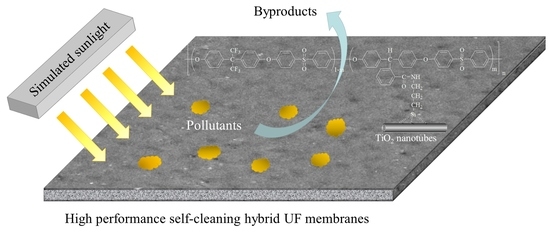
High-Performance TiO2 Nanotubes/Poly(aryl ether sulfone) Hybrid Self-Cleaning Anti-Fouling Ultrafiltration Membranes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Titanium Dioxide Nanotubes (TNTs) Photocatalysts
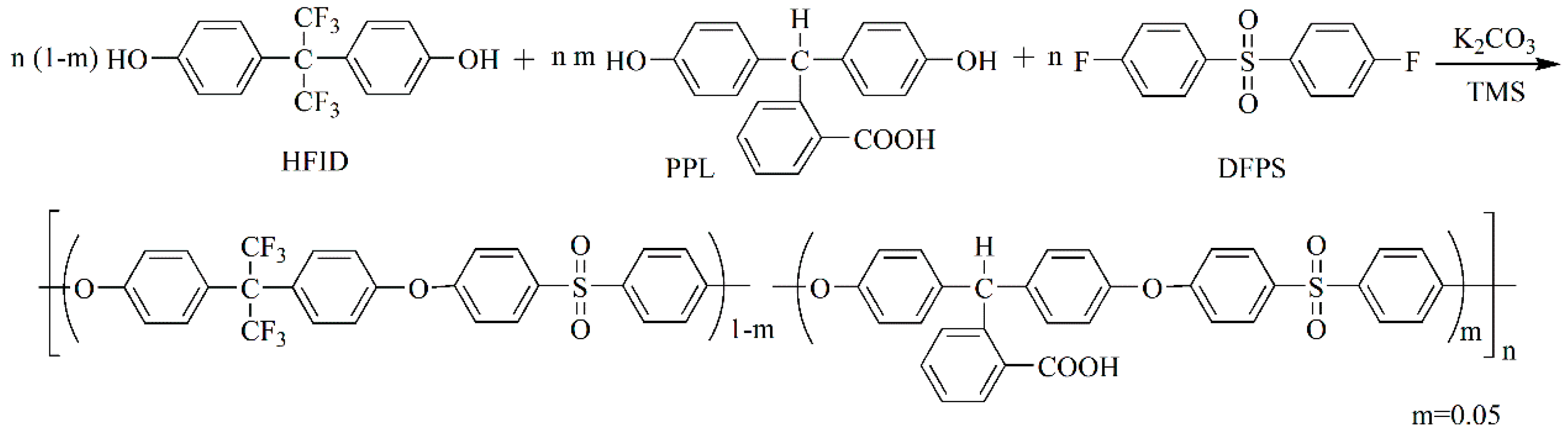
2.3. Synthesis of PES-F-COOH Copolymer
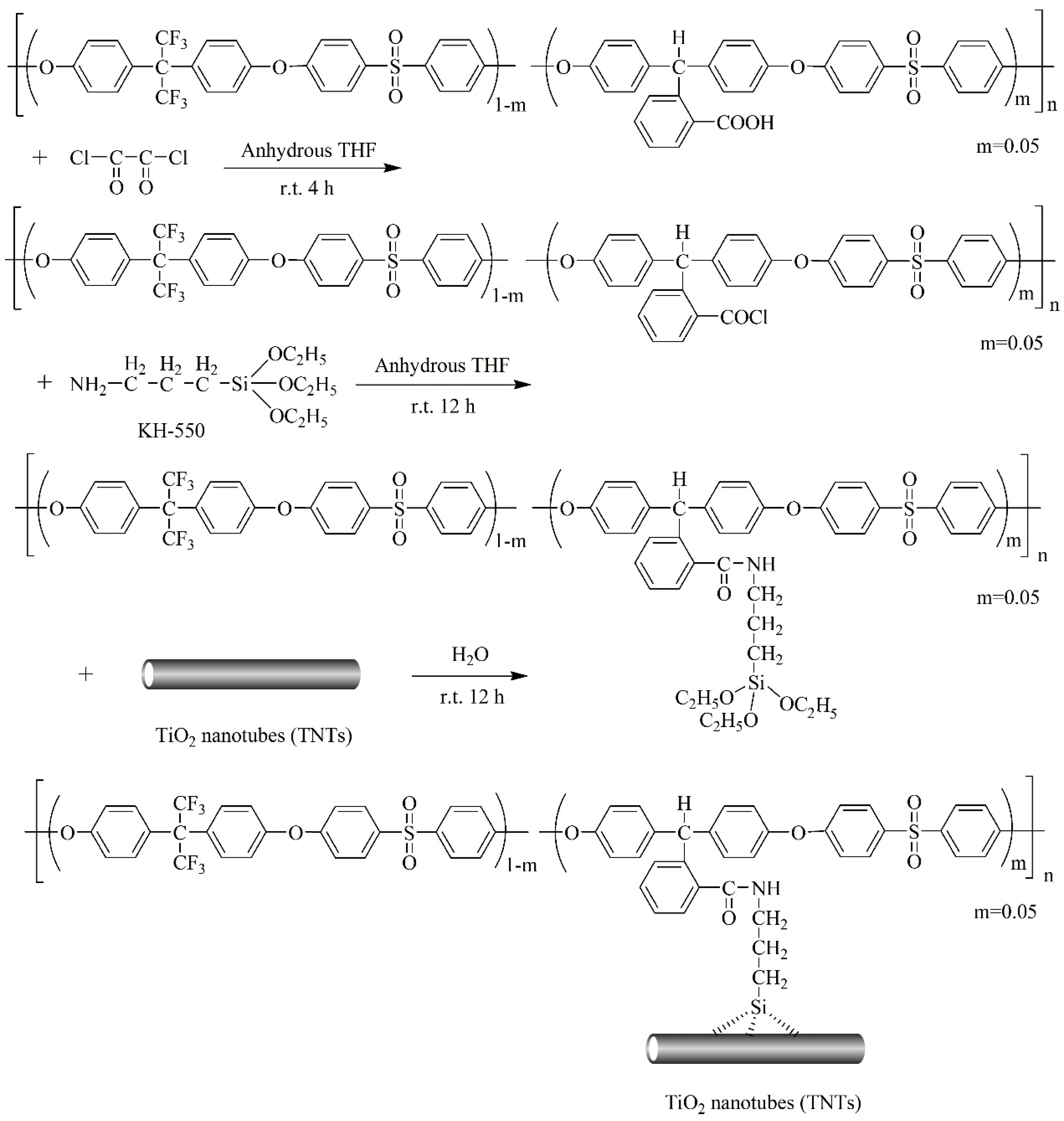
2.4. Synthesis of TNTs/PES-F-COOH Hybrid Materials
2.5. Preparation of TNTs/PES-F-COOH Ultrafiltration Membranes
2.6. Characterization
2.7. Ultrafiltration Tests
2.8. Mean Pore Size and Prosity Tests
3. Results and Discussion
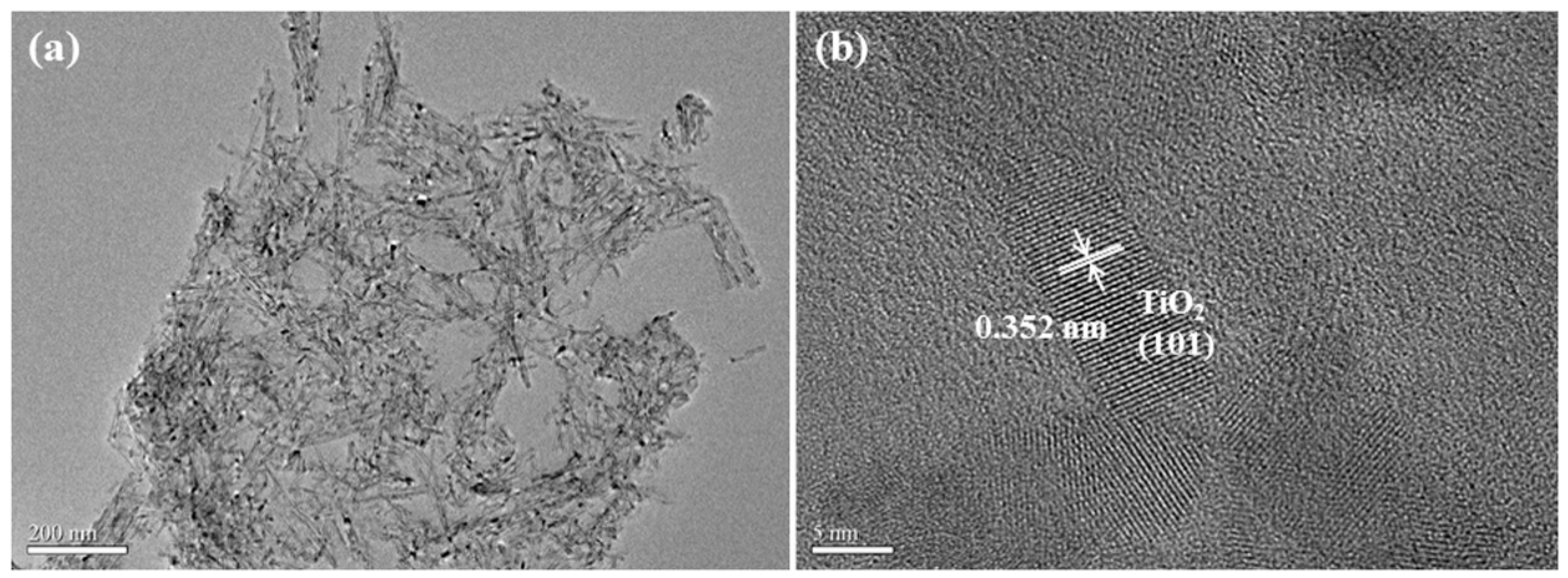
3.1. Morphology of TNTs Catalyst
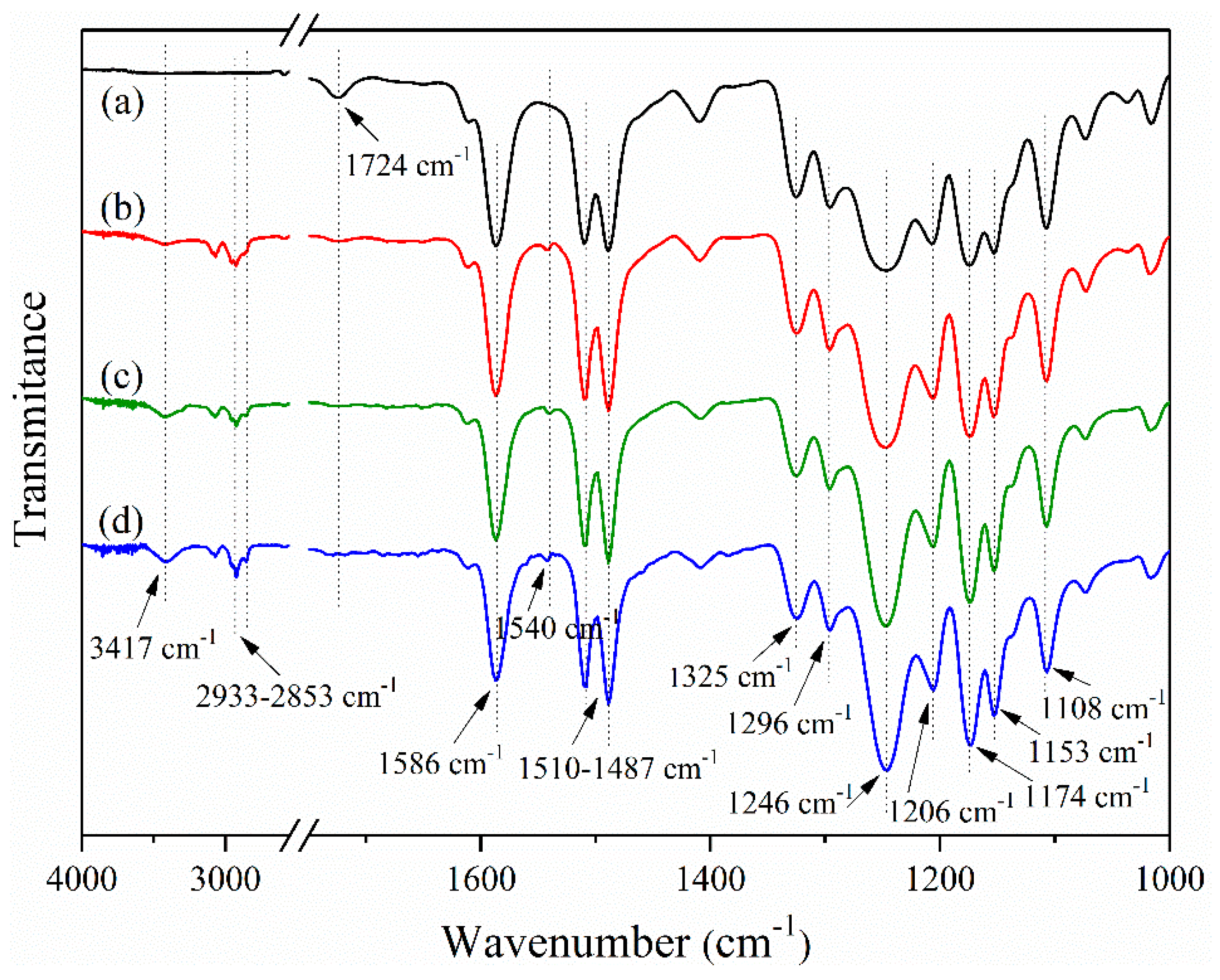
3.2. Chemical Structures of TNTs/PES-F-COOH Hybrid Materials
3.3. Morphology of UF Membranes
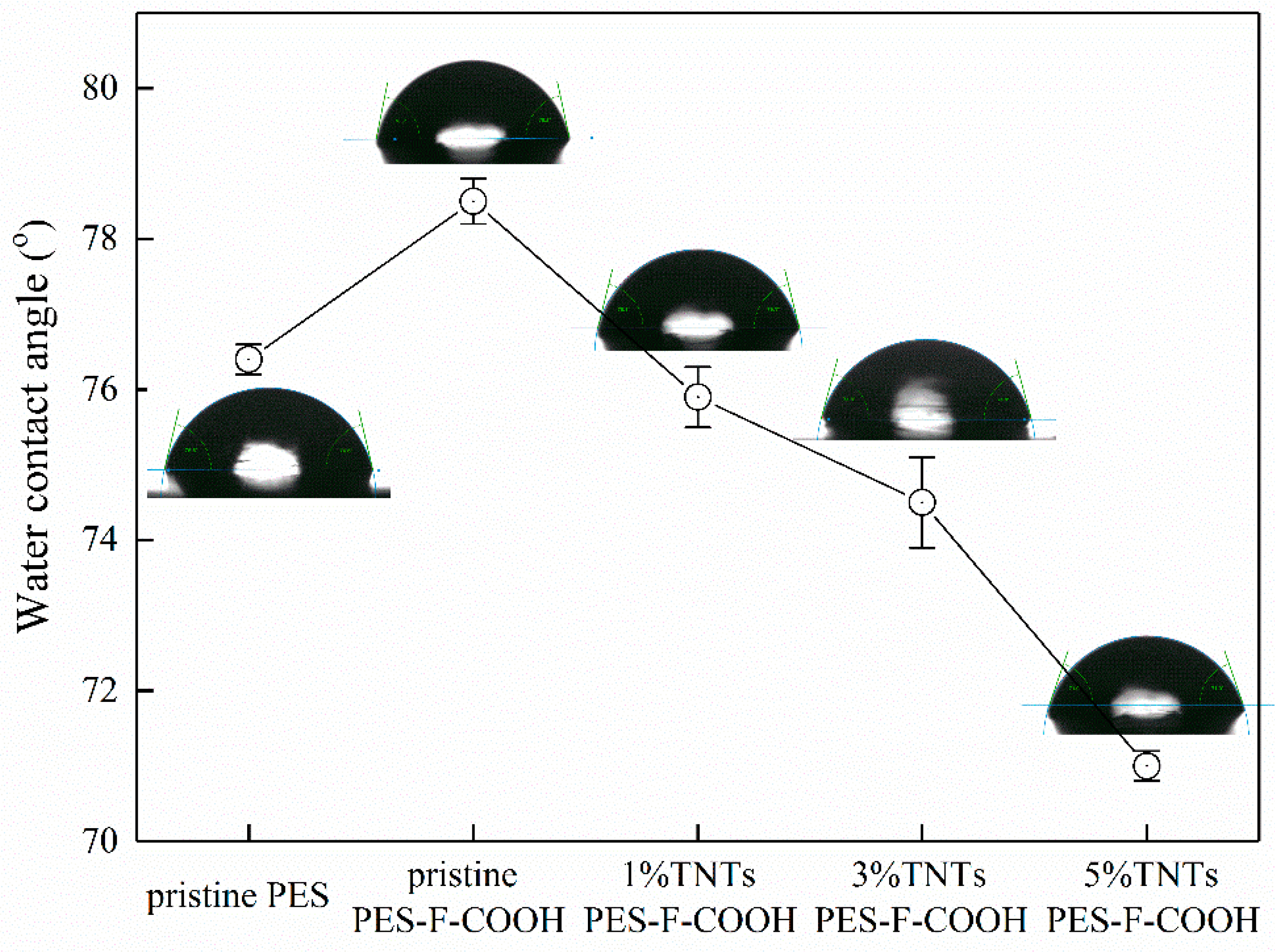
3.4. Membrane Hydrophilicity
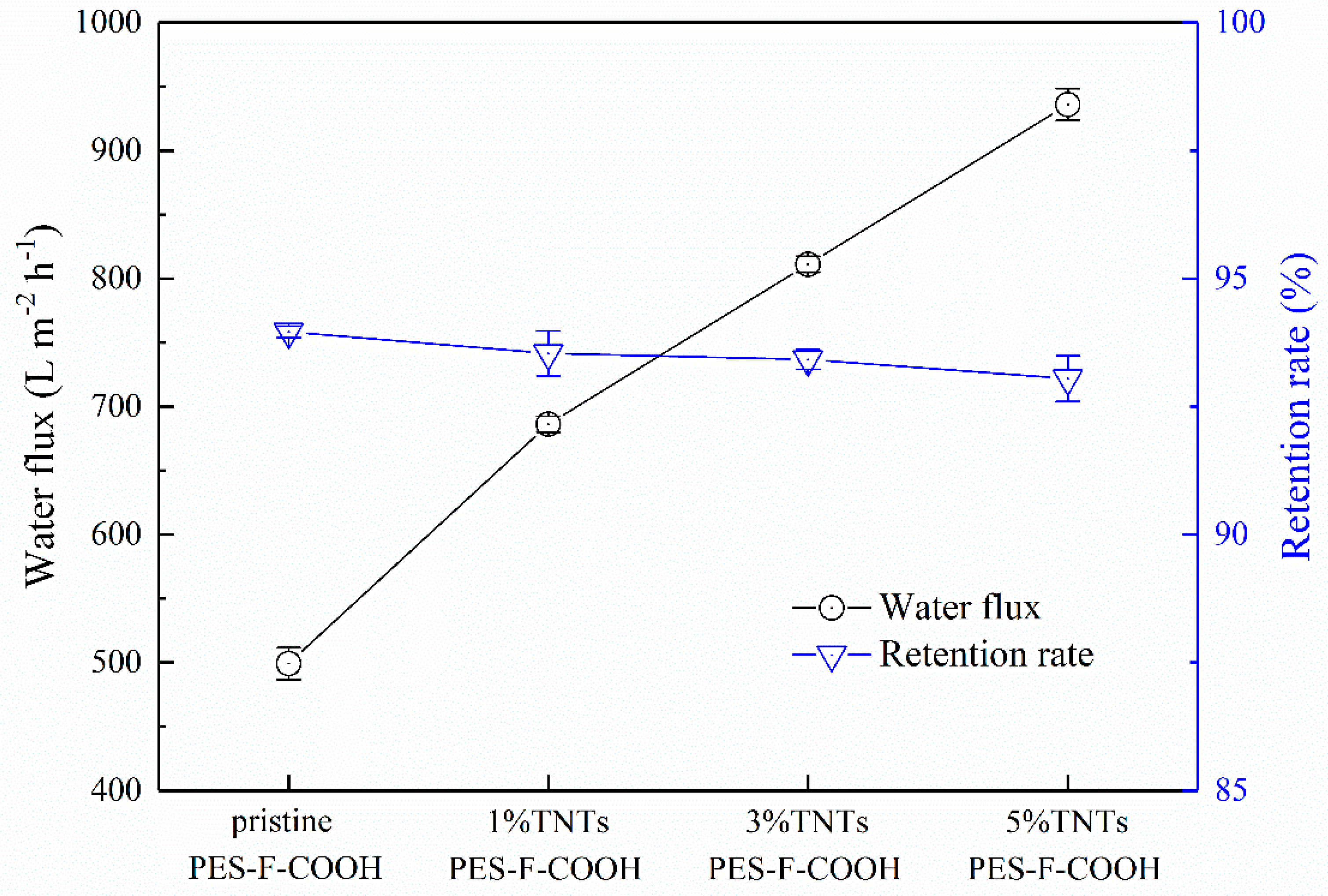
3.5. Membrane Separation Performance
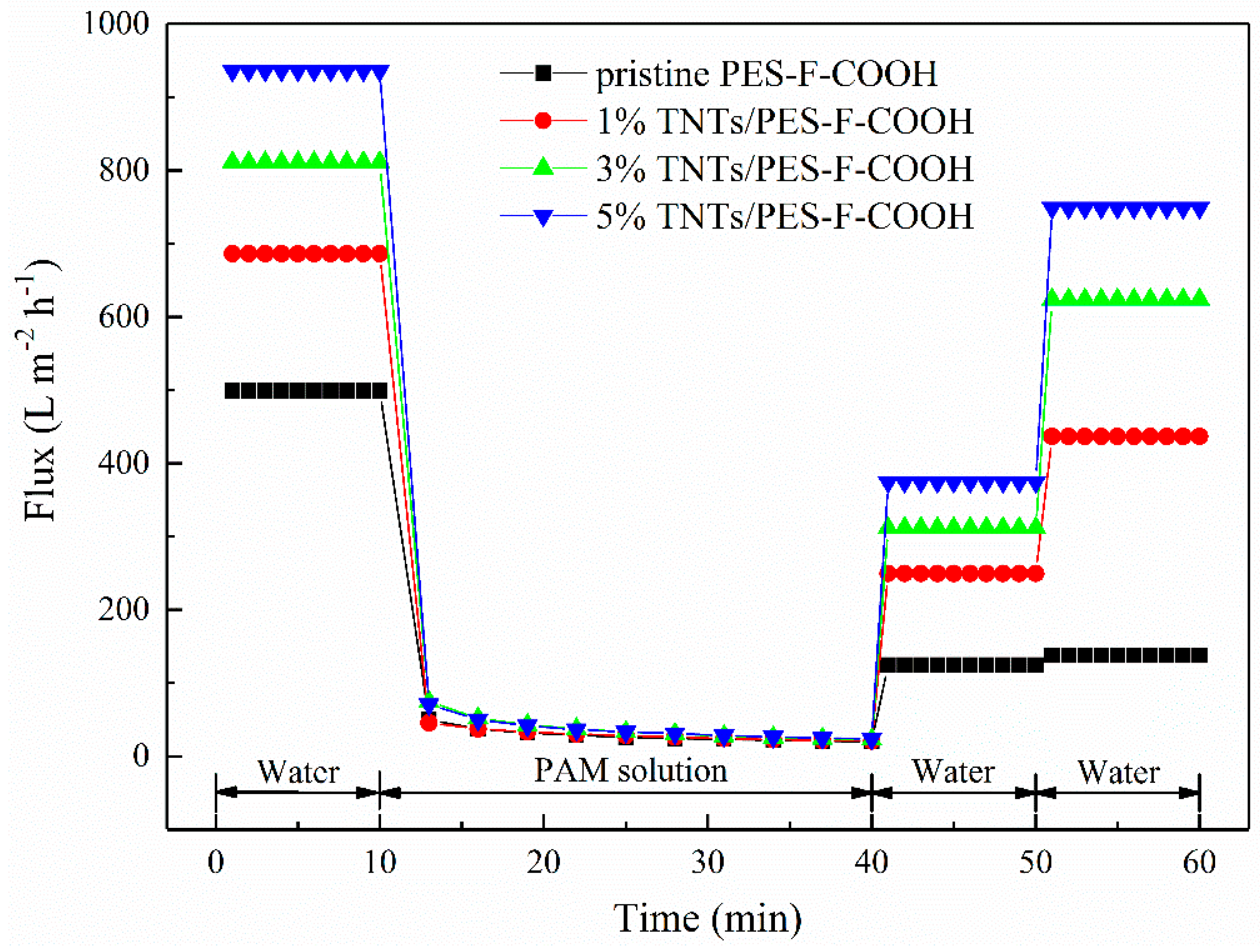
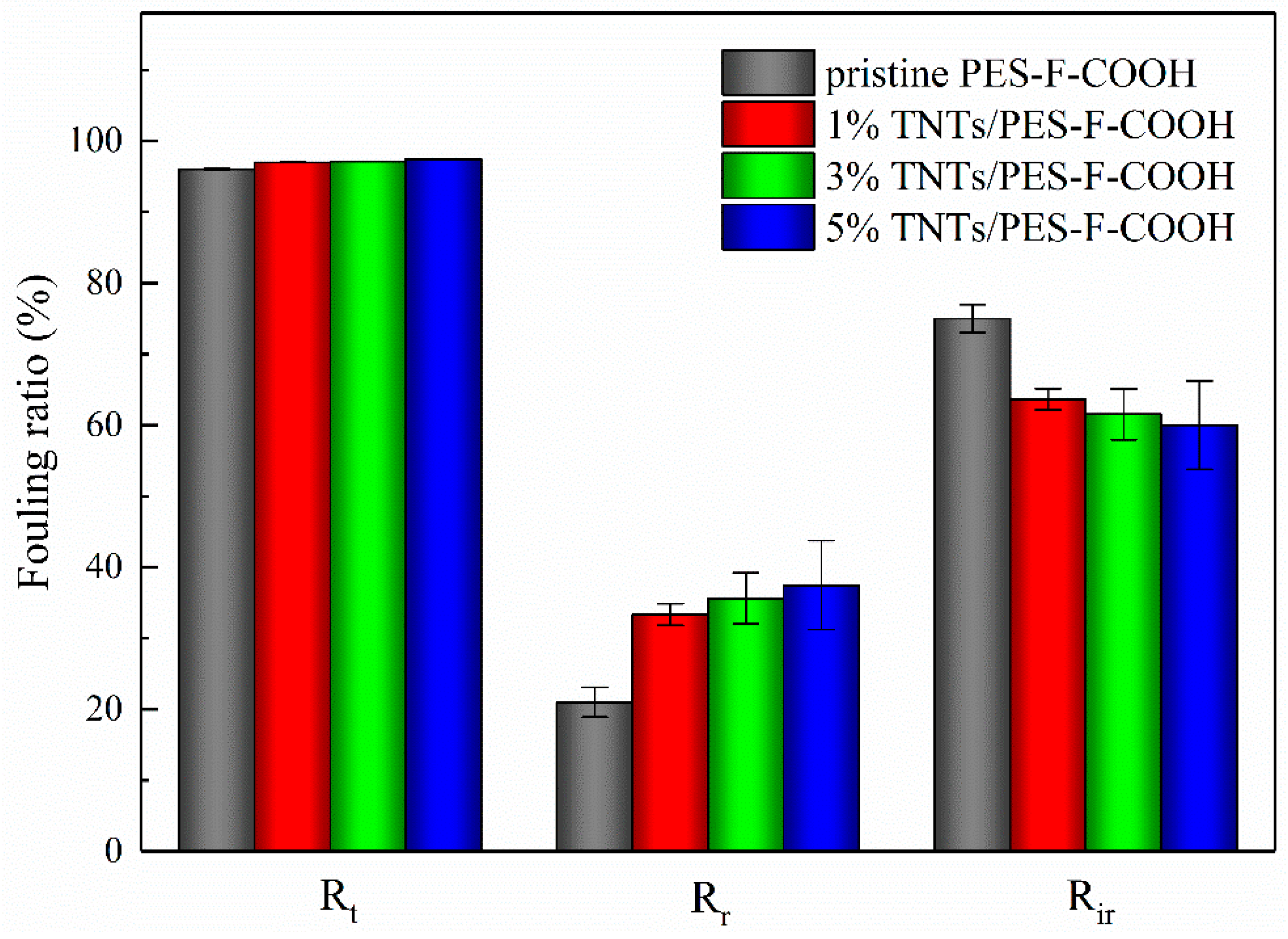
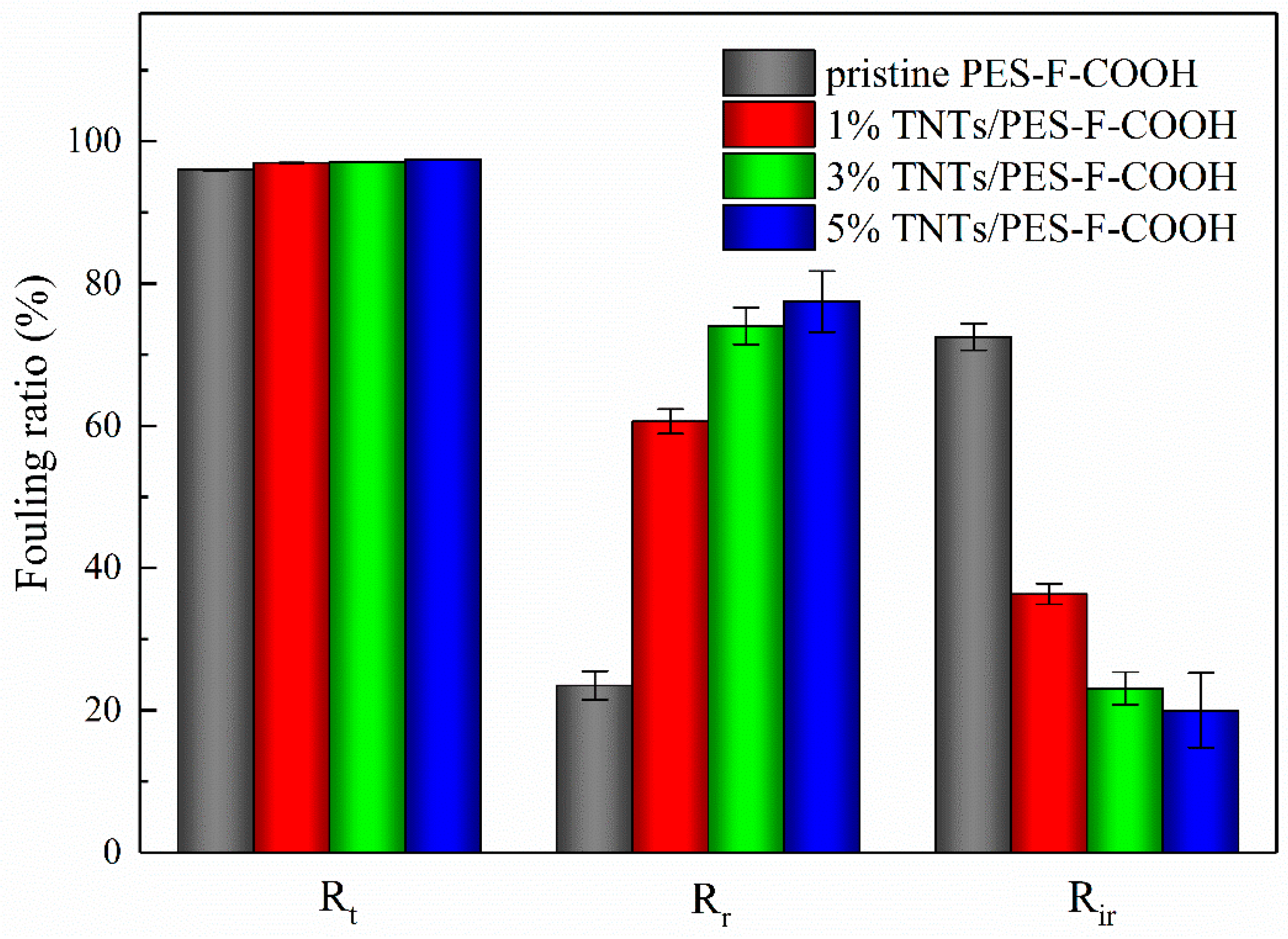
3.6. Membrane Anti-Fouling Property
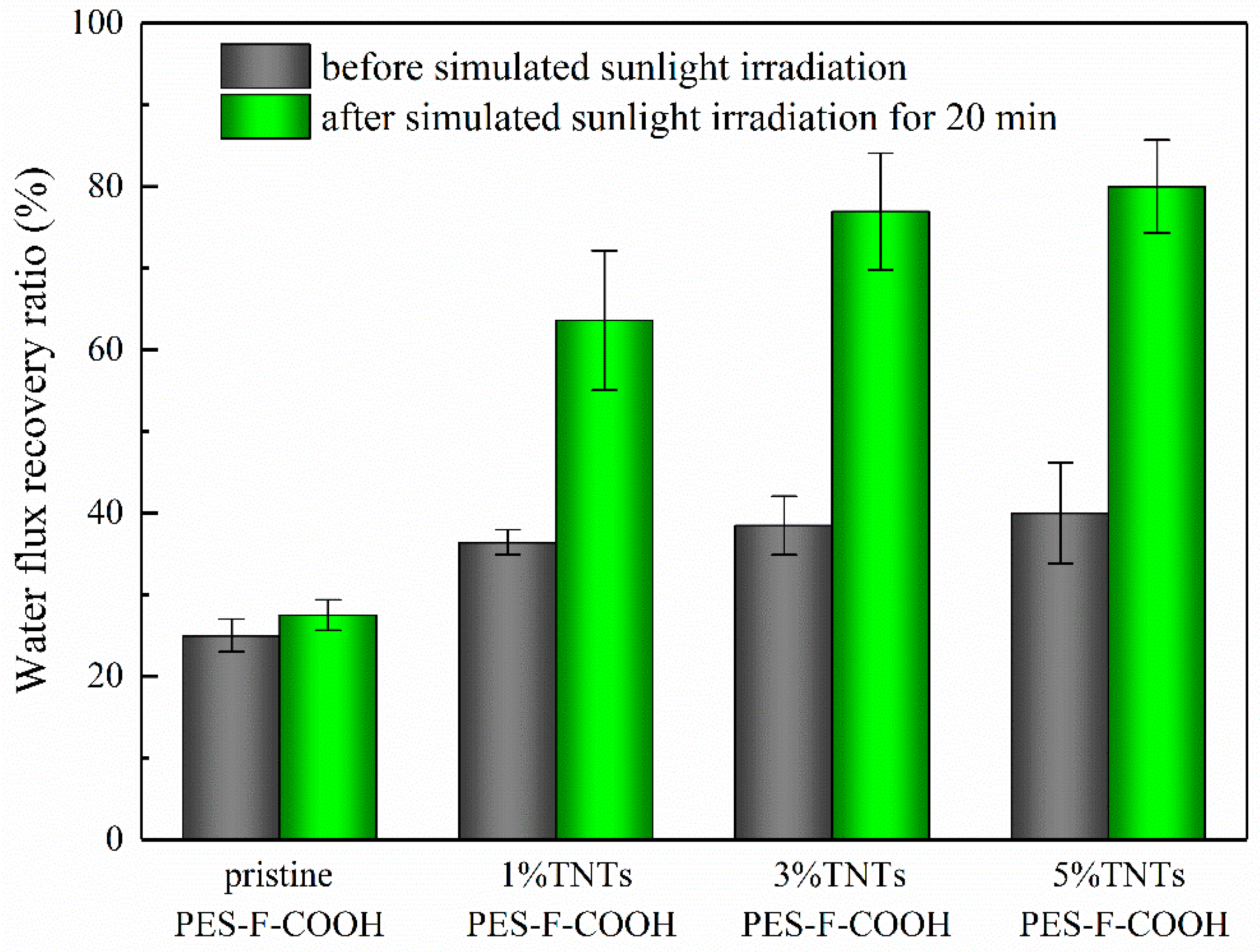
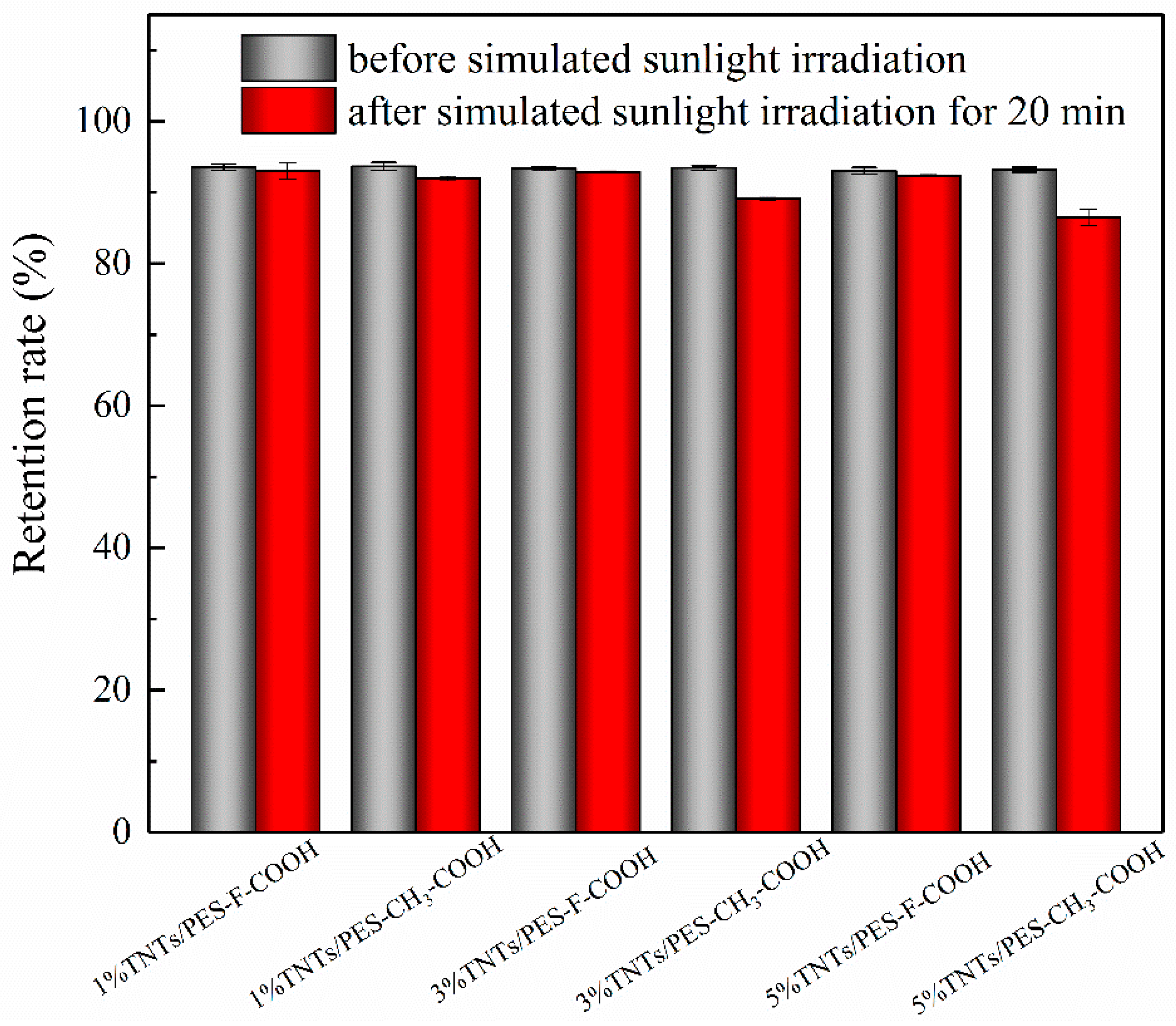
3.7. Membrane Self-Cleaning and Anti-Photocatalytic Aging Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Mariñas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nature 2008, 452, 301–310. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.W.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.; Tripathi, B.P.; Kumar, M.; Shahi, V.K. Membrane-based techniques for the separation and purification of proteins: An overview. Adv. Colloid Interface Sci. 2009, 145, 1–22. [Google Scholar] [CrossRef]
- Mohammad, A.W.; Ng, C.Y.; Lim, Y.P.; Ng, G.H. Ultrafiltration in Food Processing Industry: Review on Application, Membrane Fouling, and Fouling Control. Food Bioprocess Technol. 2012, 5, 1143–1156. [Google Scholar] [CrossRef]
- Werber, J.R.; Osuji, C.O.; Elimelech, M. Materials for next-generation desalination and water purification membranes. Nat. Rev. Mater. 2016, 1, 1–15. [Google Scholar] [CrossRef]
- Lau, W.J.; Ismail, A.F.; Misdan, N.; Kassim, M.A. A recent progress in thin film composite membrane: A review. Desalination 2012, 287, 190–199. [Google Scholar] [CrossRef]
- Liang, S.; Xiao, K.; Mo, Y.H.; Huang, X. A novel ZnO nanoparticle blended polyvinylidene fluoride membrane for anti-irreversible fouling. J. Membr. Sci. 2012, 394–395, 184–192. [Google Scholar] [CrossRef]
- Mohammad, A.W.; Teow, Y.H.; Ang, W.L.; Chung, Y.T.; Oatley-Radcliffe, D.L.; Hilal, N. Nanofiltration membranes review: Recent advances and future prospects. Desalination 2015, 356, 226–254. [Google Scholar] [CrossRef]
- Ayyaru, S.; Ahn, Y.H. Application of sulfonic acid group functionalized graphene oxide to improve hydrophilicity, permeability, and antifouling of PVDF nanocomposite ultrafiltration membranes. J. Membr. Sci. 2017, 525, 210–219. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, H.; Xia, J.; Zhang, F.F. Novel GO-blended PVDF ultrafiltration membranes. Desalination 2012, 299, 50–54. [Google Scholar] [CrossRef]
- Zhang, J.G.; Xu, Z.W.; Mai, W.; Min, C.Y. Improved hydrophilicity, permeability, antifouling and mechanical performance of PVDF composite ultrafiltration membranes tailored by oxidized low-dimensional carbon nanomaterials. J. Mater. Chem. A 2013, 1, 3101–3111. [Google Scholar] [CrossRef]
- Wu, C.J.; Li, A.; Li, L.; Zhang, L.; Wang, H.; Qi, X.H.; Zhang, Q.X. Treatment of oily water by a poly(vinyl alcohol) ultrafiltration membrane. Desalination 2008, 225, 312–321. [Google Scholar] [CrossRef]
- Um, M.J.; Yoon, S.H.; Lee, C.H.; Chung, K.Y.; Kim, J.J. Flux enhancement with gas injection in crossflow ultrafiltration of oily wastewater. Water Res. 2001, 35, 4095–4101. [Google Scholar] [CrossRef]
- Geng, Z.; Yang, X.; Boo, C.; Zhu, S.Y.; Lu, Y.; Fan, W.; Huo, M.X.; Elimelech, M.; Yang, X. Self-cleaning anti-fouling hybrid ultrafiltration membranes via side chain grafting of poly(aryl ether sulfone) and titanium dioxide. J. Membr. Sci. 2017, 529, 1–10. [Google Scholar] [CrossRef]
- Rahimpour, A. UV photo-grafting of hydrophilic monomers onto the surface of nano-porous PES membranes for improving surface properties. Desalination 2011, 265, 93–101. [Google Scholar] [CrossRef]
- Padaki, M.; Isloor, A.M.; Kumar, R.; Ismail, A.F.; Matsuura, T. Synthesis, characterization and desalination study of composite NF membranes of novel Poly[(4-aminophenyl)sulfonyl]butanediamide (PASB) and methyalated Poly[(4-aminophenyl)sulfonyl]butanediamide (mPASB) with Polysulfone (PSf). J. Membr. Sci. 2013, 428, 489–497. [Google Scholar] [CrossRef]
- Maguire-Boyle, S.J.; Barron, A.R. A new functionalization strategy for oil/water separation membranes. J. Membr. Sci. 2011, 382, 107–115. [Google Scholar] [CrossRef]
- Padaki, M.; Emadzadeh, D.; Masturra, T.; Ismail, A.F. Antifouling properties of novel PSf and TNT composite membrane and study of effect of the flow direction on membrane washing. Desalination 2015, 362, 141–150. [Google Scholar] [CrossRef]
- Zhang, R.N.; Liu, Y.N.; He, M.R.; Su, Y.L. Antifouling membranes for sustainable water purification: Strategies and mechanisms. Chem. Soc. Rev. 2016, 45, 5888–5924. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, S.L.; Zhou, Z.; Ren, J.N. Novel sulfonated thin-film composite nanofiltration membranes with improved water flux for treatment of dye solutions. J. Membr. Sci. 2012, 394, 218–229. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, C.Y.; Zhang, G.S.; Hu, L.M.; Wang, P. Photocatalytic Fe-doped TiO2/PSF composite UF membranes: Characterization and performance on BPA removal under visible-light irradiation. Chem. Eng. J. 2017, 319, 39–47. [Google Scholar] [CrossRef]
- Song, H.C.; Shao, J.H.; Wang, J.M.; Zhong, X.Q. The removal of natural organic matter with LiCl-TiO2-doped PVDF membranes by integration of ultrafiltration with photocatalysis. Desalination 2014, 344, 412–421. [Google Scholar] [CrossRef]
- Fischer, K.; Kühnert, M.; Gläser, R.; Schulze, A. Photocatalytic degradation and toxicity evaluation of diclofenac by nanotubular titanium dioxide-PES membrane in a static and continuous setup. RSC Adv. 2015, 5, 16340–16348. [Google Scholar] [CrossRef]
- Zhang, X.W.; Wang, D.K.; Costa, J.C.D. Recent progresses on fabrication of photocatalytic membranes for water treatment. Catal. Today 2014, 230, 47–54. [Google Scholar] [CrossRef]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O’Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B 2012, 125, 331–349. [Google Scholar] [CrossRef]
- Abdullah, M.; Kamarudin, S.K. Titanium dioxide nanotubes (TNT) in energy and environmental applications: An overview. Renew. Sustain. Energy Rev. 2017, 76, 212–225. [Google Scholar] [CrossRef]
- Wang, C.C.; Li, J.R.; Lv, X.L.; Zhang, Y.Q. Photocatalytic organic pollutants degradation in metal-organic frameworks. Energy Environ. Sci. 2014, 7, 2831–2867. [Google Scholar] [CrossRef]
- Kukovecz, Á.; Hodos, M.; Horváth, E.; Radnóczi, G. Oriented crystal growth model explains the formation of titania nanotubes. J. Phys. Chem. B 2005, 109, 17781–17783. [Google Scholar] [CrossRef]
- Sun, S.D.; Zhao, C.S.; Xue, J.M.; Ran, F. Modification of polyethersulfone membranes-A review of methods. Prog. Mater. Sci. 2013, 58, 76–150. [Google Scholar]
- Chen, Y.F.; Zhang, Y.T.; Liu, J.D.; Zhang, H.Q. Preparation and antibacterial property of polyethersulfone ultrafiltration hybrid membrane containing halloysite nanotubes loaded with copper ions. Chem. Eng. J. 2012, 210, 298–308. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Abdulkarim, A.A.; Ooi, B.S.; Ismail, S. Recent development in additives modifications of polyethersulfone membrane for flux enhancemen. Chem. Eng. J. 2013, 223, 246–267. [Google Scholar] [CrossRef]
- He, T.S.; Zhou, Z.F.; Xu, W.B.; Ren, F.M.; Ma, H.H.; Wang, J. Preparation and photocatalysis of TiO2-fluoropolymer electrospun fiber nanocomposites. Polymer 2009, 50, 3031–3036. [Google Scholar] [CrossRef]
- Wu, A.Y.; Kotaki, M.; Ye, L.; Lu, X. Morphology, polymorphism behavior and molecular orientation of electrospun poly(vinylidene fluoride) fibers. Polymer 2007, 48, 512–521. [Google Scholar]
- Zhang, Z.; Meng, Q.; Chung, T.C.M. Energy storage study of ferroelectric poly(vinylidene fluoride-trifluoroethylene-chlorotrifluoroethylene) terpolymers. Polymer 2009, 50, 707–715. [Google Scholar] [CrossRef]
- Song, H.C.; Shao, J.H.; He, Y.L.; Liu, B.; Zhong, X.Q. Natural organic matter removal and flux decline with PEG-TiO2-doped PVDF membranes by integration of ultrafiltration with photocatalysis. J. Membr. Sci. 2012, 405, 48–56. [Google Scholar] [CrossRef]
- Liu, N.; Chen, X.; Zhang, J. A review on TiO2-based nanotubes synthesized via hydrothermal method: Formation mechanism, structure modification, and photocatalytic application. Catal. Today 2014, 225, 34–51. [Google Scholar] [CrossRef]
- Bavykin, D.V.; Friedrich, J.M.; Walsh, F.C. Protonated titanates and TiO2 nanostructured materials: Synthesis, properties, and applications. Adv. Mater. 2006, 18, 2807–2824. [Google Scholar] [CrossRef]
- Yao, B.D.; Chan, Y.F.; Zhang, X.Y.; Zhang, W.F. Formation mechanism of TiO2 nanotubes. Appl. Phys. Lett. 2003, 82, 281–283. [Google Scholar] [CrossRef]
- Chen, Q.; Du, G.H.; Zhang, S.; Peng, L.M. The structure of trititanate nanotubes. Acta Crystallogr. Sect. B 2002, 58, 587–593. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Hu, G.Q.; Duan, X.F.; Sun, H.L. Microstructure and formation mechanism of titanium dioxide nanotubes. Chem. Phys. Lett. 2002, 365, 427–431. [Google Scholar] [CrossRef]
- Ren, J.N.; Zhang, S.L.; Liu, Y.; Wang, Y.; Pang, J.H.; Wang, Q.H.; Wang, G.B. A novel crosslinking organic-inorganic hybrid proton exchange membrane based on sulfonated poly(arylene ether sulfone) with 4-amino-phenyl pendant group for fuel cell application. J. Membr. Sci. 2013, 434, 161–170. [Google Scholar] [CrossRef]
- Rahimpour, A.; Madaeni, S.S.; Mansourpanah, Y. High performance polyethersulfone UF membrane for manufacturing spiral wound module: Preparation, morphology, performance, and chemical cleaning. Polym. Adv. Technol. 2007, 18, 403–410. [Google Scholar] [CrossRef]
- Celik, E.; Park, H.; Choi, H.; Choi, H. Carbon nanotube blended polyethersulfone membranes for fouling control in water treatment. Water Res. 2011, 45, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Esfahani, M.R.; Tyler, J.L.; Stretz, H.A.; Wells, M.J.M. Effects of a dual nanofiller, nano-TiO2 and MWCNT, for polysulfone-based nanocomposite membranes for water purification. Desalination 2015, 372, 47–56. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, J.; Zheng, Q.; Chen, X.; Zhang, H. The research of rheology and thermodynamics of organic-inorganic hybrid membrane during the membrane formation. J. Membr. Sci. 2008, 311, 200–207. [Google Scholar] [CrossRef]
- Jhaveri, J.H.; Murthy, Z.V.P. A comprehensive review on anti-fouling nanocomposite membranes for pressure driven membrane separation processes. Desalination 2016, 379, 137–154. [Google Scholar] [CrossRef]
- Madaeni, S.S.; Ghaemi, N. Characterization of self-cleaning RO membranes coated with TiO2 particles under UV irradiation. J. Membr. Sci. 2007, 303, 221–233. [Google Scholar] [CrossRef]
- Pan, H.; Zhao, X.; Fu, Z.; Tu, W. Visible-light induced photocatalysis of AgCl@Ag/titanate nanotubes/nitrogen-doped reduced graphite oxide composites. Appl. Surf. Sci. 2018, 442, 547–555. [Google Scholar] [CrossRef]
- Nishijima, K.; Fujisawa, Y.; Murakami, N.; Tsubota, T. Development of an S-doped titania nanotube (TNT) site-selectively loaded with iron(III) oxide and its photocatalytic activities. Appl. Catal. B Environ. 2008, 84, 584–590. [Google Scholar] [CrossRef]
- Li, J.H.; Yang, X.; Yu, X.D.; Xu, L.L.; Kang, W.L.; Yan, W.H.; Gao, H.F.; Liu, Z.H.; Guo, Y.H. Rare earth oxide-doped titania nanocomposites with enhanced photocatalytic activity towards the degradation of partially hydrolysis polyacrylamide. Appl. Surf. Sci. 2009, 255, 3731–3738. [Google Scholar] [CrossRef]
- Rong, X.S.; Qiu, F.X.; Zhang, C.; Fu, L.; Wang, Y.Y.; Yang, D.Y. Preparation of Ag-AgBr/TiO2-graphene and its visible light photocatalytic activity enhancement for the degradation of polyacrylamide. J. Alloy. Compd. 2015, 639, 153–161. [Google Scholar] [CrossRef]
- Sopyan, I.; Watanabe, M.; Murasawa, S.; Hashimoto, K. A film-type photocatalyst incorporating highly active TiO2 powder and fluororesin binder: Photocatalytic activity and long-term stability. J. Electroanal. Chem. 1996, 415, 183–186. [Google Scholar] [CrossRef]

| Samples | Mean Pore Size (nm) | Porosity (%) |
|---|---|---|
| pristine PES-F-COOH | 24.89 | 68.2 |
| 1%TNTs/PES-F-COOH | 25.54 | 73.1 |
| 3%TNTs/PES-F-COOH | 26.15 | 76.9 |
| 5%TNTs/PES-F-COOH | 26.80 | 80.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geng, Z.; Wang, X.; Jiang, H.; Zhang, L.; Chen, Z.; Feng, Y.; Geng, W.; Yang, X.; Huo, M.; Sun, J. High-Performance TiO2 Nanotubes/Poly(aryl ether sulfone) Hybrid Self-Cleaning Anti-Fouling Ultrafiltration Membranes. Polymers 2019, 11, 555. https://doi.org/10.3390/polym11030555
Geng Z, Wang X, Jiang H, Zhang L, Chen Z, Feng Y, Geng W, Yang X, Huo M, Sun J. High-Performance TiO2 Nanotubes/Poly(aryl ether sulfone) Hybrid Self-Cleaning Anti-Fouling Ultrafiltration Membranes. Polymers. 2019; 11(3):555. https://doi.org/10.3390/polym11030555
Chicago/Turabian StyleGeng, Zhi, Xinyu Wang, Hongchuan Jiang, Leilei Zhang, Zhiting Chen, Yong Feng, Wenzhe Geng, Xia Yang, Mingxin Huo, and Jing Sun. 2019. "High-Performance TiO2 Nanotubes/Poly(aryl ether sulfone) Hybrid Self-Cleaning Anti-Fouling Ultrafiltration Membranes" Polymers 11, no. 3: 555. https://doi.org/10.3390/polym11030555
APA StyleGeng, Z., Wang, X., Jiang, H., Zhang, L., Chen, Z., Feng, Y., Geng, W., Yang, X., Huo, M., & Sun, J. (2019). High-Performance TiO2 Nanotubes/Poly(aryl ether sulfone) Hybrid Self-Cleaning Anti-Fouling Ultrafiltration Membranes. Polymers, 11(3), 555. https://doi.org/10.3390/polym11030555