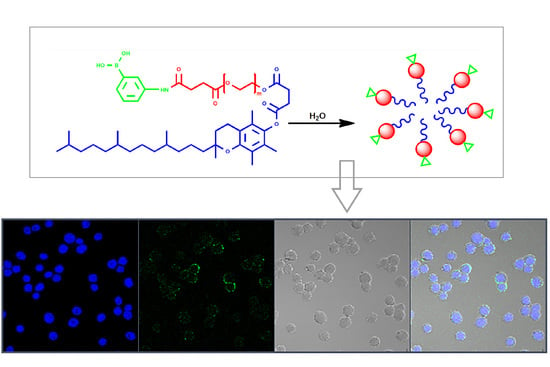
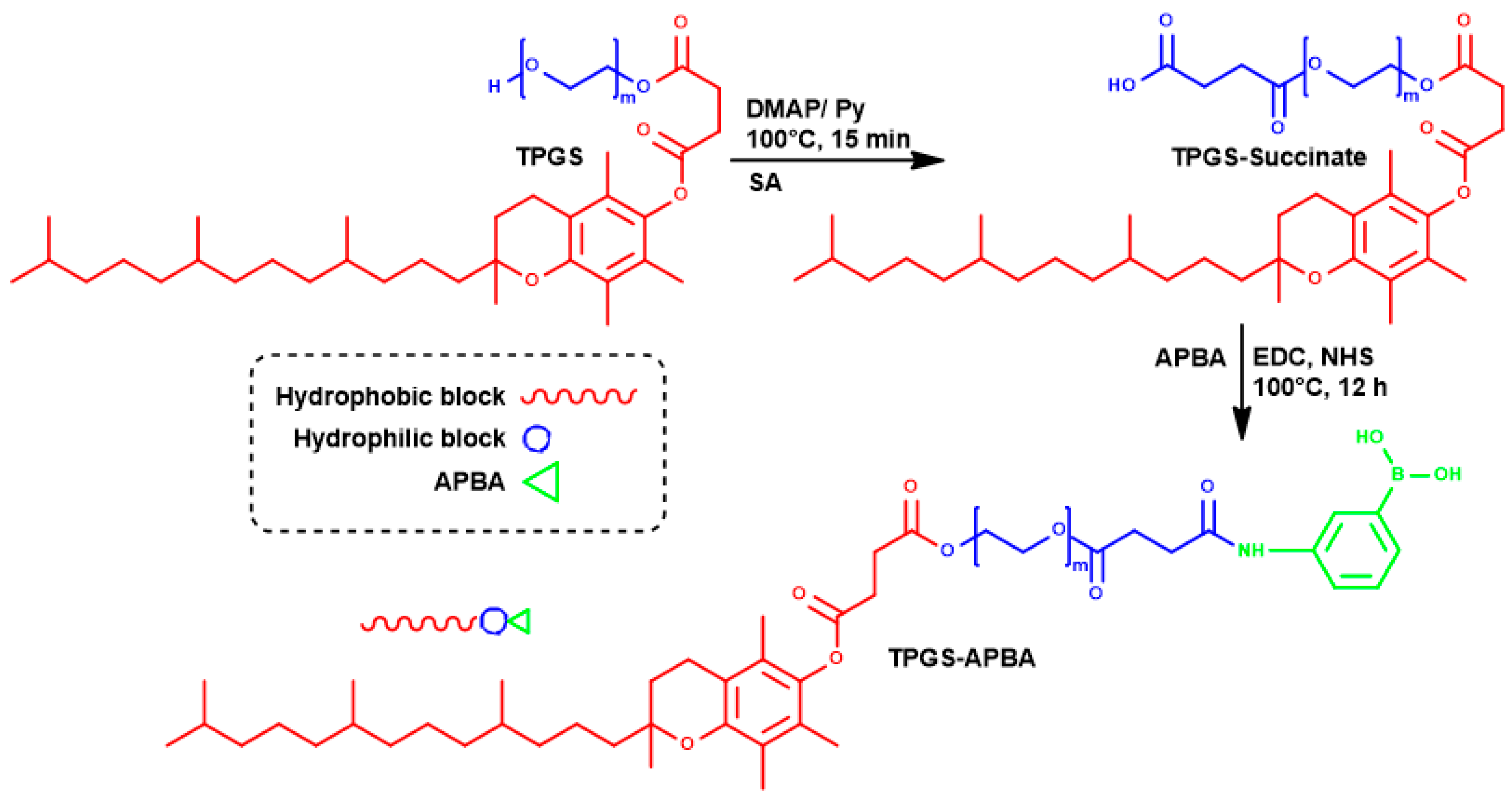
Synthesis, Colloidal Characterization and Targetability of Phenylboronic Acid Functionalized α-Tocopheryl Polyethylene Glycol Succinate in Cancer Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. Characterization
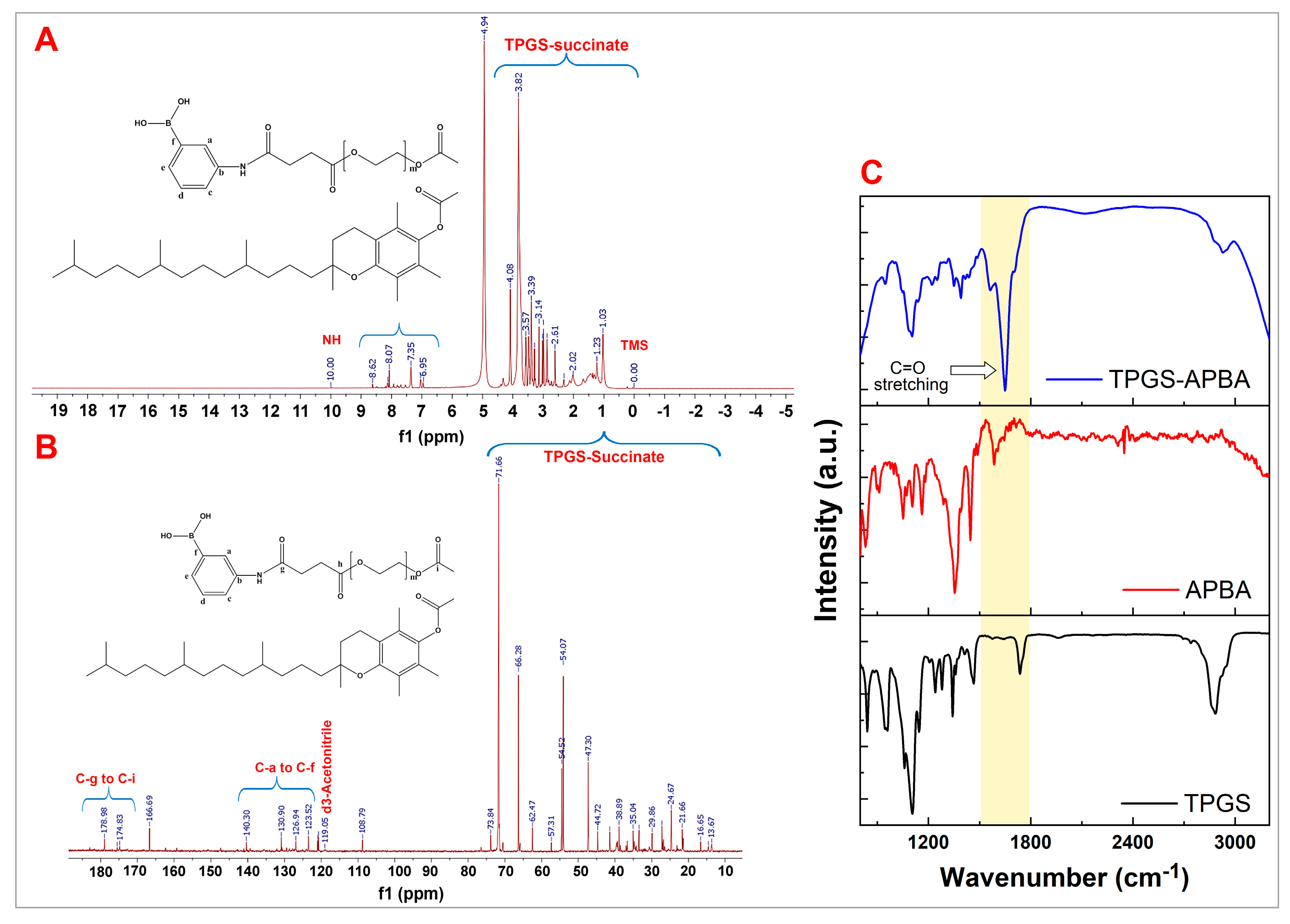
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Otsuka, H.; Uchimura, E.; Koshino, H.; Okano, A.T.; Kataoka, K. Anomalous binding profile of phenylboronic acid with N-acetylneuraminic acid (Neu5Ac) in aqueous solution with varying pH. J. Am. Chem. Soc. 2003, 125, 3493–3502. [Google Scholar] [CrossRef]
- Fukuda, M. Possible roles of tumor-associated carbohydrate antigens. Cancer Res. 1996, 56, 2237–2244. [Google Scholar] [PubMed]
- Fuster, M.M.; Esko, J.D. The sweet and sour of cancer: Glycans as novel therapeutic targets. Nat. Rev. Cancer 2005, 5, 526–542. [Google Scholar] [CrossRef]
- Ono, K.; Sanada, Y.; Kimura, Y.; Aoyama, S.; Ueda, N.; Katayama, T.; Nagahama, K. A thin hydrogel barrier linked onto cell surface sialic acids through covalent bonds induces cancer cell death in vivo. Biomater. Sci. 2020, 8, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.-W.; Yu, C.-Y.; Lin, T.-W.; Wang, P.-H.; Tsai, Y.-C. Soyasaponin I decreases the expression of α2,3-linked sialic acid on the cell surface and suppresses the metastatic potential of B16F10 melanoma cells. Biochem. Biophys. Res. Commun. 2006, 341, 614–619. [Google Scholar] [CrossRef]
- Büll, C.; Boltje, T.J.; Wassink, M.; De Graaf, A.M.A.; Van Delft, F.L.; Brok, M.H.D.; Adema, G.J. Targeting aberrant sialylation in cancer cells using a fluorinated sialic acid analog impairs adhesion, migration, and in vivo tumor growth. Mol. Cancer Ther. 2013, 12, 1935–1946. [Google Scholar] [CrossRef] [PubMed]
- Macauley, M.S.; Arlian, B.M.; Rillahan, C.D.; Pang, P.-C.; Bortell, N.; Marcondes, M.C.G.; Haslam, S.M.; Dell, A.; Paulson, J.C. Systemic blockade of sialylation in mice with a global inhibitor of sialyltransferases. J. Boil. Chem. 2014, 289, 35149–35158. [Google Scholar] [CrossRef]
- Ji, M.; Li, P.; Sheng, N.; Liu, L.; Pan, H.; Wang, C.; Cai, L.; Ma, Y. Sialic acid-targeted nanovectors with phenylboronic acid-grafted polyethylenimine robustly enhance siRNA-based cancer therapy. ACS Appl. Mater. Interfaces 2016, 8, 9565–9576. [Google Scholar] [CrossRef]
- Liang, L.; Qu, H.; Zhang, B.; Zhang, J.; Deng, R.; Shen, Y.; Xu, S.; Liang, C.; Xu, W. Tracing sialoglycans on cell membrane via surface-enhanced Raman scattering spectroscopy with a phenylboronic acid-based nanosensor in molecular recognition. Biosens. Bioelectron. 2017, 94, 148–154. [Google Scholar] [CrossRef]
- Wang, X.; Wei, B.; Cheng, X.; Wang, J.; Cheng, X. 3-Carboxyphenylboronic acid-modified carboxymethyl chitosan nanoparticles for improved tumor targeting and inhibitory. Eur. J. Pharm. Biopharm. 2017, 113, 168–177. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, W.; Xu, Y.; Zhu, S.; Wu, Y.; Chen, T.; Xiao, Y.; Lu, W.; Zhang, X.; Yu, J. Dynamic core crosslinked camptothecin prodrug micelles with reduction sensitivity and boronic acid-mediated enhanced endocytosis: An intelligent tumor-targeted delivery nanoplatform. Int. J. Pharm. 2020, 580, 119250. [Google Scholar] [CrossRef]
- Zhang, Z.; Tana, S.; Feng, S.-S. Vitamin E TPGS as a molecular biomaterial for drug delivery. Biomaterials 2012, 33, 4889–4906. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Pandey, S.; Sola, P.; Pathan, H.; Patil, R.; Ray, D.; Aswal, V.K.; Bahadur, P.; Tiwari, S. Solubilization of carbamazepine in TPGS micelles: Effect of temperature and electrolyte addition. AAPS PharmSciTech 2019, 20, 203. [Google Scholar] [CrossRef] [PubMed]
- Pathan, H.; Patil, R.; Ray, D.; Aswal, V.K.; Bahadur, P.; Tiwari, S. Structural changes in non-ionic surfactant micelles induced by ionic liquids and application thereof for improved solubilization of quercetin. J. Mol. Liq. 2019, 290, 111235. [Google Scholar] [CrossRef]
- Puig-Rigall, J.; Fernandez-Rubio, C.; González-Benito, J.; Houston, J.E.; Radulescu, A.; Nguewa, P.; Gonzalez-Gaitano, G. Structural characterization by scattering and spectroscopic methods and biological evaluation of polymeric micelles of poloxamines and TPGS as nanocarriers for miltefosine delivery. Int. J. Pharm. 2020, 578, 119057. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Liu, G.; Guo, H.; Li, C.; Zhang, Y.; Zhang, F.; Zhao, Z.; Cheng, H. Novel galactosylated biodegradable nanoparticles for hepatocyte-delivery of oridonin. Int. J. Pharm. 2016, 502, 47–60. [Google Scholar] [CrossRef]
- Zhang, X.; Peng, X.; Yu, W.; Hou, S.; Zhao, Y.; Zhang, Z.; Huang, X.; Wu, K. Alpha-tocopheryl succinate enhances doxorubicin-induced apoptosis in human gastric cancer cells via promotion of doxorubicin influx and suppression of doxorubicin efflux. Cancer Lett. 2011, 307, 174–181. [Google Scholar] [CrossRef]
- Shi, C.; Zhang, Z.; Wang, F.; Ji, X.; Zhao, Z.; Luan, Y. Docetaxel-loaded PEO–PPO–PCL/TPGS mixed micelles for overcoming multidrug resistance and enhancing antitumor efficacy. J. Mater. Chem. B 2015, 3, 4259–4271. [Google Scholar] [CrossRef]
- Assanhou, A.G.; Li, W.; Zhang, L.; Xue, L.; Kong, L.-Y.; Sun, H.; Mo, R.; Zhang, C. Reversal of multidrug resistance by co-delivery of paclitaxel and lonidamine using a TPGS and hyaluronic acid dual-functionalized liposome for cancer treatment. Biomaterials 2015, 73, 284–295. [Google Scholar] [CrossRef]
- De Melo-Diogo, D.; Pais-Silva, C.; Costa, E.C.; Louro, R.O.; Correia, I.J. D-α-tocopheryl polyethylene glycol 1000 succinate functionalized nanographene oxide for cancer therapy. Nanomedicine 2017, 12, 443–456. [Google Scholar] [CrossRef]
- Du, J.; Zheng, X.; Yong, Y.; Yu, J.; Dong, X.; Zhang, C.; Zhou, R.; Li, B.; Yan, L.; Chen, C.; et al. Design of TPGS-functionalized Cu 3 BiS 3 nanocrystals with strong absorption in the second near-infrared window for radiation therapy enhancement. Nanoscale 2017, 9, 8229–8239. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Tirosh, B.; Rubinstein, A. Increasing the affinity of cationized polyacrylamide-paclitaxel nanoparticles towards colon cancer cells by a surface recognition peptide. Int. J. Pharm. 2017, 531, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Golwala, P.; Rathod, S.; Patil, R.; Joshi, A.; Ray, D.; Aswal, V.K.; Bahadur, P.; Tiwari, S. Effect of cosurfactant addition on phase behavior and microstructure of a water dilutable microemulsion. Colloids Surf. B Biointerfaces 2020, 186, 110736. [Google Scholar] [CrossRef] [PubMed]
- Khare, V.; Sakarchi, W.A.; Gupta, P.N.; Curtis, A.D.M.; Hoskins, C. Synthesis and characterization of TPGS–gemcitabine prodrug micelles for pancreatic cancer therapy. RSC Adv. 2016, 6, 60126–60137. [Google Scholar] [CrossRef]
- Duhem, N.; Danhier, F.; Pourcelle, V.; Schumers, J.-M.; Bertrand, O.; Le Duff, C.S.; Hoeppener, S.; Schubert, U.S.; Gohy, J.-F.; Marchand-Brynaert, J.; et al. Self-assembling doxorubicin–tocopherol succinate prodrug as a new drug delivery system: Synthesis, characterization, and in vitro and in vivo anticancer activity. Bioconjugate Chem. 2013, 25, 72–81. [Google Scholar] [CrossRef]
- Bao, L.; Ding, L.; Yang, M.; Ju, H. Noninvasive imaging of sialyltransferase activity in living cells by chemoselective recognition. Sci. Rep. 2015, 5, 10947. [Google Scholar] [CrossRef]
- Huang, C.; Hu, X.; Hou, Z.; Ji, J.; Li, Z.; Luan, Y. Tailored graphene oxide-doxorubicin nanovehicles via near-infrared dye-lactobionic acid conjugates for chemo-photothermal therapy. J. Colloid Interface Sci. 2019, 545, 172–183. [Google Scholar] [CrossRef]
- Basiruddin, S.; Swain, S.K. Phenylboronic acid functionalized reduced graphene oxide based fluorescence nano sensor for glucose sensing. Mater. Sci. Eng. C 2016, 58, 103–109. [Google Scholar] [CrossRef]
- Mi, Y.; Liu, Y.; Feng, S.-S. Formulation of Docetaxel by folic acid-conjugated d-α-tocopheryl polyethylene glycol succinate 2000 (Vitamin E TPGS2k) micelles for targeted and synergistic chemotherapy. Biomaterials 2011, 32, 4058–4066. [Google Scholar] [CrossRef]
- Brandt, J.V.; Piazza, R.D.; Dos Santos, C.C.; Vega-Chacón, J.; Amantéa, B.E.; Pinto, G.C.; Magnani, M.; Piva, H.L.; Tedesco, A.C.; Primo, F.L.; et al. Synthesis and colloidal characterization of folic acid-modified PEG-b-PCL Micelles for methotrexate delivery. Colloids Surf. B Biointerfaces 2019, 177, 228–234. [Google Scholar] [CrossRef]
- Gong, J.; Huo, M.; Zhou, J.; Zhang, Y.; Peng, X.; Yu, D.; Zhang, H.; Li, J. Synthesis, characterization, drug-loading capacity and safety of novel octyl modified serum albumin micelles. Int. J. Pharm. 2009, 376, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, J.; Verma, G.; Aswal, V.K.; Patravale, V.; Hassan, P.A. Microstructure, drug binding and cytotoxicity of Pluronic P123–aerosol OT mixed micelles. RSC Adv. 2013, 3, 23080. [Google Scholar] [CrossRef]
- Karnati, K.R.; Wang, Y. Understanding the co-loading and releasing of doxorubicin and paclitaxel using chitosan functionalized single-walled carbon nanotubes by molecular dynamics simulations. Phys. Chem. Chem. Phys. 2018, 20, 9389–9400. [Google Scholar] [CrossRef] [PubMed]
- Gapinski, J.; Szymański, J.; Wilk, A.; Kohlbrecher, J.; Patkowski, A.; Hołyst, R. Size and shape of micelles studied by means of SANS, PCS, and FCS. Langmuir 2010, 26, 9304–9314. [Google Scholar] [CrossRef]
- Buwalda, S.; Al Samad, A.; El Jundi, A.; Bethry, A.; Bakkour, Y.; Coudane, J.; Nottelet, B. Stabilization of poly(ethylene glycol)-poly(epsilon-caprolactone) star block copolymer micelles via aromatic groups for improved drug delivery properties. J. Colloid Interface Sci. 2018, 514, 468–478. [Google Scholar] [CrossRef]
- Tang, X.; Zhou, S.; Tao, X.; Wang, J.; Wang, F.; Liang, Y. Targeted delivery of docetaxel via pi-pi stacking stabilized dendritic polymeric micelles for enhanced therapy of liver cancer. Mater. Sci. Eng. C 2017, 75, 1042–1048. [Google Scholar] [CrossRef]
- Guan, S.; Zhang, Q.; Bao, J.; Duan, T.; Hu, R.; Czech, T.; Tang, J. Phosphatidylserine targeting peptide-functionalized pH sensitive mixed micelles for enhanced anti-tumor drug delivery. Eur. J. Pharm. Biopharm. 2019, 147, 87–101. [Google Scholar] [CrossRef]
- Wang, X.; Wei, B.; Cheng, X.; Wang, J.; Cheng, X. Phenylboronic acid-decorated gelatin nanoparticles for enhanced tumor targeting and penetration. Nanotechnology 2016, 27, 385101. [Google Scholar] [CrossRef]
- Mozhi, A.; Ahmad, I.; Kaleem, Q.M.; Tuguntaev, R.G.; Eltahan, A.S.; Wang, C.; Yang, R.; Li, C.; Liang, X.-J. Nrp-1 receptor targeting peptide-functionalized TPGS micellar nanosystems to deliver 10-hydroxycampothecin for enhanced cancer chemotherapy. Int. J. Pharm. 2018, 547, 582–592. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, D.; Gao, Y.; Zhou, T.; Chen, M. Phenylboronic acid-functionalized unimolecular micelles based on a star polyphosphoester random copolymer for tumor-targeted drug delivery. Polym. Chem. 2020, 11, 2252–2261. [Google Scholar] [CrossRef]
- He, R.; Yin, C. Trimethyl chitosan based conjugates for oral and intravenous delivery of paclitaxel. Acta Biomater. 2017, 53, 355–366. [Google Scholar] [CrossRef] [PubMed]
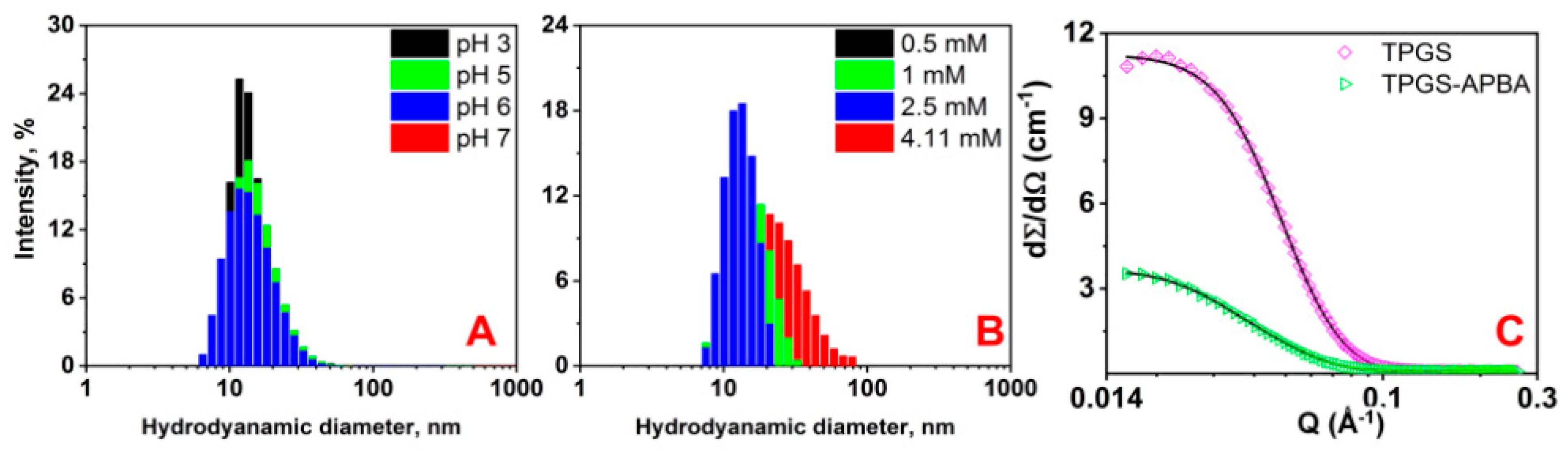
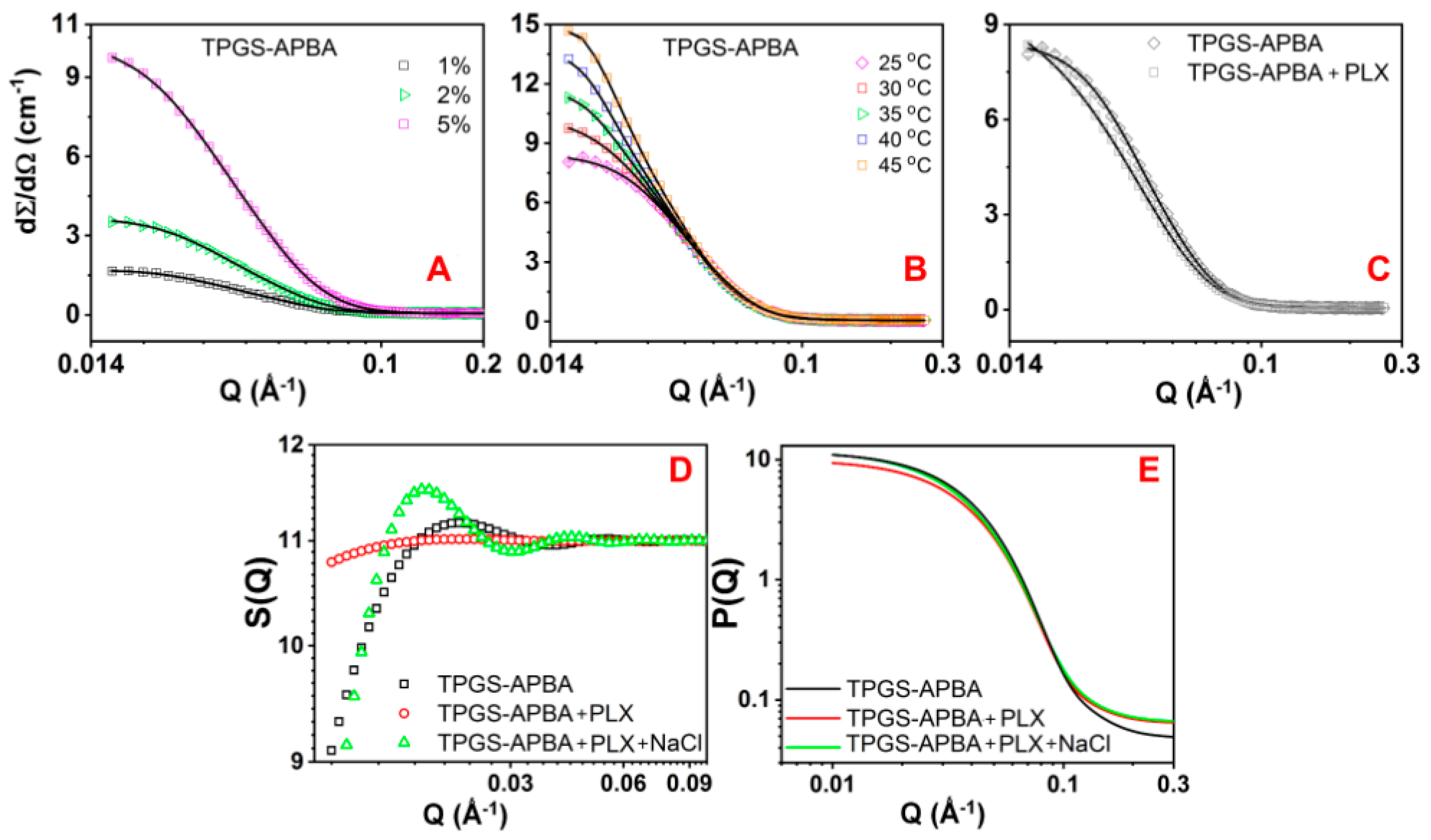
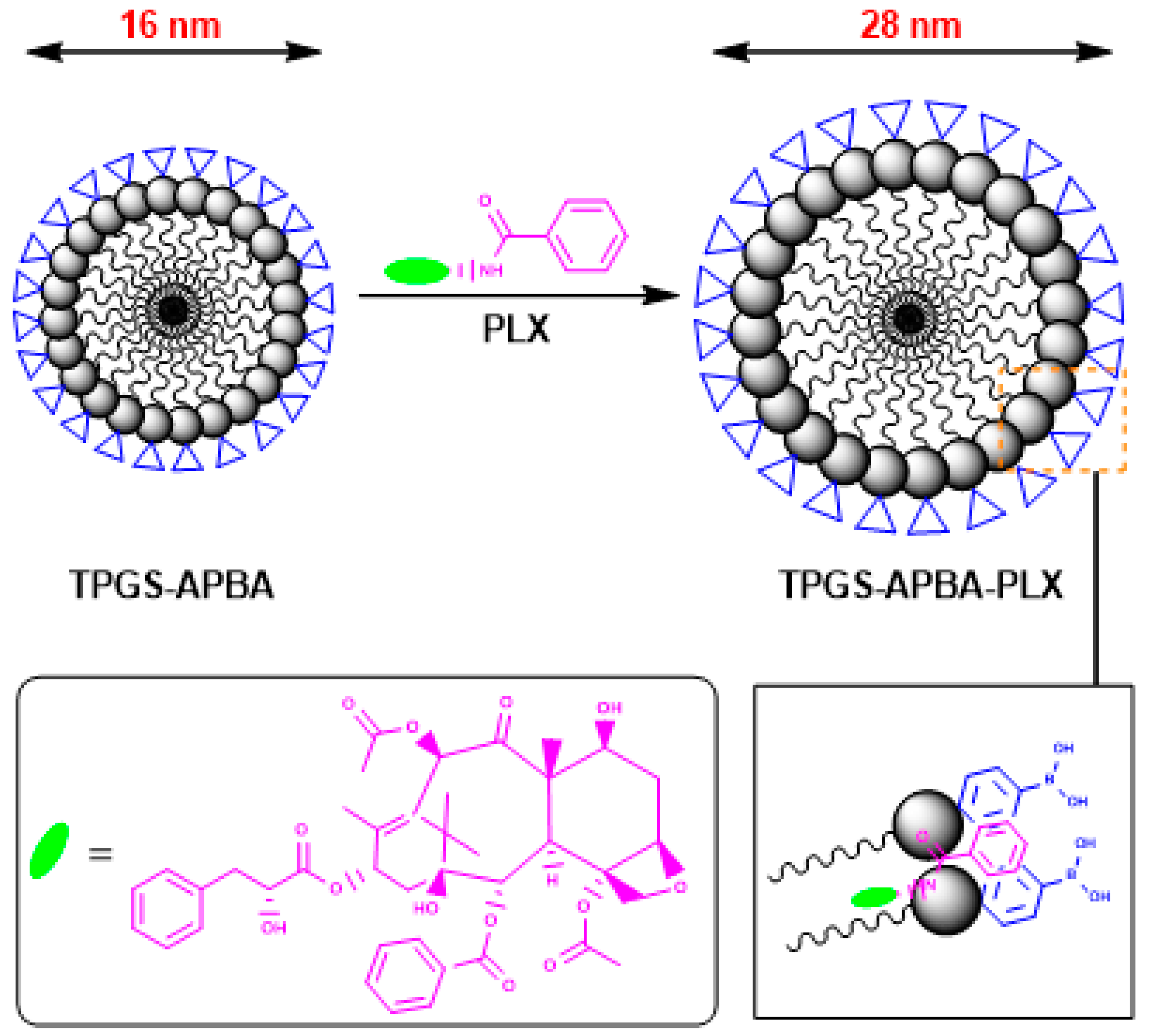

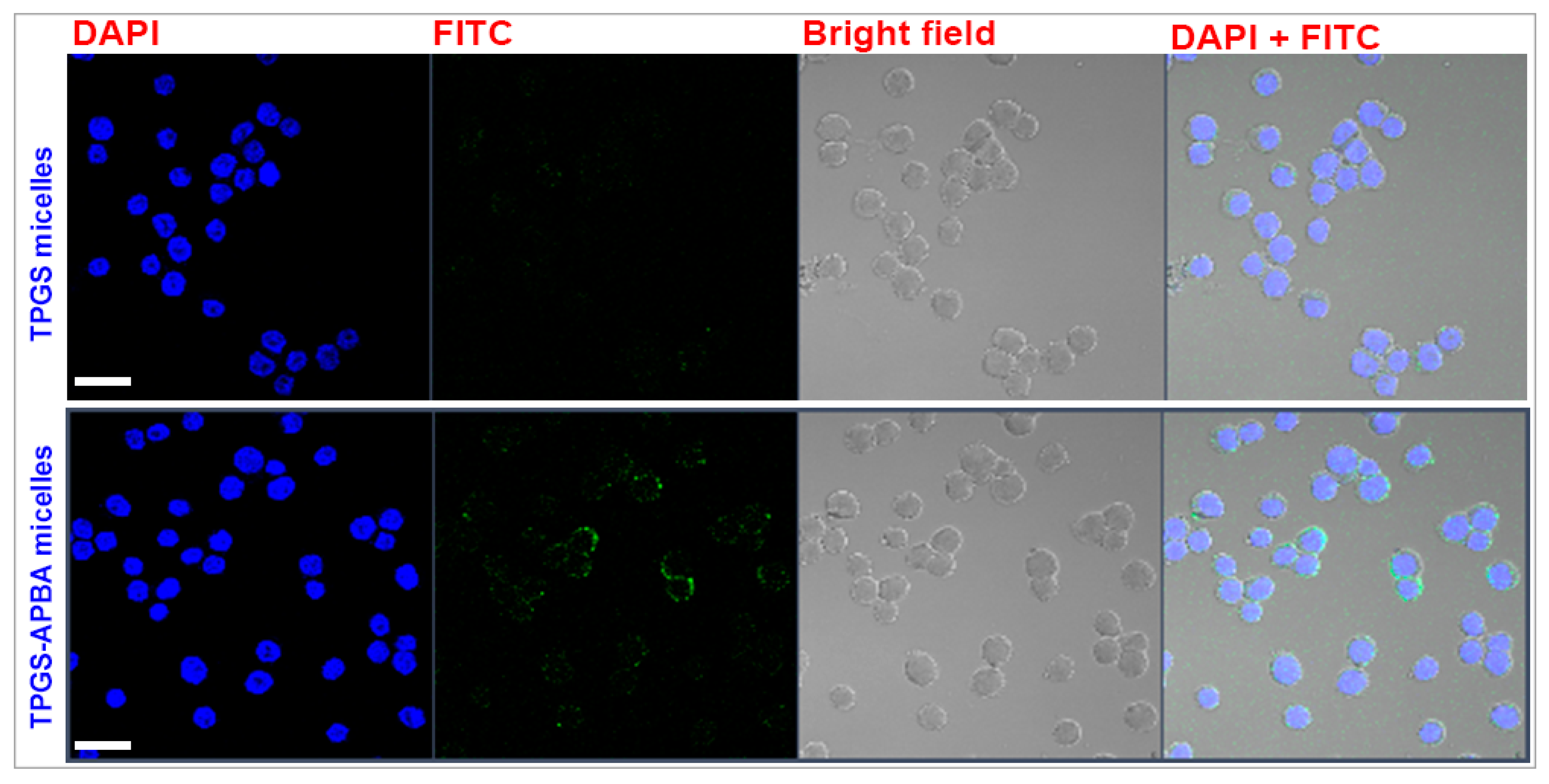

| Sample | Rc | Rhs | Φ | PD | Nagg | N (×1017) | |
|---|---|---|---|---|---|---|---|
| TPGS (2%) | 4.2 | 6.7 | 0.044 | 0.19 | 121 | 0.354 | |
| TPGS-APBA | 2% | 4.3 | 6.5 | 0.012 | 0.24 | 124 | 0.100 |
| 5% | 4.3 | 6.6 | 0.021 | 0.23 | 122 | 0.169 | |
| PLX * | 4.3 | 8.4 | 0.003 | 0.27 | 124 | 0.013 | |
| PLX * + 0.9% NaCl | 4.4 | 12.5 | 0.063 | 0.29 | 129 | 0.077 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiwari, S.; Sarolia, J.; Kansara, V.; Chudasama, N.A.; Prasad, K.; Ray, D.; Aswal, V.K.; Bahadur, P. Synthesis, Colloidal Characterization and Targetability of Phenylboronic Acid Functionalized α-Tocopheryl Polyethylene Glycol Succinate in Cancer Cells. Polymers 2020, 12, 2258. https://doi.org/10.3390/polym12102258
Tiwari S, Sarolia J, Kansara V, Chudasama NA, Prasad K, Ray D, Aswal VK, Bahadur P. Synthesis, Colloidal Characterization and Targetability of Phenylboronic Acid Functionalized α-Tocopheryl Polyethylene Glycol Succinate in Cancer Cells. Polymers. 2020; 12(10):2258. https://doi.org/10.3390/polym12102258
Chicago/Turabian StyleTiwari, Sanjay, Jayant Sarolia, Vrushti Kansara, Nishith A. Chudasama, Kamalesh Prasad, Debes Ray, Vinod K Aswal, and Pratap Bahadur. 2020. "Synthesis, Colloidal Characterization and Targetability of Phenylboronic Acid Functionalized α-Tocopheryl Polyethylene Glycol Succinate in Cancer Cells" Polymers 12, no. 10: 2258. https://doi.org/10.3390/polym12102258
APA StyleTiwari, S., Sarolia, J., Kansara, V., Chudasama, N. A., Prasad, K., Ray, D., Aswal, V. K., & Bahadur, P. (2020). Synthesis, Colloidal Characterization and Targetability of Phenylboronic Acid Functionalized α-Tocopheryl Polyethylene Glycol Succinate in Cancer Cells. Polymers, 12(10), 2258. https://doi.org/10.3390/polym12102258