TiO2 Sol-Gel Coated PAN/O-MMT Multi-Functional Composite Nanofibrous Membrane Used as the Support for Laccase Immobilization: Synergistic Effect between the Membrane Support and Enzyme for Dye Degradation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
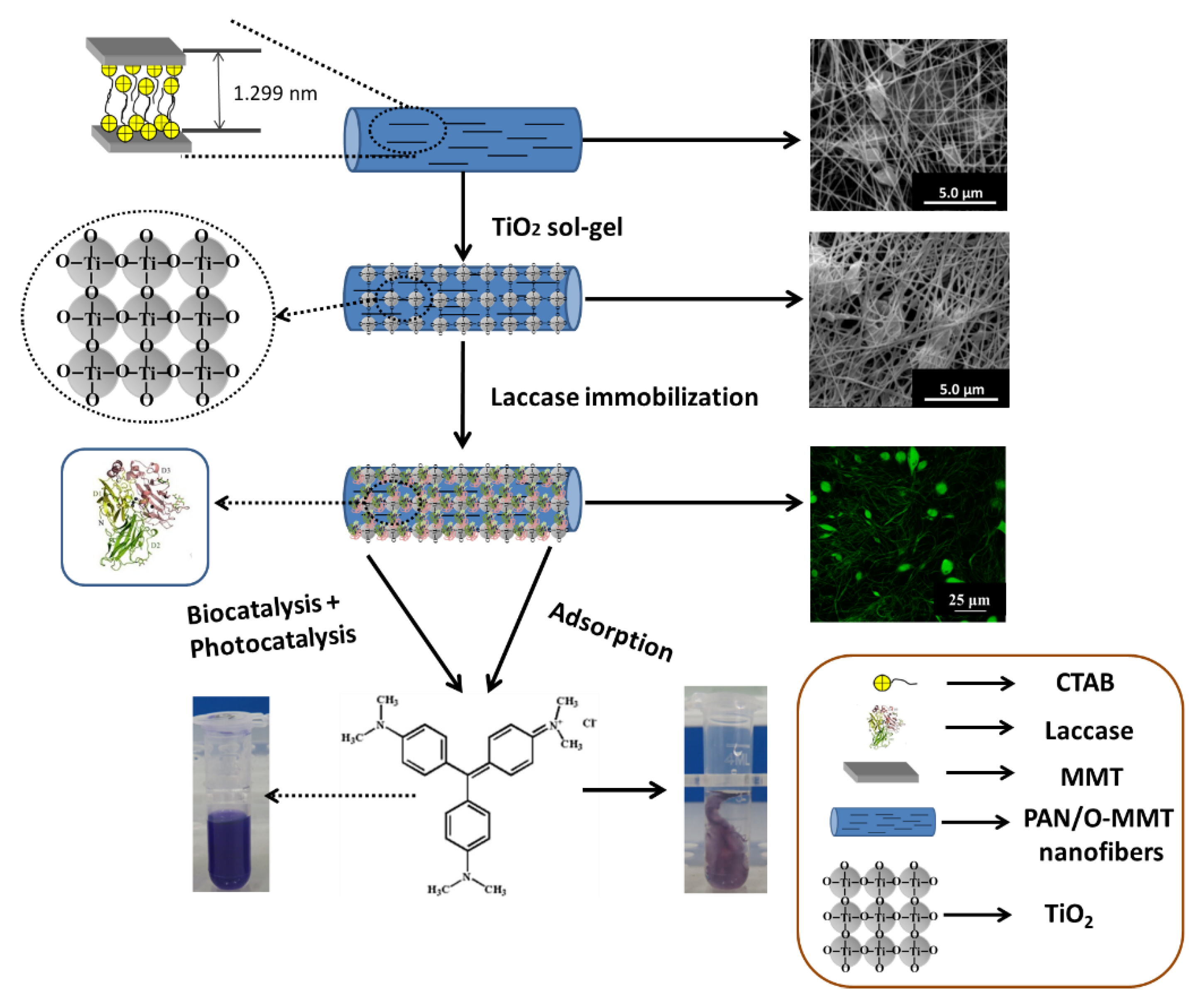
2.2. Preparation of Lac-Immobilized PAN/O-MMT/TiO2 Nanofibers
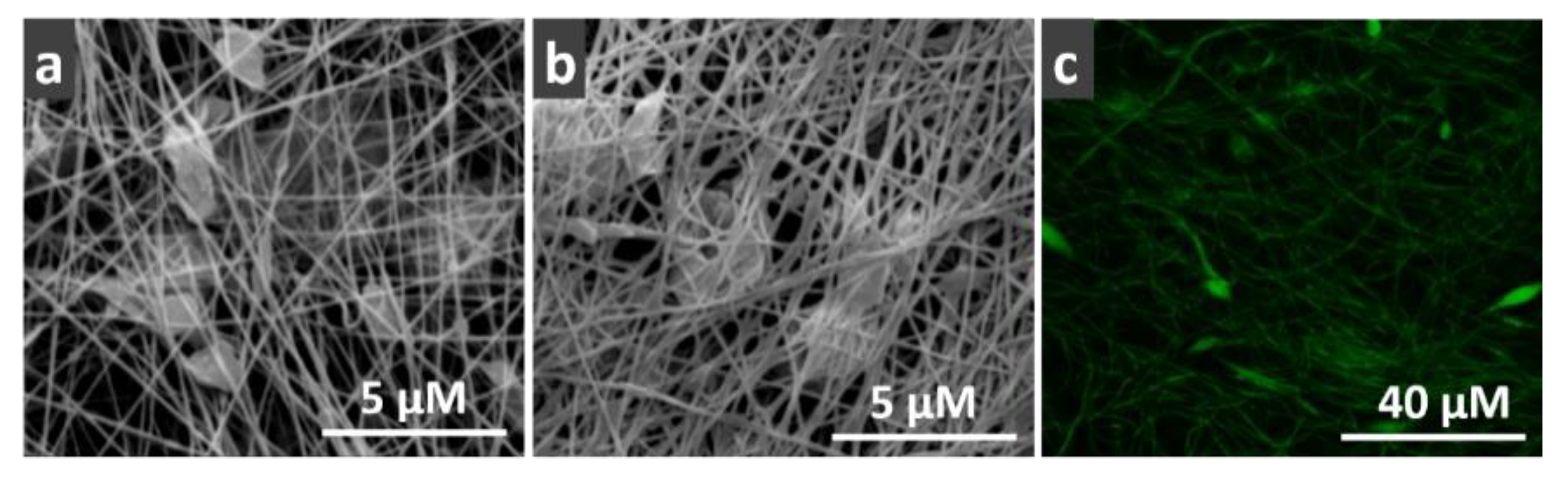
2.3. Morphology
2.4. Laccase Activity and Stability Assays
2.5. Degradation of CV
3. Results and Discussion
3.1. Morphology
3.2. Enzymatic Activity
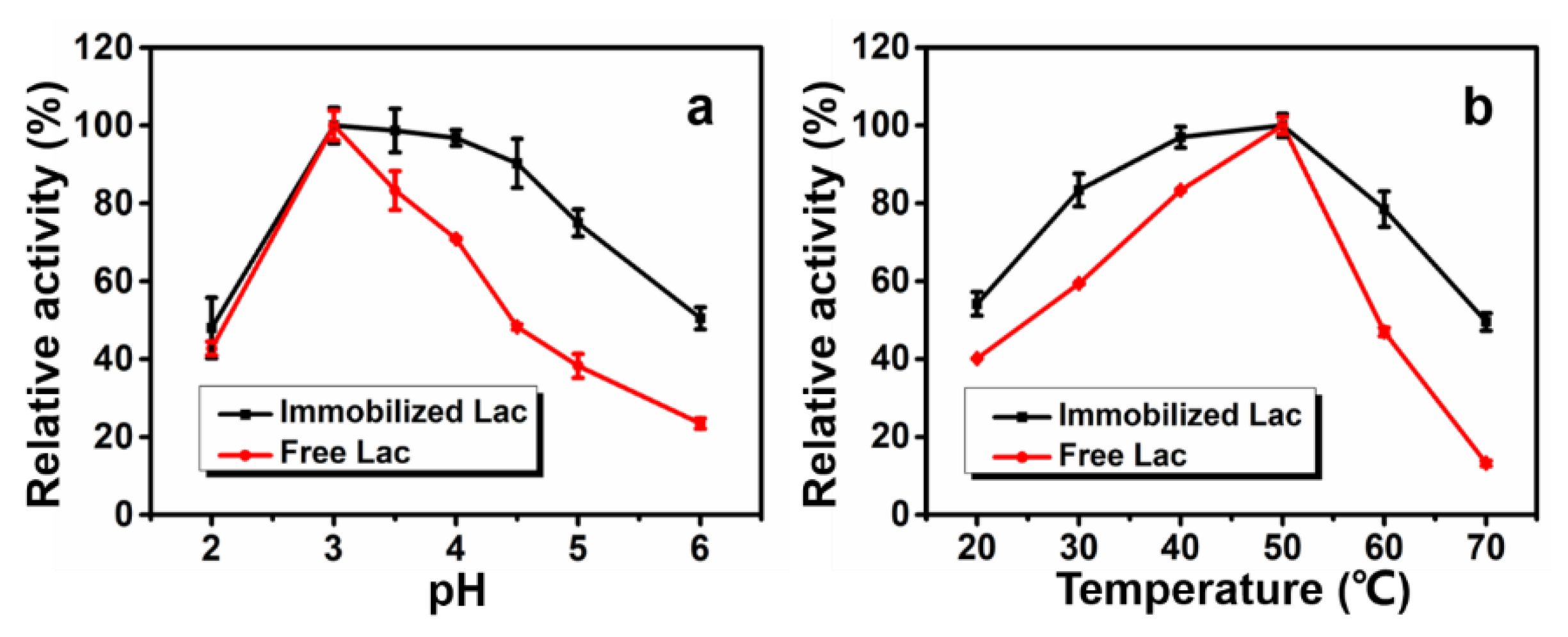
3.3. Optimum pH and Temperature
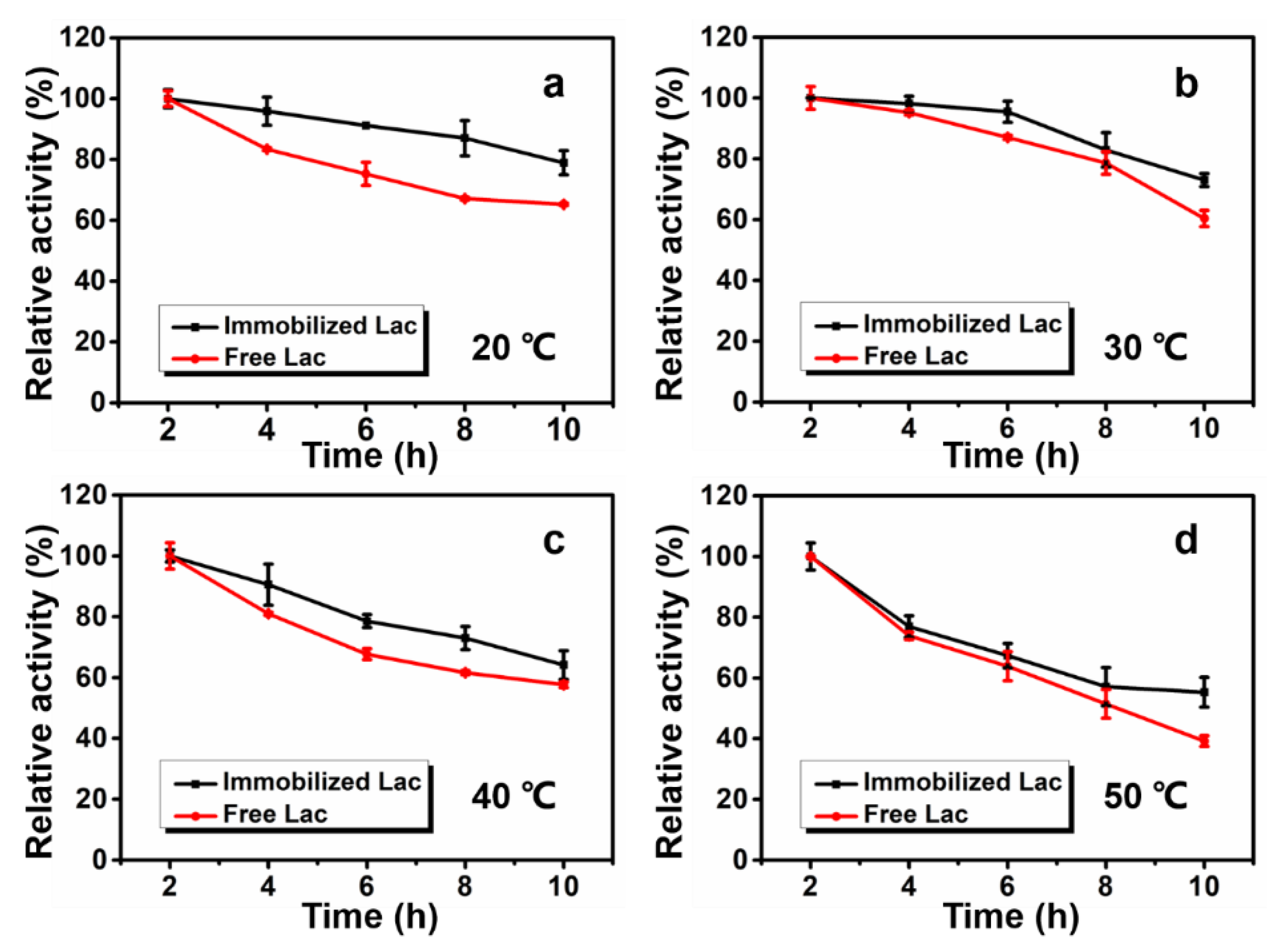
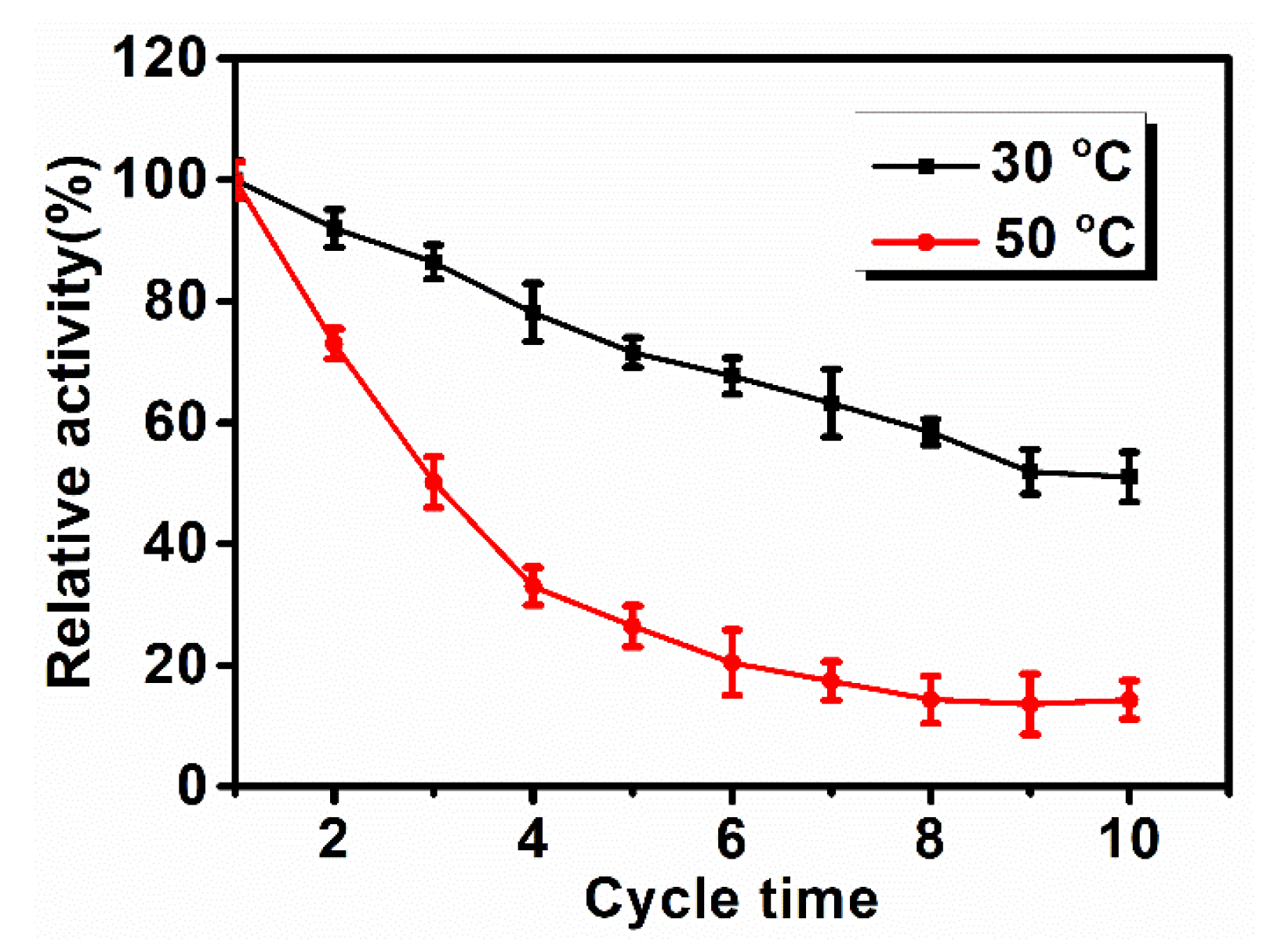
3.4. Thermal Stability and Reusability
3.5. Degradation of Crystal Violet (CV)
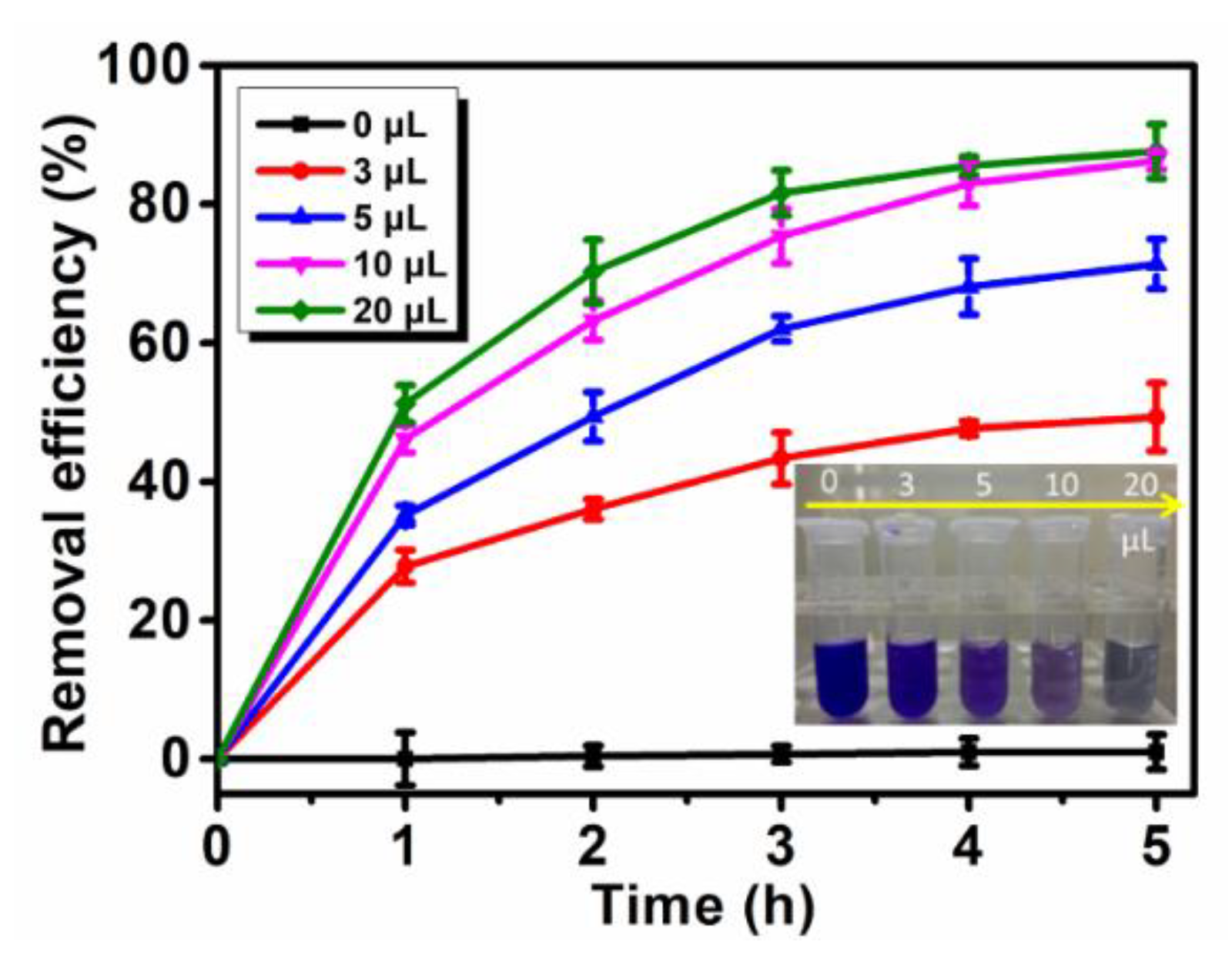
3.5.1. Effect of ABTS Concentration
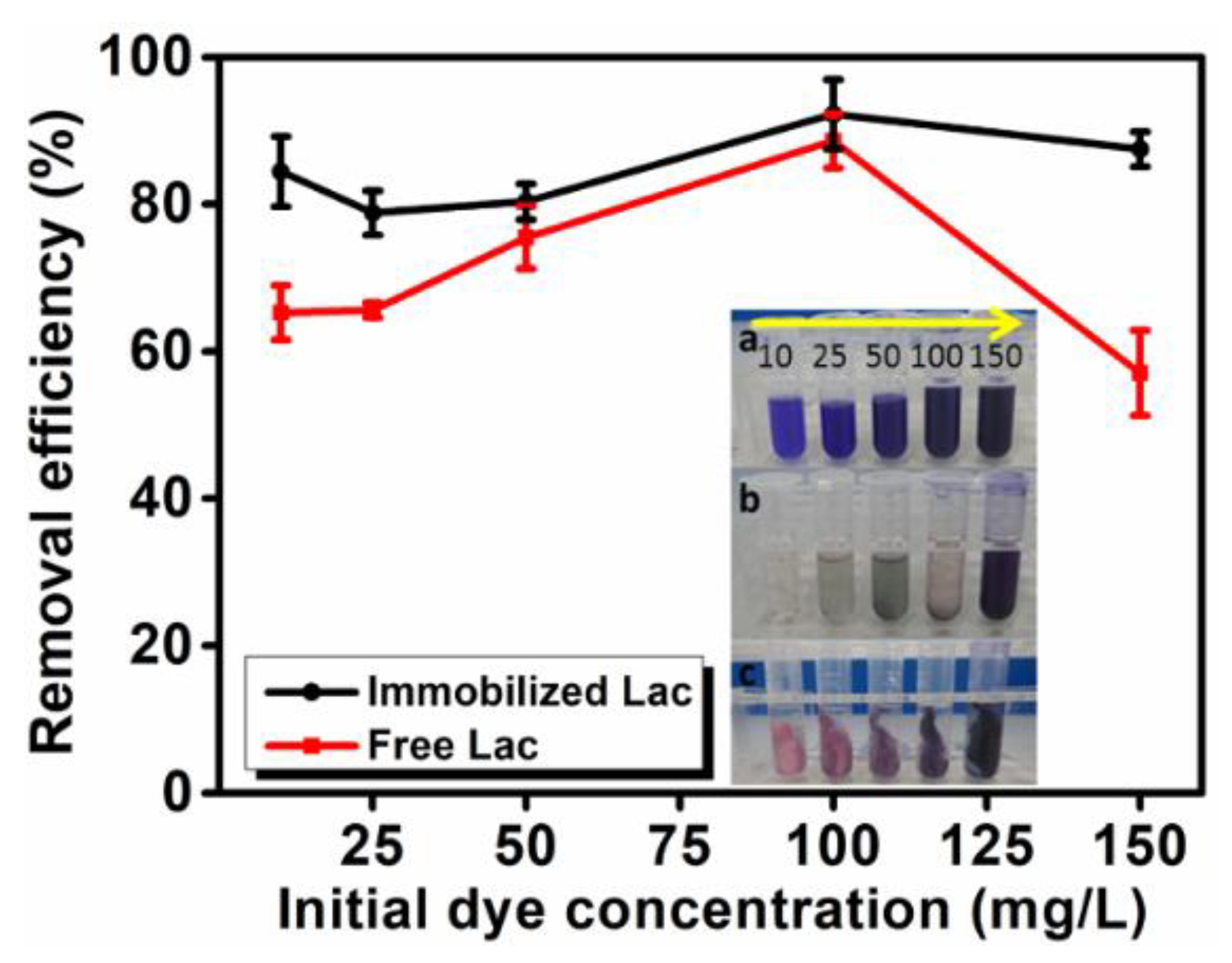
3.5.2. Effect of Initial Dye Concentration
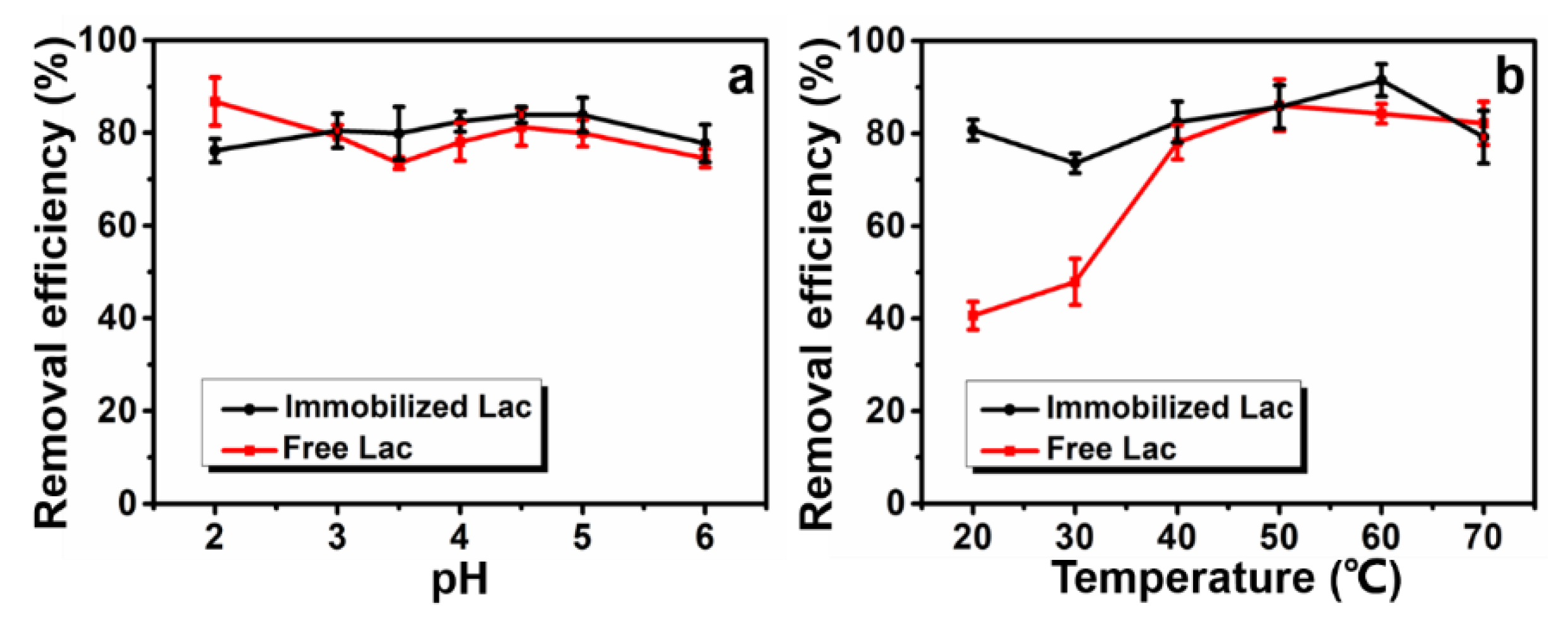
3.5.3. Effect of pH and Temperature
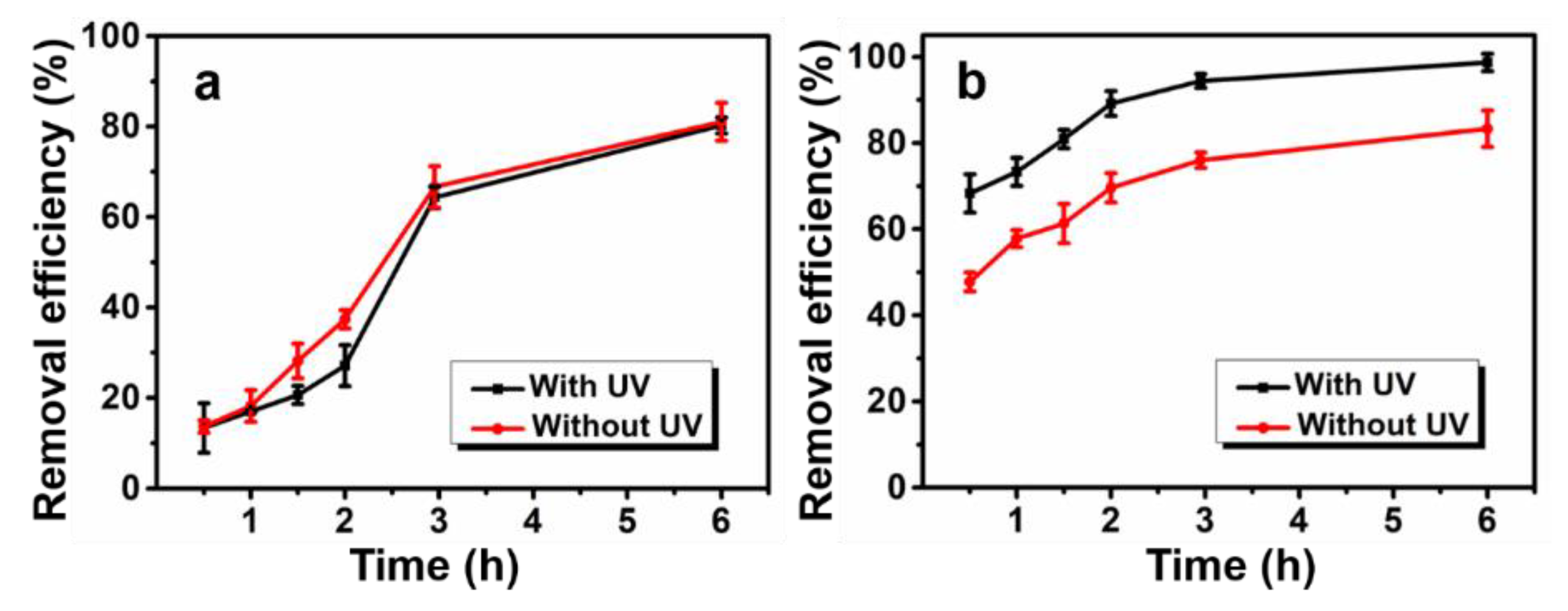
3.5.4. Effect of UV
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Umoren, S. Adsorption of methylene blue from industrial effluent using poly (vinyl alcohol). J. Mater. Environ. Sci. 2013, 4, 75–86. [Google Scholar]
- Holkar, C.; Jadhav, A.; Pinjari, D.; Mahamuni, N.; Pandit, A. A critical review on textile wastewater treatments: Possible approaches. J. Environ. Manag. 2016, 182, 351–366. [Google Scholar] [CrossRef]
- Palmieri, G.; Cennamo, G.; Sannia, G. Remazol Brilliant Blue R Decolourisation by the Fungus Pleurotus ostreatus and Its Oxidative Enzymatic System. Enzym. Microb. Technol. 2005, 36, 17–24. [Google Scholar] [CrossRef]
- Mani, S.; Bharagava, R.N. Exposure to Crystal Violet, Its Toxic, Genotoxic and Carcinogenic Effects on Environment and Its Degradation and Detoxification for Environmental Safety. Rev. Environ. Contam. Toxicol. 2015, 237, 71–104. [Google Scholar] [CrossRef]
- Fan, H.J.; Huang, S.T.; Chung, W.H.; Jan, J.L.; Lin, W.Y. Degradation pathways of Crystal Violet by Fenton and Fenton-like systems: Condition optimization and intermediate separation and identification. J. Hazard. Mater. 2009, 171, 1032–1044. [Google Scholar] [CrossRef] [PubMed]
- Azmi, W.; Sani, R.; Banerjee, U. Biodegradation of Triphenylmethane Dyes. Enzym. Microb. Technol. 1998, 22, 185–191. [Google Scholar] [CrossRef]
- Bharagava, R.N.; Mani, S.; Mulla, S.I.; Saratale, G.D. Degradation and decolourization potential of an ligninolytic enzyme producing Aeromonas hydrophila for crystal violet dye and its phytotoxicity evaluation. Ecotoxicol. Environ. Saf. 2018, 156, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Mani, S.; Bharagava, R.N. Isolation, Screening and Biochemical Characterization of Bacteria Capable of Crystal Violet Dye Decolorization. Int. J. Appl. Adv. Sci. Res. 2017, 2, 70–75. [Google Scholar]
- Gonzalez Herrera, L.F.; Sarria, V.; Sanchez, O. Degradation of Chlorophenols by Sequential Biological-Advanced Oxidative Process Using Trametes Pubescens and TiO2/UV. Bioresour. Technol. 2010, 101, 3493–3499. [Google Scholar] [CrossRef]
- Maryskova, M.; Rysova, M.; Novotny, V.; Sevcu, A. Polyamide-Laccase Nanofiber Membrane for Degradation of Endocrine-Disrupting Bisphenol A, 17α-ethinylestradiol, and Triclosan. Polymers 2019, 11, 1560. [Google Scholar] [CrossRef]
- Li, L.; Dai, W.; Yu, P.; Zhao, J.; Qu, Y. Decolorisation of synthetic dyes by crude laccase from Rigidoporus lignosus W1. J. Chem. Technol. Biotechnol. 2009, 84, 399–404. [Google Scholar] [CrossRef]
- Paździor, K.; Bilińska, L.; Ledakowicz, S. A review of the existing and emerging technologies in the combination of AOPs and biological processes in industrial textile wastewater treatment. Chem. Eng. J. 2019, 376, 120597. [Google Scholar] [CrossRef]
- Arora, D.S.; Kumar, R. Ligninolytic Fungal Laccases and Their Biotechnological Applications. Appl. Biochem. Biotechnol. 2009, 160, 1760–1788. [Google Scholar] [CrossRef] [PubMed]
- Qin, P.; Wu, Y.; Adil, B.; Wang, J.; Gu, Y.; Yu, X.; Zhao, K.; Zhang, X.; Ma, M.; Chen, Q.; et al. Optimization of Laccase from Ganoderma lucidum Decolorizing Remazol Brilliant Blue R and Glac1 as Main Laccase-Contributing Gene. Molecules 2019, 24, 3914. [Google Scholar] [CrossRef]
- Pedroza, A.; Mosqueda, R.; Alonso-Vante, N.; Rodriguez Vazquez, R. Sequential Treatment via Trametes versicolor and UV/TiO2/RuxSey to Reduce Contaminants in Waste Water Resulting From the Bleaching Process During Paper Production. Chemosphere 2007, 67, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Zhang, S.; Wang, P.; Wang, H. Degradation of high concentration 2,4-dichlorophenol by simultaneous photocatalytic-enzymatic process using TiO2/UV and laccase. J. Hazard. Mater. 2012, 205–206, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Nguyen, L.; Hou, J.; Hai, F.; Chen, V. Direct immobilization of laccase on titania nanoparticles from crude enzyme extracts of P. ostreatus culture for micro-pollutant degradation. Sep. Purif. Technol. 2017, 178, 215–223. [Google Scholar] [CrossRef]
- Tapia-Orozco, N.; Meléndez-Saavedra, F.; Figueroa, M.; Gimeno, M.; García-Arrazola, R. Removal of bisphenol A in canned liquid food by enzyme-based nanocomposites. Appl. Nanosci. 2018, 8, 1–8. [Google Scholar] [CrossRef]
- Hou, J.; Dong, G.; Luu, B.; Keller, R.; Ye, Y.; Wessling, M.; Chen, V. Hybrid membrane with TiO2 based bio-catalytic nanoparticle suspension system for the degradation of bisphenol-A. Bioresour. Technol. 2014, 169. [Google Scholar] [CrossRef]
- Sathishkumar, P.; Kamalakannan, S.; Cho, M.; Kim, J.; Hadibarata, T.; Salim, M.; Oh, B.-T. Laccase immobilization on cellulose nanofiber: The catalytic efficiency and recyclic application for simulated dye effluent treatment. J. Mol. Catal. B Enzym. 2014, 100, 111–120. [Google Scholar] [CrossRef]
- Li, G.; Nandgaonkar, A.G.; Wang, Q.; Zhang, J.; Krause, W.E.; Wei, Q.; Lucia, L.A. Laccase-immobilized bacterial cellulose/TiO2 functionalized composite membranes: Evaluation for photo- and bio-catalytic dye degradation. J. Membr. Sci. 2017, 525, 89–98. [Google Scholar] [CrossRef]
- Sanguanpak, S.; Shongkittikul, W.; Wannagon, A.; Chiemchaisri, C. Effects of TiO2 on the laccase enzyme immobilization and the bisphenol-A removal of the ceramic membranes. Desalin. Water Treat. 2017, 93, 163–170. [Google Scholar] [CrossRef][Green Version]
- Xu, H.M.; Sun, X.F.; Wang, S.Y.; Song, C.; Wang, S.G. Development of laccase/graphene oxide membrane for enhanced synthetic dyes separation and degradation. Sep. Purif. Technol. 2018, 204, 255–260. [Google Scholar] [CrossRef]
- Fathi, Z.; Doustkhah, E.; Ebrahimipour, G.; Darvishi, F. Noncovalent Immobilization of Yarrowia lipolytica Lipase on Dendritic-Like Amino Acid-Functionalized Silica Nanoparticles. Biomolecules 2019, 9, 502. [Google Scholar] [CrossRef]
- Baghban, A.; Heidarizadeh, M.; Doustkhah, E.; Rostamnia, S.; Rezaei, P.F. Covalently bonded pancreatic lipase onto the dithiocarbamate/chitosan-based magnetite: Stepwise fabrication of Fe3O4@CS/NHCS-Lip as a novel and promising nanobiocatalyst. Int. J. Biol. Macromol. 2017, 103, 1194–1200. [Google Scholar] [CrossRef]
- Heidarizadeh, M.; Doustkhah, E.; Rostamnia, S.; Rezaei, P.F.; Harzevili, F.D.; Zeynizadeh, B. Dithiocarbamate to modify magnetic graphene oxide nanocomposite (Fe3O4-GO): A new strategy for covalent enzyme (lipase) immobilization to fabrication a new nanobiocatalyst for enzymatic hydrolysis of PNPD. Int. J. Biol. Macromol. 2017, 101, 696–702. [Google Scholar] [CrossRef]
- Fathi, Z.; Doustkhah, E.; Rostamnia, S.; Darvishi, F.; Ghodsi, A.; Ide, Y. Interaction of Yarrowia lipolytica lipase with dithiocarbamate modified magnetic carbon Fe3O4@C-NHCS2H core-shell nanoparticles. Int. J. Biol. Macromol. 2018, 117, 218–224. [Google Scholar] [CrossRef]
- Xu, R.; Zhou, Q.; Li, F.; Zhang, B. Laccase immobilization on chitosan/poly(vinyl alcohol) composite nanofibrous membranes for 2,4-dichlorophenol removal. Chem. Eng. J. 2013, 222, 321–329. [Google Scholar] [CrossRef]
- Hou, J.; Dong, G.; Ye, Y.; Chen, V. Laccase immobilization on titania nanoparticles and titania-functionalized membranes. J. Membr. Sci. 2014, 452, 229–240. [Google Scholar] [CrossRef]
- Xu, R.; Chi, C.; Li, F.; Zhang, B. Laccase-Polyacrylonitrile Nanofibrous Membrane: Highly Immobilized, Stable, Reusable, and Efficacious for 2,4,6-Trichlorophenol Removal. ACS Appl. Mater. Interfaces 2013, 5, 12554–12560. [Google Scholar] [CrossRef]
- Liao, Y.; Loh, C.-H.; Tian, M.; Wang, R.; Fane, A.G. Progress in electrospun polymeric nanofibrous membranes for water treatment: Fabrication, modification and applications. Prog. Polym. Sci. 2018, 77, 69–94. [Google Scholar] [CrossRef]
- Li, D.; Xia, Y.N. Electrospinning of Nanofibers: Reinventing the Wheel? Adv. Mater. 2004, 16, 1151–1170. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef] [PubMed]
- Selloum, D.; Chaaya, A.; Bechelany, M.; Rouessac, V.; Miele, P.; Tingry, S. A highly efficient gold/electrospun PAN fiber material for improved laccase biocathodes for biofuel cell applications. J. Mater. Chem. A 2014, 2, 2794–2800. [Google Scholar] [CrossRef]
- Xu, R.; Si, Y.; Wu, X.; Li, F.; Zhang, B. Triclosan removal by laccase immobilized on mesoporous nanofibers: Strong adsorption and efficient degradation. Chem. Eng. J. 2014, 255, 63–70. [Google Scholar] [CrossRef]
- Ge, J.C.; Choi, N.J. Performance of electrospun nanofibrous membranes for trapping of BTX aromatic hydrocarbons and heavy metal ions: Mechanisms, isotherms and kinetics. J. Clean. Prod. 2019, 217, 388–397. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, W.; Zhang, Q.; Ghiladi, R.; Wei, Q. Preparation of Photodynamic P(MMA -co- MAA) Composite Nanofibers Doped with MMT: A Facile Method for Increasing Antimicrobial Efficiency. Appl. Surf. Sci. 2018, 457, 247–255. [Google Scholar] [CrossRef]
- Zhang, G.K.; Ding, X.; He, F.; Yu, X.; Zhou, J.; Hu, Y.; Xie, J. Low-Temperature Synthesis and Photocatalytic Activity of TiO2 Pillared Montmorillonite. Langmuir ACS J. Surf. Colloids 2008, 24, 1026–1030. [Google Scholar] [CrossRef]
- Wang, Q.; Peng, L.; Du, Y.; Xu, J.; Cai, Y.; Feng, Q.; Huang, F.; Wei, Q. Fabrication of hydrophilic nanoporous PMMA/O-MMT composite microfibrous membrane and its use in enzyme immobilization. J. Porous Mater. 2012, 20, 457–464. [Google Scholar] [CrossRef]
- Li, G.; Nandgaonkar, A.; Lu, K.; Krause, W.; Wei, Q.; Lucia, L. Laccase immobilized on PAN/O-MMT composite nanofibers support for substrate bioremediation: A: De novo adsorption and biocatalytic synergy. RSC Adv. 2016, 6, 41420–41427. [Google Scholar] [CrossRef]
- Wan, L.S.; Ke, B.B.; Xu, Z.K. Electrospun nanofibrous membranes filled with carbon nanotubes for redox enzyme immobilization. Enzym. Microb. Technol. 2008, 42, 332–339. [Google Scholar] [CrossRef]
- Wang, F.; Guo, C.; Liu, H.Z.; Liu, C.Z. Immobilization of Pycnoporus sanguineus laccase by metal affinity adsorption on magnetic chelator particles. J. Chem. Technol. Biotechnol. 2008, 83, 97–104. [Google Scholar] [CrossRef]
- Li, N.; Xia, Q.; Li, Y.; Hou, X.; Niu, M.; Ping, Q.; Xiao, H. Immobilizing Laccase on Modified Cellulose/CF Beads to Degrade Chlorinated Biphenyl in Wastewater. Polymers 2018, 10, 798. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Yuan, B.; Zhang, Y.; Guo, M. Immobilization of laccase on magnetic silica nanoparticles and its application in the oxidation of guaiacol, a phenolic lignin model compound. RSC Adv. 2015, 5, 99439–99447. [Google Scholar] [CrossRef]
- Quintanilla, F.; Duarte-Vázquez, M.; García-Almendárez, B.; Tinoco, R.; Vazquez-Duhalt, R.; Regalado, C. Polyehtylene glycol improves phenol removal by immobiized turnip peroxidase. Bioresour. Technol. 2008, 99, 8605–8611. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, C. Dependence of the mutual diffusion coefficient on the dye concentration in the polymer/dye system. Macromolecules 1990, 23, 1218–1220. [Google Scholar] [CrossRef]
| Lacs | Enzyme Loading (mg/g Membrane) | Retention Activity (%) | Km (µmol/mL) | Vmax (µmol/mg·min) |
|---|---|---|---|---|
| Free Lac | - | - | 0.12 | 595.24 |
| Immobilized Lac | 342 | 82.5 | 0.35 | 292.71 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Wang, T.; Lv, Z.; Cui, M.; Zhao, Z.; Cao, X.; Wei, Q. TiO2 Sol-Gel Coated PAN/O-MMT Multi-Functional Composite Nanofibrous Membrane Used as the Support for Laccase Immobilization: Synergistic Effect between the Membrane Support and Enzyme for Dye Degradation. Polymers 2020, 12, 139. https://doi.org/10.3390/polym12010139
Wang Q, Wang T, Lv Z, Cui M, Zhao Z, Cao X, Wei Q. TiO2 Sol-Gel Coated PAN/O-MMT Multi-Functional Composite Nanofibrous Membrane Used as the Support for Laccase Immobilization: Synergistic Effect between the Membrane Support and Enzyme for Dye Degradation. Polymers. 2020; 12(1):139. https://doi.org/10.3390/polym12010139
Chicago/Turabian StyleWang, Qingqing, Tingting Wang, Zihao Lv, Mengting Cui, Ziqiang Zhao, Xiuming Cao, and Qufu Wei. 2020. "TiO2 Sol-Gel Coated PAN/O-MMT Multi-Functional Composite Nanofibrous Membrane Used as the Support for Laccase Immobilization: Synergistic Effect between the Membrane Support and Enzyme for Dye Degradation" Polymers 12, no. 1: 139. https://doi.org/10.3390/polym12010139
APA StyleWang, Q., Wang, T., Lv, Z., Cui, M., Zhao, Z., Cao, X., & Wei, Q. (2020). TiO2 Sol-Gel Coated PAN/O-MMT Multi-Functional Composite Nanofibrous Membrane Used as the Support for Laccase Immobilization: Synergistic Effect between the Membrane Support and Enzyme for Dye Degradation. Polymers, 12(1), 139. https://doi.org/10.3390/polym12010139




