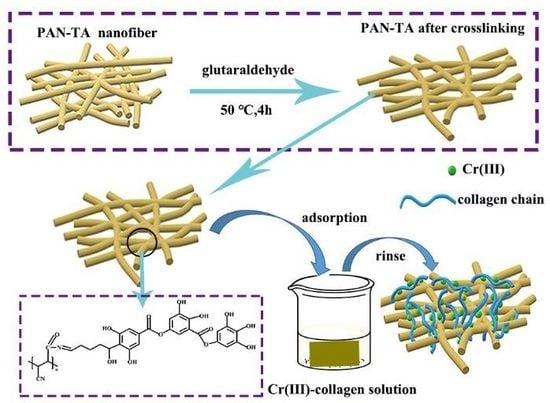
Fabrication of PAN Electrospun Nanofibers Modified by Tannin for Effective Removal of Trace Cr(III) in Organic Complex from Wastewater
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
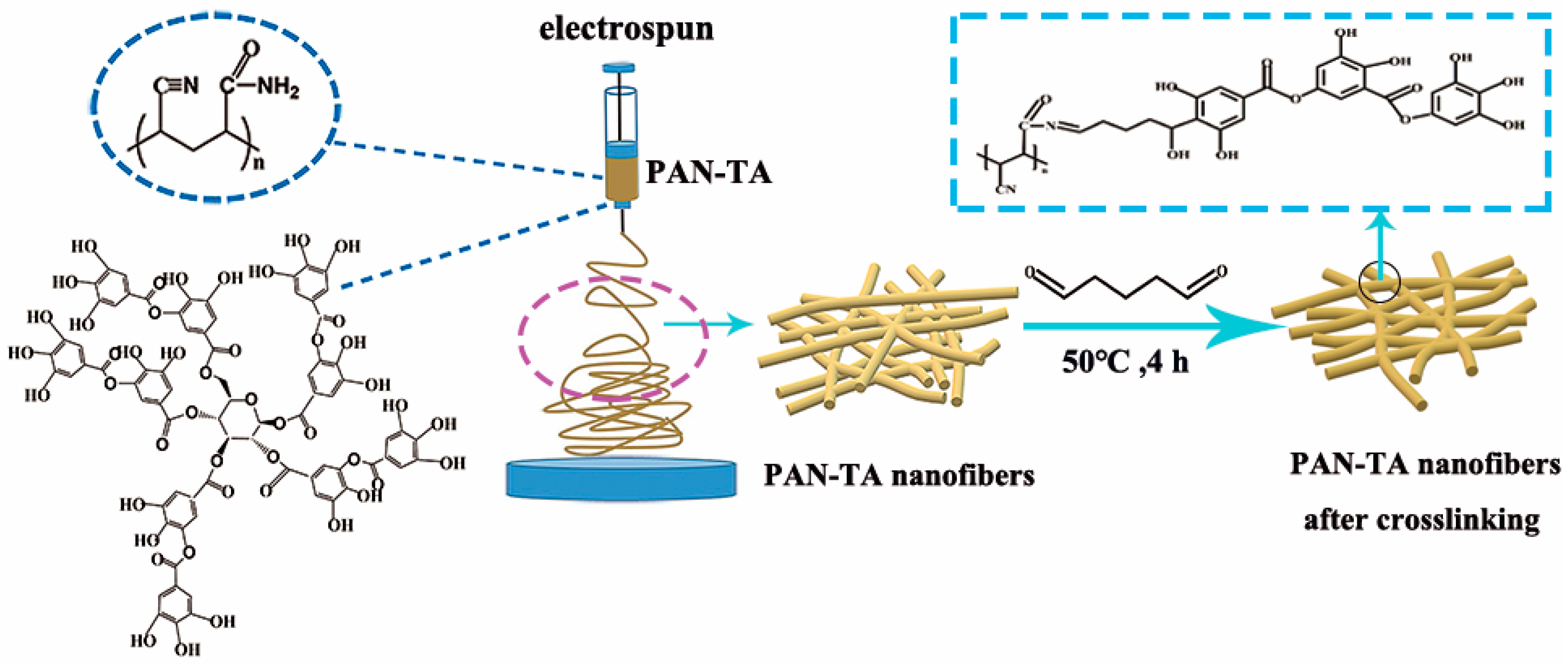
2.2. Preparation of Electrospun PAN–TA Nanofibers
2.3. Characterization
2.4. Adsorption Studies of Electrospun PAN–TA Nanofiber Membrane
2.4.1. Influential Factors on Adsorption Capacity
2.4.2. Kinetic Study
2.4.3. Adsorption Isotherm and Thermodynamics
2.4.4. Stability of the PAN–TA Nanofiber Adsorbent
3. Results and Discussion
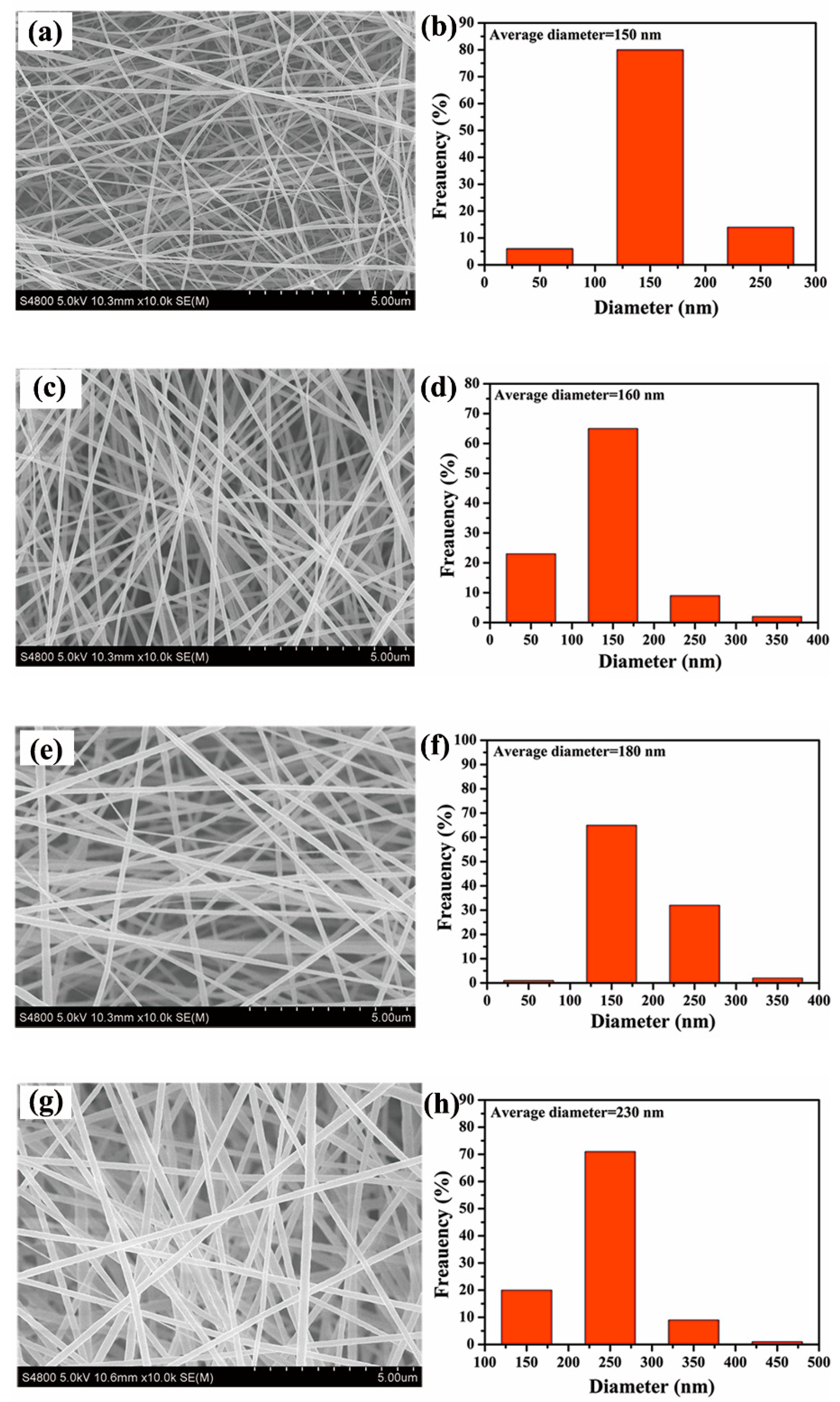
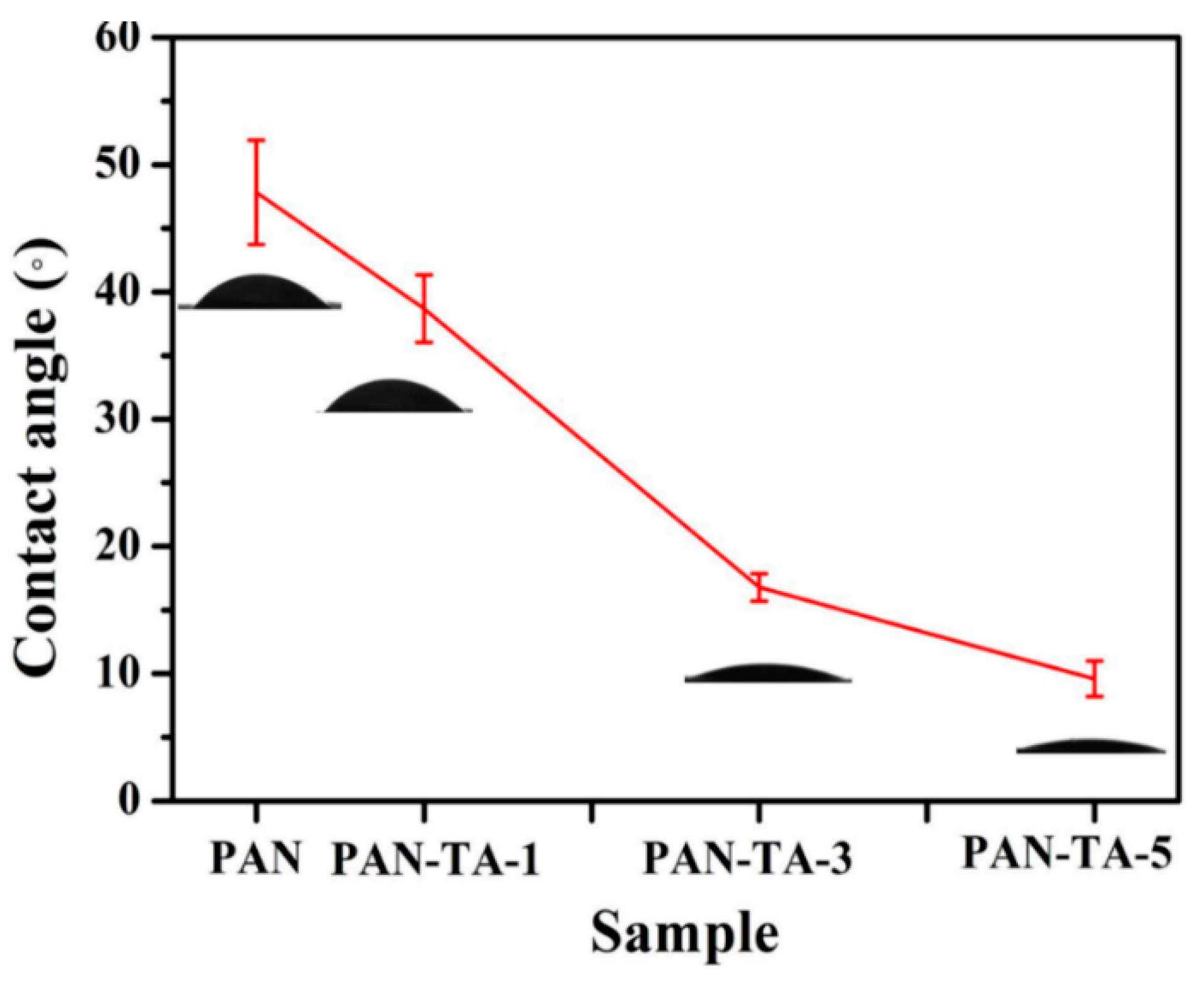
3.1. Preparation and Morphology
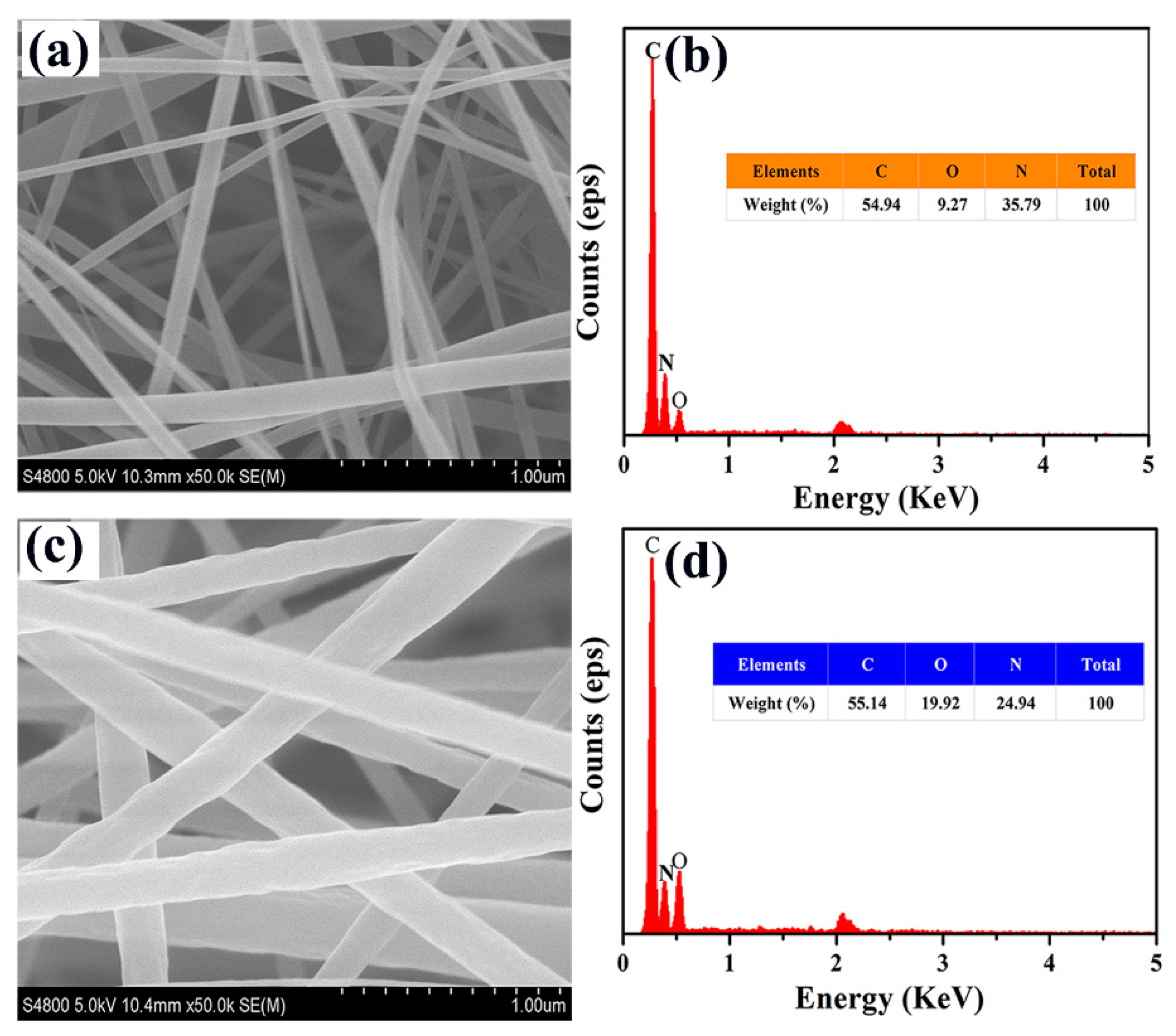
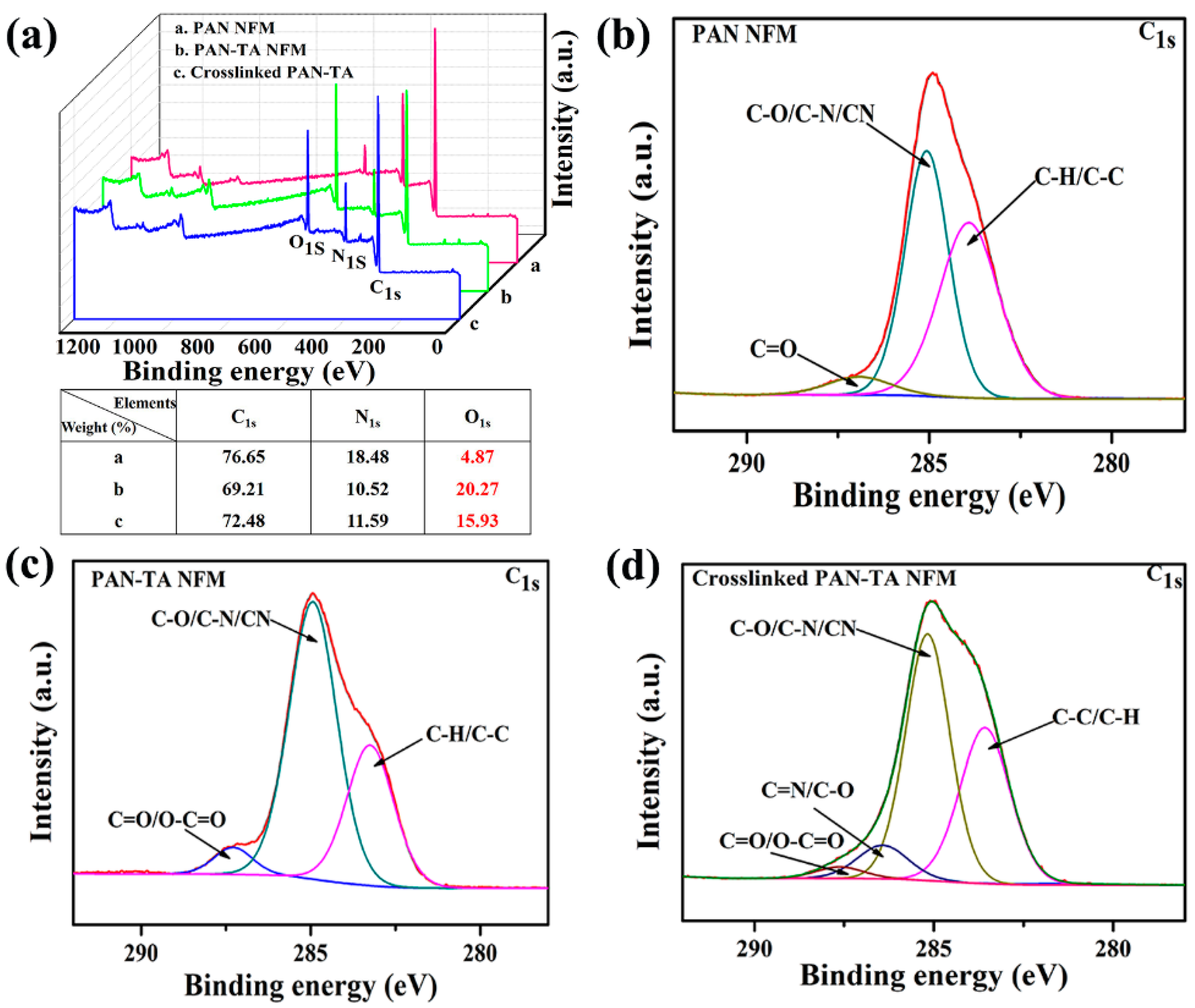
3.2. Composition Characterization
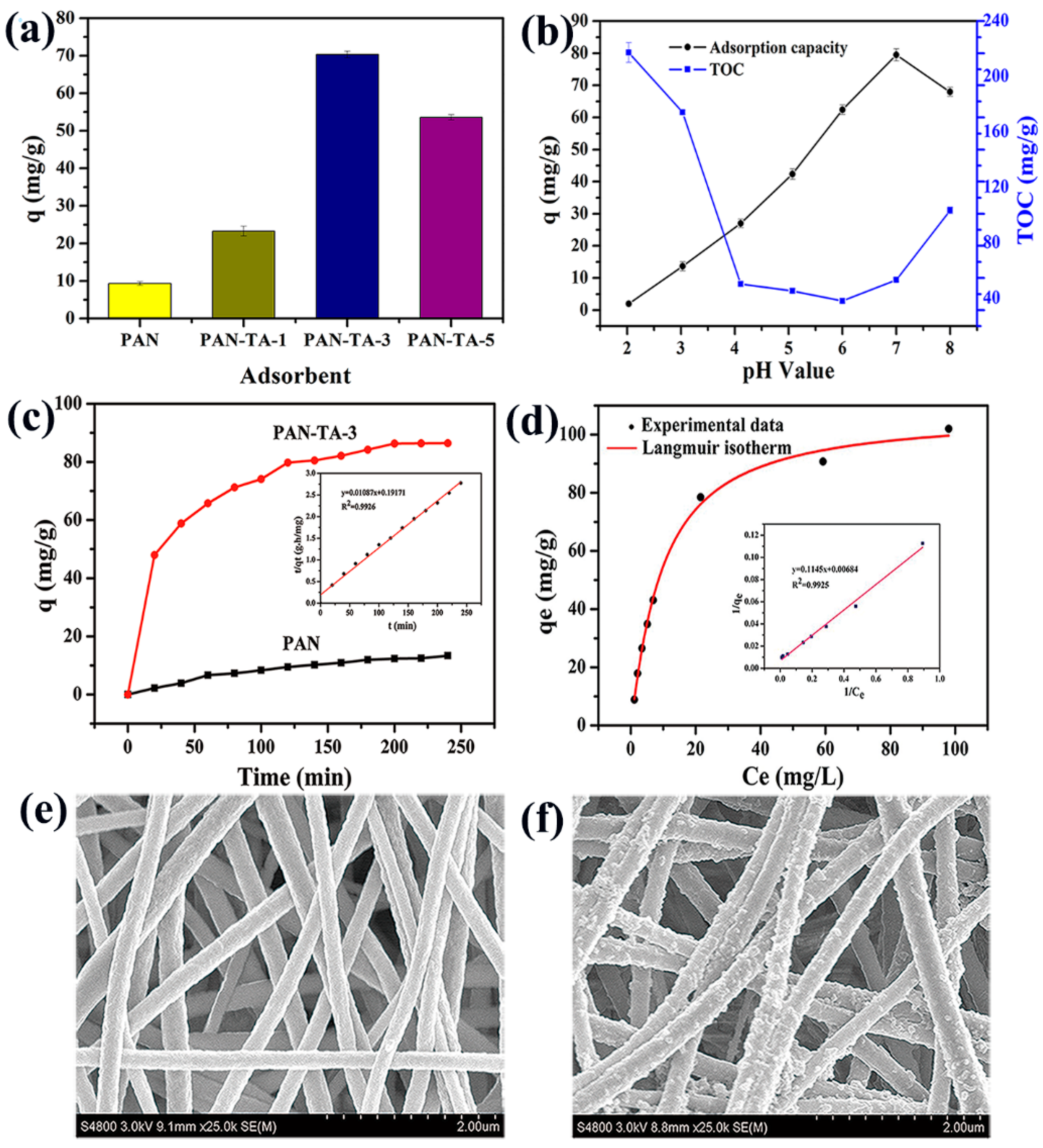
3.3. Batch Adsorption Experiments
3.3.1. Initial pH Effects
3.3.2. Kinetic Study
3.3.3. Adsorption Isotherms and Thermodynamics
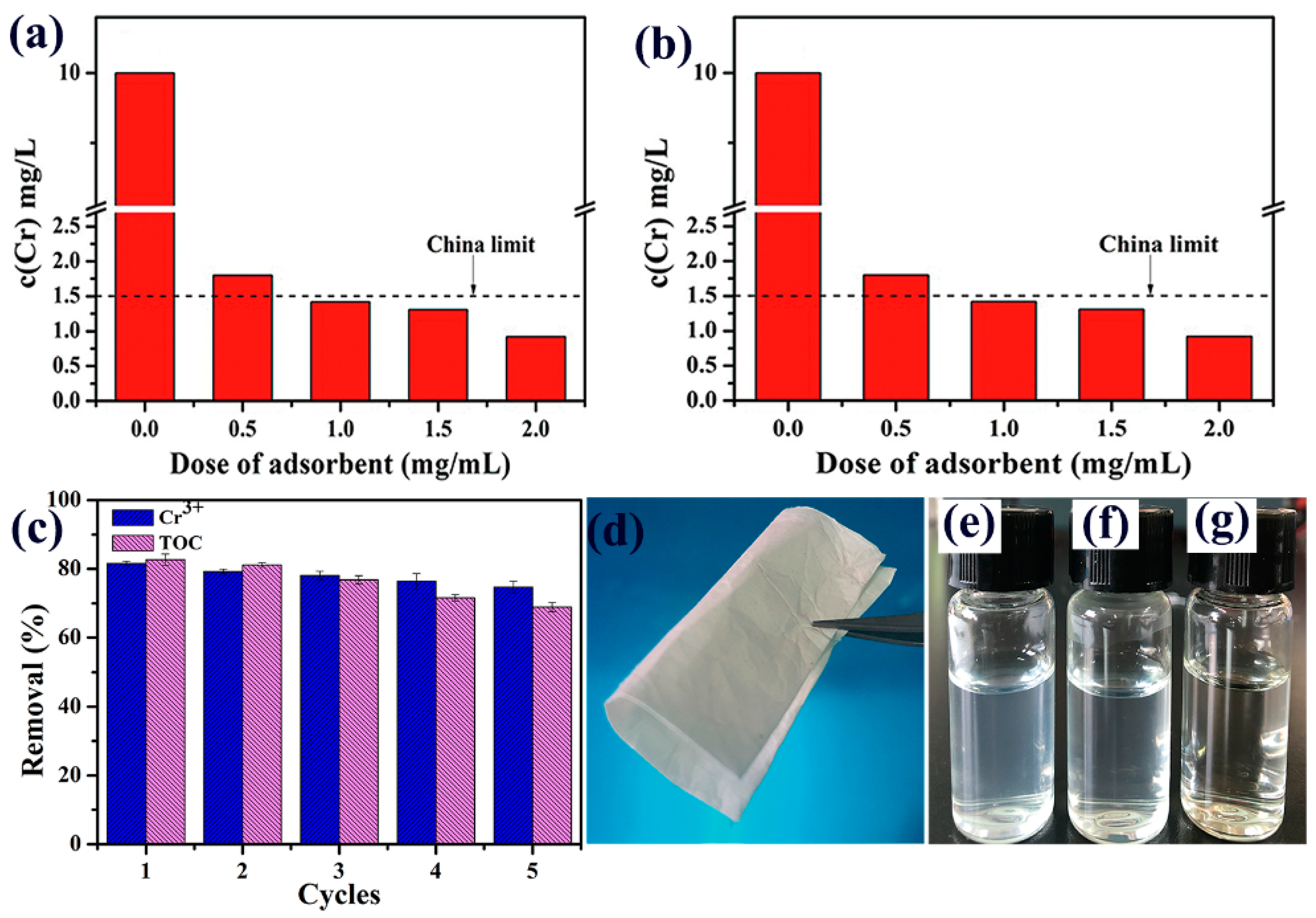
3.3.4. Trace Cr(III) in Complex Removal
3.3.5. Adsorption Cycles
3.4. Stability of PAN–TA-3 Nanofiber Adsorbent
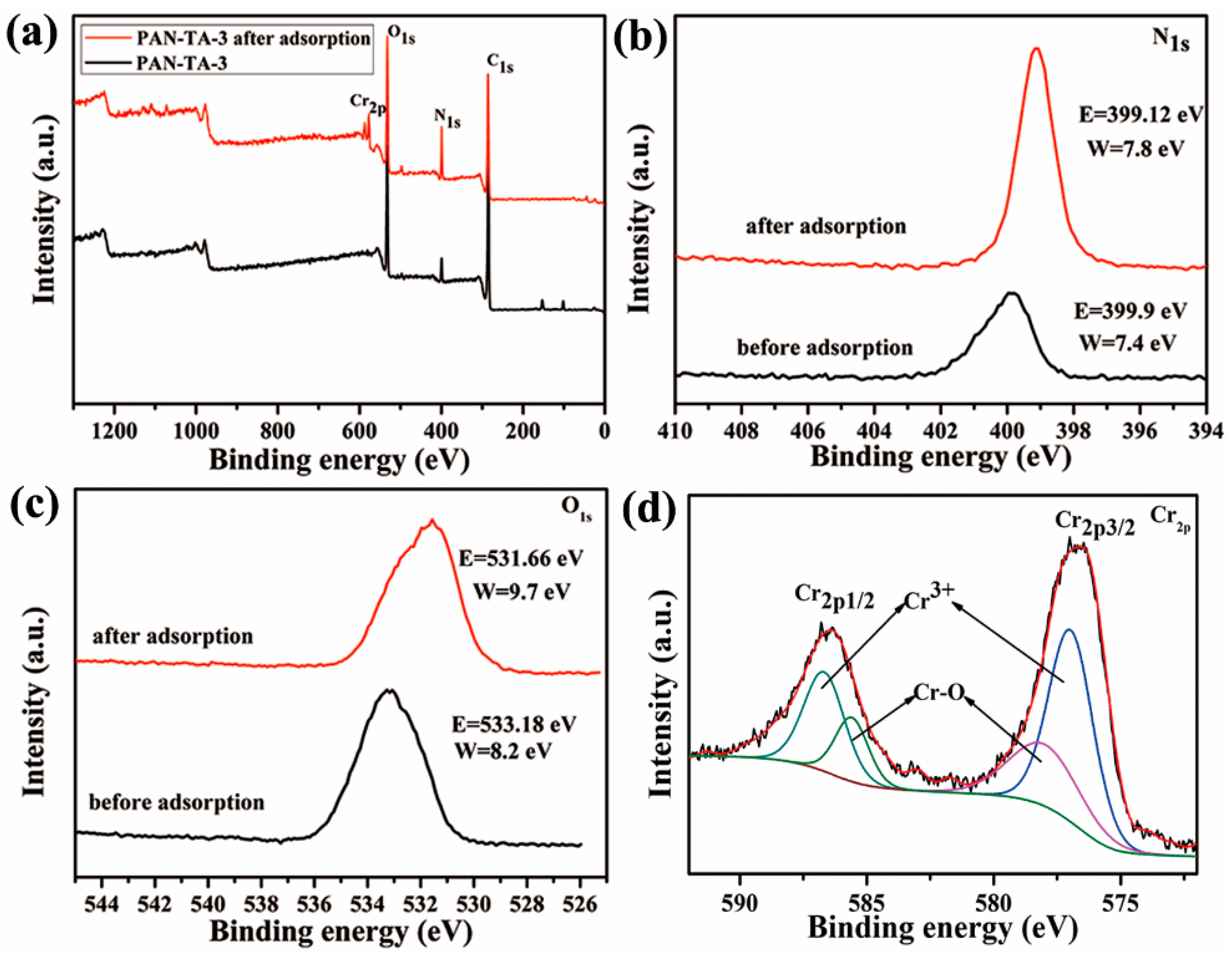
3.5. Adsorption Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Huang, X.; Liao, X.; Shi, B. Tannin-immobilized mesoporous silica bead (BT-SiO2) as an effective adsorbent of Cr(III) in aqueous solutions. J. Hazard. Mater. 2010, 173, 33–39. [Google Scholar] [CrossRef]
- Choi, K.; Lee, S.; Park, J.O.; Park, J.A.; Cho, S.H.; Lee, S.Y.; Lee, J.H.; Choi, J.W. Chromium removal from aqueous solution by a PEI-silica nanocomposite. Sci. Rep. 2018, 8, 1438. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Huang, X.; Tang, Z.; Huang, Q.; Niu, F.; Wang, X. Polymer-based nanocomposites for heavy metal ions removal from aqueous solution: A review. Polym. Chem. 2018, 9, 3562–3582. [Google Scholar] [CrossRef]
- Burakov, A.E.; Galunin, E.V.; Burakova, I.V.; Kucherova, A.E.; Agarwal, S.; Tkachev, A.G.; Gupta, V.K. Adsorption of heavy metals on conventional and nanostructured materials for wastewater treatment purposes: A review. Ecotoxicol. Environ. Saf. 2018, 148, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Crisostomo, C.A.B.; Lima, F.A.; Dias, R.M.; Cardoso, V.L.; de Resende, M.M. Joint Assessment of Bioreduction of Chromium(VI) and of Removals of Both Total Chromium and Total Organic Carbon (TOC) in Sequential Hybrid Bioreactors. Water Air Soil Pollut. 2016, 227, 51. [Google Scholar] [CrossRef]
- Ma, H.; Li, H.; Lian, K.; Ma, X. Adsorptive Removal of Trivalent Chromium in Aqueous Solution Using Precipitate Produced from Aluminum Tanning Wastewater. Water Air Soil Pollut. 2014, 225, 1956. [Google Scholar] [CrossRef]
- Zhao, R.; Li, X.; Sun, B.; Li, Y.; Li, Y.; Yang, R.; Wang, C. Branched polyethylenimine grafted electrospun polyacrylonitrile fiber membrane: A novel and effective adsorbent for Cr(vi) remediation in wastewater. J. Mater. Chem. A 2017, 5, 1133–1144. [Google Scholar] [CrossRef]
- Miretzky, P.; Cirelli, A.F. Cr(VI) and Cr(III) removal from aqueous solution by raw and modified lignocellulosic materials: A review. J. Hazard. Mater. 2010, 180, 1–19. [Google Scholar] [CrossRef]
- Wang, D.; He, S.; Shan, C.; Ye, Y.; Ma, H.; Zhang, X.; Zhang, W.; Pan, B. Chromium speciation in tannery effluent after alkaline precipitation: Isolation and characterization. J. Hazard. Mater. 2016, 316, 169–177. [Google Scholar] [CrossRef]
- Zhou, W.; Long, W.; Xu, T.; Peng, L.; Zhang, W. Organic ligands unexpectedly increase the toxicity of chromium(III) for luminescent bacteria. Environ. Chem. Lett. 2019, 17, 1849–1855. [Google Scholar] [CrossRef]
- Rajput, S.; Pittman, C.U., Jr.; Mohan, D. Magnetic magnetite (Fe3O4) nanoparticle synthesis and applications for lead (Pb2+) and chromium (Cr6+) removal from water. J. Colloid Interface Sci. 2016, 468, 334–346. [Google Scholar] [CrossRef] [PubMed]
- Santhosh, C.; Velmurugan, V.; Jacob, G.; Jeong, S.K.; Grace, A.N.; Bhatnagar, A. Role of nanomaterials in water treatment applications: A review. Chem. Eng. J. 2016, 306, 1116–1137. [Google Scholar] [CrossRef]
- Hu, Y.; Wu, X.; He, X.; Xing, D. Phosphorylated polyacrylonitrile-based electrospun nanofibers for removal of heavy metal ions from aqueous solution. Polym. Adv. Technol. 2019, 30, 545–551. [Google Scholar] [CrossRef]
- Dehghani, M.H.; Sanaei, D.; Ali, I.; Bhatnagar, A. Removal of chromium(VI) from aqueous solution using treated waste newspaper as a low-cost adsorbent: Kinetic modeling and isotherm studies. J. Mol. Liq. 2016, 215, 671–679. [Google Scholar] [CrossRef]
- Taha, A.A.; Qiao, J.L.; Li, F.T.; Zhang, B.R. Preparation and application of amino functionalized mesoporous nanofiber membrane via electrospinning for adsorption of Cr3+ from aqueous solution. J. Environ. Sci. 2012, 24, 610–616. [Google Scholar] [CrossRef]
- Chaúque, E.F.C.; Dlamini, L.N.; Adelodun, A.A.; Greyling, C.J.; Ngila, J.C. Modification of electrospun polyacrylonitrile nanofibers with EDTA for the removal of Cd and Cr ions from water effluents. App. Surf. Sci. 2016, 369, 19–28. [Google Scholar] [CrossRef]
- Lou, H.Q.; Cao, X.Z.; Yan, X.; Wang, L.; Chen, Z.B. Adsorption performance of Cd(II), Cr(III), Cu(II), Ni(II), Pb(II) and Zn(II) by aminated solution-blown polyacrylonitrile micro/nanofibers. Water Sci. Technol. 2018, 2017, 378. [Google Scholar] [CrossRef]
- Lofrano, G.; Meric, S.; Zengin, G.E.; Orhon, D. Chemical and biological treatment technologies for leather tannery chemicals and wastewaters: A review. Sci. Total Environ. 2013, 461, 265–281. [Google Scholar] [CrossRef]
- Walsh, A.R.; O’Halloran, J. Chromium Speciation in Tannery Effluent--I. An Assessment of Techniques and the Role of organic Cr(III) complexes. Science 1996, 30, 2393–2400. [Google Scholar] [CrossRef]
- Guo, Z.R.; Zhang, G.; Fang, J.; Dou, X. Enhanced chromium recovery from tanning wastewater. J. Clean. Prod. 2006, 14, 75–79. [Google Scholar] [CrossRef]
- Ye, Y.; Shan, C.; Zhang, X.; Liu, H.; Wang, D.; Lv, L.; Pan, B. Water Decontamination from Cr(III)-Organic Complexes Based on Pyrite/H2O2: Performance, Mechanism, and Validation. Environ. Sci. Technol. 2018, 52, 10657–10664. [Google Scholar] [CrossRef] [PubMed]
- TSileika, S.; Barrett, D.G.; Zhang, R.; Lau, K.H.A.; Messersmith, P.B. Colorless Multifunctional Coatings Inspired by Polyphenols Found in Tea, Chocolate, and Wine. Angew. Chem. Int. Ed. 2013, 52, 10766–10770. [Google Scholar] [CrossRef] [PubMed]
- Hirotaka, E.; Richardson, J.J.; Kang, L.; Best, J.P.; Koeverden, M.P.; Van Such, G.K.; Jiwei, C.; Frank, C. One-step assembly of coordination complexes for versatile film and particle engineering. Science 2013, 341, 154–157. [Google Scholar]
- Ma, S.; Lee, H.; Liang, Y.; Zhou, F. Astringent Mouthfeel as a Consequence of Lubrication Failure. Angew. Chem. Int. Ed. 2016, 55, 5793–5797. [Google Scholar] [CrossRef] [PubMed]
- Qiang, T.; Bu, Q.; Ren, L.; Wang, X. Adsorption behaviors of Cr(III) on carboxylated collagen fiber. J. Appl. Polym.Sci. 2014, 131, 40285. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Rejeena, S.R.; Tharun, A.R. Preparation, characterization and adsorption behavior of tannin-modified poly(glycidylmethacrylate)-grafted zirconium oxide-densified cellulose for the selective separation of bovine serum albumin. Colloids Surf. B 2012, 93, 49–58. [Google Scholar] [CrossRef]
- Meng, J.; Lin, X.; Zhou, J.; Zhang, R.; Chen, Y.; Long, X.; Shang, R.; Luo, X. Preparation of tannin-immobilized gelatin/PVA nanofiber band for extraction of uranium (VI) from simulated seawater. Ecotoxicol. Environ. Saf. 2019, 170, 9–17. [Google Scholar] [CrossRef]
- Meng, J.; Lin, X.; Li, H.; Zhang, Y.; Luo, X. Adsorption capacity of kelp-like electrospun nanofibers immobilized with bayberry tannin for uranium(VI) extraction from seawater. RSC Adv. 2019, 9, 8091–8103. [Google Scholar] [CrossRef]
- Shubo, D.; Renbi, B. Removal of trivalent and hexavalent chromium with aminated polyacrylonitrile fibers: Performance and mechanisms. Water Res. 2004, 38, 2424–2432. [Google Scholar]
- Nthumbi, R.M.; Adelodun, A.A.; Ngila, J.C. Electrospun and functionalized PVDF/PAN composite for the removal of trace metals in contaminated water. Phys. Chem. Earth 2017, 100, 225–235. [Google Scholar] [CrossRef]
- Haddad, M.Y.; Alharbi, H.F. Enhancement of heavy metal ion adsorption using electrospun polyacrylonitrile nanofibers loaded with ZnO nanoparticles. J. Appl. Polym. Sci. 2019, 136, 47209. [Google Scholar] [CrossRef]
- Almasian, A.; Jalali, M.L.; Fard, G.C.; Maleknia, L. Surfactant grafted PDA-PAN nanofiber: Optimization of synthesis, characterization and oil absorption property. Chem. Eng. J. 2017, 326, 1232–1241. [Google Scholar] [CrossRef]
- Chauhan, D.; Afreen, S.; Mishra, S.; Sankararamakrishnan, N. Synthesis, characterization and application of zinc augmented aminated PAN nanofibers towards decontamination of chemical and biological contaminants. J. Ind. Eng. Chem. 2017, 55, 50–64. [Google Scholar] [CrossRef]
- Feng, Q.; Wu, D.; Zhao, Y.; Wei, A.; Wei, Q.; Fong, H. Electrospun AOPAN/RC blend nanofiber membrane for efficient removal of heavy metal ions from water. J. Hazard. Mater. 2018, 344, 819–828. [Google Scholar] [CrossRef]
- Yuji, A.; Sparks, D.L.; Davis, J.A. Arsenate adsorption mechanisms at the allophane-water interface. Environ. Sci. Technol. 2005, 39, 2537–2544. [Google Scholar]
- Yang, Y.H.; Shu, D.T.; Fu, T.D.; Zhang, H.Y. Equilibrium and Kinetics Studies for Adsorption of Cu(II) from Aqueous Solution by Modified Phosphogypusum. Adv. Mater. Res. 2012, 518, 369–375. [Google Scholar] [CrossRef]
- Aliabadi, M.; Irani, M.; Ismaeili, J.; Piri, H.; Parnian, M.J. Electrospun nanofiber membrane of PEO/Chitosan for the adsorption of nickel, cadmium, lead and copper ions from aqueous solution. Chem. Eng. J. 2013, 220, 237–243. [Google Scholar] [CrossRef]
- Price, M.L.; Butler, L.G. Rapid visual estimation and spectrophotometric determination of tannin content of sorghum grain. J. Agric. Food Chem. 1977, 25, 1268–1273. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Munir, M.M.; Suryamas, A.B.; Iskandar, F.; Okuyama, K. Scaling law on particle-to-fiber formation during electrospinning. Polymer 2009, 50, 4935–4943. [Google Scholar] [CrossRef]
- Sharma, D.K.; Shen, J.; Li, F. Reinforcement of Nafion into Polyacrylonitrile (PAN) to fabricate them into nanofiber mats by electrospinning: Characterization of enhanced mechanical and adsorption properties. RSC Adv. 2014, 4, 39110–39117. [Google Scholar] [CrossRef]
- Ge, J.; Zong, D.; Jin, Q.; Yu, J.; Ding, B. Biomimetic and Superwettable Nanofibrous Skins for Highly Efficient Separation of Oil-in-Water Emulsions. Adv. Funct. Mater. 2018, 28, 1705051–1705061. [Google Scholar] [CrossRef]
- Weber, F.; Barrantes, A.; Tiainen, H. Silicic Acid-Mediated Formation of Tannic Acid Nanocoatings. Langmuir 2019, 35, 3327–3336. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.-J.; Yu, S.-W. Application of novel hybrid bioadsorbent, tannin/chitosan/sericite, for the removal of Pb(II) toxic ion from aqueous solution. Korean J. Chem. Eng. 2018, 35, 2198–2206. [Google Scholar] [CrossRef]
- Zhou, Z.; Lai, C.; Zhang, L.; Yong, Q.; Hou, H.; Reneker, D.H.; Hao, F. Development of carbon nanofibers from aligned electrospun polyacrylonitrile nanofiber bundles and characterization of their microstructural, electrical, and mechanical properties. Polymer 2009, 50, 2999–3006. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, M.; Zhao, J.; Chen, J.; Zeng, G.; Huang, H.; Tian, J.; Wen, Y.; Zhang, X.; Wei, Y. Facile preparation of polyethylenimine-tannins coated SiO2 hybrid materials for Cu2+ removal. Appl. Surf. Sci. 2018, 427, 535–544. [Google Scholar] [CrossRef]
- Ramírez-Estrada, A.; Mena-Cervantes, V.Y.; Fuentes-García, J.; Vazquez-Arenas, J.; Palma-Goyes, R.; Flores-Vela, A.I.; Vazquez-Medina, R.; Altamirano, R.H. Cr(III) removal from synthetic and real tanning effluents using an electro-precipitation method. J. Environ. Chem. Eng. 2018, 6, 1219–1225. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, Y.; Jin, L.; Wang, Y.; Qin, M. Adsorption of Cu (II), Pb (II) and Cr (VI) from aqueous solutions using black wattle tannin-immobilized nanocellulose. J. Hazard. Mater. 2017, 339, 91–99. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, Q.; Liu, B.; Hu, T.; Liu, W.; Yao, J. Green synthesis of tannin-hexamethylendiamine based adsorbents for efficient removal of Cr(VI). J. Hazard. Mater. 2018, 352, 27–35. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, Y.; Kuang, Y.; Yang, R.; Ma, J.; Zhao, S.; Liao, Y.; Mao, H. Adsorptive removal of Cr3+ from aqueous solutions using chitosan microfibers immobilized with plant polyphenols as biosorbents with high capacity and selectivity. Appl. Surf. Sci. 2017, 404, 418–425. [Google Scholar] [CrossRef]
- Debnath, S.; Ghosh, U.C. Kinetics, isotherm and thermodynamics for Cr(III) and Cr(VI) adsorption from aqueous solutions by crystalline hydrous titanium oxide. J. Chem. Thermodyn. 2008, 40, 67–77. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Z.; Wang, J.; Li, Q.; Wang, X. Capsular polypyrrole hollow nanofibers: An efficient recyclable adsorbent for hexavalent chromium removal. J. Mater. Chem. A 2015, 3, 15124–15132. [Google Scholar] [CrossRef]
- Kumar, A.S.K.; Jiang, S.-J.; Tseng, W.-L. Effective adsorption of chromium(VI)/Cr(III) from aqueous solution using ionic liquid functionalized multiwalled carbon nanotubes as a super sorbent. J. Mater. Chem. A 2015, 3, 7044–7057. [Google Scholar] [CrossRef]

| Adsorbent | Pseudo-First-Order Model | Pseudo-Second-Order Model | ||||
|---|---|---|---|---|---|---|
| qe (mg g−1) | k1 (h−1) | R2 | qe (mg g−1) | k2 (g mg−1 h−1) | R2 | |
| PAN–TA-3 | 96.76 | 0.0018 | 0.8681 | 91.996 | 0.69 × 10−3 | 0.9926 |
| Adsorbent | Langmuir Isotherm | Freundlich Isotherm | ||||
|---|---|---|---|---|---|---|
| qmax (mg g−1) | b (L mg−1) | R2 | KF | n | R2 | |
| PAN–TA-3 | 147.06 | 0.059 | 0.9925 | 12.53 | 1.95 | 0.9049 |
| Adsorbent | Adsorbate | qm (mg g−1) | Conditions | Reference | ||
|---|---|---|---|---|---|---|
| pH | Dosage (g L−1) | Initial Concentration (mg L−1) | ||||
| Tannin-immobilized mesoporous silica bead | Cr(III) | 67.6 | 5.5 | 1 | 100 | [1] |
| Tannin-immobilized nanocellulose | Cu(II) | 46.14 | 6 | 0.5 | 50 | [48] |
| Cr(VI) | 59 | 2 | 0.5 | 50 | ||
| Polyethylenimine-tannins coated SiO2 hybrid materials | Cu(II) | 100.1 | 7 | 0.4 | 100 | [46] |
| Tannin hexamethylendiamine based adsorbents | Cr(VI) | 283.3 | 2.5 | 0.5 | 160 | [49] |
| Chitosan microfibers immobilized with plant polyphenols | Cr(III) | 20.9 | 5.5 | 2 | 104 | [50] |
| PAN–TA-3 electrospun nanofibers | Cr(III)-collagen complex | 79.48 | 7 | 0.5 | Cr(III) 50, collagen 200 | This paper |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Xue, C.-H.; Ma, H.-R.; Ding, Y.-R.; Jia, S.-T. Fabrication of PAN Electrospun Nanofibers Modified by Tannin for Effective Removal of Trace Cr(III) in Organic Complex from Wastewater. Polymers 2020, 12, 210. https://doi.org/10.3390/polym12010210
Zhang J, Xue C-H, Ma H-R, Ding Y-R, Jia S-T. Fabrication of PAN Electrospun Nanofibers Modified by Tannin for Effective Removal of Trace Cr(III) in Organic Complex from Wastewater. Polymers. 2020; 12(1):210. https://doi.org/10.3390/polym12010210
Chicago/Turabian StyleZhang, Jing, Chao-Hua Xue, Hong-Rui Ma, Ya-Ru Ding, and Shun-Tian Jia. 2020. "Fabrication of PAN Electrospun Nanofibers Modified by Tannin for Effective Removal of Trace Cr(III) in Organic Complex from Wastewater" Polymers 12, no. 1: 210. https://doi.org/10.3390/polym12010210
APA StyleZhang, J., Xue, C.-H., Ma, H.-R., Ding, Y.-R., & Jia, S.-T. (2020). Fabrication of PAN Electrospun Nanofibers Modified by Tannin for Effective Removal of Trace Cr(III) in Organic Complex from Wastewater. Polymers, 12(1), 210. https://doi.org/10.3390/polym12010210