceta 4 K × 4 K camera with a resolution ratio ≤0.3 nm.
3. Results and Discussion
3.1. Synthesis and Characterization of AxS48O46S48Ax Pentablock Terpolymers
AxS48O46S48Ax pentablock terpolymers with different lengths x of PAA blocks were synthesized via a two-step ATRP process with Br-PEO46-Br as the macroinitiator, St as the first monomer, and tBA as the second monomer, followed with the hydrolysis of PtBA blocks, as shown in Scheme 1. Four AxS48O46S48Ax pentablock terpolymers with x = 15, 40, 60, and 90 were obtained in the present work. Figure 1 shows the 1H NMR spectra of AxS48O46S48Ax and corresponding parent TxS48O46S48Tx pentablock terpolymers. The characteristic peak of –C(CH3)3 with chemical shift of ca. 1.35 ppm, which can be clearly observed for TxS48O46S48Tx (Figure 1a), completely disappeared for AxS48O46S48Ax (Figure 1b), indicating that the PtBA blocks were completely hydrolyzed and turned into PAA blocks. The degrees of polymerization of S (48) and T (x) blocks were determined from the 1H NMR spectra using the known value of the O block (46). Figure 2 shows the GPC traces of four TxS48O46S48Tx pentablock polymers, which exhibited narrow dispersities (Mw/Mns < 1.20). Note that it was difficult to determine the Mw/Mns of AxS48O46S48Ax using GPC because of the strong polarity of the PAA block, which would be strongly adsorbed onto the GPC column during the GPC measurement, leading to the wrong results of molecular weight and dispersity. The Mw/Mns of TxS48O46S48Tx were used to present the Mw/Mns of AxS48O46S48Ax, which were expected to be in the same range as that of the corresponding TxS48O46S48Tx, as discussed in our previous work [36]. Table 1 summarizes the number-average molecular weight (Mn) and dispersity (Mw/Mn) of AxS48O46S48Ax pentablock terpolymers studied in the present work.
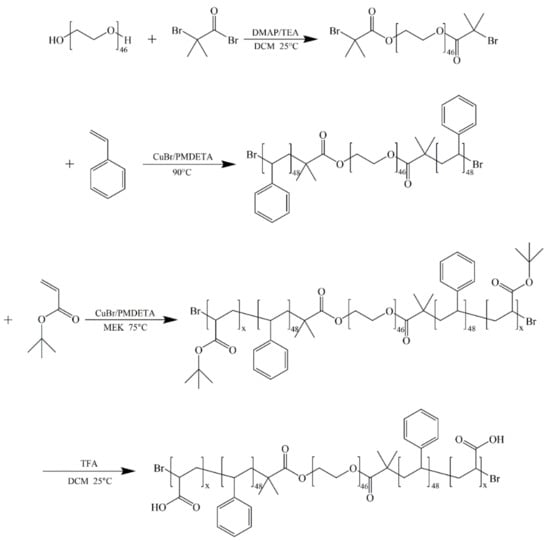
Scheme 1.
Synthesis route of AxS48O46S48Ax pentablock terpolymers.
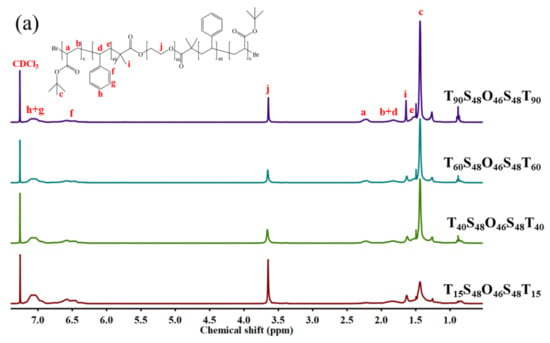
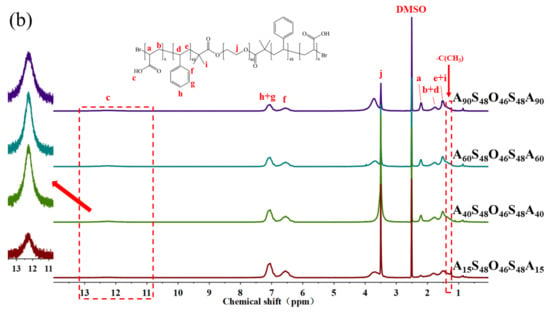
Figure 1.
1H NMR spectra of (a) TxS48O46S48Tx (x = 15, 40, 60, 90) in CDCl3 and (b) AxS48O46S48Ax (x = 15, 40, 60, 90) in DMSO-d6.
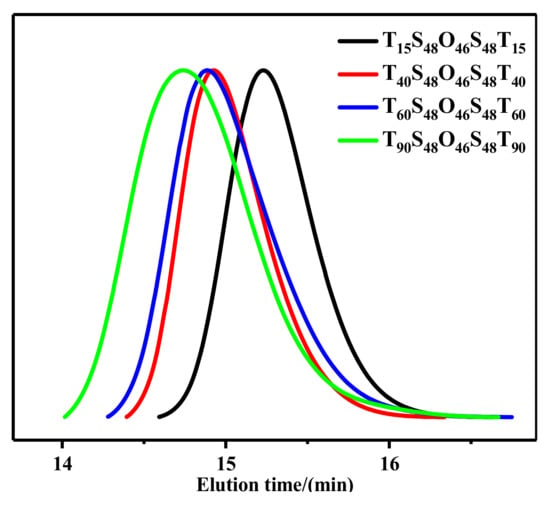
Figure 2.
GPC traces of TxS48O46S48Tx (x = 15, 40, 60, 90) pentablock terpolymers.

Table 1.
Number-average molecular weight, dispersity, volume fraction, and micelle morphology of AxS48O46S48Ax pentablock terpolymers.
3.2. Morphologies and Structures of AxS48O46S48Ax Micelles in Dilute Aqueous Solutions
Because of the amphiphilic nature of AxS48O46S48Ax pentablock terpolymers, they formed micelles in dilute aqueous solutions. Note that the PS block is hydrophobic and the PEO and PAA blocks are hydrophilic. Figure 3 shows the representative cryo-TEM images of A15S48O46S48A15, A40S48O46S48A40, A60S48O46S48A60, and A90S48O46S48A90 micelles in dilute aqueous solutions. Additional cryo-TEM images were also given in Figure S2. The observed morphologies were well reproducible. It can be seen that A15S48O46S48A15 pentablock terpolymers formed spherical vesicles with ordered porous membranes in aqueous solutions, which were rarely reported for block copolymer micelles (Figure 3A,B and Figure S2A). The preparation of porous vesicles usually required sophisticated design of polymer chains or a complicated preparation process. Kim et al. [37] reported the fabrication of porous supramolecular microcapsules by using specially designed dumbbell-shaped rod amphiphiles, which consisted of a phenylacetylene segment in the middle with hydrophilic polyether dendrons and hydrophobic alkane branches on each side. Xu et al. [38] reported the fabrication of polystyrene-b-poly(4-vinylpyridine) (PS-b-P4VP) block copolymer vesicles with porous membranes by using an emulsion solvent evaporation method with a high-boiling non-solvent droplet as the liquid core template. By varying the block length and ratio of PS and P4VP blocks as well as the additives, the spherical, toroid, and onion mesopores in the membranes were obtained for the PS-b-P4VP vesicles. Wong et al. [39] prepared the vesicles with inverse hexagonal pores in the membranes with poly(glycosyloxyethyl methacrylate)-b-poly(benzyl acrylate)-b-poly(4-vinylpyridine) triblock terpolymer (PGlcEMA-b-PBzA-b-P4VP) by liquid–liquid phase separation and polymer microphase separation processes. First, PGlcEMA-b-PBzA-b-P4VP was dissolved in DMF/MeOH mixed solutions and formed polymer/DMF-rich droplets dispersed in MeOH mixture, where DMF was the good solvent and MeOH was the poor solvent for all three blocks. During the dialysis against MeOH, the PGlcEMA-b-PBzA-b-P4VP chains migrated to the interface and formed the membranes to stabilize the droplets, and ultimately, underwent phase separation into a three-layer inverse hexagonal phase when DMF content continuously decreased. Herein, vesicles with ordered porous vesicles were obtained by directly dialyzing A15S48O46S48A15 THF solution against deionized water. From Figure 3A, partial collapse of membranes was clearly observed, which was a typical phenomenon for vesicles and resulted from the pressure difference between the interior and exterior of the vesicles during sample preparation for TEM observation [1]. The diameters of A15S48O46S48A15 vesicles were about 200–300 nm and the diameters of pores in the vesicle membranes were about 7 nm. The hydrophobic PS blocks formed the walls of vesicles and the hydrophilic PEO and PAA blocks on the membrane surfaces stabilized the vesicles, as illustrated in Scheme 2. Increasing the length x of PAA blocks from 15 to 40, the long double nanotubes were mostly observed for A40S48O46S48A40 aqueous solutions (Figure 3C,D and Figure S2B). Interestingly, the inner nanotubes were almost exactly located in the middle of outer nanotubes. The diameter of the outer nanotube was about 95 nm and the diameter of the inner nanotube was about 34 nm. The wall thicknesses of the outer and inner nanotubes were almost the same, i.e., about 7 ± 0.8 nm. The gap between the outer nanotubes and inner nanotubes was about 24 nm. The walls of the nanotubes were composed of PS blocks, which appeared dark in cryo-TEM images. Similarly, the hydrophilic PEO and PAA blocks on the surface of nanotubes stabilized the long double nanotubes in aqueous solutions. Because of the low molecular weight (5000 g/mol), the PEO block cannot be observed in cryo-TEM images [40]. The PAA chains were also invisible as they were highly swollen in aqueous solution [41] and also, too short to be identified. Such double nanotubes were, to our best knowledge, the first reported for block copolymer micelles in aqueous solutions. Besides the long double nanotubes, a few spherical vesicles were also observed for A40S48O46S48A40 (Figure 3D). When the length x of the PAA block increased to 60, the long nanotubes were observed for A60S48O46S48A60 aqueous solutions (Figure 3E,F and Figure S2C,D). However, these long nanotubes were different with those observed for A40S48O46S48A40. The diameter of the outer nanotube increased to 110~130 nm and the inner nanotubes ruptured into many short nanotubes. Modulation of inner short nanotubes was also observed (Figure 3F). The wall thicknesses of outer long nanotubes and inner short nanotubes or modulated nanotubes were the same as those of A40S48O46S48A40 nanotubes, i.e., about 7 nm. Expansion and distortion of the nanotubes were also observed, as shown in Figure S2C,D. Possibly, the increased length of the PAA block resulted in stronger repulsion interactions between the outer and inner nanotubes, causing the rupture of the inner nanotubes. The extended chain lengths (RE) of PEO and PAA blocks can be estimated by using Equation (1), given as [35]:
where n is the degree of polymerization of block chain, l is the length of covalent bond, and θ is the angle of bonds. The lengths l of C–C and C–O bonds are 0.154 and 0.143 nm, respectively. The angle θ of C–C–C and C–O–C are 109.5° and 108°, respectively. RE of the PEO block was then calculated to be about 16 nm, which was the same for A40S48O46S48A40 and A60S48O46S48A60. As a result, the longest length of the hydrophilic PEO block on the surface of nanotubes was about 8 nm because the PEO block was located in the middle of A40S48O46S48A40 and A60S48O46S48A60 pentablock terpolymers and had to fold during the formation of nanotube micelles. For A40S48O46S48A40, RE of each PAA block was about 10 nm. From Figure 3C,D, the gap between the outer nanotubes and inner nanotubes was about 24 nm, which was larger than twice of RE of the PAA or PEO block. In other words, the hydrophilic PEO and PAA block chains on the surfaces of outer and inner nanotubes did not touch each other for the long double nanotubes of A40S48O46S48A40. However, RE of the PAA block increased to about 16 nm for A60S48O46S48A60. The gap of 24 nm between the outer and inner nanotubes was less than twice of RE of the PAA block, i.e., about 32 nm, which meant that the hydrophilic PAA block chains on the surface of outer and inner nanotubes would touch each other. As a result, the size of outer nanotubes increased, and the inner nanotubes ruptured to form short tubes in order to avoid such touch and release the energy penalty for A60S48O46S48A60. Further increasing the length of the PAA block to x = 90, spherical micelles and some large compound vesicles with spherical micelles inside were observed for A90S48O46S48A90 aqueous solutions, as shown in Figure 3G,H. The diameters of the spherical micelles were in the range of 100–200 nm. The corona chains of spherical micelles can be clearly observed from the magnified cryo-TEM image shown in Figure S2E and the inset of Figure 3G. The length of corona chain was measured to be about 20 nm, which was slightly smaller than the extended chain length RE of the PAA block, i.e., about 23 nm. The sizes of large compound vesicles were about 700–800 nm and the sizes of spherical micelles inside the vesicles were about 100–200 nm. More large compound vesicles were shown in Figure S2F. Probably, these large compound vesicles were the intermediate between the long nanotubes observed for A60S48O46S48A60 and the spherical micelles shown in Figure 3G. The rupture of the large compound vesicles would release the inside spherical micelles.
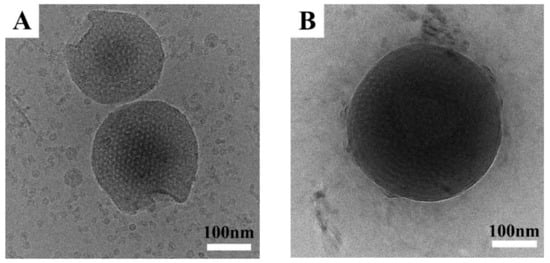
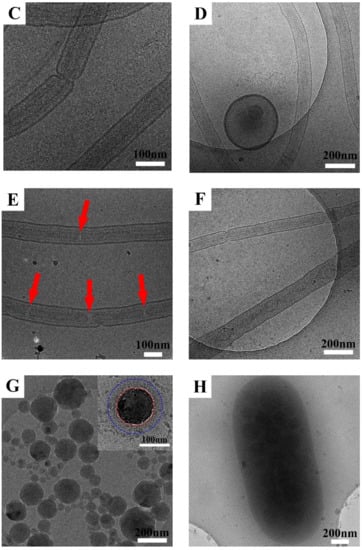
Figure 3.
Cryo-TEM images of (A) and (B) A15S48O46S48A15, (C) and (D) A40S48O46S48A40, (E) and (F) A60S48O46S48A60, (G) and (H) A90S48O46S48A90 micelles in dilute aqueous solutions. The red arrows in Figure 3E point out the fractures of inner nanotubes.
Scheme 2.
The schematic morphologies and possible chain structures of the observed AxS48O46S48Ax micelles in dilute aqueous solutions. (a) x = 15, (b) x = 40, (c) x = 60, (d) x = 90.
3.3. Dynamic Light Scattering Measurements of AxS48O46S48Ax Micelles in Dilute Aqueous Solutions
The self-assembly behavior of AxS48O46S48Ax micelles in dilute aqueous solutions was investigated by dynamic light scattering (DLS). Figure 4 shows the normalized electric field autocorrelation functions g(1)(t) of AxS48O46S48Ax micelles in dilute aqueous solution at 25 °C and scattering angle θ of 90°. These decay curves of g(1)(t) can be described by using a single exponential decay function plus a stretched exponential function given as [42,43]:
where the pre-factors Afast and Aslow are the amplitudes of the fast and slow relaxation modes, respectively, and the sum of Afast and Aslow is close to 1. τfast and τslow represent the relaxation times of the fast and slow modes, respectively. The reciprocal of τ is the relaxation rate Γ (Γ = 1/τ). The stretched exponent, γ, (0 < γ ≤ 1) is inversely proportional to the width of the distribution of the relaxation time. A larger value of γ implies a narrower distribution of the relaxation time. Equation (2) has been widely used to describe micelles with anisotropic shapes in solutions. Fast and slow relaxation modes have been applied to distinguish the difference of motion modes for the solutions containing objects with anisotropic shapes or with diverse sizes and morphologies [42,44,45]. The fitting results were summarized in Table 2.
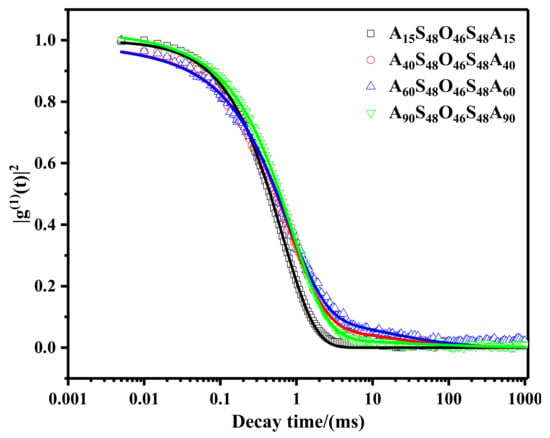
Figure 4.
Normalized electric field autocorrelation function g(1)(t) of AxS48O46S48Ax micelles (x = 15, 40, 60, 90) in dilute aqueous solutions measured by DLS at 25 °C and scattering angle θ of 90°. The solid lines well fit with Equation (2) with R2 ≥ 0.999.

Table 2.
The fitting parameters of Equation (2) for g(1)(t) of AxS48O46S48Ax micelles in dilute aqueous solutions at 25 °C and scattering angle of 90°.
It can be seen that g(1)(t) of A15S48O46S48A15 micelles in dilute aqueous solution can be well described by a single exponential decay function, which indicated that the A15S48O46S48A15 micelles were spherical in shape with relatively narrow size distribution. This result was consistent with the observation of cryo-TEM for A15S48O46S48A15 micelles, as shown in Figure 3A. The hydrodynamic radius (Rh) of A15S48O46S48A15 micelles can be estimated from τfast value by using the Stokes–Einstein equation:
where kB is the Boltzmann constant (1.38 × 10−23 J/K), T is the Kelvin temperature, η is the viscosity of water at 25 °C, i.e., 0.89 × 10−3 N·s·m−2, and D = 1/(q2 × τfast) is the mutual diffusion coefficient of the micelles in solution with scattering vector q. The scattering vector q can be calculated by , where n is the refractive index of the solution, λ is the wavelength of laser light in vacuum, and θ is the scattering angle. The value of Rh was calculated to be about 110 nm, which was in good agreement with those obtained from cryo-TEM images, i.e., 100–150 nm. For A40S48O46S48A40 and A60S48O46S48A60 micelles in dilute aqueous solutions, g(1)(t)s were well fitted by Equation (2) with stretched exponential function, indicating the existence of anisotropic micelles in solutions. The fitting values of Afast, Aslow, and γ were similar for A40S48O46S48A40 and A60S48O46S48A60 micelles, suggesting that the micelle morphologies and size distributions of the two samples were similar. The slight increase in Afast and τfast of A40S48O46S48A40 micelles might be attributed to the existence of small spherical vesicles in the solutions as showed in Figure 3D, which were not observed for A60S48O46S48A60 micelle solutions. Furthermore, τslow of A60S48O46S48A60 micelles was slightly larger than that of A40S48O46S48A40 micelles, which might be attributed to the larger diameter of the long outer nanotubes and the modulation of nanotubes, as shown in Figure 3E,F and Figure S2C,D. For A90S48O46S48A90 micelle solutions, the hydrodynamic radius estimated from τfast was about 125 nm, which was slightly larger than the size of spherical micelles but much smaller than the size of large compound vesicles obtained by cryo-TEM. The slow mode presented by τslow could be attributed to the large compound vesicles with spherical micelles inside. Afast was much larger than Aslow, suggesting that the spherical micelles were the majority in A90S48O46S48A90 micelle solutions. Furthermore, the small value of γ suggested a board size distribution of micelles, which was consistent with the cryo-TEM observation shown in Figure 3G,H.
3.4. Possible Chain Structure of AxS48O46S48Ax Micelles in Dilute Aqueous Solutions
For AxS48O46S48Ax micelles in dilute aqueous solutions, spherical vesicles with ordered porous membranes, a long double nanotube, a long nanotube with an inner modulated nanotube or short nanotube, and spherical micelles or large compound vesicles with spherical micelles inside were observed for A15S48O46S48A15, A40S48O46S48A40, A60S48O46S48A60, and A90S48O46S48A90 in dilute aqueous solutions, respectively, with increasing the length x of the PAA block. Scheme 2 shows the schematic morphologies and possible chain structures of the observed AxS48O46S48Ax micelles. The hydrophobic PS blocks formed the walls of vesicles and nanotubes as well as the core of spherical micelles. The hydrophilic PEO and PAA block chains were on the surfaces of vesicle membranes, nanotubes, and spherical micelles, stabilizing the micelles in aqueous solutions. The middle PEO block chain also folded because of the chain connection restriction. PAA is a weak polyanion with pKa of ~5 and the ionization degree of the PAA chain is strongly dependent on the pH value of solutions [46,47]. In deionized water, the PAA chains are partly ionized. Figure 5 shows the zeta potential ξ of AxS48O46S48Ax micelles in dilute aqueous solutions. The negative value of ξ indicated that the PAA block chains were on the surfaces of the micelles and ionized in aqueous solutions. The value of ξ for block polymer micelles in dilute aqueous solutions was affected by the pH of solutions [48], the nanostructure of micelles, and the density of charged block chains on the outer surfaces of micelles [49]. Therefore, the values of ξ of AxS48O46S48Ax micelles in dilute aqueous solutions were related with the morphologies and chain structures of the micelles. It can be seen from Figure 5 that the absolute value of ξ first decreased from about 43.7 to 9.8 mV when increasing PAA block length x from 15 to 60 and then, increased again to 22.5 mV for x of 90. ξ of −43.7 mV revealed large amounts of ionized PAA chains located on the outer surfaces of A15S48O46S48A15 vesicles. With increasing x to 40 and 60, the long double nanotubes were formed for A40S48O46S48A40 and A60S48O46S48A60, causing the reduction in specific surface area and the decrease in ionized PAA chains on the outer surfaces of the outer nanotubes. Furthermore, the ionized PAA chains on the surface of inner nanotubes did not contribute to the value of ξ. For A40S48O46S48A40 micelles, the remaining vesicles besides nanotubes result in a larger variation of the Zeta potential. On the other hand, for A60S48O46S48A60 micelles, the deformations, fractures, and knots of nanotubes make the error bar of ξ even broader. For A90S48O46S48A90 micelles with x of 90, ξ increased again to about 22.5 mV. The formation of spherical micelles increased the corona surface area where the ionized PAA chains were located. However, ξ of A90S48O46S48A90 micelles was still smaller than that of A15S48O46S48A15 vesicles, which might be attributed to the formation of large compound vesicles with spherical micelles inside. Similarly, the spherical micelles inside the vesicles did not contribute to the value of ξ. Furthermore, the coexistence of different micelle structures and containing micelles in micelles might result in a large error bar when determining ξ for A40S48O46S48A40, A60S48O46S48A60, and A90S48O46S48A90 micelles in aqueous solutions.
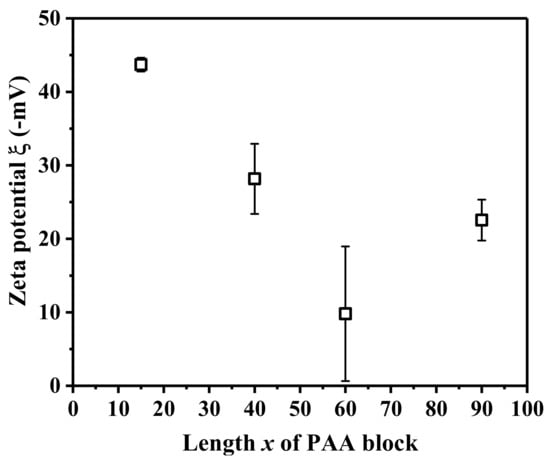
Figure 5.
Zeta potential ξ of AxS48O46S48Ax with various length x of PAA block (x = 15, 40, 60, 90) micelles in aqueous solutions at 25 °C.
4. Conclusions
In summary, four AxS48O46S48Ax pentablock terpolymers with different lengths x of PAA block (x = 15, 40, 60, and 90) were synthesized via a two-step ATRP process with Br-PEO46-Br as the macroinitiator, St as the first monomer, and tBA as the second monomer, followed with the hydrolysis of PtBA blocks. The AxS48O46S48Ax pentablock terpolymers formed micelles with various morphologies in dilute aqueous solutions depending on the length x of the PAA block. The morphology of AxS48O46S48Ax micelles changes from spherical vesicles with ordered porous membranes to long double nanotubes, then to long nanotubes with inner modulated nanotubes or short nanotubes, and finally, to spherical micelles or large compound vesicles with spherical micelles inside with increasing the length x of the PAA block from 15 to 90. The hydrophobic PS blocks formed the walls of vesicles and nanotubes as well as the core of spherical micelles. The hydrophilic PEO and partially ionized the PAA block chains located on the surfaces of vesicle membranes, nanotubes, and spherical micelles, leading to the negative zeta potential of the AxS48O46S48Ax micelles and stabilizing the micelles in dilute aqueous solutions. The porous vesicles and double layer nanotubes might have potential applications as drug delivery and material transporting media.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4360/12/10/2183/s1. Figure S1. 1H NMR spectrum of Br-PEO46-Br macroinitiator. Figure S2. Additional cryo-TEM images of (A) A15S48O46S48A15, (B) A40S48O46S48A40, (C and D) A60S48O46S48A60, (E and F) A90S48O46S48A90 micelles in dilute aqueous solutions.
Author Contributions
Conceptualization, J.G. and B.D.; Methodology, J.G., K.A., C.L. and B.D.; Data curation, J.G. and B.D.; Project administration, B.D.; Writing—original draft, J.G. and B.D.; Writing—review and editing, J.N., J.X. and B.D. All authors have read and agreed to the published version of the manuscript.
Funding
The authors thank the National Natural Science Foundation of China (Nos. 21674097, 21875214 and 21774111), and the second level of 2016 Zhejiang Province 151 Talent Project for financial support.
Acknowledgments
Thanks to Lingyun Wu in the Center of Cryo-Electron Microscopy (CCEM), Zhejiang University for her technical assistance with Cryo-TEM measurements.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mai, Y.; Eisenberg, A. Self-Assembly of Block Copolymers. Chem. Soc. Rev. 2012, 41, 5969–5985. [Google Scholar] [CrossRef] [PubMed]
- Alexandridis, P.; Lindman, B. Amphiphilic Block Copolymers: Self-Assembly and Applications, 1st ed.; Elsevier: Amsterdam, TheNetherlands, 2000. [Google Scholar]
- Service, R.F. How Far Can We Push Chemical Self-Assembly? Science 2005, 309, 95. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Hernandez, J.; Chécot, F.; Gnanou, Y.; Lecommandoux, S. Toward “Smart” Nano-Objects by Self-Assembly of Block Copolymers in Solution. Prog. Polym. Sci. 2005, 30, 691–724. [Google Scholar] [CrossRef]
- Yuan, L.; Chen, W.; Li, J.; Hu, J.; Yan, J.; Yang, D. PEG-b-PtBA-b-PHEMA Well-Defined Amphiphilic Triblock Copolymer: Synthesis, Self-Assembly, and Application in Drug Delivery. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 4579–4588. [Google Scholar] [CrossRef]
- Ghaemy, M.; Ziaei, S.; Alizadeh, R. Synthesis of pH-Sensitive Amphiphilic Pentablock Copolymers via Combination of Ring-Opening and Atom Transfer Radical Polymerization for Drug Delivery. Eur. Polym. J. 2014, 58, 103–114. [Google Scholar] [CrossRef]
- Lazzari, M.; López-Quintela, M.A. Block Copolymers as a Tool for Nanomaterial Fabrication. Adv. Mater. 2003, 15, 1583–1594. [Google Scholar] [CrossRef]
- Bastakoti, B.P.; Inuoe, M.; Yusa, S.; Liao, S.-H.; Wu, K.C.-W.; Nakashima, K.; Yamauchi, Y. A Block Copolymer Micelle Template for Synthesis of Hollow Calcium Phosphate Nanospheres with Excellent Biocompatibility. Chem. Commun 2012, 48, 6532–6534. [Google Scholar] [CrossRef]
- Zhai, S.; Manako, Y.; Yusa, S.-I.; Nakashima, K. Synthesis of Nanometer-Sized Hollow Calcium Tungstate Particles by Using Micelles of Poly(Styrene-b-acrylic acid-b-ethylene oxide) as a Soft Template. Chem. Lett. 2013, 42, 735–737. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, D.; Yoon, Y.J.; Pang, X.; Wang, Z.; Jung, J.; He, Y.; Harn, Y.W.; He, M.; Zhang, S.; et al. Hairy Uniform Permanently Ligated Hollow Nanoparticles with Precise Dimension Control and Tunable Optical Properties. J. Am. Chem. Soc. 2017, 139, 12956–12967. [Google Scholar] [CrossRef]
- Mei, S.; Qi, H.; Zhou, T.; Li, C.Y. Precisely Assembled Cyclic Gold Nanoparticle Frames by 2D Polymer Single-Crystal Templating. Angew. Chem. Int. Ed. 2017, 56, 13645–13649. [Google Scholar] [CrossRef]
- Taubert, A.; Napoli, A.; Meier, W. Self-Assembly of Reactive Amphiphilic Block Copolymers as Mimetics for Biological Membranes. Curr. Opin. Chem. Biol. 2004, 8, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Clodt, J.I.; Filiz, V.; Rangou, S.; Buhr, K.; Abetz, C.; Höche, D.; Hahn, J.; Jung, A.; Abetz, V. Double Stimuli-Responsive Isoporous Membranes via Post-Modification of pH-Sensitive Self-Assembled Diblock Copolymer Membranes. Adv. Funct. Mater. 2013, 23, 731–738. [Google Scholar] [CrossRef]
- Kreuzer, L.P.; Widmann, T.; Hohn, N.; Wang, K.; Bießmann, L.; Peis, L.; Moulin, J.; Hilderbrand, V.; Laschewsky, A.; Papadakis, C.M.; et al. Swelling and Exchange Behavior of Poly(Sulfobetaine)-Based Block Copolymer Thin Films. Macromolecules 2019, 52, 3486–3498. [Google Scholar] [CrossRef]
- Giacomelli, C.; Schmidt, V.; Aissou, K.; Borsali, R. Block Copolymer Systems: From Single Chain to Self-Assembled Nanostructures. Langmuir 2010, 26, 15734–15744. [Google Scholar] [CrossRef] [PubMed]
- Brendel, J.C.; Schacher, F.H. Block Copolymer Self-Assembly in Solution-Quo Vadis? Chem. Asian J. 2018, 13, 230–239. [Google Scholar] [CrossRef]
- Bhargava, P.; Zheng, J.X.; Li, P.; Quirk, R.P.; Harris, F.W.; Cheng, S.Z.D. Self-Assembled Polystyrene-block-Poly(ethylene oxide) Micelle Morphologies in Solution. Macromolecules 2006, 39, 4880–4888. [Google Scholar] [CrossRef]
- Zhang, L.; Lin, J.; Lin, S. Self-Assembly Behavior of Amphiphilic Block Copolymer/Nanoparticle Mixture in Dilute Solution Studied by Self-Consistent-Field Theory/Density Functional Theory. Macromolecules 2007, 40, 5582–5592. [Google Scholar] [CrossRef]
- Israelachvili, J.N.; Mitchell, D.J.; Ninham, B.W. Theory of Self-Assembly of Hydrocarbon Amphiphiles into Micelles and Bilayers. J. Chem. Soc. Faraday Trans. 1976, 72, 1525–1568. [Google Scholar]
- Nagarajan, R.; Ganesh, K. Block Copolymer Self-Assembly in Selective Solvents: Theory of Solubilization in Spherical Micelles. Macromolecules 1989, 22, 4312–4325. [Google Scholar] [CrossRef]
- Letchford, K.; Burt, H. A Review of the Formation and Classification of Amphiphilic Block Copolymer Nanoparticulate Structures: Micelles, Nanospheres, Nanocapsules and Polymersomes. Eur. J. Pharm. Biopharm 2007, 65, 259–269. [Google Scholar] [CrossRef]
- Smart, T.; Lomas, H.; Massignani, M.; Flores-Merino, M.V.; Perez, L.R.; Battaglia, G. Block Copolymer Nanostructures. Nano Today 2008, 3, 38–46. [Google Scholar] [CrossRef]
- Blanazs, A.; Madsen, J.; Battaglia, G.; Ryan, A.J.; Armes, S.P. Mechanistic Insights for Block Copolymer Morphologies: How Do Worms Form Vesicles? J. Am. Chem. Soc. 2011, 133, 16581–16587. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Jiang, W. Self-Assembly of ABC Triblock Copolymer into Giant Segmented Wormlike Micelles in Dilute Solution. Macromolecules 2005, 38, 9315–9323. [Google Scholar] [CrossRef]
- Shen, X.; Huo, F.; Kang, H.; Zhang, S.; Li, J.; Zhang, W. Modification of Block Copolymer Vesicles: What Will Happen When AB Diblock Copolymer is Block-Extended to an ABC Triblock Terpolymer? Polym. Chem. 2015, 6, 3407–3414. [Google Scholar] [CrossRef]
- Lutz, J. Writing on Polymer Chains. Acc. Chem. Res. 2013, 46, 2696–2705. [Google Scholar] [CrossRef] [PubMed]
- Bates, F.S.; Hillmyer, M.A.; Lodge, T.P.; Bates, C.M.; Delaney, K.T.; Fredrickson, G.H. Multiblock Polymers: Panacea or Pandora’s Box? Science 2012, 336, 434–440. [Google Scholar] [CrossRef]
- Zhang, L.; Cheng, Z.; Zhou, N.; Zhang, R.; Zhu, X. Synthesis of Amphiphilic ABCBA-Type Pentablock Copolymer from Consecutive ATRPs and Self-Assembly in Aqueous Solution. Macromol. Symp. 2008, 261, 54–63. [Google Scholar] [CrossRef]
- Thünemann, A.F.; Kubowicz, S.; von Berlepsch, H.; Möhwald, H. Two-Compartment Micellar Assemblies Obtained via Aqueous Self-Organization of Synthetic Polymer Building Blocks. Langmuir 2006, 22, 2506–2510. [Google Scholar] [CrossRef]
- Parekh, P.; Ohno, S.; Yusa, S.; Lage, E.V.; Casas, M.; Sández-Macho, I.; Aswal, V.K.; Bahadur, P. Surface and Aggregation Behavior of Pentablock Copolymer PNIPAM7-F127-PNIPAM7 in Aqueous Solutions. J. Phys. Chem. B 2016, 120, 7569–7578. [Google Scholar] [CrossRef]
- Determan, M.D.; Cox, J.P.; Seifert, S.; Thiyagarajan, P.; Mallapragada, S.K. Synthesis and Characterization of Temperature and pH-Responsive Pentablock Copolymers. Polymer 2005, 46, 6933–6946. [Google Scholar] [CrossRef]
- Determan, M.D.; Guo, L.; Thiyagarajan, P.; Mallapragada, S.K. Supramolecular Self-Assembly of Multiblock Copolymers in Aqueous Solution. Langmuir 2006, 22, 1469–1473. [Google Scholar] [CrossRef] [PubMed]
- Mei, A.; Guo, X.; Ding, Y.; Zhang, X.; Xu, J.; Fan, Z.; Du, B. PNIPAm-PEO-PPO-PEO-PNIPAm Pentablock Terpolymer: Synthesis and Chain Behavior in Aqueous Solution. Macromolecules 2010, 43, 7312–7320. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, T.; Mei, A.; Chen, T.; Ding, Y.; Zhang, X.; Xu, J.; Fan, Z.; Du, B. Solution Behaviors and Microstructures of PNIPAm-P123-PNIPAm Pentablock Terpolymers in Dilute and Concentrated Aqueous Solutions. Phys. Chem. Chem. Phys. 2013, 15, 8276–8286. [Google Scholar] [CrossRef]
- Lv, C.; Zhang, Z.; Gao, J.; Xue, J.; Li, J.; Nie, J.; Xu, J.; Du, B. Self-Assembly of Thermosensitive Amphiphilic Pentablock Terpolymer PNIPAMx-b-PtBA90-b-PPO36-b-PtBA90-b-PNIPAMx in Dilute Aqueous Solution. Macromolecules 2018, 51, 10136–10149. [Google Scholar] [CrossRef]
- Lv, C.; Wang, R.; Gao, J.; Ding, N.; Dong, S.; Nie, J.; Xu, J.; Du, B. PAA-b-PPO-b-PAA Triblock Copolymers with Enhanced Phase Separation and Inverse Order-to-Order Phase Transition upon Increasing Temperature. Polymer 2019, 185, 121982. [Google Scholar] [CrossRef]
- Kim, J.; Lee, E.; Lim, Y.; Lee, M. Supramolecular Capsules with Gated Pores from an Amphiphilic Rod Assembly. Angew. Chem. Int. Ed. 2008, 47, 4662–4666. [Google Scholar] [CrossRef]
- Xu, J.; Li, J.; Yang, Y.; Wang, K.; Xu, N.; Li, J.; Liang, R.; Shen, L.; Xie, X.; Tao, J.; et al. Block Copolymer Capsules with Structure-Dependent Release Behavior. Angew. Chem. Int. Ed. 2016, 55, 14633–14637. [Google Scholar] [CrossRef]
- Wong, C.K.; Heidelmann, M.; Dulle, M.; Qiang, X.; Förster, S.; Stenzel, M.H.; Gröschel, A.H. Vesicular Polymer Hexosomes Exhibit Topological Defects. J. Am. Chem. Soc. 2020, 142, 10989–10995. [Google Scholar] [CrossRef]
- Won, Y.Y.; Brannan, A.K.; Davis, H.T.; Bates, F.S. Cryogenic Transmission Electron Microscopy (Cryo-TEM) of Micelles and Vesicles Formed in Water by Poly(ethylene oxide)-Based Block Copolymers. J. Phys. Chem. B 2002, 106, 3354–3364. [Google Scholar] [CrossRef]
- Hales, K.; Chen, Z.; Wooley, K.L.; Pochan, D.J. Nanoparticles with Tunable Internal Structure from Triblock Copolymers of PAA-b-PMA-b-PS. Nano Lett. 2008, 8, 2023–2026. [Google Scholar] [CrossRef]
- Nystrom, B.; Walderhaug, H.; Hansen, F.K. Dynamic Crossover Effects Observed in Solutions of a Hydrophobically Associating Water-Soluble Polymer. J. Phys. Chem. 1993, 97, 7743–7752. [Google Scholar]
- Ngai, K.L. Dynamics of Semidilute Solutions of Polymers and Associating Polymers. Adv. Colloid Interface Sci. 1996, 64, 1–43. [Google Scholar]
- He, W.; Xu, J.; Du, B.; Fan, Z.; Sun, F. Effect of pH on the Micellar Morphology of Semicrystalline PCL-b-PEO Block Copolymers in Aqueous Solution. Macromol. Chem. Phys. 2012, 213, 952–964. [Google Scholar]
- Ke, X.; Wang, L.; Xu, J.; Du, B.; Tu, Y.; Fan, Z. Effect of Local Chain Deformability on the Temperature-Induced Morphological Transitions of Polystyrene-b-poly(N-isopropylacrylamide) Micelles in Aqueous Solution. Soft Matter 2014, 10, 5201–5211. [Google Scholar] [PubMed]
- Geng, Y.; Ahmed, F.; Bhasin, N.; Discher, D.E. Visualizing Worm Micelle Dynamics and Phase Transitions of a Charged Diblock Copolymer in Water. J. Phys. Chem. B 2005, 109, 3772–3779. [Google Scholar] [PubMed]
- Yang, S.; Wen, G.; Pispas, S.; You, K. Effects of Spreading and Subphase Conditions on the Interfacial Behavior of an Amphiphilic Copolymer Poly(n-butylacrylate)-b-poly(acrylic acid). Polymer 2019, 172, 66–74. [Google Scholar]
- Xie, D.; Rezende, C.A.; Liu, G.; Pispas, S.; Zhang, G.; Lee, L.-T. Effect of Hydrogen-Bonding Complexation on the Interfacial Behavior of Poly(isoprene)-b-poly(ethylene oxide) and Poly(isoprene)-b-poly(acrylic acid) Langmuir Monolayers. J. Phys. Chem. B 2009, 113, 739–744. [Google Scholar]
- Gontsarik, M.; Yaghmur, A.; Ren, Q.; Maniura-Weber, K.; Salentinig, S. From Structure to Function: pH-Switchable Antimicrobial Nano-Self-Assemblies. ACS Appl. Mater. Interfaces 2019, 11, 2821–2829. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).