Synergistic Effects on Incorporation of β-Tricalcium Phosphate and Graphene Oxide Nanoparticles to Silk Fibroin/Soy Protein Isolate Scaffolds for Bone Tissue Engineering
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Silk Fibroin Solution
2.2. Fabrication of SF/SPI-Based Composite Scaffolds
2.3. Characterization of the SF/SPI-Based Composite Scaffolds
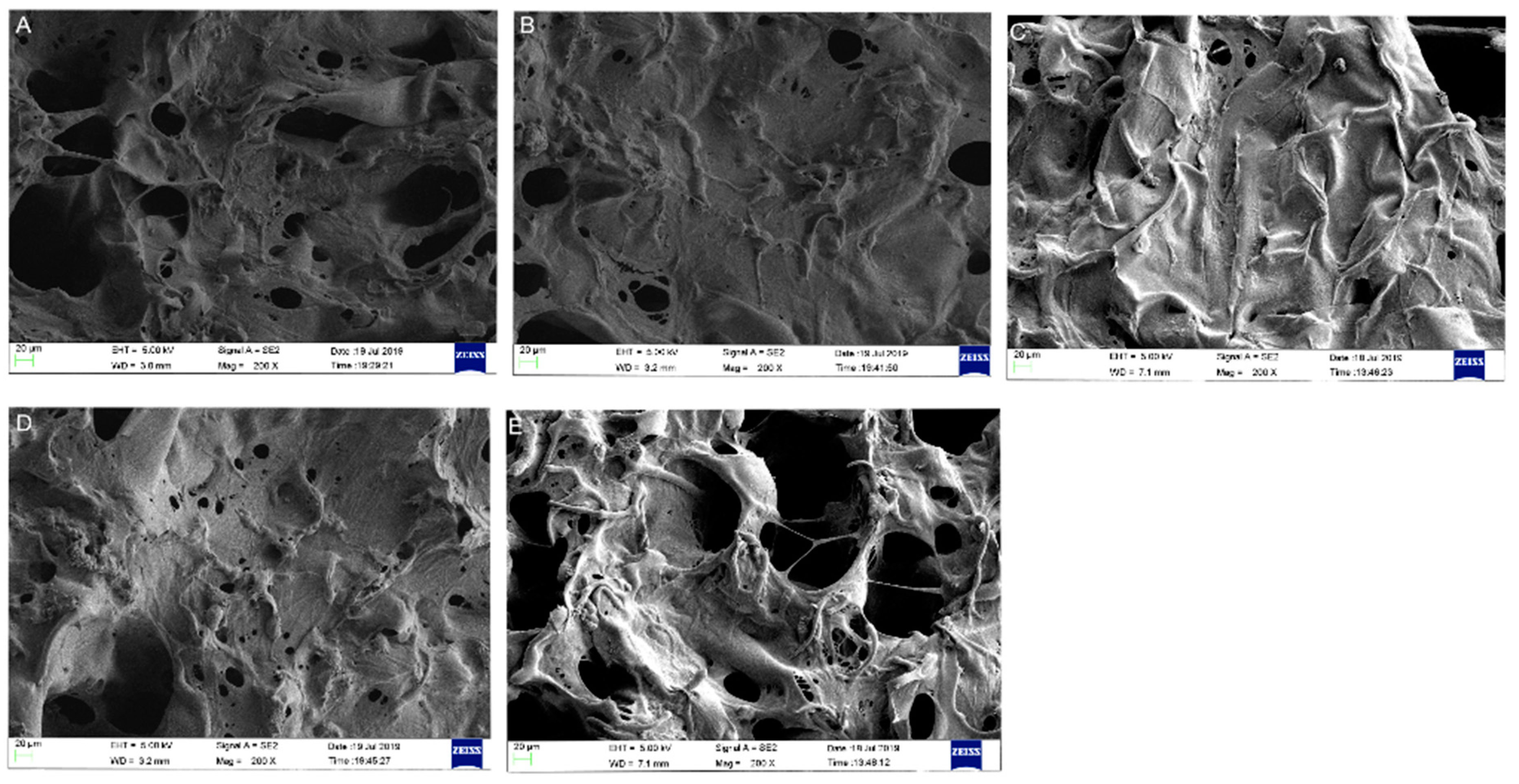
2.3.1. Morphologies of the SF/SPI-Based Composite Scaffolds
2.3.2. Compositions of the SF/SPI-Based Composite Scaffolds
2.3.3. Pore Size and Porosity of the SF/SPI-Based Composite Scaffolds
2.3.4. Water Adsorption of the SF/SPI-Based Composite Scaffolds
2.3.5. In Vitro Mineralization of the SF/SPI-Based Composite Scaffolds
2.3.6. Mechanical Properties of the SF/SPI-Based Composite Scaffolds
2.4. In Vitro Biocompatibility and Osteogenic Evaluation of the SF/SPI-Based Composite Scaffolds
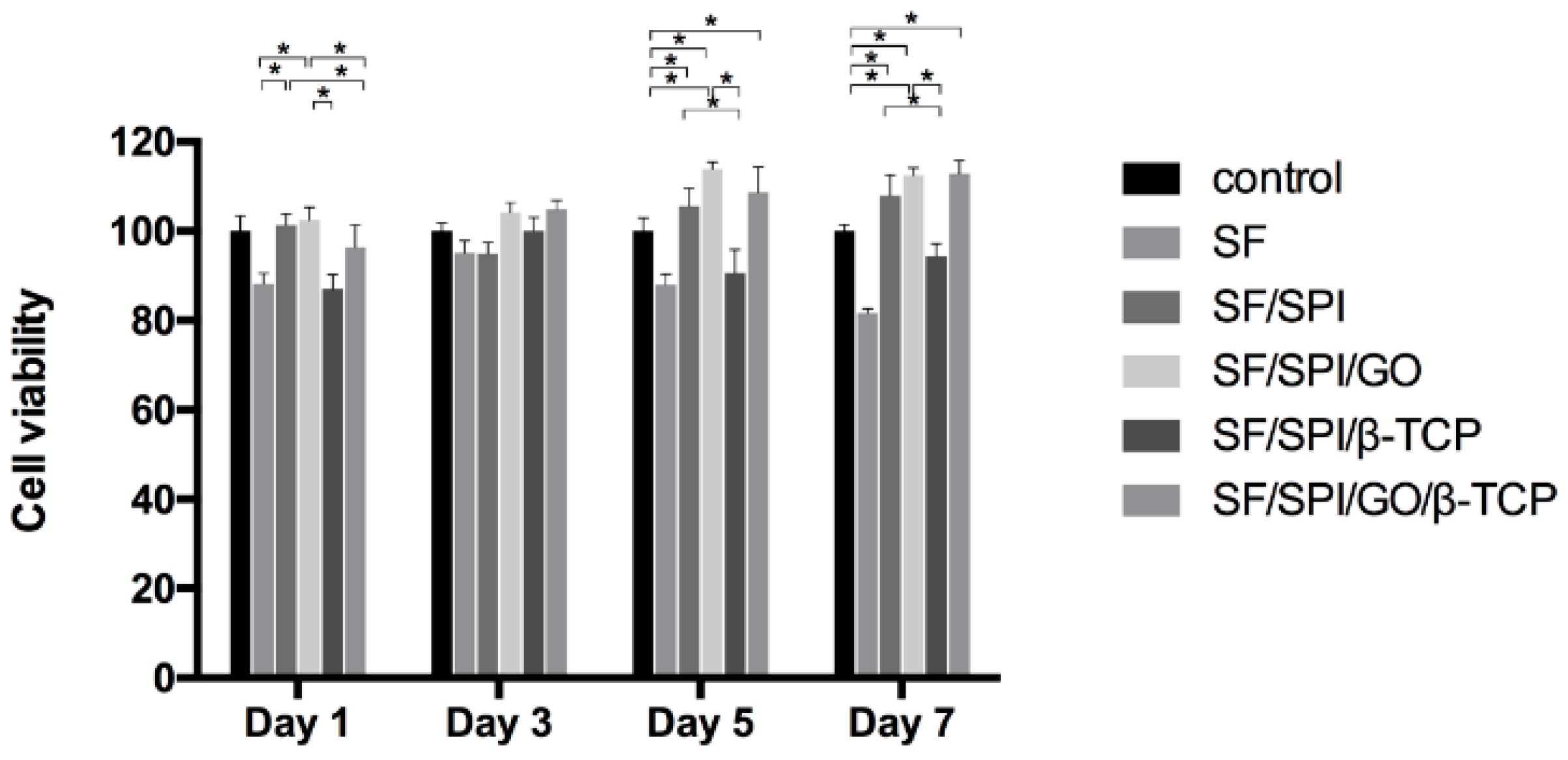
2.4.1. Morphology and Viability of BMMCs on the SF/SPI-Based Composite Scaffolds
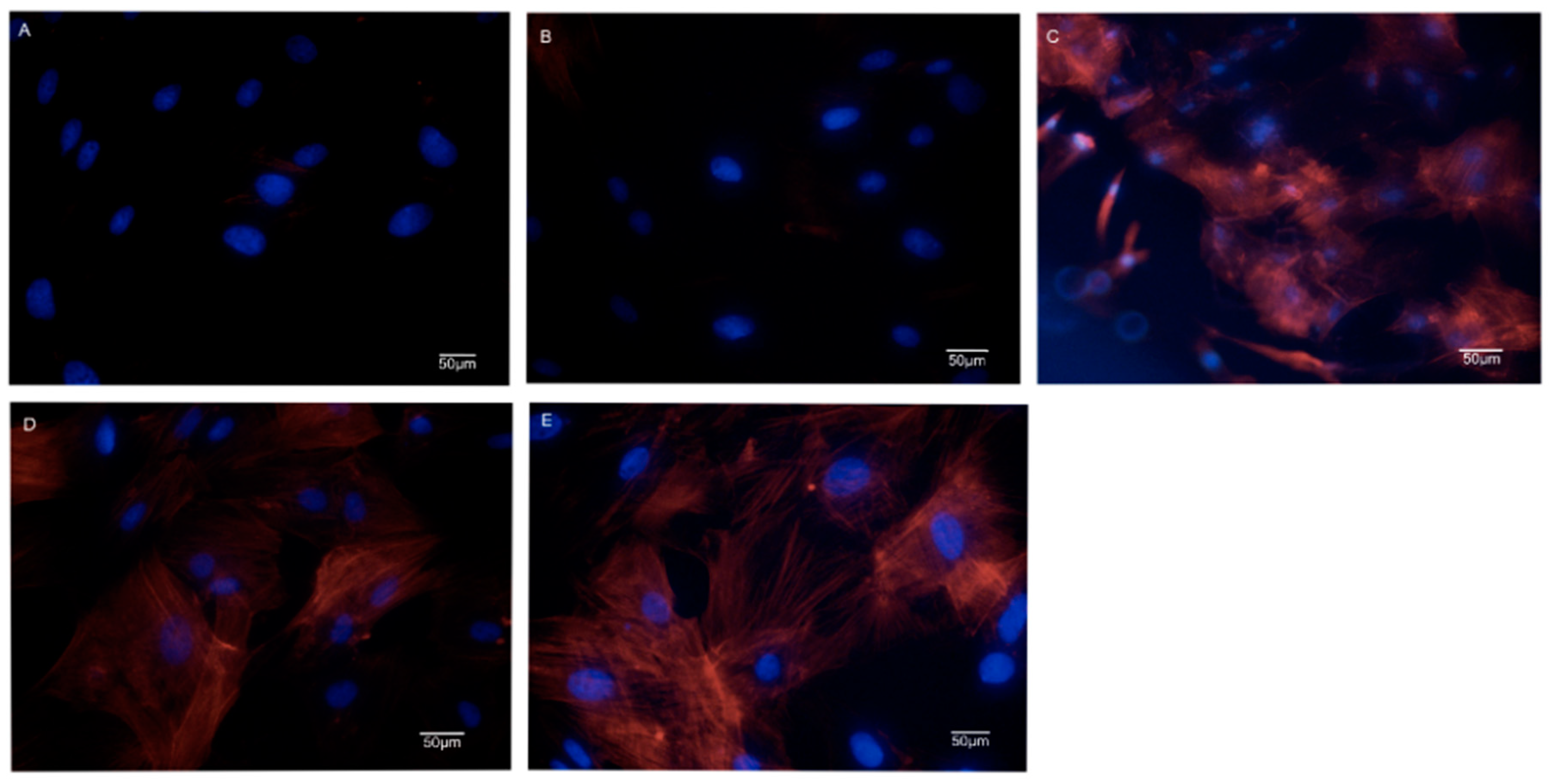
2.4.2. Cell Cytoskeletal Organization of BMSCs on the SF/SPI-Based Composite Scaffolds
2.4.3. Alkaline Phosphatase Activity of BMSCs on the SF/SPI-Based Composite Scaffolds
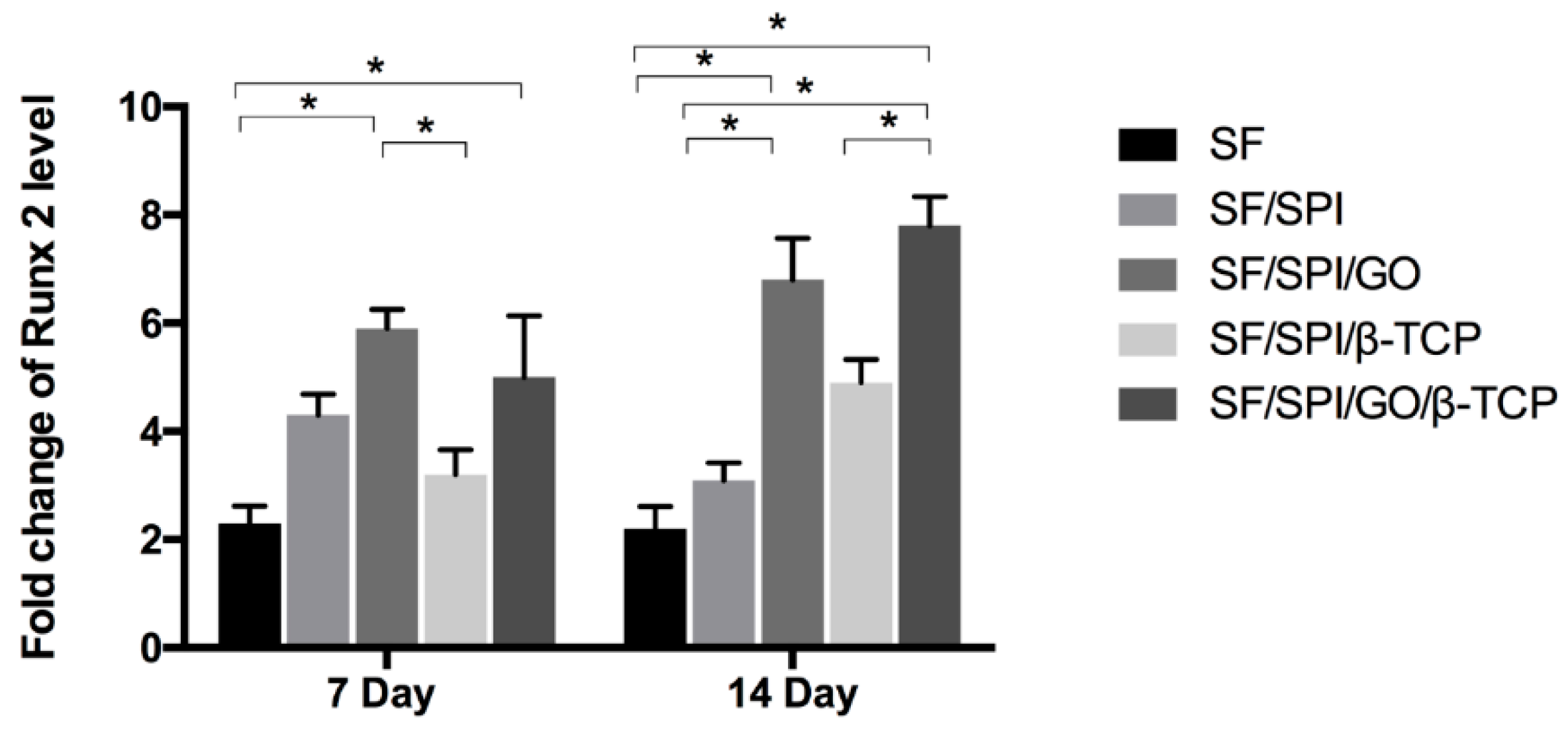
2.4.4. Osteogenesis-Related Gene Expression of BMSCs on the SF/SPI-Based Composite Scaffolds
2.5. Statistics Analysis
3. Results
3.1. Physical and Chemical Properties of the SF/SPI-Based Composite Scaffolds
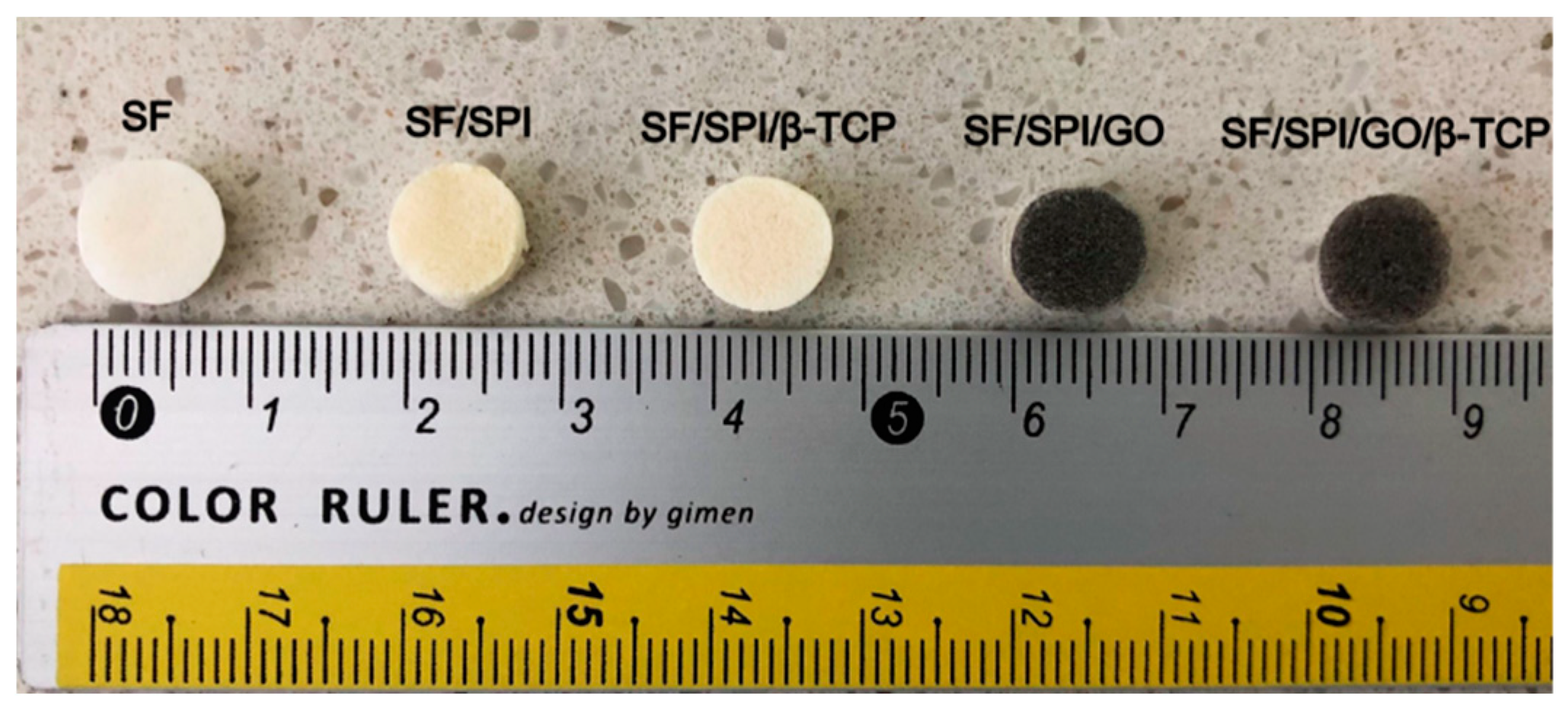
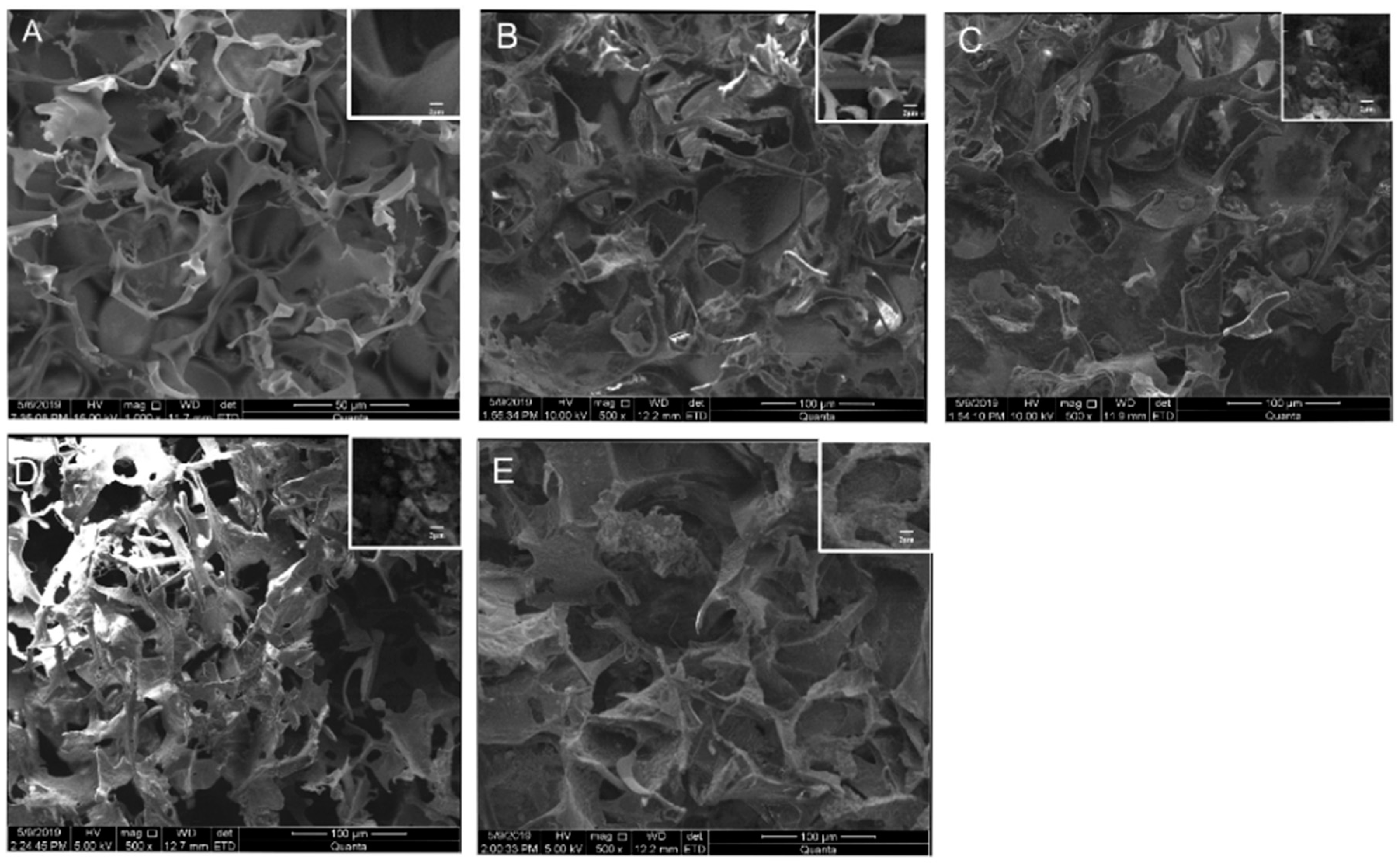
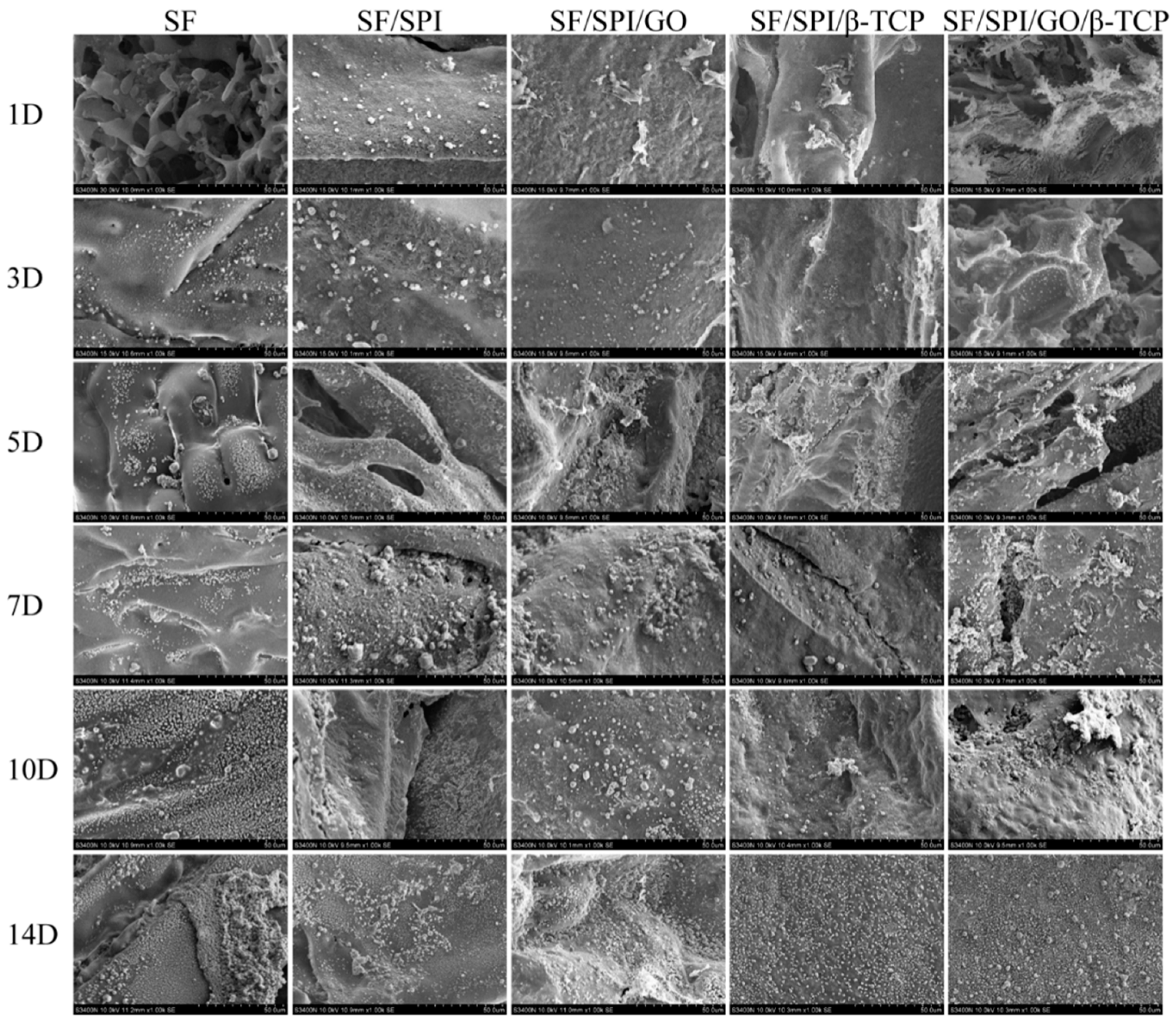
3.1.1. Morphologies of the SF/SPI-Based Composite Scaffolds
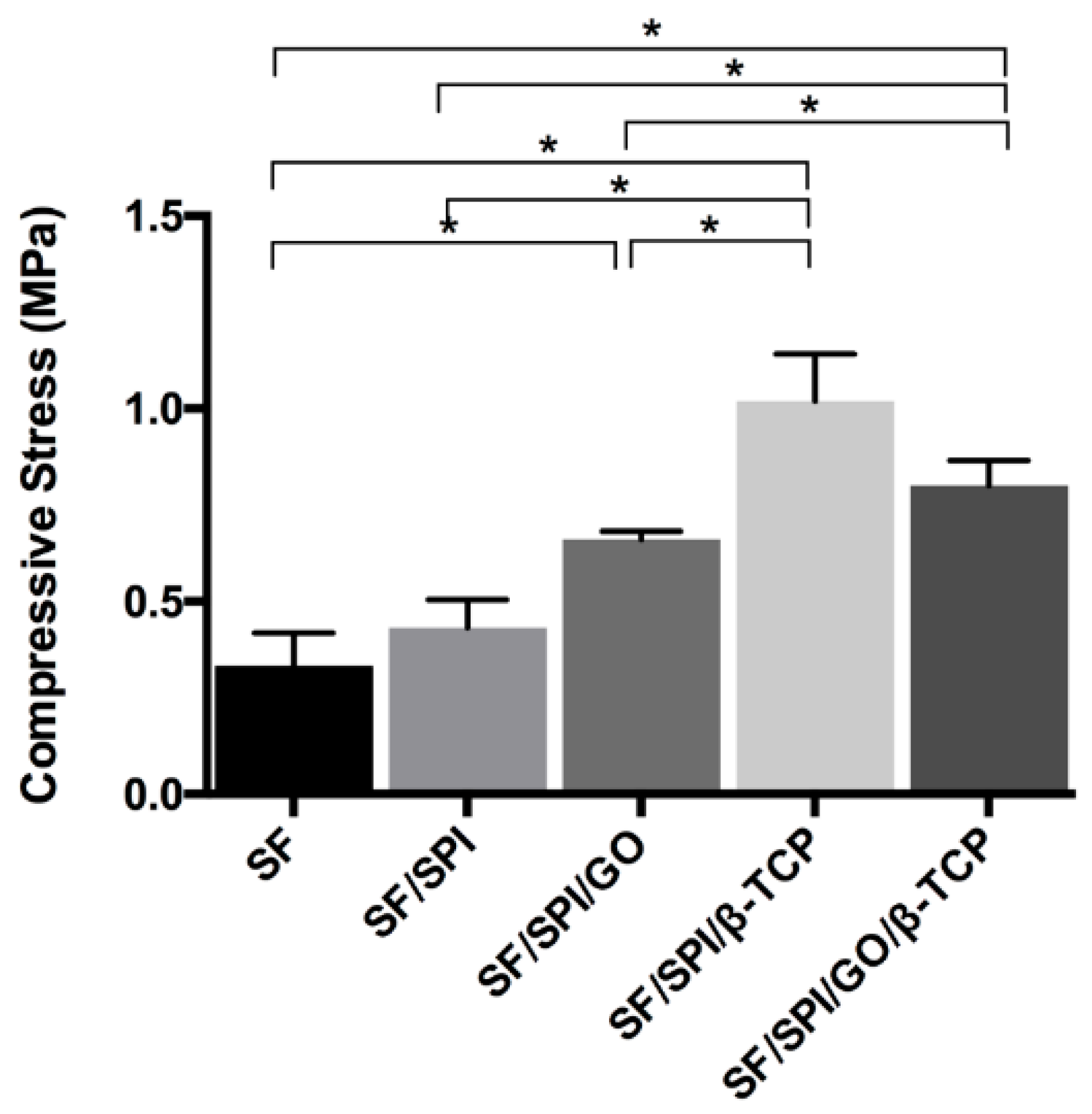
3.1.2. Mechanical Properties of the SF/SPI-Based Composite Scaffolds
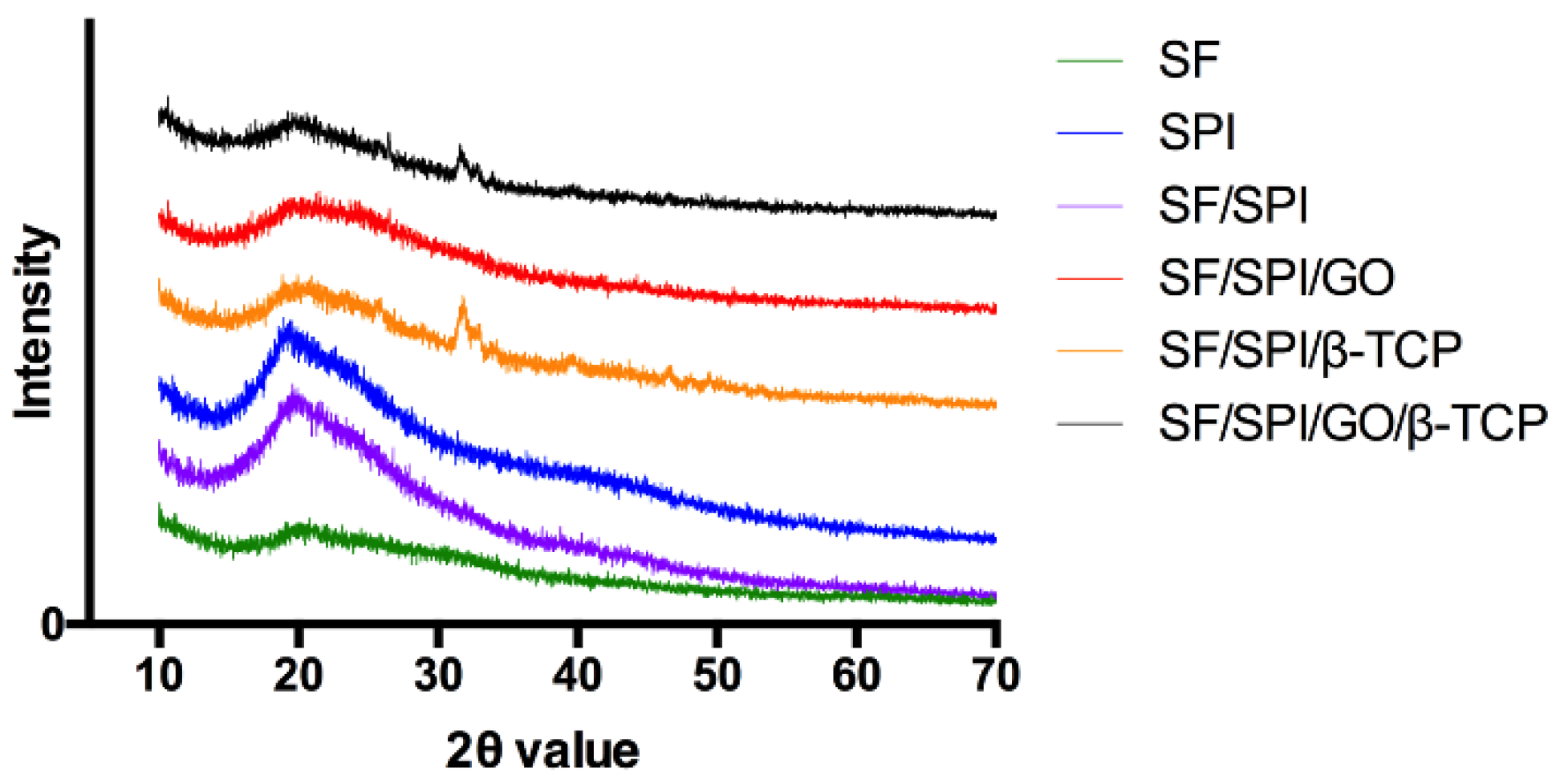
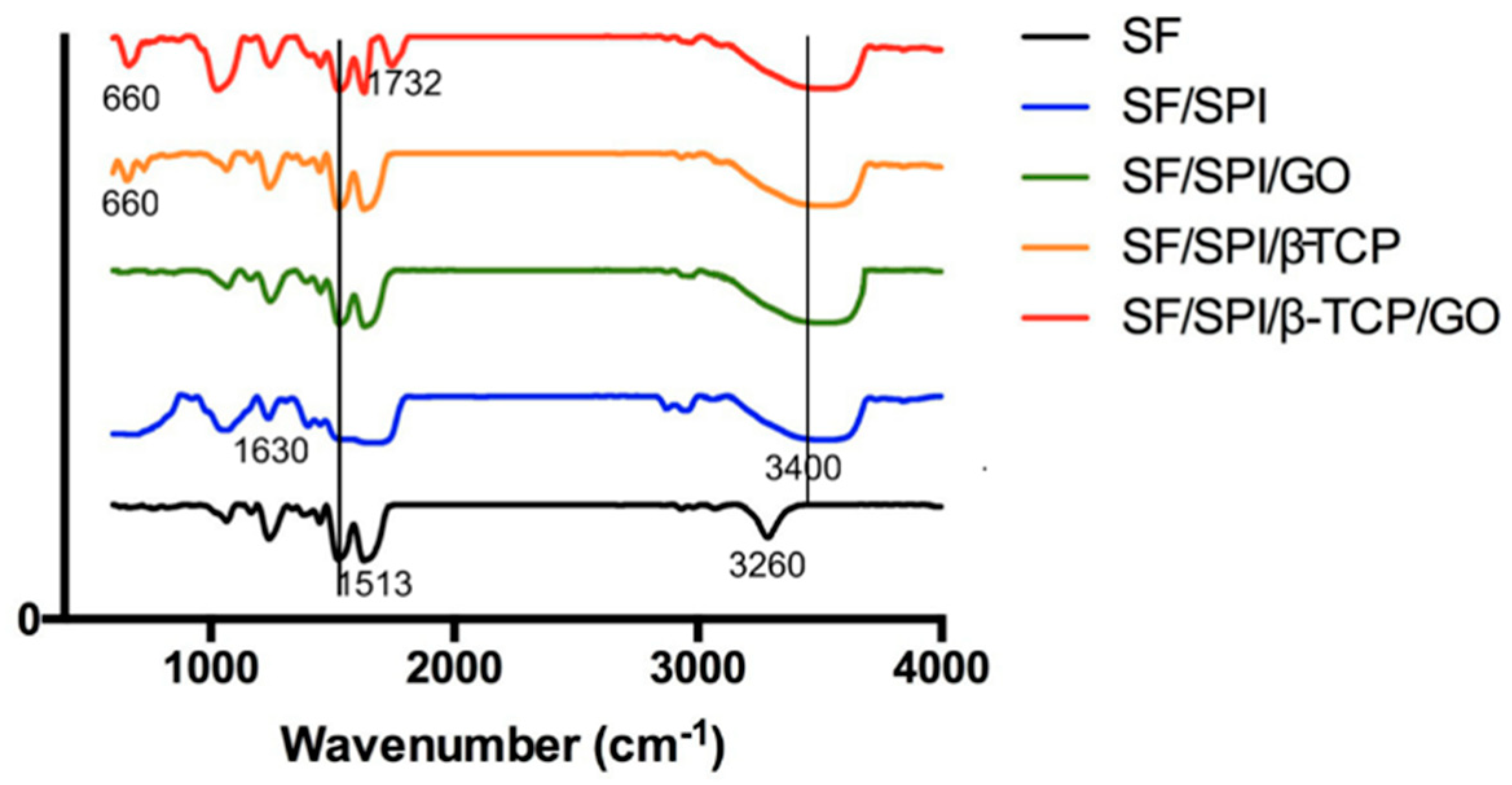
3.1.3. Chemical Constituents of the SF/SPI-Based Composite Scaffolds
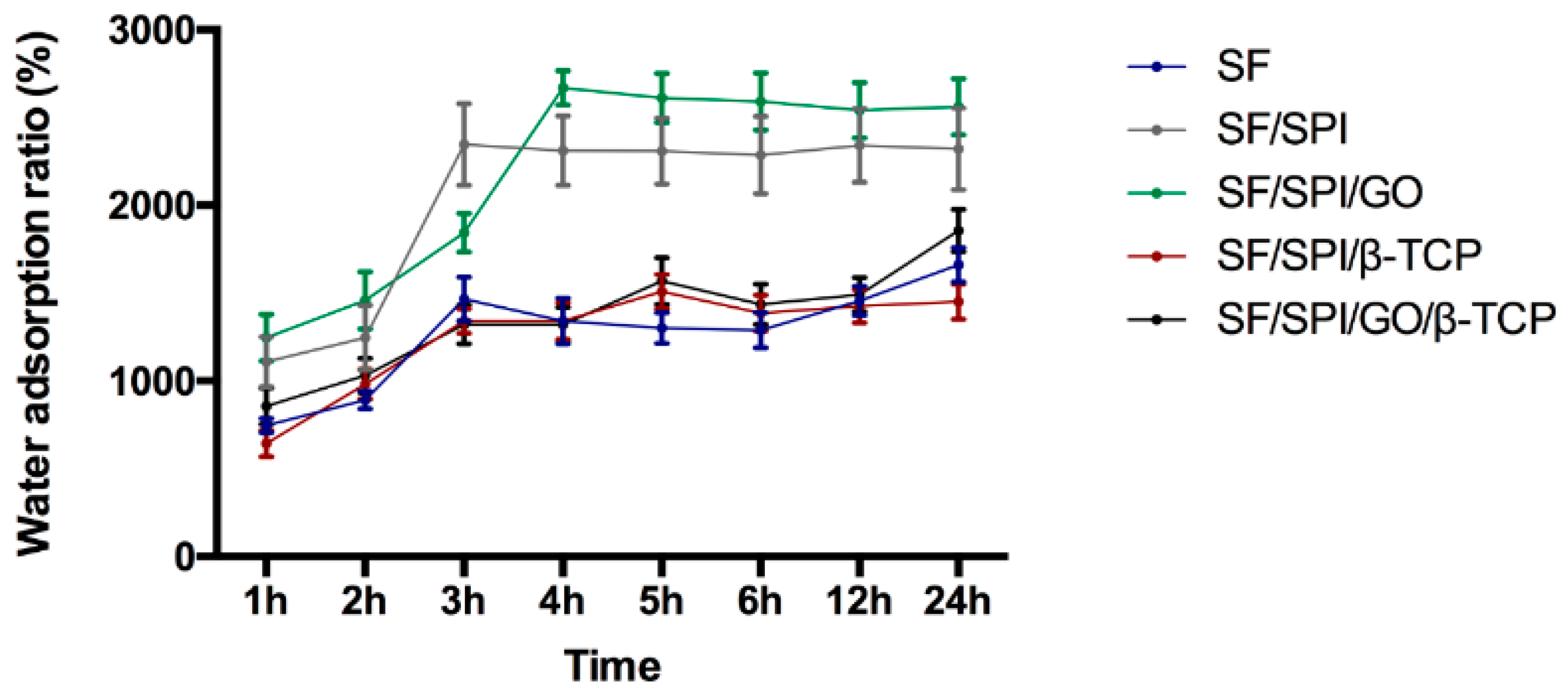
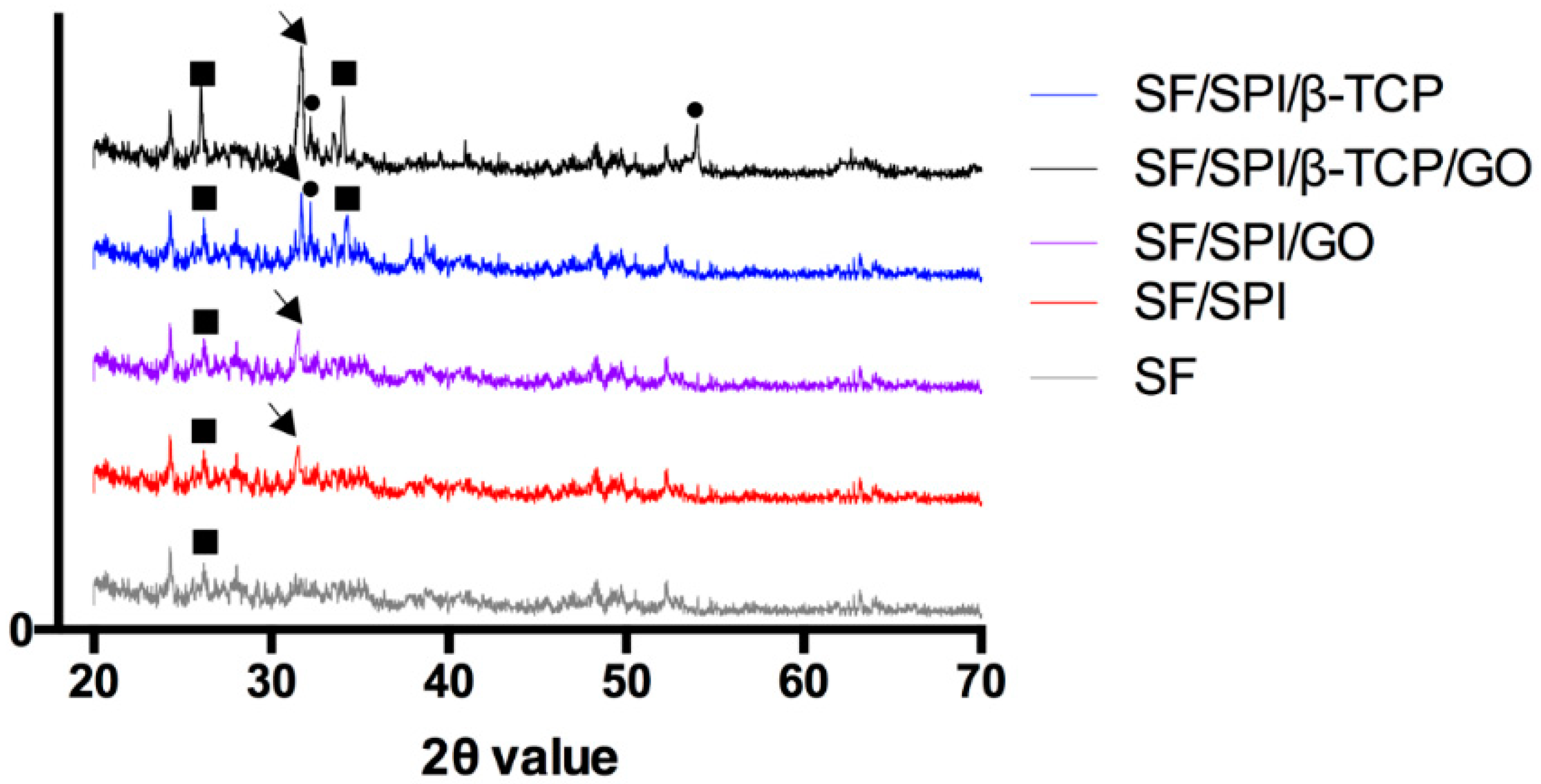
3.2. In Vitro Biomineralization Capability of the SF/SPI-Based Composite Scaffolds
3.3. Morphologies and Proliferation of BMSCs on the SF/SPI-Based Composite Scaffolds
3.4. ALP Activities of BMSCs on the SF/SPI-Based Composite Scaffolds
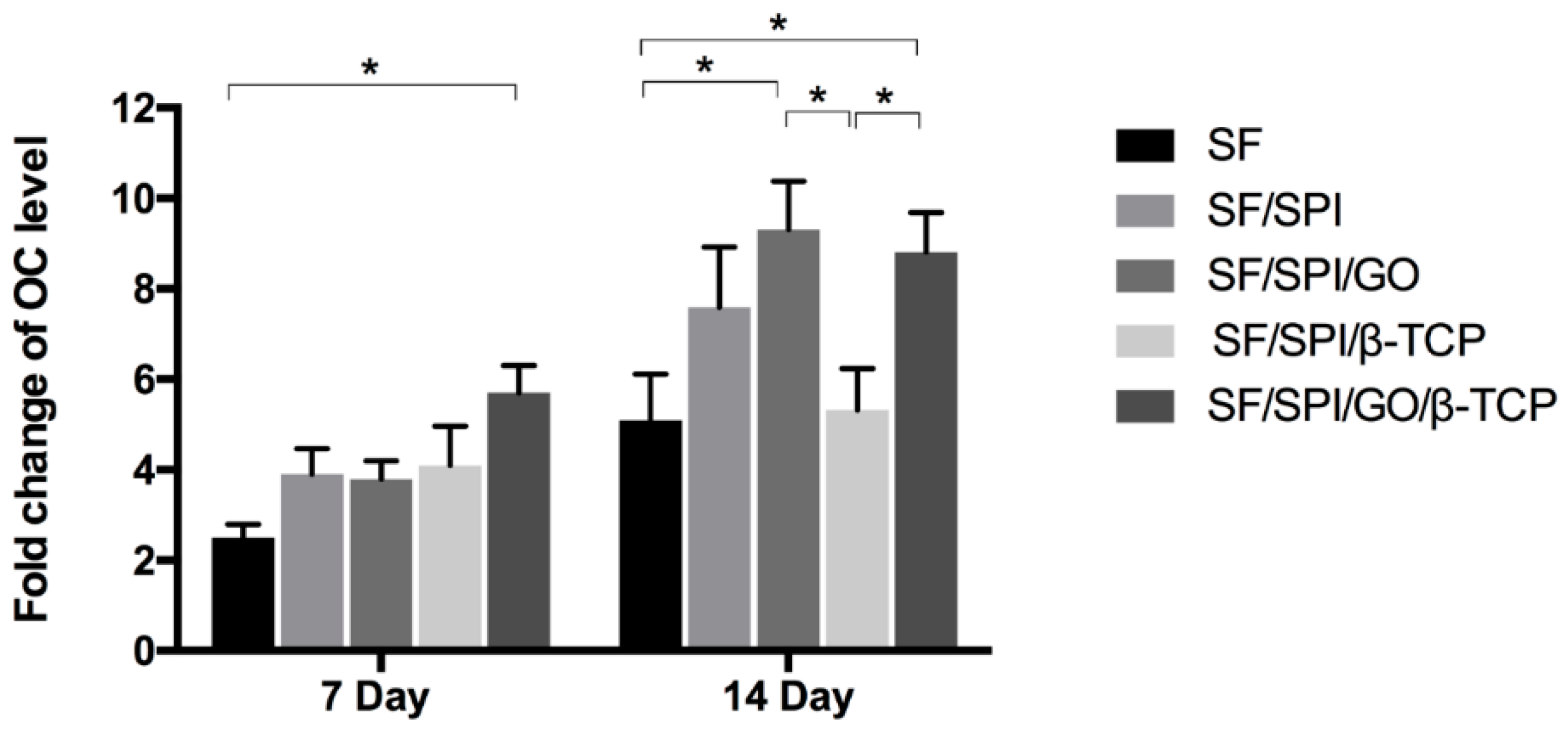
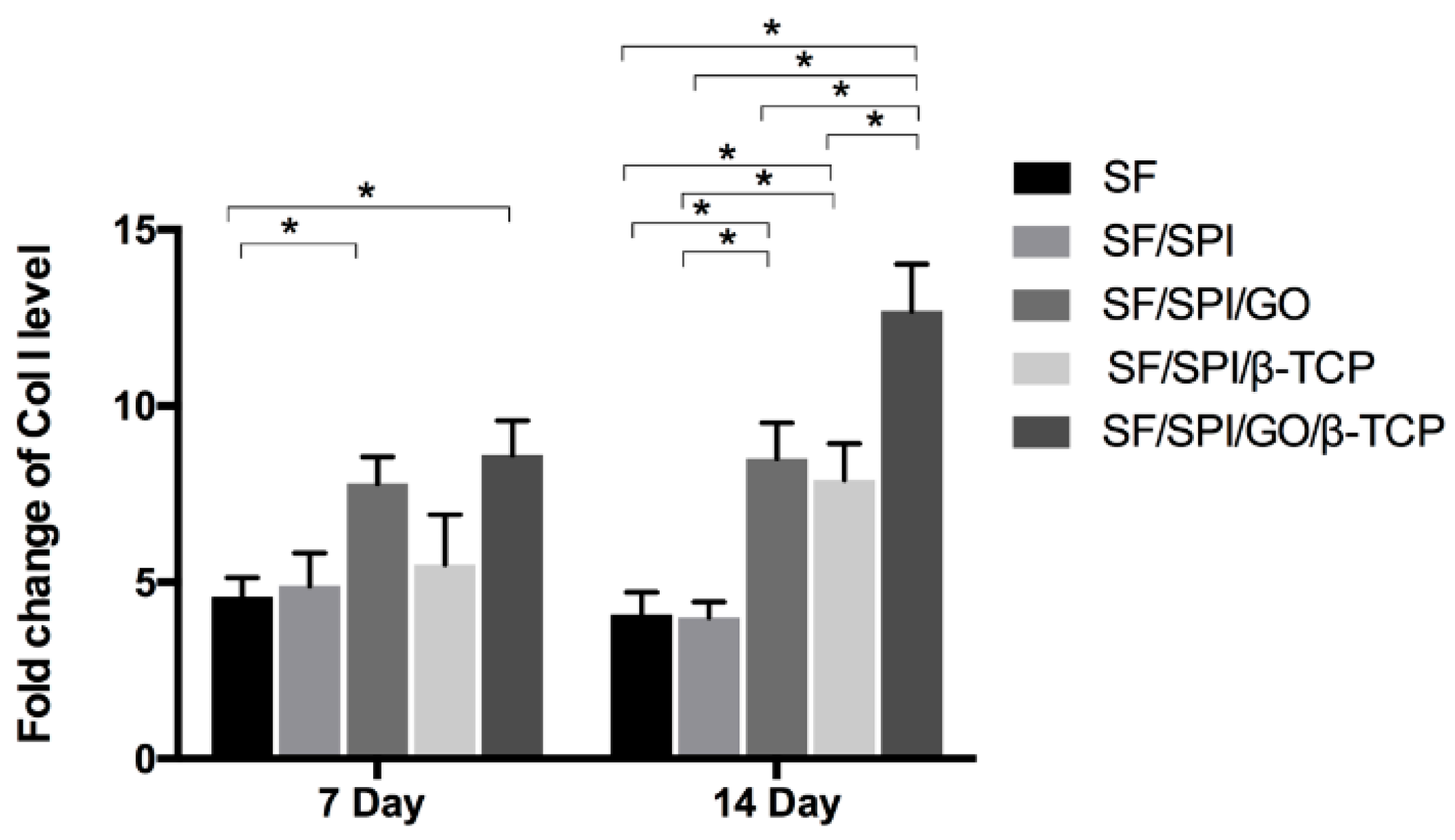
3.5. Osteogenesis-Related Gene Expression of BMSCs on the SF/SPI-Based Composite Scaffolds
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tatara, A.M.; Shah, S.R.; Demian, N.; Ho, T.; Shum, J.; van den Beucken, J.J.J.P.; Jansen, J.A.; Wong, M.E.; Mikos, A.G. Reconstruction of large mandibular defects using autologous tissues generated from in vivo bioreactors. Acta Biomater. 2016, 45, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Hosseinpour, S.; Ghazizadeh Ahsaie, M.; Rezai Rad, M.; Baghani, M.T.; Motamedian, S.R.; Khojasteh, A. Application of selected scaffolds for bone tissue engineering: A systematic review. Oral Maxillofac. Surg. 2017, 21, 109–129. [Google Scholar] [CrossRef] [PubMed]
- Thibaudeau, L.; Taubenberger, A.V.; Holzapfel, B.M.; Quent, V.M.; Fuehrmann, T.; Hesami, P.; Brown, T.D.; Dalton, P.D.; Power, C.A.; Hollier, B.G.; et al. A tissue-engineered humanized xenograft model of human breast cancer metastasis to bone. Dis. Model. Mech. 2014, 7, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Place, E.S.; Evans, N.D.; Stevens, M.M. Complexity in biomaterials for tissue engineering. Nat. Mater. 2009, 8, 457–470. [Google Scholar] [CrossRef]
- Gu, Y.; Bai, Y.; Zhang, D. Osteogenic stimulation of human dental pulp stem cells with a novel gelatin-hydroxyapatite-tricalcium phosphate scaffold. J. Biomed. Mater. Res. A 2018, 106, 1851–1861. [Google Scholar] [CrossRef]
- Kundu, B.; Rajkhowa, R.; Kundu, S.C.; Wang, X. Silk fibroin biomaterials for tissue regenerations. Adv. Drug Deliv. Rev. 2013, 65, 457–470. [Google Scholar] [CrossRef]
- Jin, H.-J.; Chen, J.; Karageorgiou, V.; Altman, G.H.; Kaplan, D.L. Human bone marrow stromal cell responses on electrospun silk fibroin mats. Biomaterials 2004, 25, 1039–1047. [Google Scholar] [CrossRef]
- Liu, F.; Chen, Q.; Liu, C.; Ao, Q.; Tian, X.; Fan, J.; Tong, H.; Wang, X. Natural polymers for organ 3D bioprinting. Polymers 2018, 10, 1278. [Google Scholar] [CrossRef]
- Gaffney-Stomberg, E.; Cao, J.J.; Lin, G.G.; Wulff, C.R.; Murphy, N.E.; Young, A.J.; McClung, J.P.; Pasiakos, S.M. Dietary protein level and source differentially affect bone metabolism, strength, and intestinal calcium transporter expression during ad libitum and food-restricted conditions in male rats. J. Nutr. 2014, 144, 821–829. [Google Scholar] [CrossRef]
- Dirkes, R.K.; Richard, M.W.; Meers, G.M.; Butteiger, D.N.; Krul, E.S.; Thyfault, J.P.; Rector, R.S.; Hinton, P.S. Soy protein isolate suppresses bone resorption and improves trabecular microarchitecture in spontaneously hyperphagic, rapidly growing male OLETF rats. Curr. Dev. Nutr. 2018, 2, nzy010. [Google Scholar] [CrossRef]
- Chen, J.-R.; Lazarenko, O.P.; Blackburn, M.L.; Badger, T.M.; Ronis, M.J.J. Soy Protein Isolate Inhibits High Fat Diet-Induced Senescence Pathways in Osteoblasts to Maintain Bone Acquisition in Male Rats. Endocrinology 2015, 156, 475–487. [Google Scholar] [CrossRef]
- Luo, Y.; Shen, H.; Fang, Y.; Cao, Y.; Huang, J.; Zhang, M.; Dai, J.; Shi, X.; Zhang, Z. Enhanced proliferation and osteogenic differentiation of mesenchymal stem cells on graphene oxide-incorporated electrospun poly(lactic-co-glycolic acid) nanofibrous mats. ACS Appl. Mater. Interfaces 2015, 7, 6331–6339. [Google Scholar] [CrossRef]
- Nayak, T.R.; Andersen, H.; Makam, V.S.; Khaw, C.; Bae, S.; Xu, X.; Ee, P.-L.R.; Ahn, J.-H.; Hong, B.H.; Pastorin, G.; et al. Graphene for controlled and accelerated osteogenic differentiation of human mesenchymal stem cells. ACS Nano 2011, 5, 4670–4678. [Google Scholar] [CrossRef]
- Guo, W.; Wang, S.; Yu, X.; Qiu, J.; Li, J.; Tang, W.; Li, Z.; Mou, X.; Liu, H.; Wang, Z. Construction of a 3D rGO-collagen hybrid scaffold for enhancement of the neural differentiation of mesenchymal stem cells. Nanoscale 2016, 8, 1897–1904. [Google Scholar] [CrossRef]
- Wen, J.H.; Vincent, L.G.; Fuhrmann, A.; Choi, Y.S.; Hribar, K.C.; Taylor-Weiner, H.; Chen, S.; Engler, A.J. Interplay of matrix stiffness and protein tethering in stem cell differentiation. Nat. Mater. 2014, 13, 979–987. [Google Scholar] [CrossRef]
- Mohammadrezaei, D.; Golzar, H.; Rezai Rad, M.; Omidi, M.; Rashedi, H.; Yazdian, F.; Khojasteh, A.; Tayebi, L. In vitro effect of graphene structures as an osteoinductive factor in bone tissue engineering: A systematic review. J. Biomed. Mater. Res. A 2018, 106, 2284–2343. [Google Scholar] [CrossRef]
- Shin, S.R.; Li, Y.-C.; Jang, H.L.; Khoshakhlagh, P.; Akbari, M.; Nasajpour, A.; Zhang, Y.S.; Tamayol, A.; Khademhosseini, A. Graphene-based materials for tissue engineering. Adv. Drug Deliv. Rev. 2016, 105, 255–274. [Google Scholar] [CrossRef]
- Kolanthai, E.; Sindu, P.A.; Khajuria, D.K.; Veerla, S.C.; Kuppuswamy, D.; Catalani, L.H.; Mahapatra, D.R. Graphene oxide-a tool for the preparation of chemically crosslinking free alginate-chitosan-collagen scaffolds for bone tissue engineering. ACS Appl. Mater. Interfaces 2018, 10, 12441–12452. [Google Scholar] [CrossRef]
- Lee, J.H.; Ryu, M.Y.; Baek, H.-R.; Lee, K.M.; Seo, J.-H.; Lee, H.-K.; Ryu, H.-S. Effects of porous beta-tricalcium phosphate-based ceramics used as an E. coli-derived rhBMP-2 carrier for bone regeneration. J. Mater. Sci. Mater. Med. 2013, 24, 2117–2127. [Google Scholar] [CrossRef]
- Duan, R.; Barbieri, D.; Luo, X.; Weng, J.; de Bruijn, J.D.; Yuan, H. Submicron-surface structured tricalcium phosphate ceramic enhances the bone regeneration in canine spine environment. J. Orthop. Res. 2016, 34, 1865–1873. [Google Scholar] [CrossRef]
- Bose, S.; Tarafder, S. Calcium phosphate ceramic systems in growth factor and drug delivery for bone tissue engineering: A review. Acta Biomater. 2012, 8, 1401–1421. [Google Scholar] [CrossRef]
- Liu, F.; Liu, Y.; Li, X.; Wang, X.; Li, D.; Chung, S.; Chen, C.; Lee, I.-S. Osteogenesis of 3D printed macro-pore size biphasic calcium phosphate scaffold in rabbit calvaria. J. Biomater. Appl. 2019, 33, 1168–1177. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zheng, Z.; Li, G.; Kaplan, D.L.; Wang, X. Control of silk microsphere formation using polyethylene glycol (PEG). Acta Biomater. 2016, 39, 156–168. [Google Scholar] [CrossRef]
- Wang, X.; Li, D.; Wang, W.; Feng, Q.; Cui, F.; Xu, Y.; Song, X.; van der Werf, M. Crosslinked collagen/chitosan matrices for artificial livers. Biomaterials 2003, 24, 3213–3220. [Google Scholar] [CrossRef]
- Wang, X.; Yan, Y.; Lin, F.; Xiong, Z.; Wu, R.; Zhang, R.; Lu, Q. Preparation and characterization of a collagen/chitosan/heparin matrix for an implantable bioartificial liver. J. Biomater. Sci. Polym. E 2005, 16, 1063–1080. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Chen, Y.-P.; Han, J.; Mo, J.; Dong, P.-F.; Zhuo, Y.-H.; Feng, Y. Biocompatiable silk fibroin/carboxymethyl chitosan/strontium substituted hydroxyapatite/cellulose nanocrystal composite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2019, 136, 1247–1257. [Google Scholar] [CrossRef]
- Zou, L.; Zhang, Y.; Liu, X.; Chen, J.; Zhang, Q. Biomimetic mineralization on natural and synthetic polymers to prepare hybrid scaffolds for bone tissue engineering. Colloids Surf. B Biointerfaces 2019, 178, 222–229. [Google Scholar] [CrossRef]
- Deen, I.; Rosei, F. Silk fibroin-derived polypeptides additives to promote hydroxyapatite nucleation in dense collagen hydrogels. PLoS ONE 2019, 14, e0219429. [Google Scholar] [CrossRef]
- Park, H.J.; Min, K.D.; Lee, M.C.; Kim, S.H.; Lee, O.J.; Ju, H.W.; Moon, B.M.; Lee, J.M.; Park, Y.R.; Kim, D.W.; et al. Fabrication of 3D porous SF/beta-TCP hybrid scaffolds for bone tissue reconstruction. J. Biomed. Mater. Res. A 2016, 104, 1779–1787. [Google Scholar] [CrossRef]
- Garrido, T.; Etxabide, A.; Guerrero, P.; de la Caba, K. Characterization of agar/soy protein biocomposite films: Effect of agar on the extruded pellets and compression moulded films. Carbohydr. Polym. 2016, 151, 408–416. [Google Scholar] [CrossRef]
- Lee, D.H.; Tripathy, N.; Shin, J.H.; Song, J.E.; Cha, J.G.; Min, K.D.; Park, C.H.; Khang, G. Enhanced osteogenesis of beta-tricalcium phosphate reinforced silk fibroin scaffold for bone tissue biofabrication. Int. J. Biol. Macromol. 2017, 95, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Baheiraei, N.; Nourani, M.R.; Mortazavi, S.M.J.; Movahedin, M.; Eyni, H.; Bagheri, F.; Norahan, M.H. Development of a bioactive porous collagen/beta-tricalcium phosphate bone graft assisting rapid vascularization for bone tissue engineering applications. J. Biomed. Mater. Res. A 2018, 106, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Shamekhi, M.A.; Mirzadeh, H.; Mahdavi, H.; Rabiee, A.; Mohebbi-Kalhori, D.; Baghaban Eslaminejad, M. Graphene oxide containing chitosan scaffolds for cartilage tissue engineering. Int. J. Biol. Macromol. 2019, 127, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Cheng, B.; Chen, K.; Cui, W.; Qi, J.; Li, X.; Deng, L. Enhanced osteogenesis of bone marrow-derived mesenchymal stem cells by a functionalized silk fibroin hydrogel for bone defect repair. Adv. Healthc. Mater. 2019, 8, e1801043. [Google Scholar] [CrossRef] [PubMed]
- Chien, K.B.; Makridakis, E.; Shah, R.N. Three-dimensional printing of soy protein scaffolds for tissue regeneration. Tissue Eng. Part C Methods 2013, 19, 417–426. [Google Scholar] [CrossRef]
- Chien, K.B.; Shah, R.N. Novel soy protein scaffolds for tissue regeneration: Material characterization and interaction with human mesenchymal stem cells. Acta Biomater. 2012, 8, 694–703. [Google Scholar] [CrossRef]
- Chen, B.-Q.; Kankala, R.K.; Chen, A.-Z.; Yang, D.-Z.; Cheng, X.-X.; Jiang, N.-N.; Zhu, K.; Wang, S.-B. Investigation of silk fibroin nanoparticle-decorated poly (l-lactic acid) composite scaffolds for osteoblast growth and differentiation. Int. J. Nanomed. 2017, 12, 1877–1890. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Castilho, M.; Moseke, C.; Ewald, A.; Gbureck, U.; Groll, J.; Pires, I.; Tessmar, J.; Vorndran, E. Direct 3D powder printing of biphasic calcium phosphate scaffolds for substitution of complex bone defects. Biofabrication 2014, 6, 15006. [Google Scholar] [CrossRef]
- Li, J.; Zhi, W.; Xu, T.; Shi, F.; Duan, K.; Wang, J.; Mu, Y.; Weng, J. Ectopic osteogenesis and angiogenesis regulated by porous architecture of hydroxyapatite scaffolds with similar interconnecting structure in vivo. Regen. Biomater. 2016, 3, 285–297. [Google Scholar] [CrossRef]
- Mygind, T.; Stiehler, M.; Baatrup, A.; Li, H.; Zou, X.; Flyvbjerg, A.; Kassem, M.; Bunger, C. Mesenchymal stem cell ingrowth and differentiation on coralline hydroxyapatite scaffolds. Biomaterials 2007, 28, 1036–1047. [Google Scholar] [CrossRef] [PubMed]
- Deligianni, D.D.; Katsala, N.D.; Koutsoukos, P.G.; Missirlis, Y.F. Effect of surface roughness of hydroxyapatite on human bone marrow cell adhesion, proliferation, differentiation and detachment strength. Biomaterials 2001, 22, 87–96. [Google Scholar] [CrossRef]
- Ionita, M.; Pandele, M.A.; Iovu, H. Sodium alginate/graphene oxide composite films with enhanced thermal and mechanical properties. Carbohydr. Polym. 2013, 94, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Bayraktar, H.H.; Morgan, E.F.; Niebur, G.L.; Morris, G.E.; Wong, E.K.; Keaveny, T.M. Comparison of the elastic and yield properties of human femoral trabecular and cortical bone tissue. J. Biomech. 2004, 37, 27–35. [Google Scholar] [CrossRef]
- Wang, X.; Ma, J.; Wang, Y.; He, B. Structural characterization of phosphorylated chitosan and their applications as effective additives of calcium phosphate cements. Biomaterials 2001, 22, 2247–2255. [Google Scholar] [CrossRef]
- Wang, X.; Ma, J.; Wang, Y.; He, B. Bone repair in radii and tibias of rabbits with phosphorylated chitosan reinforced calcium phosphate cements. Biomaterials 2002, 23, 4167–4176. [Google Scholar] [CrossRef]
- Wang, X.; Ma, J.; Feng, Q.; Cui, F. Skeletal repair in of rabbits with calcium phosphate cements incorporated phosphorylated chitin reinforced. Biomaterials 2002, 23, 4591–4600. [Google Scholar] [CrossRef]
- Wang, X.; Ma, J.; Feng, Q.; Cui, F. In vivo testing of S-chitosan enhanced calcium phosphate cements. J. Bioact. Compat. Polym. 2003, 18, 259–271. [Google Scholar] [CrossRef]
- Wang, X.; Ma, J.; Feng, Q.; Cui, F. The effects of S-chitosan on the physical properties of calcium phosphate cements. J. Bioact. Compat. Polym. 2003, 18, 45–57. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Zhu, P.; Masuda, Y.; Koumoto, K. The effect of surface charge on hydroxyapatite nucleation. Biomaterials 2004, 25, 3915–3921. [Google Scholar] [CrossRef] [PubMed]
- Turk, S.; Altinsoy, I.; Celebi Efe, G.; Ipek, M.; Ozacar, M.; Bindal, C. 3D porous collagen/functionalized multiwalled carbon nanotube/chitosan/hydroxyapatite composite scaffolds for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 92, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Wang, X. Overview on biocompatibilities of implantable biomaterials. In Advances in Biomaterials Science and Biomedical Applications in Biomedicine; Lazinica, R., Ed.; In Tech: Rijeka, Croatia, 2013; pp. 111–155. [Google Scholar]
- Zhao, X.; Liu, L.; Wang, J.; Xu, Y.F.; Zhang, W.M.; Khang, G.; Wang, X. In vitro vascularization of a combined system based on a 3D bioprinting technique. J. Tissue Eng. Regen. Med. 2014, 10, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.; Wang, X. Biodegradable polymers and stem cells for bioprinting. Molecules 2016, 21, 539. [Google Scholar] [CrossRef] [PubMed]
- Wang, X. Spatial effects of stem cell engagement in 3D printing constructs. J. Stem Cells Res. Rev. Rep. 2014, 1, 5–9. [Google Scholar]
- Wang, X.; Xu, H. Incorporation of DMSO and dextran-40 into a gelatin/alginate hydrogel for controlled assembled cell cryopreservation. Cryobiology 2010, 61, 345–351. [Google Scholar] [CrossRef]
- Saravanan, S.; Chawla, A.; Vairamani, M.; Sastry, T.P.; Subramanian, K.S.; Selvamurugan, N. Scaffolds containing chitosan, gelatin and graphene oxide for bone tissue regeneration in vitro and in vivo. Int. J. Biol. Macromol. 2017, 104, 1975–1985. [Google Scholar] [CrossRef]
- Meskinfam, M.; Bertoldi, S.; Albanese, N.; Cerri, A.; Tanzi, M.C.; Imani, R.; Baheiraei, N.; Farokhi, M.; Fare, S. Polyurethane foam/nano hydroxyapatite composite as a suitable scaffold for bone tissue regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 82, 130–140. [Google Scholar] [CrossRef]
- Wang, X.; Ao, Q.; Tian, X.; Fan, J.; Wei, Y.; Hou, W.; Tong, H.; Bai, S. 3D bioprinting technologies for hard tissue and organ engineering. Materials 2016, 9, 802. [Google Scholar] [CrossRef]
- Wang, X.; Ao, Q.; Tian, X.; Fan, J.; Wei, Y.; Tong, H.; Hou, W.; Bai, S. Gelatin-based hydrogels for organ 3D bioprinting. Polymers 2017, 9, 401. [Google Scholar] [CrossRef]
- Li, S.; Tian, X.; Fan, J.; Tong, H.; Ao, Q.; Wang, X. Chitosans for tissue repair and organ three-dimensional (3D) bioprinting. Micromachines 2019, 10, 765. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, C. Fibrin hydrogels for endothelialized liver tissue engineering with a predesigned vascular network. Polymers 2018, 10, 1048. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, X. Hared tissue and organ manufacturing. In Organ Manufacturing; Wang, X., Ed.; Nova Science Publishers Inc.: New York, NY, USA, 2015; pp. 301–333. [Google Scholar]
- Liu, L.; Zhou, X.; Xu, Y.; Zhang, W.M.; Liu, C.-H.; Wang, X.H. Controlled release of growth factors for regenerative medicine. Curr. Pharm. Des. 2015, 21, 1627–1632. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, X. 3D biomimetic models for drug delivery and regenerative medicine. Curr. Pharm. Des. 2015, 21, 1618–1626. [Google Scholar] [CrossRef] [PubMed]
- Wang, X. Advanced polymers for three-dimensional (3D) organ bioprinting. Micromachines 2019, 10, 814. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, C. 3D bioprinting of adipose-derived stem cells for organ manufacturing. In Enabling Cutting Edge Technology for Regenerative Medicine; Springer: Singapore, 2018; Chapter 1; pp. 3–14. [Google Scholar]
- Wang, X. Bioartificial organ manufacturing technologies. Cell Transplant. 2018, 27, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Tatavarty, R.; Ding, H.; Lu, G.; Taylor, R.J.; Bi, X. Synergistic acceleration in the osteogenesis of human mesenchymal stem cells by graphene oxide-calcium phosphate nanocomposites. Chem. Commun. 2014, 50, 8484–8487. [Google Scholar] [CrossRef]
- Bressan, E.; Ferroni, L.; Gardin, C.; Sbricoli, L.; Gobbato, L.; Ludovichetti, F.S.; Tocco, I.; Carraro, A.; Piattelli, A.; Zavan, B. Graphene based scaffolds effects on stem cells commitment. J. Transl. Med. 2014, 12, 296. [Google Scholar] [CrossRef]
- Wei, G.; Gong, C.; Hu, K.; Wang, Y.; Zhang, Y. Biomimetic hydroxyapatite on graphene supports for biomedical applications: A review. Nanomaterials 2019, 9, 1435. [Google Scholar] [CrossRef]
- LeGeros, R.Z. Calcium phosphate-based osteoinductive materials. Chem. Rev. 2008, 108, 4742–4753. [Google Scholar] [CrossRef]

| Genes | Forward Primer | Reverse Primer |
|---|---|---|
| Runx2 | CGCCTCACAAACAACCACAG | TCACTGTGCTGAAGAGGCTG |
| OC | CATGAAGGCTTTGTCAGACT | CTCTCTCTGCTCACTCTGCT |
| Col I | CCACCCCAGGGATAAAAACT | GGAGAGGAGTGCCAACTCCAG |
| GAPDH | AGTGCCAGCCTCGTCTCATA | GATGGTGATGGGTTTCCCGT |
| SF | SF/SPI | SF/SPI/GO | SF/SPI/β-TCP | SF/SPI/β-TCP/GO | |
|---|---|---|---|---|---|
| Pore size (μm) | 117.76 ± 6.33 | 113.37 ± 6.33 | 108 ± 6.33 | 232.53 ± 4.09 | 194 ± 6.18 |
| Porosity (%) | 79.32 ± 1.62 | 82.28 ± 2.15 | 87.66 ± 2.77 | 82.63 ± 1.04 | 80.45 ± 2.04 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, F.; Liu, C.; Zheng, B.; He, J.; Liu, J.; Chen, C.; Lee, I.-s.; Wang, X.; Liu, Y. Synergistic Effects on Incorporation of β-Tricalcium Phosphate and Graphene Oxide Nanoparticles to Silk Fibroin/Soy Protein Isolate Scaffolds for Bone Tissue Engineering. Polymers 2020, 12, 69. https://doi.org/10.3390/polym12010069
Liu F, Liu C, Zheng B, He J, Liu J, Chen C, Lee I-s, Wang X, Liu Y. Synergistic Effects on Incorporation of β-Tricalcium Phosphate and Graphene Oxide Nanoparticles to Silk Fibroin/Soy Protein Isolate Scaffolds for Bone Tissue Engineering. Polymers. 2020; 12(1):69. https://doi.org/10.3390/polym12010069
Chicago/Turabian StyleLiu, Fan, Chen Liu, Bowen Zheng, Jia He, Jun Liu, Cen Chen, In-seop Lee, Xiaohong Wang, and Yi Liu. 2020. "Synergistic Effects on Incorporation of β-Tricalcium Phosphate and Graphene Oxide Nanoparticles to Silk Fibroin/Soy Protein Isolate Scaffolds for Bone Tissue Engineering" Polymers 12, no. 1: 69. https://doi.org/10.3390/polym12010069
APA StyleLiu, F., Liu, C., Zheng, B., He, J., Liu, J., Chen, C., Lee, I.-s., Wang, X., & Liu, Y. (2020). Synergistic Effects on Incorporation of β-Tricalcium Phosphate and Graphene Oxide Nanoparticles to Silk Fibroin/Soy Protein Isolate Scaffolds for Bone Tissue Engineering. Polymers, 12(1), 69. https://doi.org/10.3390/polym12010069








