Synthesis of Molecularly Imprinted Polymers for the Selective Extraction of Polymyxins from Environmental Water Samples
Abstract
1. Introduction
2. Materials and Methods
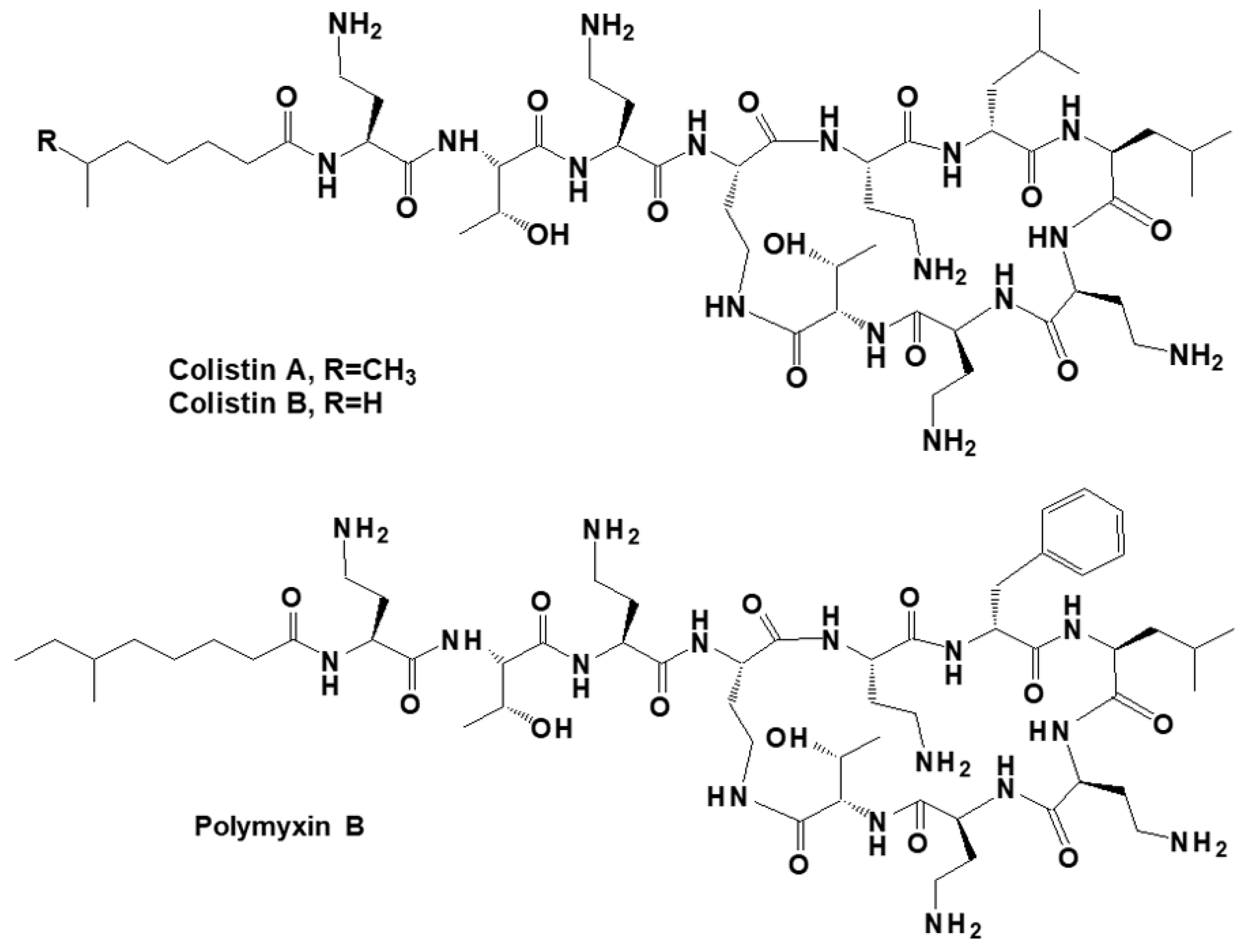
2.1. Reagents and Materials
2.2. Polymer Preparation
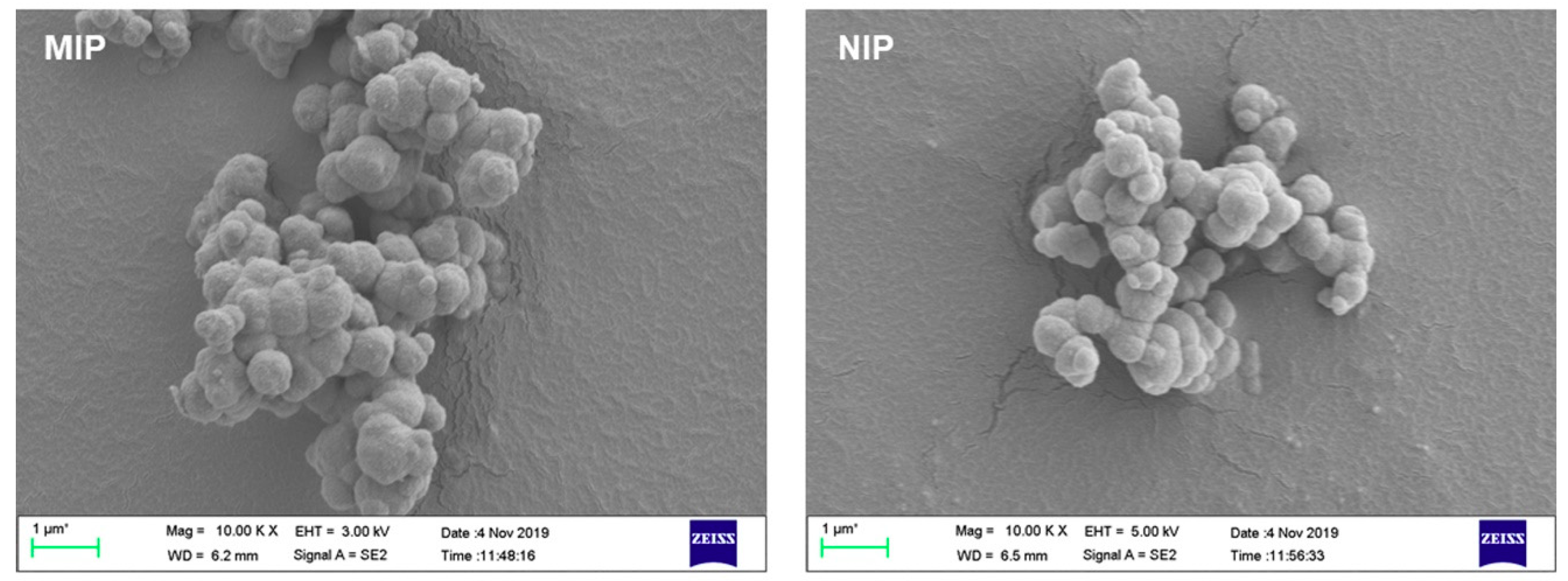
2.3. Characterization Techniques
2.4. Sample preparation
2.5. HPLC Analysis
3. Results and Discussion
3.1. Preparation and Characterization of Imprinted Polymers
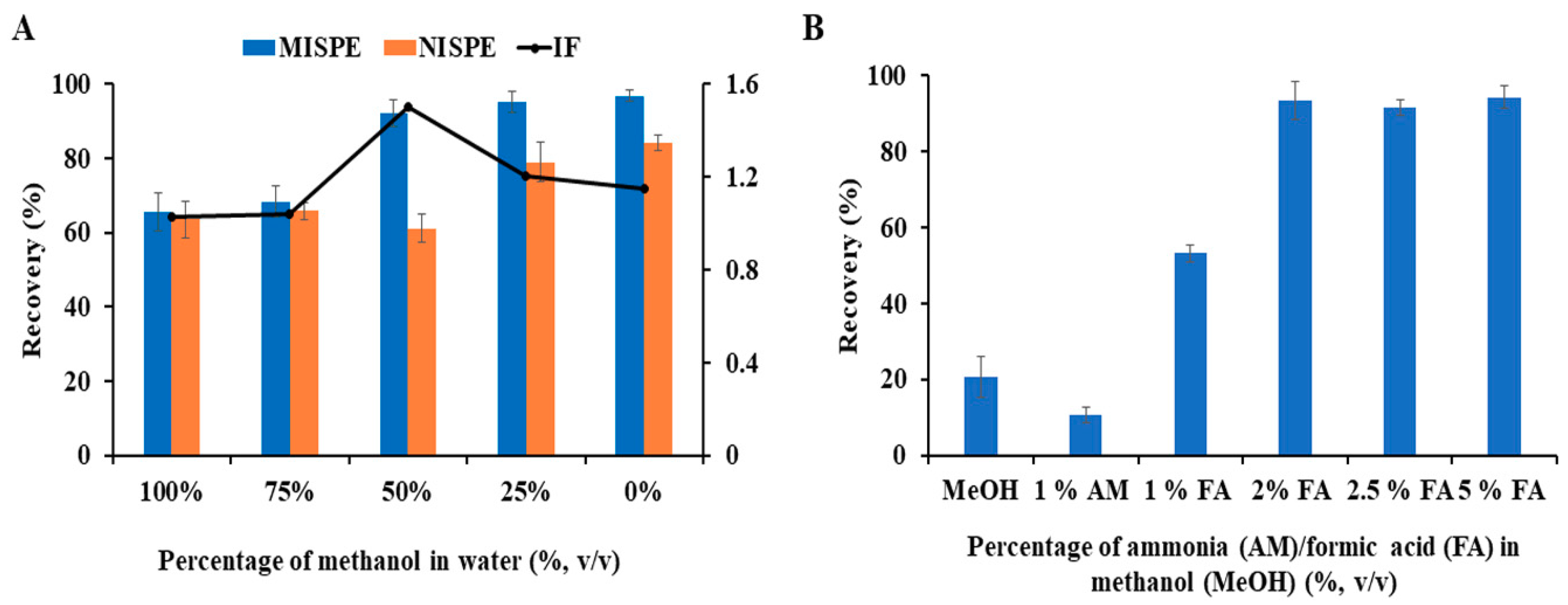
3.2. Optimization of MISPE Conditions
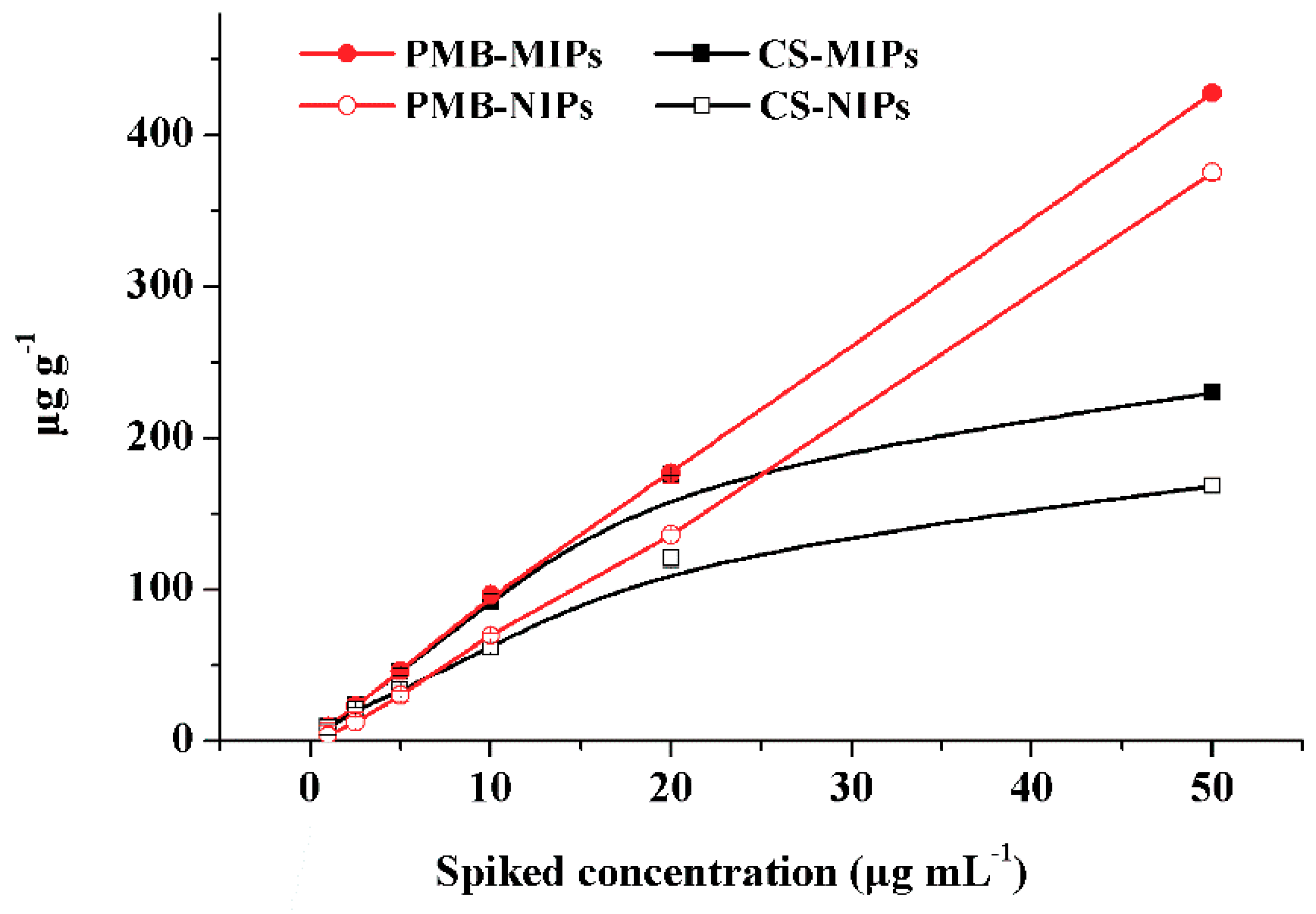
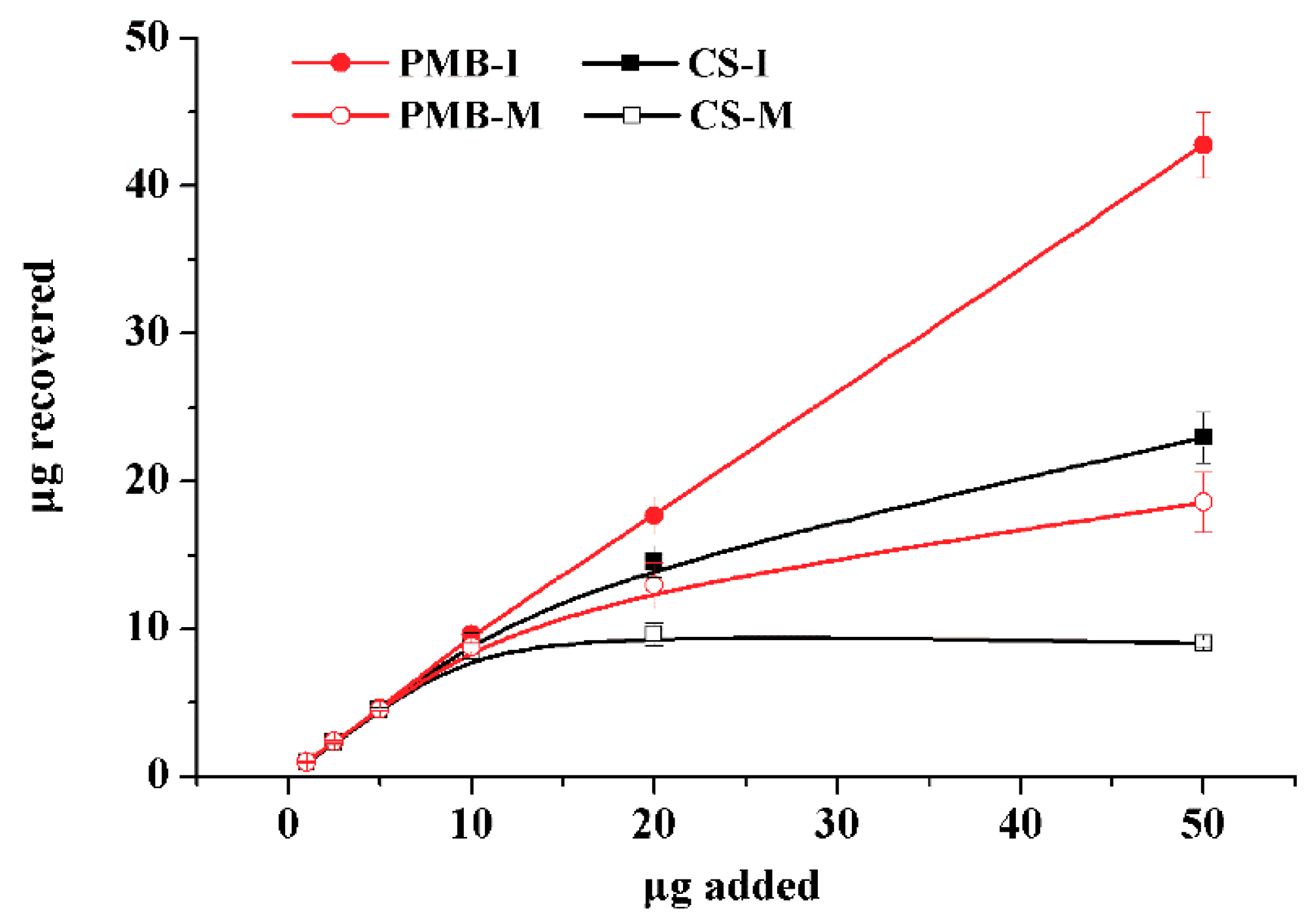
3.3. Cross-Reactivity and Competition for the Binding Sites
3.4. Breakthrough Volume and Reusability
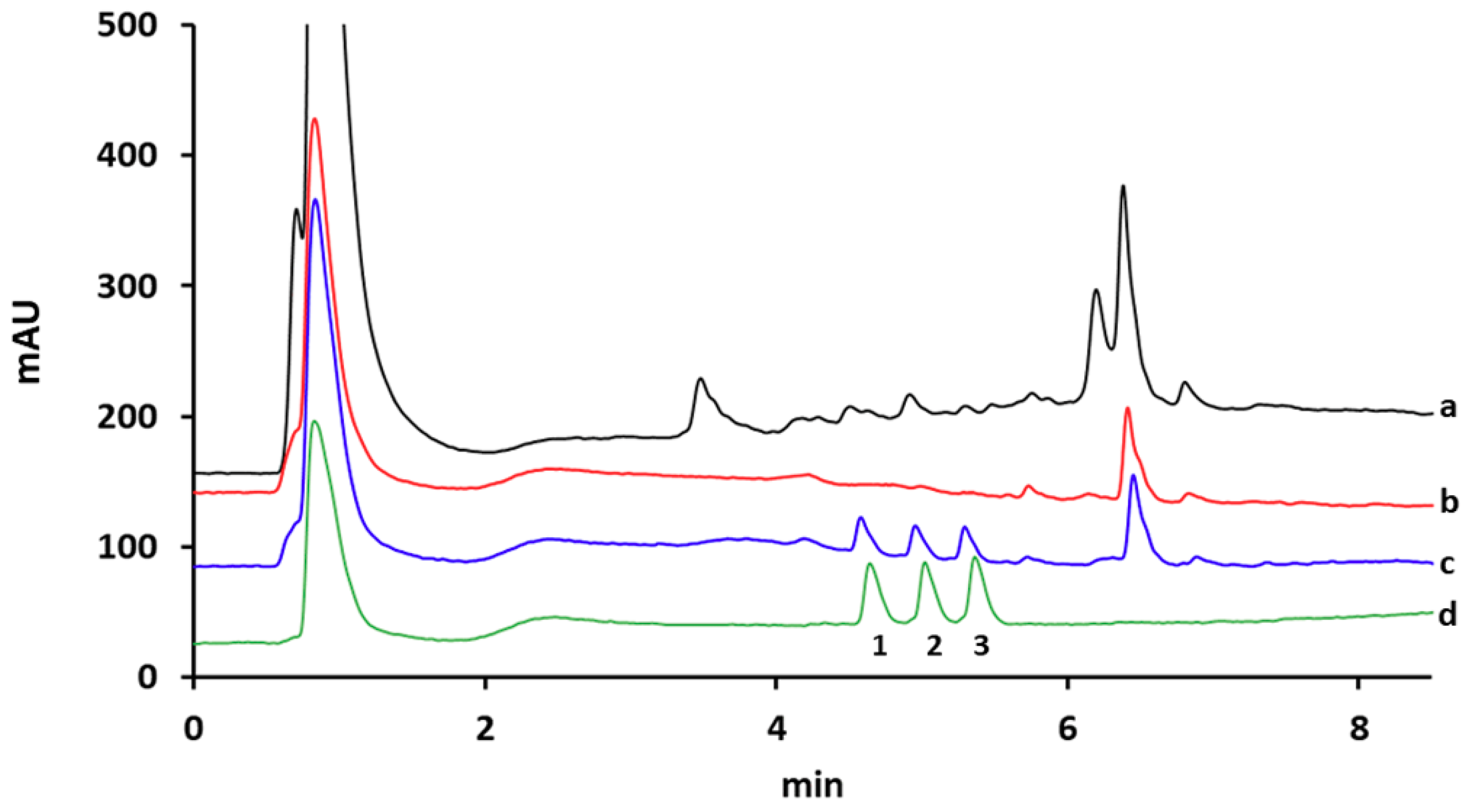
3.5. Analytical Performance and Application of the Proposed MISPE Procedure to the Extraction of Polymyxins from Environmental Water Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Boison, J.O.; Lee, S.; Matus, J. A multi-residue method for the determination of seven polypeptide drug residues in chicken muscle tissues by LC-MS/MS. Anal. Bioanal. Chem. 2015, 407, 4065–4078. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Tian, X.; Ren, C.; Huang, H.; Zhang, X.; Gong, X.; Liu, H.; Yu, Z.; Zhang, L. Analysis of colistin A and B in fishery products by ultra performance liquid chromatography with positive electrospray ionization tandem mass spectrometry. J. Chromatogr. B 2012, 899, 14–20. [Google Scholar] [CrossRef]
- Smith, G.W.; Ronette, G.; Craigmill, A.L.; Webb, A.I.; Riviere, J.E. Extralabel intramammary use of drugs in dairy cattle. J. Am. Vet. Med. A 2005, 226, 1994–1996. [Google Scholar]
- Callens, B.; Persoons, D.; Maes, D.; Laanen, M.; Postma, M.; Boyen, F.; Haesebrouck, F.; Butaye, P.; Catry, B.; Dewulf, J. Prophylactic and metaphylactic antimicrobial use in Belgian fattening pig herds. Prev. Vet. Med. 2012, 106, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Katsunuma, Y.; Hanazumi, M.; Fujisaki, H.; Minato, H.; Hashimoto, Y.; Yonemochi, C. Associations between the use of antimicrobial agents for growth promotion and the occurrence of antimicrobial-resistant Escherichia coli and enterococci in the feces of livestock and livestock farmers in Japan. J. Gen. Appl. Microbiol. 2007, 53, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Kempf, I.; Fleury, M.A.; Drider, D.; Bruneau, M.; Sanders, P.; Chauvin, C.; Madec, J.Y.; Jouy, E. What do we know about resistance to colistin in Enterobacteriaceae in avian and pig production in Europe? Int. J. Antimicrob. Agents 2013, 42, 379–383. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Wang, Y.; Walsh, T.R.; Yi, L.-X.; Zhang, R.; Spencer, J.; Doi, Y.; Tian, G.; Dong, B.; Huang, X.; et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: A microbiological and molecular biological study. Lancet Infect. Dis. 2016, 16, 161–168. [Google Scholar] [CrossRef]
- Tsai, I.L.; Sun, H.Y.; Chen, G.Y.; Lin, S.W.; Kuo, C.H. Simultaneous quantification of antimicrobial agents for multidrug-resistant bacterial infections in human plasma by ultra-high-pressure liquid chromatography–tandem mass spectrometry. Talanta 2013, 116, 593–603. [Google Scholar] [CrossRef]
- Hembach, N.; Schmid, F.; Alexander, J.; Hiller, C.; Rogall, E.T.; Schwartz, T. Occurrence of the mcr-1 Colistin Resistance Gene and other Clinically Relevant Antibiotic Resistance Genes in Microbial Populations at Different Municipal Wastewater Treatment Plants in Germany. Front. Microbiol. 2017, 8, 1282. [Google Scholar] [CrossRef]
- Zurfuh, K.; Poirel, L.; Nordmann, P.; Nüesch-Inderbinen, M.; Hächler, H.; Stephan, R. Occurrence of the Plasmid-Borne mcr-1 Colistin Resistance Gene in Extended-Spectrum-β-Lactamase-Producing Enterobacteriaceae in River Water and Imported Vegetable Samples in Switzerland. Antimicrob. Agents Chem. 2016, 60, 2594. [Google Scholar] [CrossRef]
- Caltagirone, M.; Nucleo, E.; Spalla, M.; Zara, F.; Novazzi, F.; Marchetti, V.M.; Piazza, A.; Bitar, I.; De Cicco, M.; Paolucci, S.; et al. Occurrence of Extended Spectrum β-Lactamases, KPC-Type, and MCR-1.2-Producing Enterobacteriaceae from Wells, River Water, and Wastewater Treatment Plants in Oltrepò Pavese Area, Northern Italy. Front. Microbiol. 2017, 8, 2232. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Agriculture of China. NO. 2428 Announcement: Ban of Colistin Sulfate for Promoting in Animal Growth. 2016. Available online: http://www.360doc.com/content/16/0730/14/94334_579558101.shtml (accessed on 28 October 2019).
- European Medicines Agency. Updated Advice on the Use of Colistin Products in Animals within the European Union: Development of Resistance and Possible Impact on Human and Animal Health. May 26, 2016. EMA/231573/2016. 2016. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/updated-advice-use-colistin-products-animals-within-european-union-development-resistance-possible_en.pdf (accessed on 28 October 2019).
- Hu, F.Y.; He, L.M.; Yang, J.W.; Bian, K.; Wang, Z.N.; Yang, H.C.; Liu, Y.H. Determination of 26 veterinary antibiotics residues in water matrices by lyophilization in combination with LC–MS/MS. J. Chromatogr. B 2014, 949, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.M.; Han, Y.Q.; Hong, X.; Hu, X.Z.; Peng, X.T. Rapid and sensitive detection of the phenoxy acid herbicides in environmental water samples by magnetic solid-phase extraction combined with LC-MS/MS. J. Sep. Sci. 2018, 41, 2221–2228. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Li, X.; Zheng, K.; Ke, Y.; Wang, Y.; Wang, L.; Yu, F.; Xia, X. Determination of colistin in animal tissues, egg, milk, and feed by ultra-high performance liquid chromatography-tandem mass spectrometry. Food Chem. 2018, 248, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, A.; Widmer, M. Quantitative analysis of polypeptide antibiotic residues in a variety of food matrices by liquid chromatography coupled to tandem mass spectrometry. Anal. Chim. Acta 2013, 797, 81–88. [Google Scholar] [CrossRef]
- Song, X.; Xie, J.; Zhang, M.; Zhang, Y.; Li, J.; Huang, Q.; He, L. Simultaneous determination of eight cyclopolypeptide antibiotics in feed by high performance liquid chromatography coupled with evaporation light scattering detection. J. Chromatogr. B 2018, 1076, 103–109. [Google Scholar] [CrossRef]
- Van Poucke, C.; De Keyser, K.; Baltusnikiene, A.; McEvoy, J.D.G.; Van Peteghem, C. Liquid chromatographic–tandem mass spectrometric detection of banned antibacterial growth promoters in animal feed. Anal. Chim. Acta 2003, 483, 99–109. [Google Scholar] [CrossRef]
- Chepyala, D.; Tsai, I.L.; Sun, H.-Y.; Lin, S.-W.; Kuo, C.H. Development and validation of a high-performance liquid chromatography-fluorescence detection method for the accurate quantification of colistin in human plasma. J. Chromatogr. B 2015, 980, 48–54. [Google Scholar] [CrossRef]
- Gikas, E.; Bazoti, F.N.; Katsimardou, M.; Anagnostopoulos, D.; Papanikolaou, K.; Inglezos, I.; Skoutelis, A.; Daikos, G.L.; Tsarbopoulos, A. Determination of colistin A and colistin B in human plasma by UPLC–ESI high resolution tandem MS: Application to a pharmacokinetic study. J. Pharmaceut. Biomed. Anal. 2013, 83, 228–236. [Google Scholar] [CrossRef]
- Lu, W.; Liu, J.; Li, J.; Wang, X.; Lv, M.; Cui, R.; Chen, L. Dual-template molecularly imprinted polymers for dispersive solid-phase extraction of fluoroquinolones in water samples coupled with high performance liquid chromatography. Analyst 2019, 144, 1292–1302. [Google Scholar] [CrossRef]
- Zhong, M.; Wang, Y.H.; Wang, L.; Long, R.Q.; Chen, C.L. Synthesis and characterization of magnetic molecularly imprinted polymers for enrichment of sanguinarine from the extraction wastewater of M. cordata. J. Ind. Eng. Chem. 2018, 66, 107–115. [Google Scholar] [CrossRef]
- Piletska, E.V.; Guerreiro, A.R.; Romero-Guerra, M.; Chianella, I.; Turner, A.P.F.; Piletsky, S.A. Design of molecular imprinted polymers compatible with aqueous environment. Anal. Chim. Acta 2008, 607, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Zhang, F.; Luo, X.; Yang, B.; Jin, G.; Yan, J.; Liang, X. Synthesis of molecularly imprinted polymer sorbents and application for the determination of aminoglycosides antibiotics in honey. J. Chromatogr. A 2013, 1313, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Miguel, E.M.; Souza, C.F.; Silva, A.R.; Aucélio, R.Q. Thioglycolic acid-CdTe quantum dots sensing and molecularly imprinted polymer based solid phase extraction for the determination of kanamycin in milk, vaccine and stream water samples. Sens. Actuators B Chem. 2017, 246, 444–454. [Google Scholar] [CrossRef]
- Yao, R.; Yu, Z.; Wu, M.; Yu, H. Preparation and evaluation of molecularly imprinted membrane of teicoplanin. Anal. Methods 2018, 10, 5416–5422. [Google Scholar] [CrossRef]
- Dirion, B.; Cobb, Z.; Schillinger, E.; Andersson, L.I.; Sellergren, B. Water-Compatible Molecularly Imprinted Polymers Obtained via High-Throughput Synthesis and Experimental Design. J. Am. Chem. Soc. 2003, 125, 15101–15109. [Google Scholar] [CrossRef]
- Tamayo, F.G.; Casillas, J.L.; Martin-Esteban, A. Highly selective fenuron-imprinted polymer with a homogeneous binding site distribution prepared by precipitation polymerisation and its application to the clean-up of fenuron in plant samples. Anal. Chim. Acta 2003, 482, 165–173. [Google Scholar] [CrossRef]
- Díaz-Álvarez, M.; Barahona, F.; Turiel, E.; Martín-Esteban, A. Supported liquid membrane-protected molecularly imprinted beads for micro-solid phase extraction of sulfonamides in environmental waters. J. Chromatogr. A 2014, 1357, 158–164. [Google Scholar] [CrossRef]
- Wang, J.; Cormack, P.A.G.; Sherrington, D.C.; Khoshdel, E. Monodisperse, Molecularly Imprinted Polymer Microspheres Prepared by Precipitation Polymerization for Affinity Separation Applications. Angew. Chem. Inte. Edit. 2003, 42, 5336–5338. [Google Scholar] [CrossRef]
- Li, W.; Stöver, H.D.H. Monodisperse Cross-Linked Core−Shell Polymer Microspheres by Precipitation Polymerization. Macromolecules 2000, 33, 4354–4360. [Google Scholar] [CrossRef]
- Andrea, K.; Kasiakou, S.K.; Tam, V.H.; Falagas, M.E. Polymyxin B: Similarities to and differences from colistin (polymyxin E). Expert Rev. Anti-Infect. Ther. 2007, 5, 811. [Google Scholar]
- Commission of the European Communities. Official Journal of the European Communities, European Commission Decision 2002/657/EC. 2002, pp. 8–36. Available online: http://www.doc88.com/p-485336286714.html (accessed on 28 October 2019).
| Water Source | Analyte a | Intra-Day Recovery (RSD b, %, n = 3) | LOD c | LOQ d | LOQ d | ||
|---|---|---|---|---|---|---|---|
| I | II | III | (RSD, %, n = 9) | (µg L−1) | (µg L−1) | ||
| River | CSA | 74.7 (3.7) | 65.9 (4.5) | 75.7 (3.2) | 72.1 (7.3) | 2.0 | 6.5 |
| CSB | 77.2 (3.6) | 72.1 (3.8) | 72.1 (1.9) | 73.8 (4.6) | 1.5 | 5.0 | |
| PMB | 83.7 (3.2) | 76.9 (9.7) | 83.6 (1.4) | 81.4 (6.3) | 1.0 | 3.0 | |
| Spring | CSA | 72.3 (2.1) | 71.6 (4.7) | 76.8 (1.2) | 73.6 (4.2) | 1.0 | 3.0 |
| CSB | 74.6 (2.2) | 81.8 (3.9) | 74.4 (3.0) | 76.9 (5.5) | 1.0 | 3.0 | |
| PMB | 85.5 (5.3) | 83.1 (4.3) | 80.9 (10.2) | 83.2 (6.4) | 1.0 | 3.0 | |
| Lake | CSA | 83.1 (3.9) | 84.4 (6.2) | 86.4 (9.5) | 84.6 (6.3) | 2.0 | 6.0 |
| CSB | 85.6 (3.7) | 86.7 (2.3) | 85.1 (3.6) | 85.8 (3.1) | 1.5 | 5.0 | |
| PMB | 83.8 (3.1) | 90.1 (1.8) | 84.5 (2.3) | 86.2 (4.0) | 1.0 | 3.0 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, X.; Turiel, E.; He, L.; Martín-Esteban, A. Synthesis of Molecularly Imprinted Polymers for the Selective Extraction of Polymyxins from Environmental Water Samples. Polymers 2020, 12, 131. https://doi.org/10.3390/polym12010131
Song X, Turiel E, He L, Martín-Esteban A. Synthesis of Molecularly Imprinted Polymers for the Selective Extraction of Polymyxins from Environmental Water Samples. Polymers. 2020; 12(1):131. https://doi.org/10.3390/polym12010131
Chicago/Turabian StyleSong, Xuqin, Esther Turiel, Limin He, and Antonio Martín-Esteban. 2020. "Synthesis of Molecularly Imprinted Polymers for the Selective Extraction of Polymyxins from Environmental Water Samples" Polymers 12, no. 1: 131. https://doi.org/10.3390/polym12010131
APA StyleSong, X., Turiel, E., He, L., & Martín-Esteban, A. (2020). Synthesis of Molecularly Imprinted Polymers for the Selective Extraction of Polymyxins from Environmental Water Samples. Polymers, 12(1), 131. https://doi.org/10.3390/polym12010131







