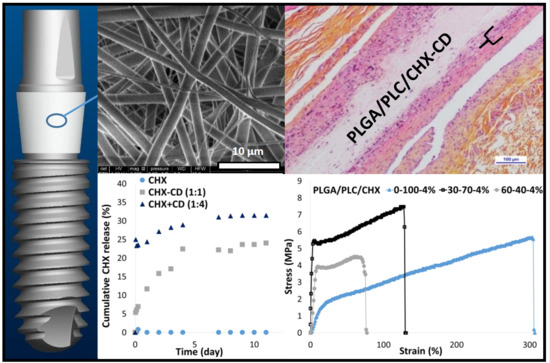
Electrospun Bioresorbable Membrane Eluting Chlorhexidine for Dental Implants
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. CHX Complexation with CD
2.2.1. Membrane Electrospinning
2.2.2. Membrane Sterilization
2.3. Scanning Electron Microscopy (SEM)–Energy Dispersive X-ray Spectroscopy (EDS)
2.4. X-ray Photoelectron Spectroscopy (XPS)
2.5. Fourier-Transform Infrared Spectroscopy (FTIR)
2.6. Differential Scanning Calorimetry (DSC)
2.7. In Vitro Drug Release Study
2.8. Mechanical Characterization
2.9. In Vivo Test
2.9.1. Animal Protocol
2.9.2. Histologic Preparation
2.9.3. Histopathologic Evaluation
3. Results and Discussion
3.1. Membrane Formulation Design
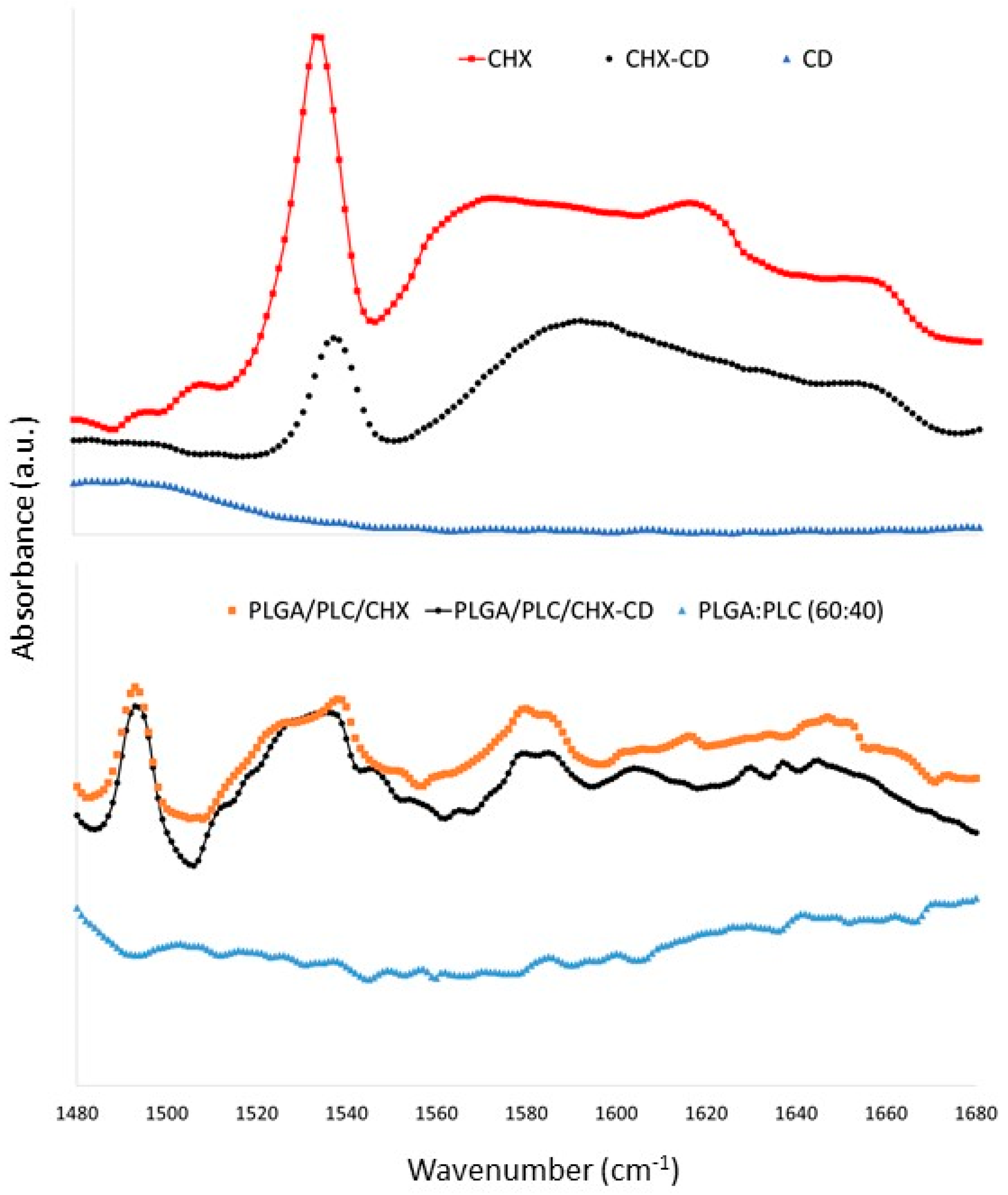
3.2. Membrane Characterizations
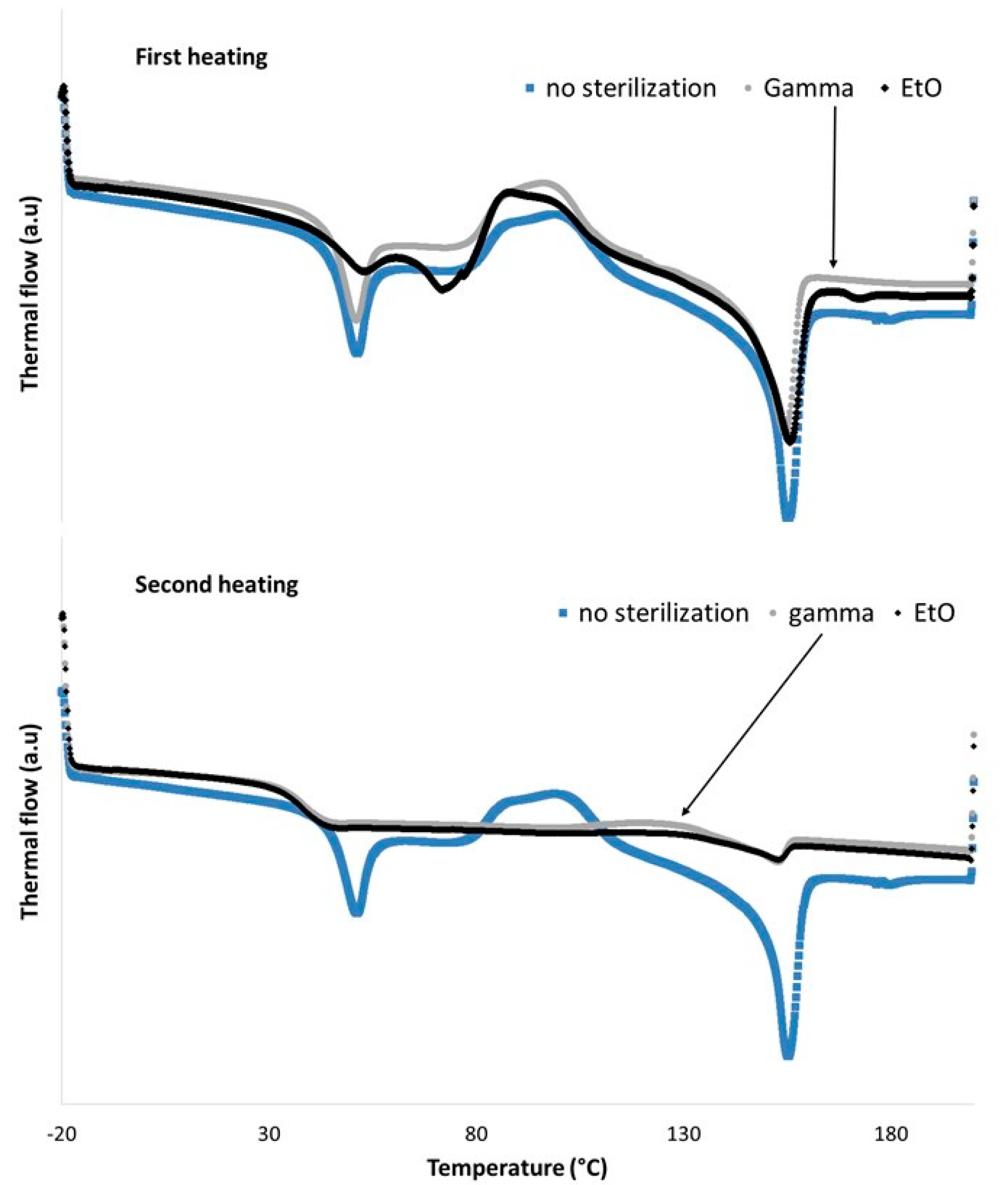
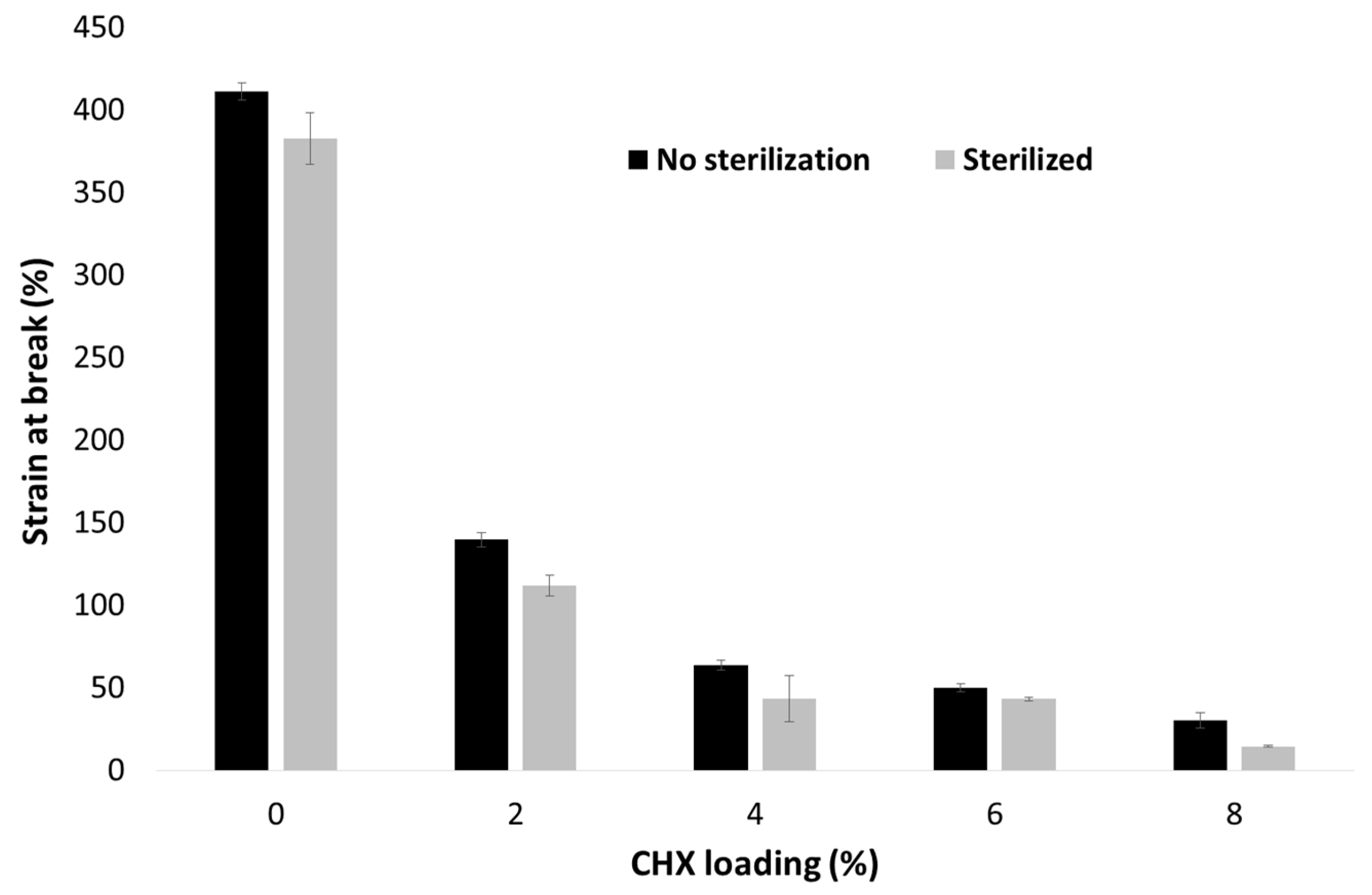
3.3. Selection of the Sterilization Method
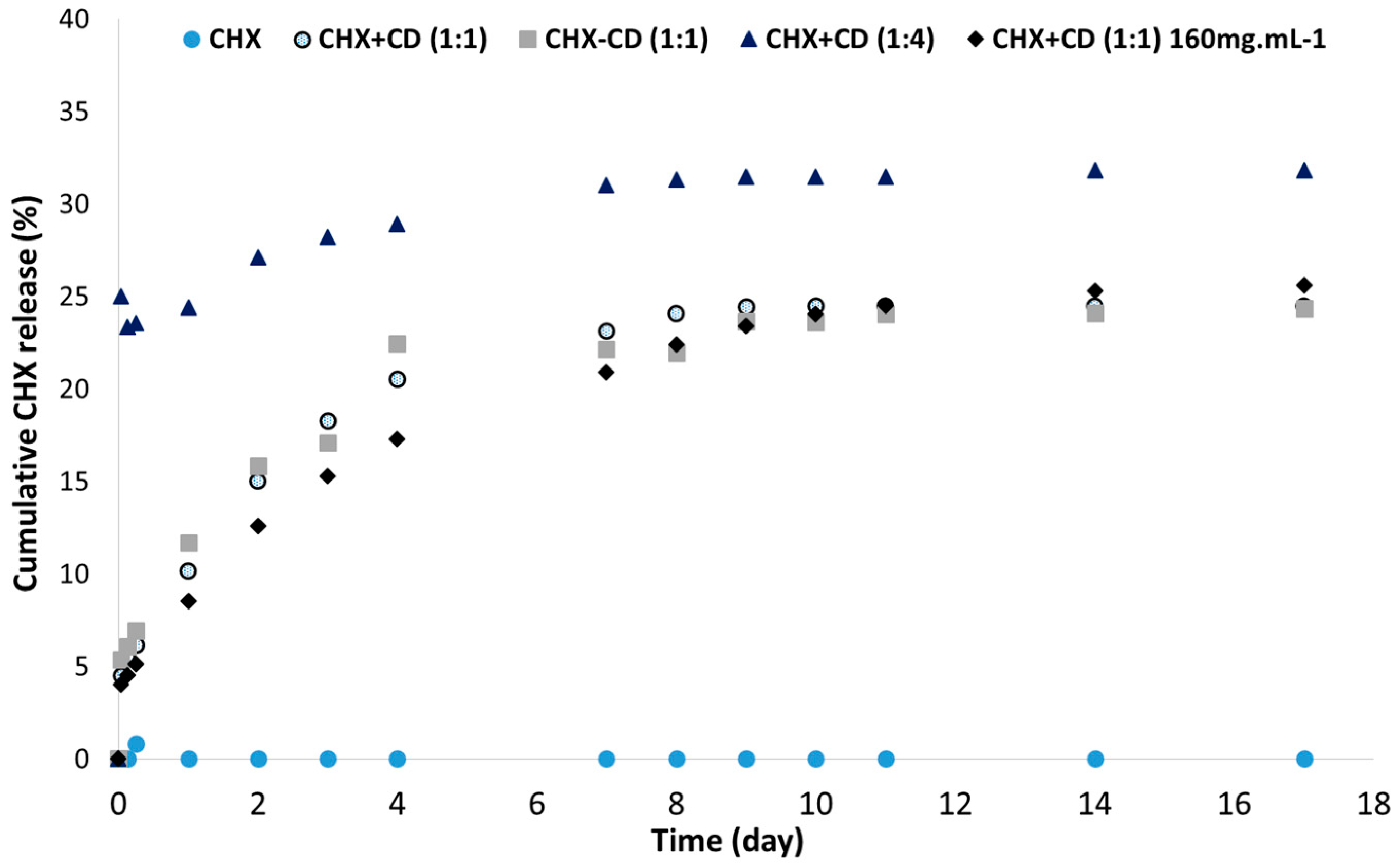
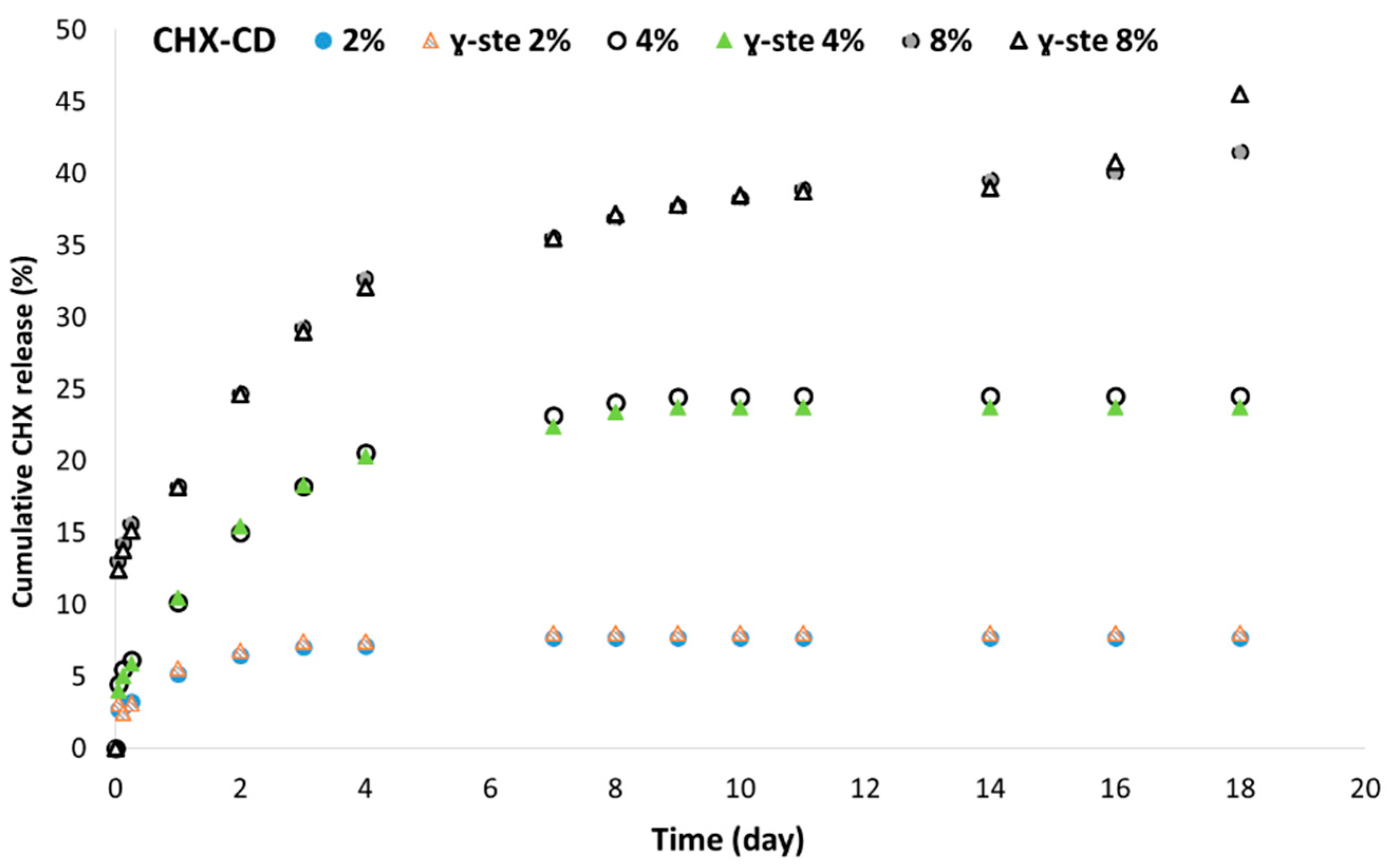
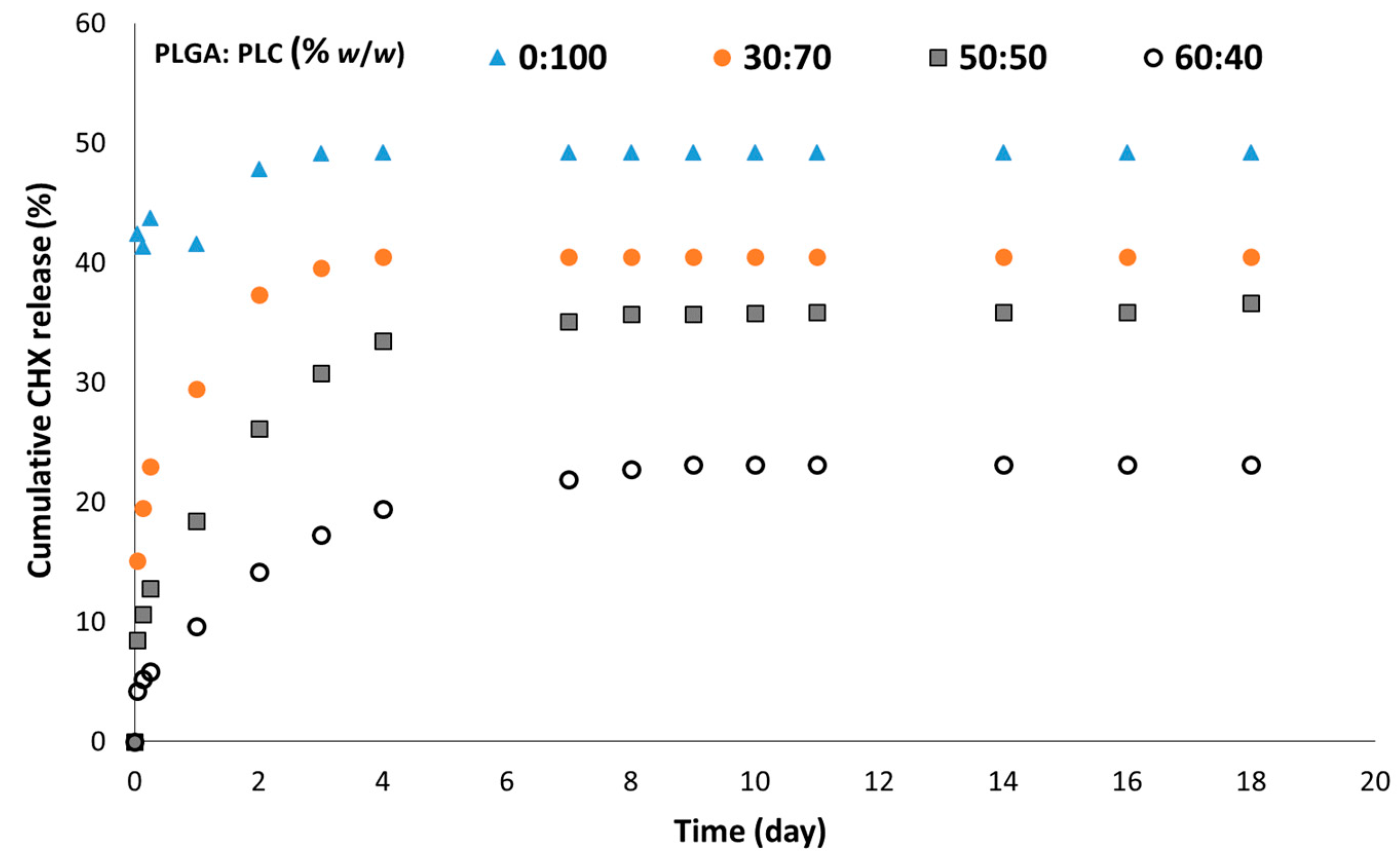
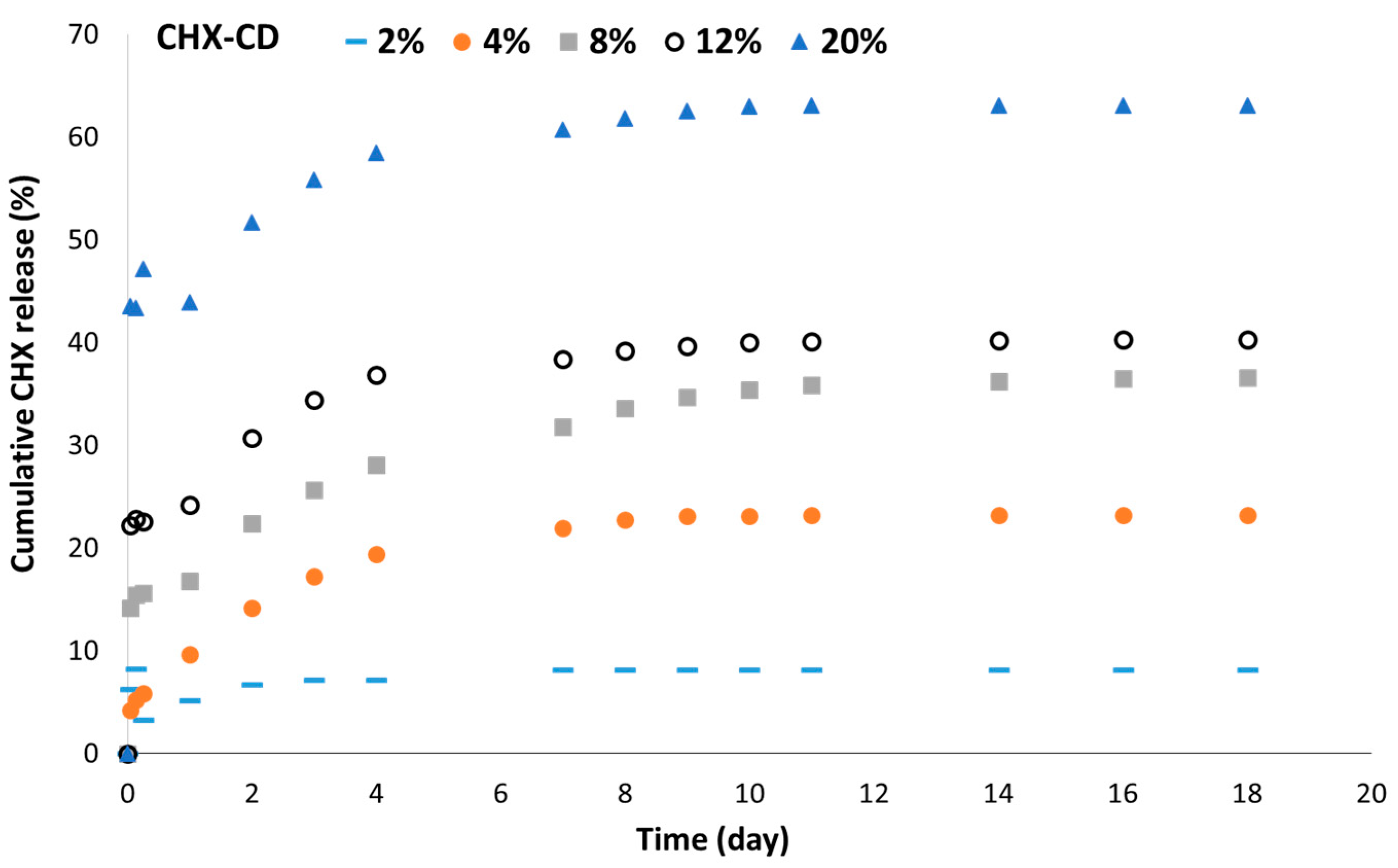
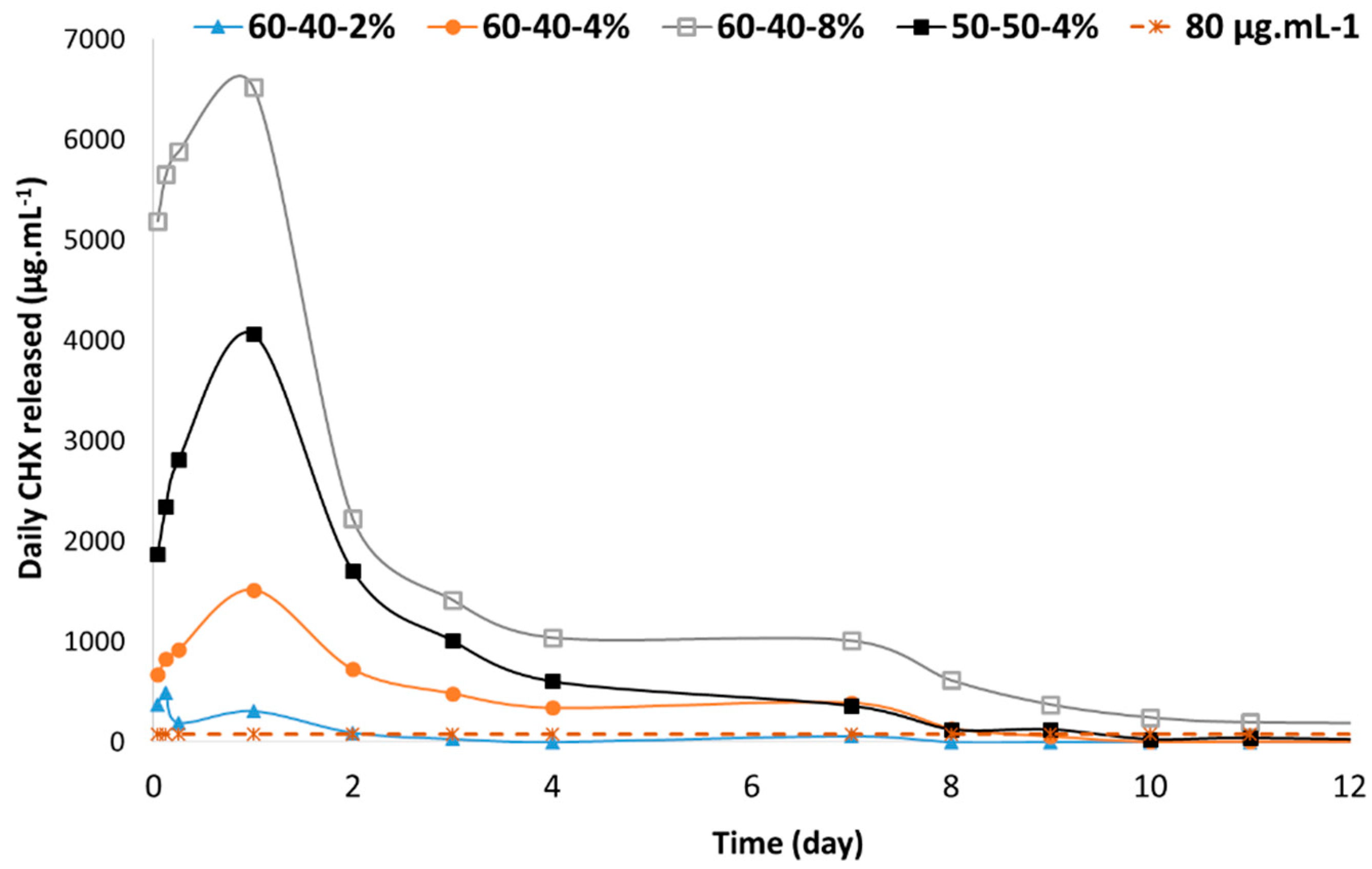
3.4. In Vitro CHX Release
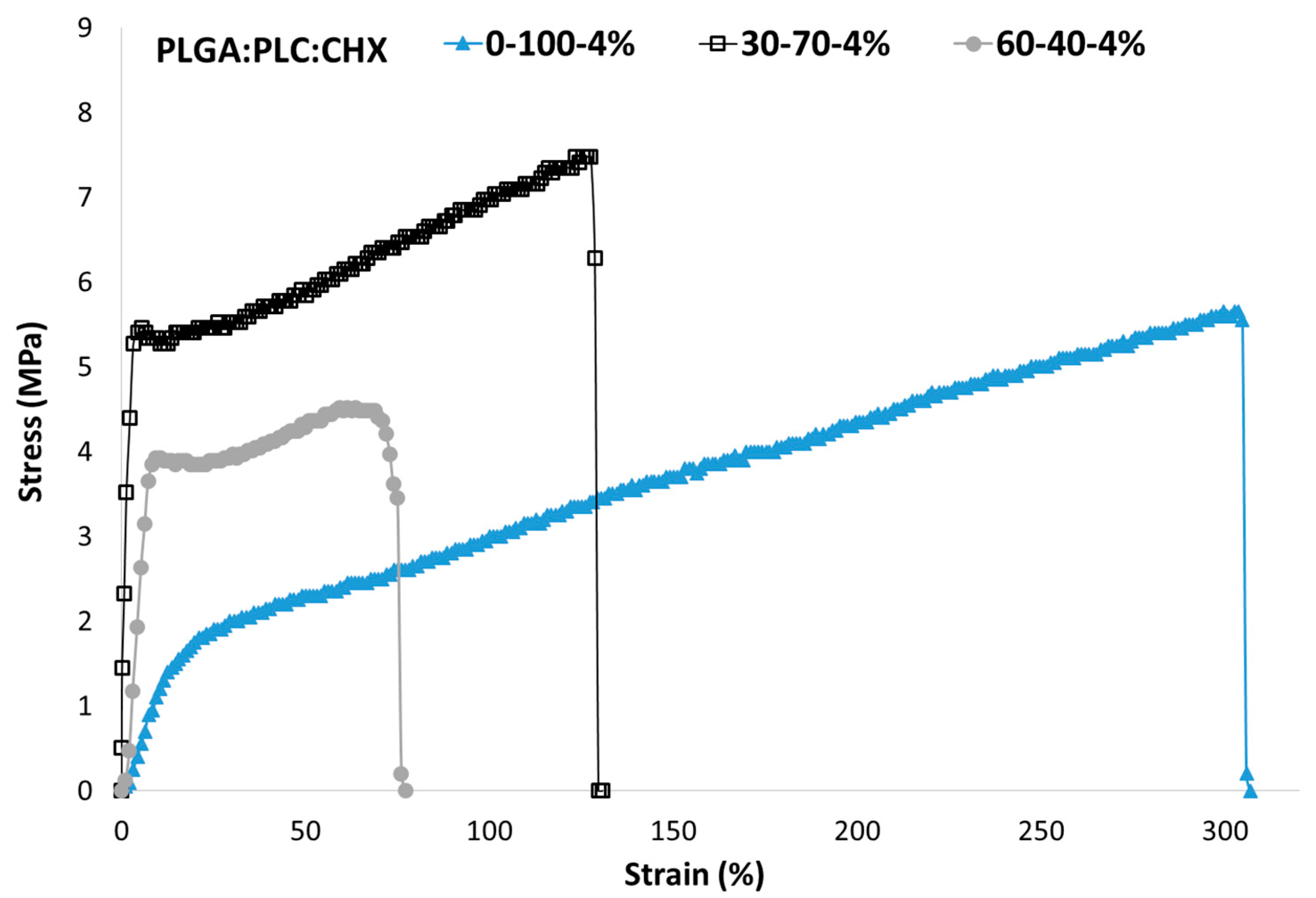
3.5. Mechanical Properties
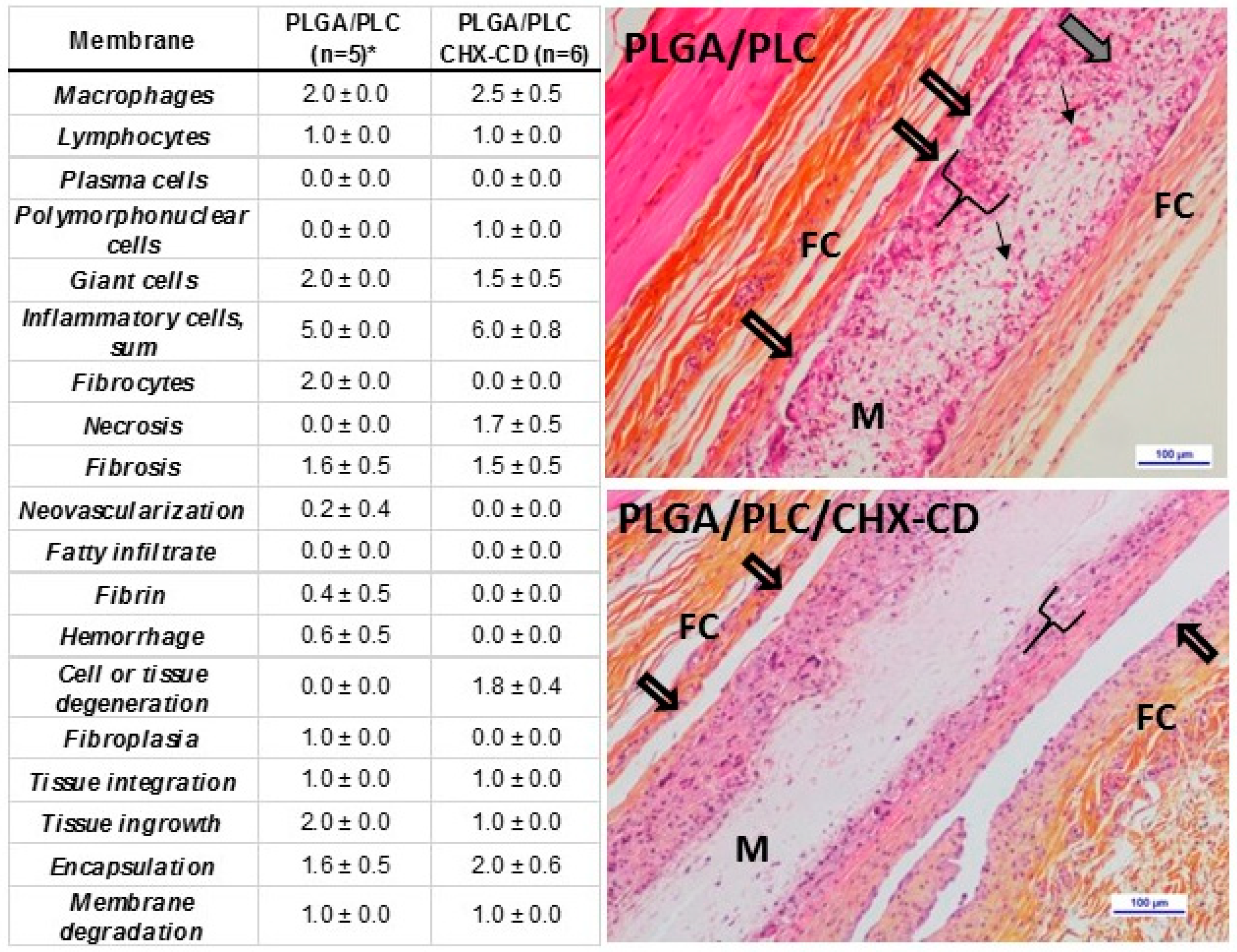
3.6. In Vivo Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Buser, D.; Janner, S.F.M.; Wittneben, J.-G.; Brägger, U.; Ramseier, C.A.; Salvi, G.E. 10-year survival and success rates of 511 titanium implants with a sandblasted and acid-etched surface: A retrospective study in 303 partially edentulous patients. Clin. Implant Dent. Relat. Res. 2012, 14, 839–851. [Google Scholar] [CrossRef] [PubMed]
- van Velzen, F.J.J.; Ofec, R.; Schulten, E.A.J.M.; Ten Bruggenkate, C.M. 10-year survival rate and the incidence of peri-implant disease of 374 titanium dental implants with a SLA surface: A prospective cohort study in 177 fully and partially edentulous patients. Clin. Oral Implants Res. 2015, 26, 1121–1128. [Google Scholar] [CrossRef] [PubMed]
- Shahi, R.G.; Albuquerque, M.T.P.; Münchow, E.A.; Blanchard, S.B.; Gregory, R.L.; Bottino, M.C. Novel bioactive tetracycline-containing electrospun polymer fibers as a potential antibacterial dental implant coating. Odontology 2017, 105, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Salvi, G.E.; Cosgarea, R.; Sculean, A. Prevalence and Mechanisms of Peri-implant Diseases. J. Dent. Res. 2017, 96, 31–37. [Google Scholar] [CrossRef]
- Romeo, E.; Lops, D.; Chiapasco, M.; Ghisolfi, M.; Vogel, G. Therapy of peri-implantitis with resective surgery. A 3-year clinical trial on rough screw-shaped oral implants. Part II: Radiographic outcome. Clin. Oral Implants Res. 2007, 18, 179–187. [Google Scholar] [CrossRef]
- Sahrmann, P.; Luso, S.; Mueller, C.; Ender, A.; Attin, T.; Stawarczyk, B.; Schmidlin, P.R. Titanium Implant Characteristics After Implantoplasty: An In Vitro Study on Two Different Kinds of Instrumentation. Int. J. Oral Maxillofac. Implants 2019, 34, 1299–1305. [Google Scholar] [CrossRef]
- Heitz-Mayfield, L.J.A.; Needleman, I.; Salvi, G.E.; Pjetursson, B.E. Consensus statements and clinical recommendations for prevention and management of biologic and technical implant complications. Int. J. Oral Maxillofac. Implants 2014, 29 (Suppl. 2014), 346–350. [Google Scholar] [CrossRef]
- Gristina, A.G. Biomaterial-centered infection: Microbial adhesion versus tissue integration. Science 1987, 237, 1588–1595. [Google Scholar] [CrossRef]
- Zhuang, Y.; Lin, K.; Yu, H. Advance of Nano-Composite Electrospun Fibers in Periodontal Regeneration. Front. Chem. 2019, 7, 495. [Google Scholar] [CrossRef]
- Reise, M.; Wyrwa, R.; Müller, U.; Zylinski, M.; Völpel, A.; Schnabelrauch, M.; Berg, A.; Jandt, K.D.; Watts, D.C.; Sigusch, B.W. Release of metronidazole from electrospun poly(L-lactide-co-D/L-lactide) fibers for local periodontitis treatment. Dent. Mater. 2012, 28, 179–188. [Google Scholar] [CrossRef]
- Tijing, L.; Woo, Y.C.; Yao, M.; Ren, J.; Shon, H.K. Electrospinning for Membrane Fabrication: Strategies and Applications. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Amsterdam, The Netherland, 2017; ISBN 978-0-12-409547-2. [Google Scholar]
- Nam, J.; Huang, Y.; Agarwal, S.; Lannutti, J. Materials selection and residual solvent retention in biodegradable electrospun fibers. J. Appl. Polym. Sci. 2008, 107, 1547–1554. [Google Scholar] [CrossRef]
- Dai, Z.; Ronholm, J.; Tian, Y.; Sethi, B.; Cao, X. Sterilization techniques for biodegradable scaffolds in tissue engineering applications. J. Tissue Eng. 2016, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Rediguieri, C.F.; Sassonia, R.C.; Dua, K.; Kikuchi, I.S.; de Jesus Andreoli Pinto, T. Impact of sterilization methods on electrospun scaffolds for tissue engineering. Eur. Polym. J. 2016, 82, 181–195. [Google Scholar] [CrossRef]
- Zeng, P.; Rao, A.; Wiedmann, T.S.; Bowles, W. Solubility properties of chlorhexidine salts. Drug Dev. Ind. Pharm. 2009, 35, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Zhang, X.; Shahid, S.; Cattell, M.J.; Gould, D.J.; Sukhorukov, G.B. Electrospun poly(lactic acid) fibers containing novel chlorhexidine particles with sustained antibacterial activity. Biomater. Sci. 2016, 5, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Pokrowiecki, R. The paradigm shift for drug delivery systems for oral and maxillofacial implants. Drug Deliv. 2018, 25, 1504–1515. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.; Ingle, N.P.; Montero, G.A.; Kim, S.H.; King, M.W. Bioresorbable elastomeric vascular tissue engineering scaffolds via melt spinning and electrospinning. Acta Biomater. 2010, 6, 1958–1967. [Google Scholar] [CrossRef]
- D’Amato, A.R.; Bramson, M.T.K.; Puhl, D.L.; Johnson, J.; Corr, D.T.; Gilbert, R.J. Solvent retention in electrospun fibers affects scaffold mechanical properties. Electrospinning 2018, 2, 15–28. [Google Scholar] [CrossRef]
- D’Amato, A.R.; Schaub, N.J.; Cardenas, J.M.; Fiumara, A.S.; Troiano, P.M.; Fischetti, A.; Gilbert, R.J. Removal of Retained Electrospinning Solvent Prolongs Drug Release from Electrospun PLLA Fibers. Polym. Guildf 2017, 123, 121–127. [Google Scholar] [CrossRef]
- Yue, I.C.; Poff, J.; Cortés, M.E.; Sinisterra, R.D.; Faris, C.B.; Hildgen, P.; Langer, R.; Shastri, V.P. A novel polymeric chlorhexidine delivery device for the treatment of periodontal disease. Biomaterials 2004, 25, 3743–3750. [Google Scholar] [CrossRef]
- Zeng, P.; Zhang, G.; Rao, A.; Bowles, W.; Wiedmann, T.S. Concentration dependent aggregation properties of chlorhexidine salts. Int. J. Pharm. 2009, 367, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Lavoine, N.; Tabary, N.; Desloges, I.; Martel, B.; Bras, J. Controlled release of chlorhexidine digluconate using β-cyclodextrin and microfibrillated cellulose. Colloids Surf. B Biointerfaces 2014, 121, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Sousa, F.B.D.; Denadai, Â.M.L.; Lula, I.S.; Nascimento, C.S., Jr.; Neto, N.S.G.F.; Lima, A.C.; Almeida, W.B.D.; Sinisterra, R.D. Supramolecular Self-Assembly of Cyclodextrin and Higher Water Soluble Guest: Thermodynamics and Topological Studies. J. Am. Chem. Soc. 2008, 130, 8426–8436. [Google Scholar] [CrossRef] [PubMed]
- Cortés, M.E.; Sinisterra, R.D.; Avila-Campos, M.J.; Tortamano, N.; Rocha, R.G. The Chlorhexidine: Beta;-Cyclodextrin Inclusion Compound: Preparation, Characterization and Microbiological Evaluation. J. Incl. Phenom. 2001, 40, 297–302. [Google Scholar] [CrossRef]
- Narayanan, G.; Shen, J.; Boy, R.; Gupta, B.S.; Tonelli, A.E. Aliphatic Polyester Nanofibers Functionalized with Cyclodextrins and Cyclodextrin-Guest Inclusion Complexes. Polymers 2018, 10, 428. [Google Scholar] [CrossRef]
- Topuz, F.; Uyar, T. Electrospinning of Cyclodextrin Functional Nanofibers for Drug Delivery Applications. Pharmaceutics 2019, 11, 6. [Google Scholar] [CrossRef]
- Pupe, C.G.; Villardi, M.; Rodrigues, C.R.; Rocha, H.V.A.; Maia, L.C.; de Sousa, V.P.; Cabral, L.M. Preparation and evaluation of antimicrobial activity of nanosystems for the control of oral pathogens Streptococcus mutans and Candida albicans. Int. J. Nanomed. 2011, 6, 2581–2590. [Google Scholar]
- Jiang, H.; Fang, D.; Hsiao, B.S.; Chu, B.; Chen, W. Optimization and characterization of dextran membranes prepared by electrospinning. Biomacromolecules 2004, 5, 326–333. [Google Scholar] [CrossRef]
- Fernández, J.; Etxeberria, A.; Sarasua, J.-R. Synthesis, structure and properties of poly(L-lactide-co-ε-caprolactone) statistical copolymers. J. Mech. Behav. Biomed. Mater. 2012, 9, 100–112. [Google Scholar] [CrossRef]
- Davison, L.; Themistou, E.; Buchanan, F.; Cunningham, E. Low temperature gamma sterilization of a bioresorbable polymer, PLGA. Radiat. Phys. Chem. 2018, 143, 27–32. [Google Scholar] [CrossRef]
- Rychter, M.; Baranowska-Korczyc, A.; Milanowski, B.; Jarek, M.; Maciejewska, B.M.; Coy, E.L.; Lulek, J. Cilostazol-Loaded Poly(ε-Caprolactone) Electrospun Drug Delivery System for Cardiovascular Applications. Pharm. Res. 2018, 35, 32. [Google Scholar] [CrossRef] [PubMed]
- Augustine, R.; Saha, A.; Jayachandran, V.P.; Thomas, S.; Kalarikkal, N. Dose-Dependent Effects of Gamma Irradiation on the Materials Properties and Cell Proliferation of Electrospun Polycaprolactone Tissue Engineering Scaffolds. Int. J. Polym. Mater. Polym. Biomater. 2015, 64, 526–533. [Google Scholar] [CrossRef]
- Cottam, E.; Hukins, D.W.L.; Lee, K.; Hewitt, C.; Jenkins, M.J. Effect of sterilisation by gamma irradiation on the ability of polycaprolactone (PCL) to act as a scaffold material. Med. Eng. Phys. 2009, 31, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Holy, C.E.; Cheng, C.; Davies, J.E.; Shoichet, M.S. Optimizing the sterilization of PLGA scaffolds for use in tissue engineering. Biomaterials 2000, 22, 25–31. [Google Scholar] [CrossRef]
- Selim, M.; Bullock, A.J.; Blackwood, K.A.; Chapple, C.R.; MacNeil, S. Developing biodegradable scaffolds for tissue engineering of the urethra. BJU Int. 2011, 107, 296–302. [Google Scholar] [CrossRef]
- Chou, S.-F.; Carson, D.; Woodrow, K.A. Current strategies for sustaining drug release from electrospun nanofibers. J. Control Release 2015, 220, 584–591. [Google Scholar] [CrossRef]
- Priyadarshini, B.M.; Mitali, K.; Lu, T.B.; Handral, H.K.; Dubey, N.; Fawzy, A.S. PLGA nanoparticles as chlorhexidine-delivery carrier to resin-dentin adhesive interface. Dent. Mater. 2017, 33, 830–846. [Google Scholar] [CrossRef]
- Natu, M.V.; de Sousa, H.C.; Gil, M.H. Effects of drug solubility, state and loading on controlled release in bicomponent electrospun fibers. Int. J. Pharm. 2010, 397, 50–58. [Google Scholar] [CrossRef]
- do Amorim, C.V.G.; Aun, C.E.; Mayer, M.P.A. Susceptibility of some oral microorganisms to chlorhexidine and paramonochlorophenol. Braz. Oral Res. 2004, 18, 242–246. [Google Scholar] [CrossRef]
- Niu, Z.; Wang, X.; Meng, X.; Guo, X.; Jiang, Y.; Xu, Y.; Li, Q.; Shen, C. Controllable fiber orientation and nonlinear elasticity of electrospun nanofibrous small diameter tubular scaffolds for vascular tissue engineering. Biomed. Mater. 2019, 14, 035006. [Google Scholar] [CrossRef]
- Jundziłł, A.; Pokrywczyńska, M.; Adamowicz, J.; Kowalczyk, T.; Nowacki, M.; Bodnar, M.; Marszałek, A.; Frontczak-Baniewicz, M.; Mikułowski, G.; Kloskowski, T.; et al. Vascularization Potential of Electrospun Poly(L-Lactide-co-Caprolactone) Scaffold: The Impact for Tissue Engineering. Med. Sci. Monit. 2017, 23, 1540–1551. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chou, S.-F.; Woodrow, K.A. Relationships between mechanical properties and drug release from electrospun fibers of PCL and PLGA blends. J. Mech. Behav. Biomed. Mater. 2017, 65, 724–733. [Google Scholar] [CrossRef] [PubMed]
- Soliman, S.; Sant, S.; Nichol, J.W.; Khabiry, M.; Traversa, E.; Khademhosseini, A. Controlling the porosity of fibrous scaffolds by modulating the fiber diameter and packing density. J. Biomed. Mater. Res. A 2011, 96, 566–574. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Hong, Y. Enhancing cell infiltration of electrospun fibrous scaffolds in tissue regeneration. Bioact. Mater. 2016, 1, 56–64. [Google Scholar] [CrossRef] [PubMed]
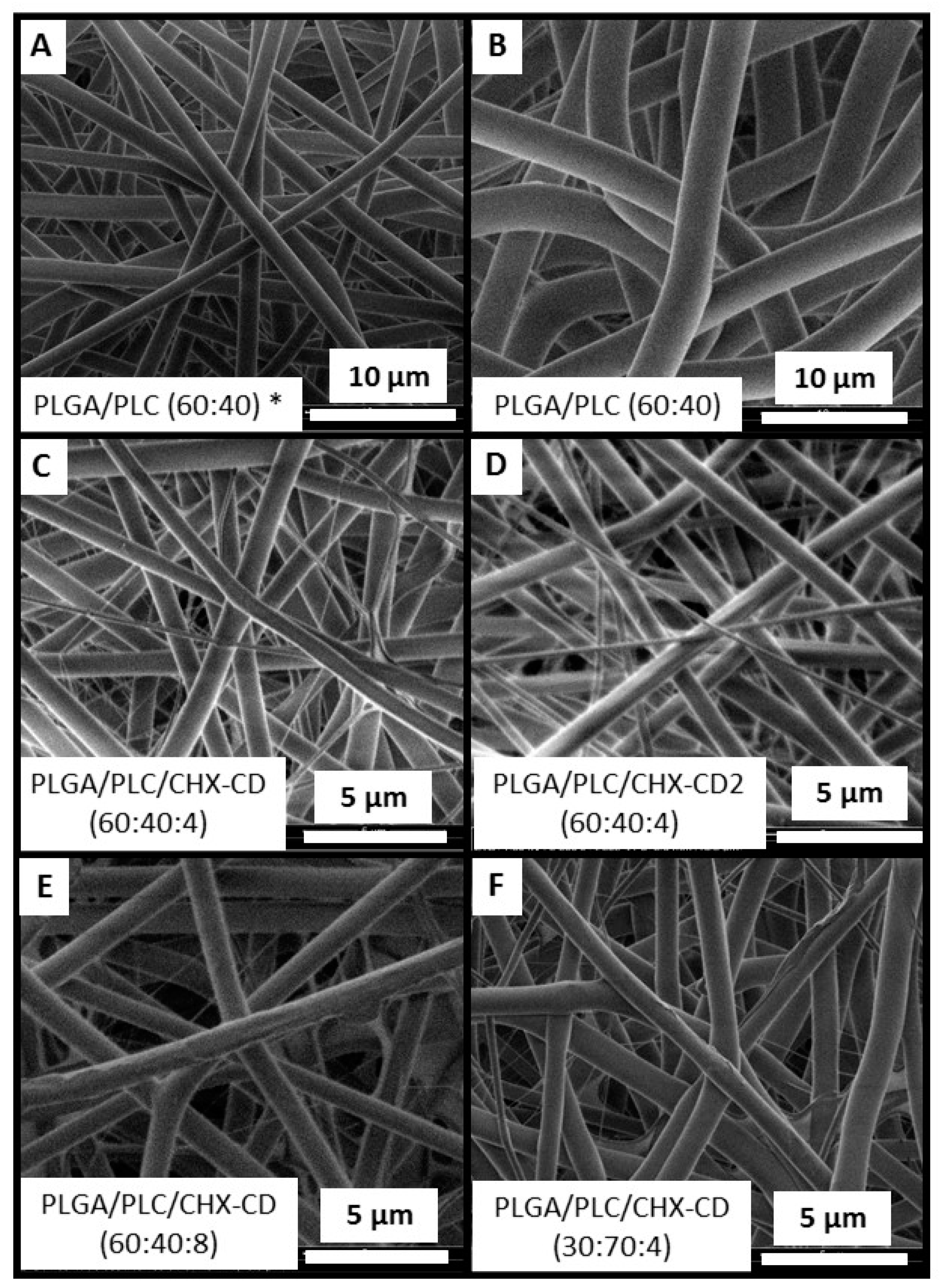
| Sample | Concentration (mg·mL−1) | PLGA (%) | PLC (%) | CHX (%) | Ratio CD | F (at %) | Fiber Ø (nm) | Thickness (µm) |
|---|---|---|---|---|---|---|---|---|
| A | 75 | 100 | 0 | 0 | 0 | 0.85 | 1877 ± 331 | 239 ± 20 |
| B | 80 | 0 | 100 | 10 | 0 | 0 | 1264 ± 138 | 217 ± 21 |
| C | 100 | 60 | 40 | 0 | 0 | 0 | 1458 ± 174 * | 259 ± 23 |
| D | 100 | 60 | 40 | 0 | 1 | <0.1 | 1532 ± 237 * | 268 ± 57 |
| E | 100 | 60 | 40 | 0 | 0 | 0 | 2996 ± 189 | 227 ± 12 |
| F | 100 | 30 | 70 | 4 | 1 | 0 | 746 ± 297 | 199 ± 19 |
| G | 100 | 50 | 50 | 4 | 1 | 0 | 897 ± 450 | 285 ± 41 |
| H | 100 | 60 | 40 | 4 | 1 | 0 | 655 ± 366 | 265 ± 30 |
| I | 100 | 60 | 40 | 6 | 1 | 0 | 664 ± 320 | 290 ± 37 |
| J | 100 | 60 | 40 | 8 | 1 | <0.1 | 824 ± 369 | 351 ± 31 |
| K | 100 | 60 | 40 | 20 | 1 | 0.75 | 967 ± 173 | 398 ± 10 |
| L | 100 | 60 | 40 | 4 | CD2 | 0 | 642 ± 270 | 234 ± 18 |
| M | 160 | 60 | 40 | 4 | 1 | 0.4 | 2283 ± 223 | 409 ± 39 |
| N | 100 | 60 | 40 | 4 | 4 | 1.05 | 889 ± 343 | 296 ± 26 |
| Sample | C (%) | O (%) | N (%) | Cl (%) | O/C (%) | N/Cl (%) |
|---|---|---|---|---|---|---|
| PLGA/PLC (60:40) | 65.1 ± 1.2 | 34.5 ± 1.3 | 0.5 ± 1.3 | - | 0.53 ± 0.03 | - |
| PLGA/PLC/CD (60:40:4) | 59.7 ± 0.1 | 40.1 ± 0.1 | 0.2 ± 0.1 | - | 0.67 ± 0.01 | - |
| PLGA/PLC/CHX-CD (60:40:4) | 62.0 ± 0.1 | 36.2 ± 0.4 | 1.7 ± 0.3 | 0.2 ± 0.1 | 0.59 ± 0.01 | 6.92 ± 0.82 |
| CHX | 70.6 ± 0.2 | 8.9 ± 0.7 | 17.0 ± 0.6 | 3.5 ± 0.3 | 0.13 ± 0.01 | 4.88 ± 0.21 |
| CD | 57.8 ± 0.4 | 42.2 ± 0.4 | - | - | 0.73 ± 0.01 | - |
| CHX-CD | 56.6 ± 2.2 | 33.4 ± 1.6 | 7.9 ± 0.5 | 2.1 ± 0.1 | 0.59 ± 0.05 | 3.73 ± 0.08 |
| Sample | C–C/C–H | C–O/C–N | C=O/O–C–O/ O–C–N | O–C=O/O–C=N/ N–C=O/N–C=N |
|---|---|---|---|---|
| PLGA/PLC (60:40)-pt1 | 43 | 25 | 5 | 27 |
| PLGA/PLC (60:40)-pt2 | 43 | 27 | 2 | 28 |
| PLGA/PLC/CD (60:40:4)-pt1 | 27 | 45 | 18 | 10 |
| PLGA/PLC/CD (60:40:4)-pt2 | 27 | 45 | 18 | 10 |
| PLGA/PLC/CHX-CD (60:40:4)-pt1 | 34 | 35 | 13 | 18 |
| PLGA/PLC/CHX-CD (60:40:4)-pt2 | 30 | 34 | 18 | 18 |
| CHX-pt1 | 68 | 20 | 2 | 10 |
| CHX-pt2 | 65 | 23 | 2 | 10 |
| CD-pt 1 | 14 | 60 | 21 | 5 |
| CD-pt 2 | 14 | 65 | 17 | 4 |
| CHX-CD-pt 1 | 22 | 53 | 21 | 4 |
| CHX-CD-pt 2 | 24 | 56 | 17 | 3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pouponneau, P.; Perrey, O.; Brunon, C.; Grossiord, C.; Courtois, N.; Salles, V.; Alves, A. Electrospun Bioresorbable Membrane Eluting Chlorhexidine for Dental Implants. Polymers 2020, 12, 66. https://doi.org/10.3390/polym12010066
Pouponneau P, Perrey O, Brunon C, Grossiord C, Courtois N, Salles V, Alves A. Electrospun Bioresorbable Membrane Eluting Chlorhexidine for Dental Implants. Polymers. 2020; 12(1):66. https://doi.org/10.3390/polym12010066
Chicago/Turabian StylePouponneau, Pierre, Ophélie Perrey, Céline Brunon, Carol Grossiord, Nicolas Courtois, Vincent Salles, and Antoine Alves. 2020. "Electrospun Bioresorbable Membrane Eluting Chlorhexidine for Dental Implants" Polymers 12, no. 1: 66. https://doi.org/10.3390/polym12010066
APA StylePouponneau, P., Perrey, O., Brunon, C., Grossiord, C., Courtois, N., Salles, V., & Alves, A. (2020). Electrospun Bioresorbable Membrane Eluting Chlorhexidine for Dental Implants. Polymers, 12(1), 66. https://doi.org/10.3390/polym12010066