Bentonite Modified by Allylamine Polymer for Adsorption of Amido Black 10B
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Modification of Bent
2.3. Characterizations
2.4. Batch Adsorption Experiments
3. Results and Discussion
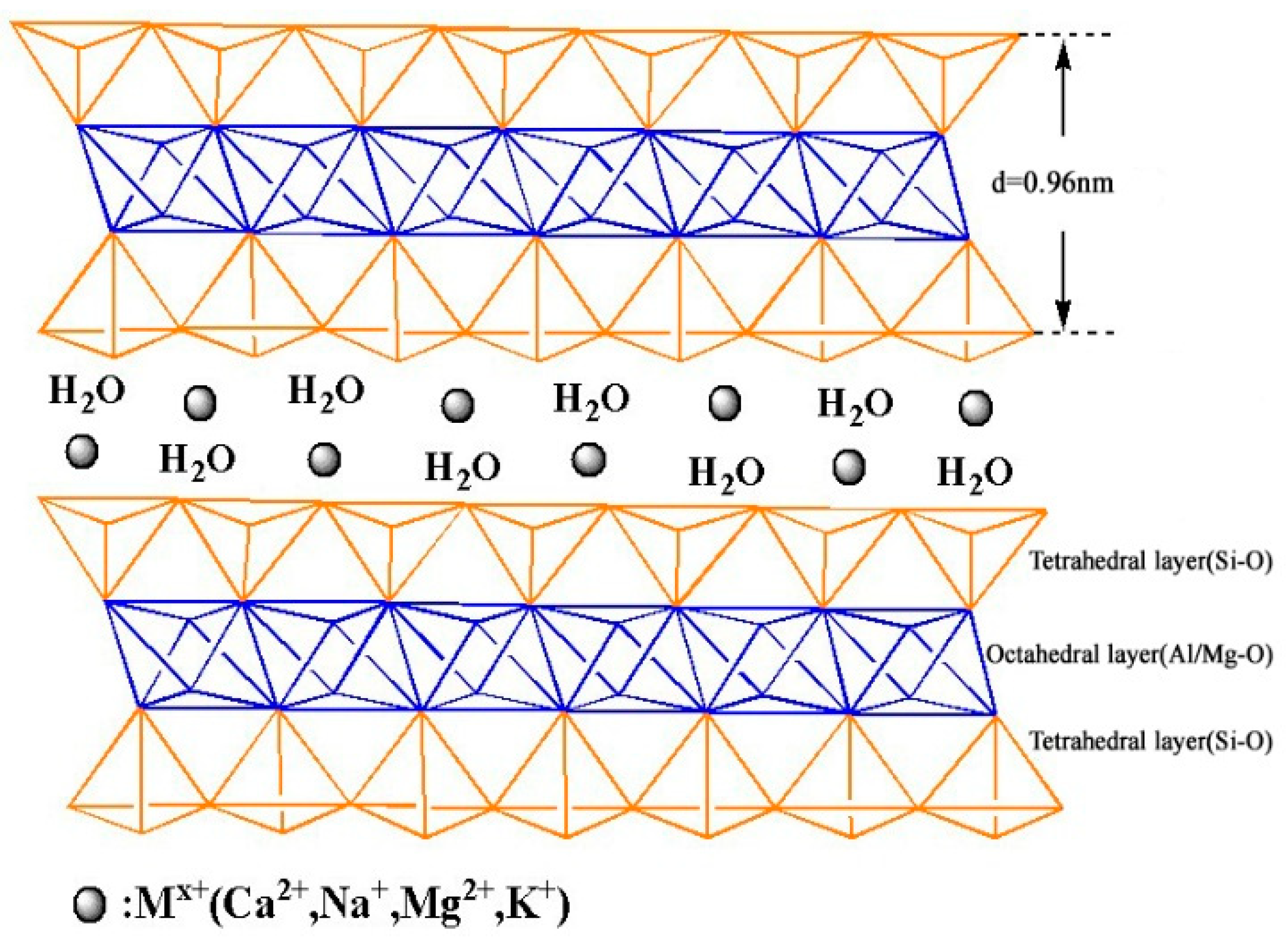
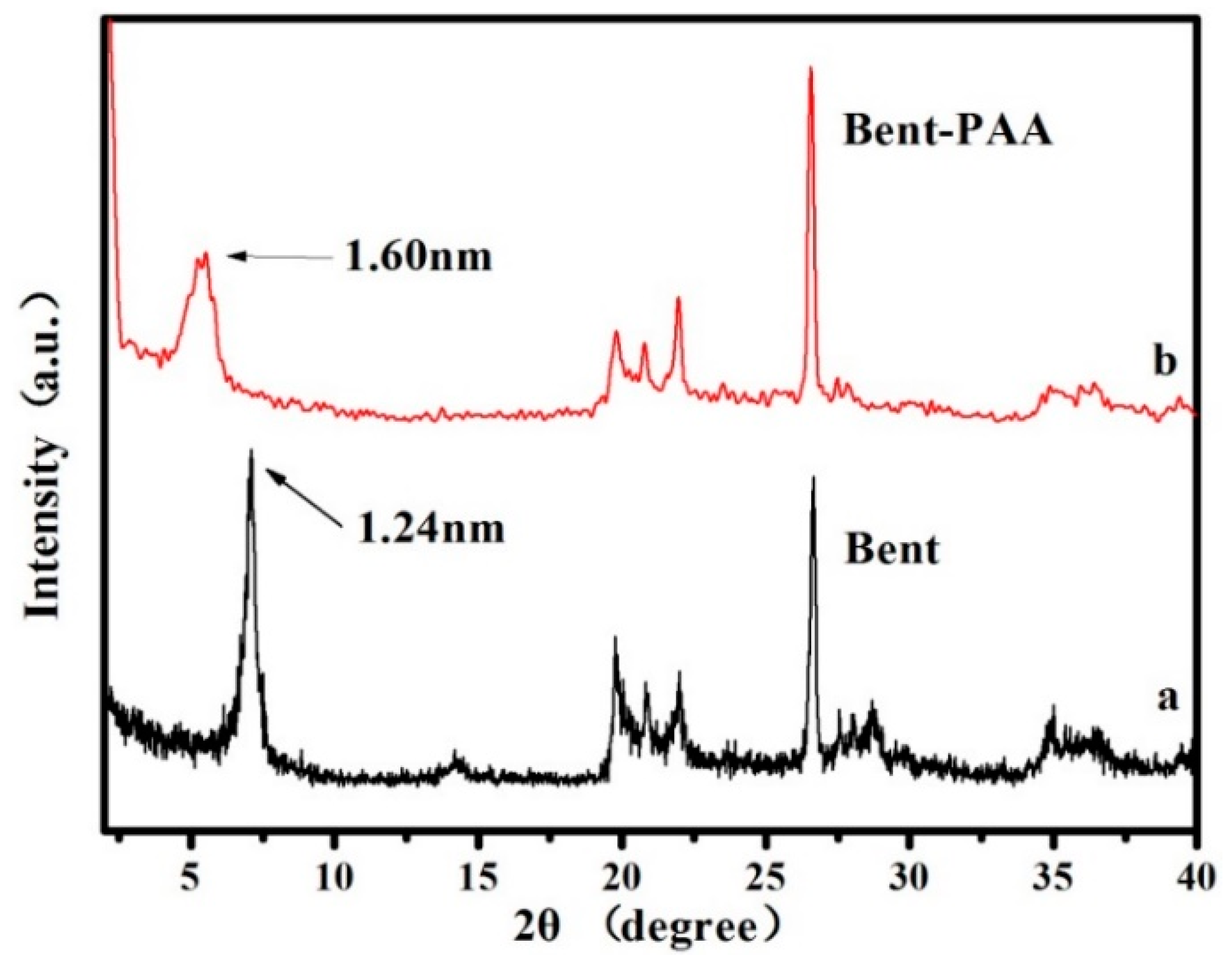
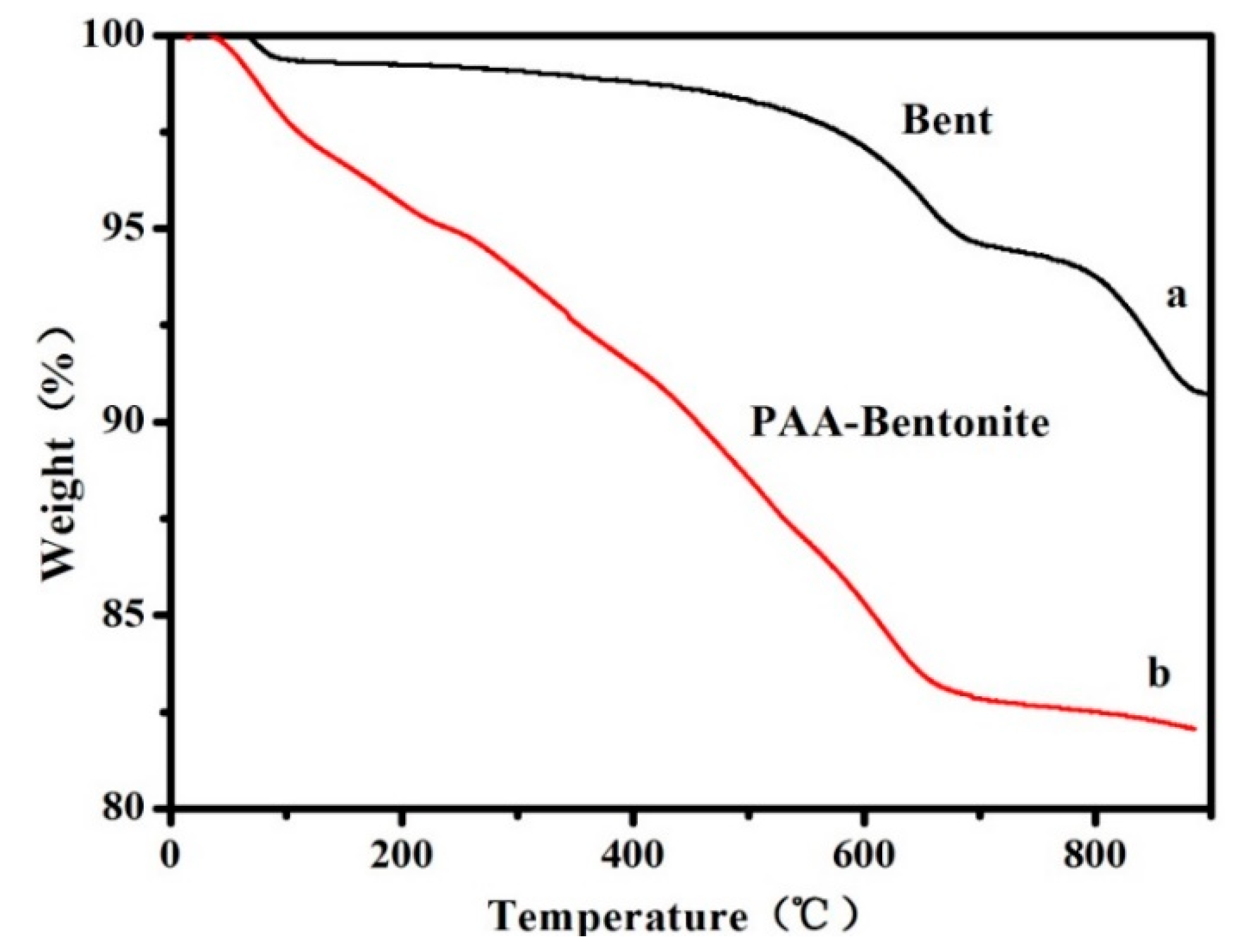
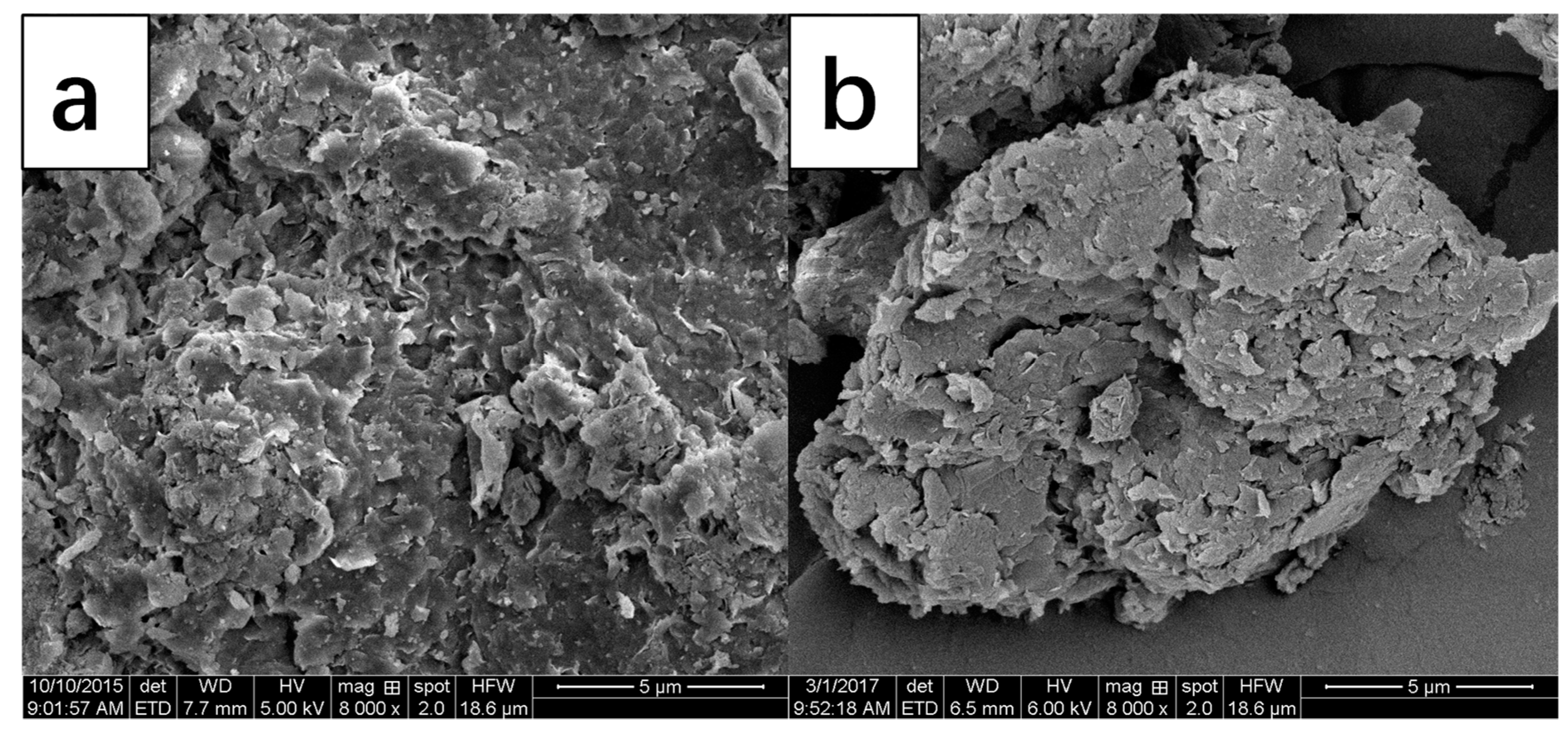
3.1. Bent-PAA Characterization
3.2. Adsorption Results
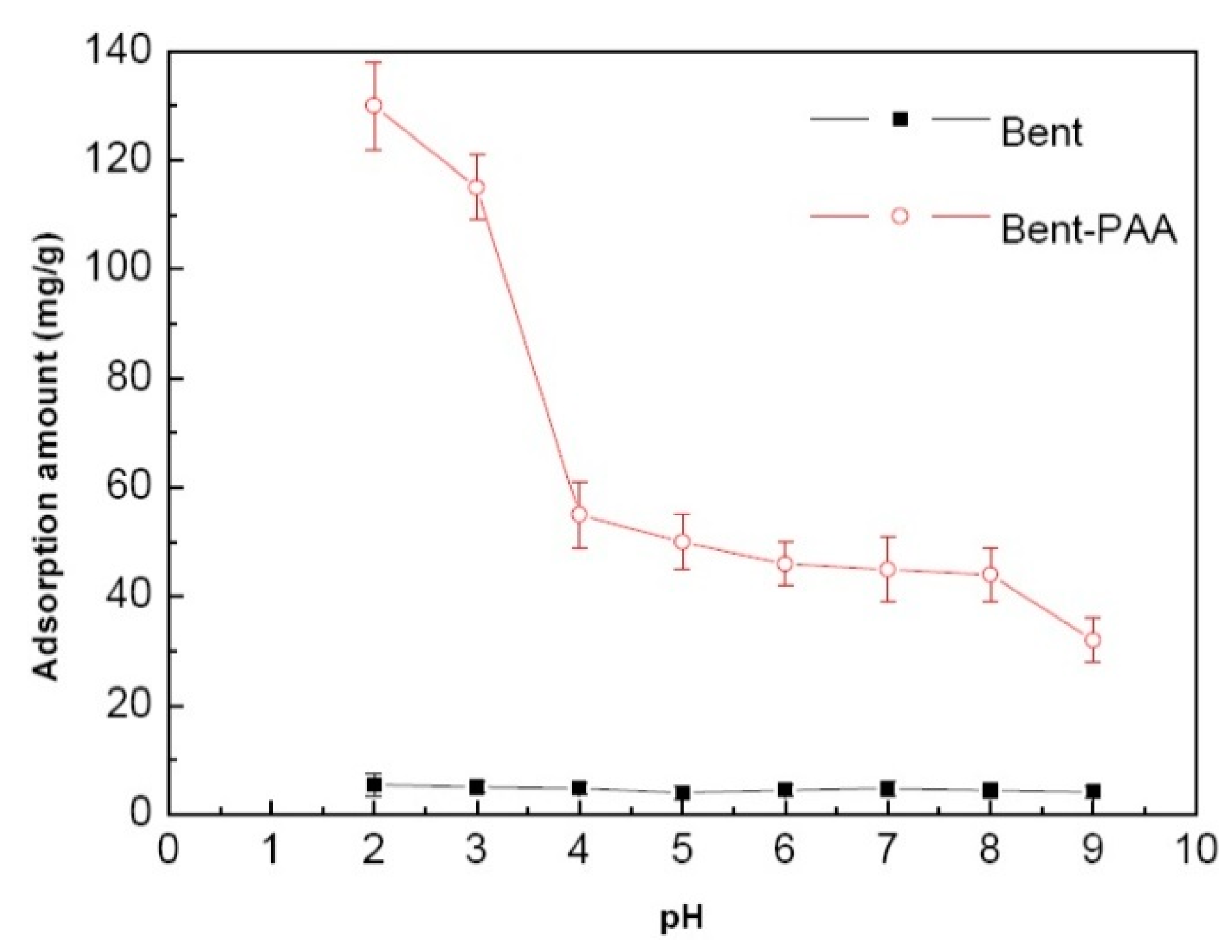
3.2.1. Effect of pH
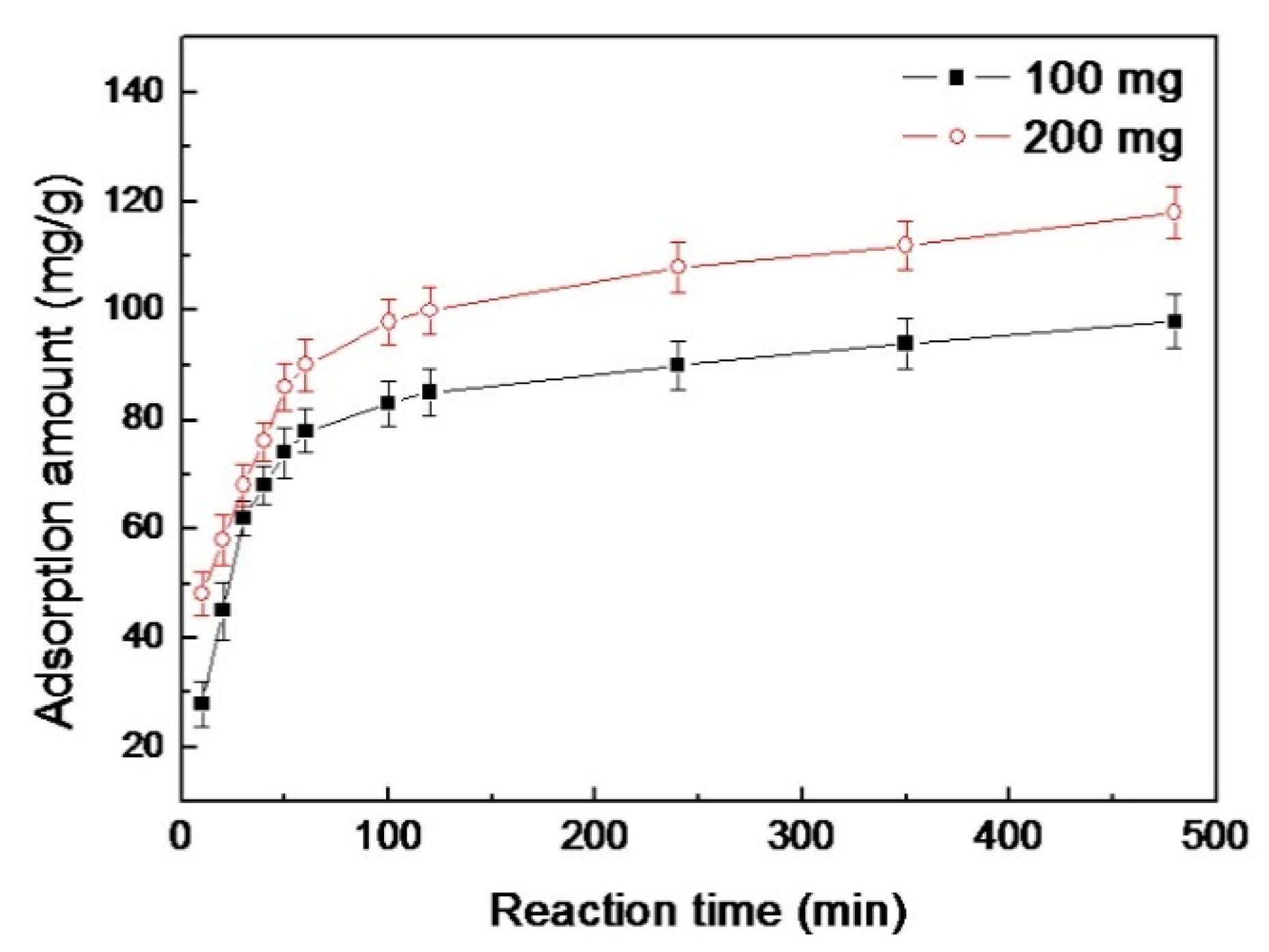
3.2.2. Effect of Contact Time
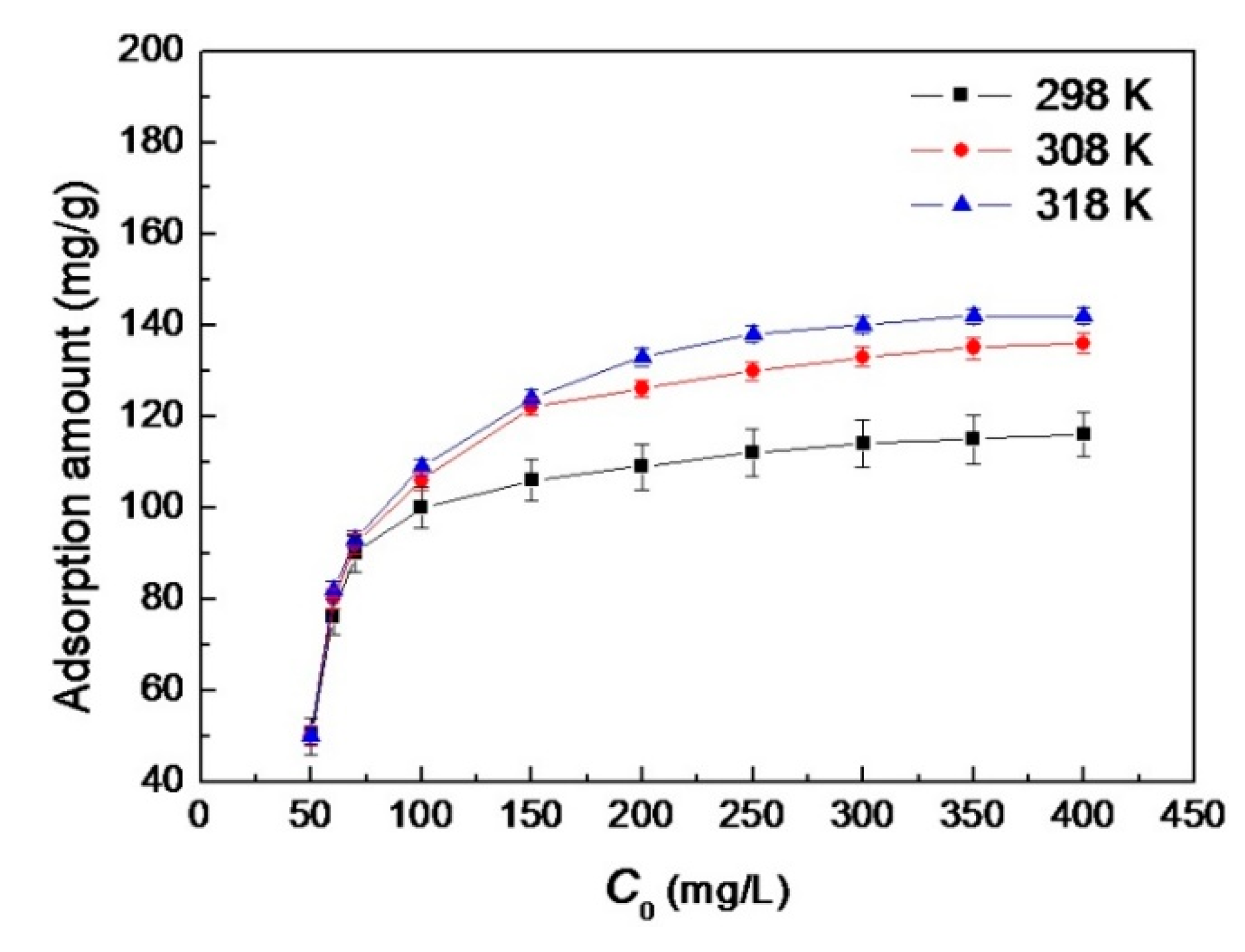
3.2.3. Effect of Initial Concentration of AB10B
3.2.4. Effect of Temperature
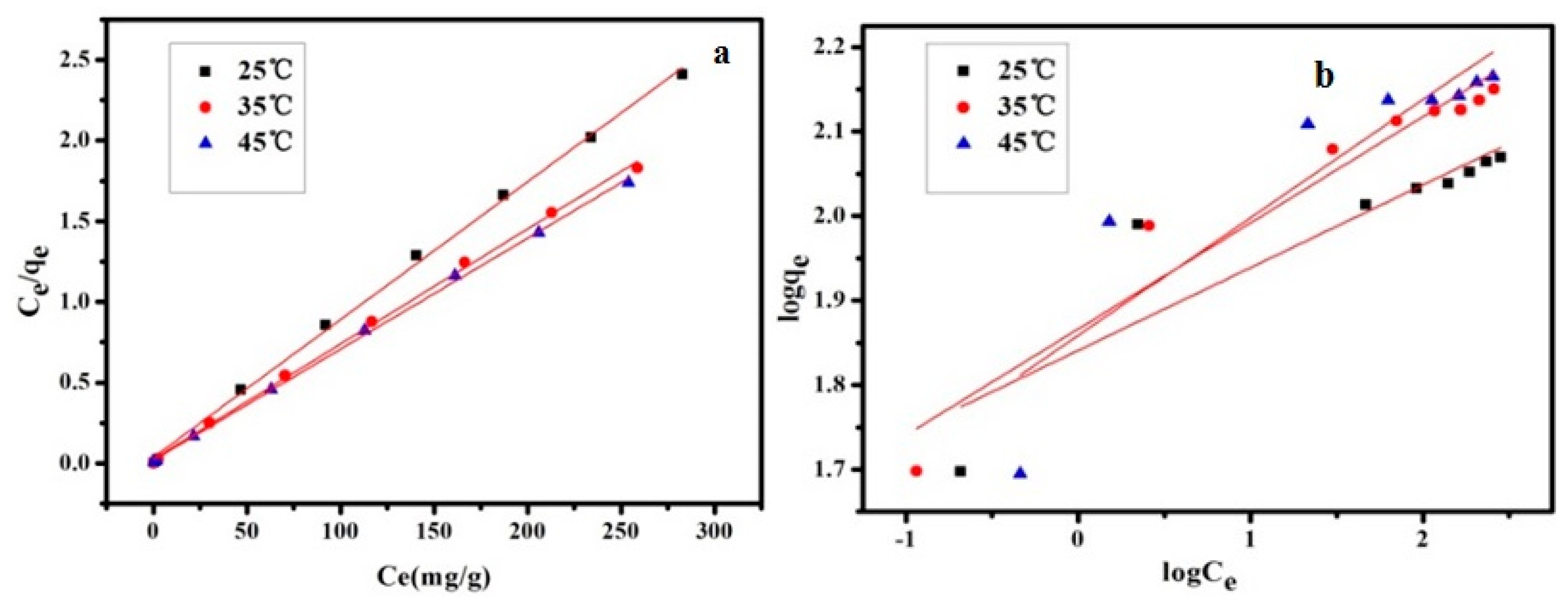
3.2.5. Adsorption Isotherm
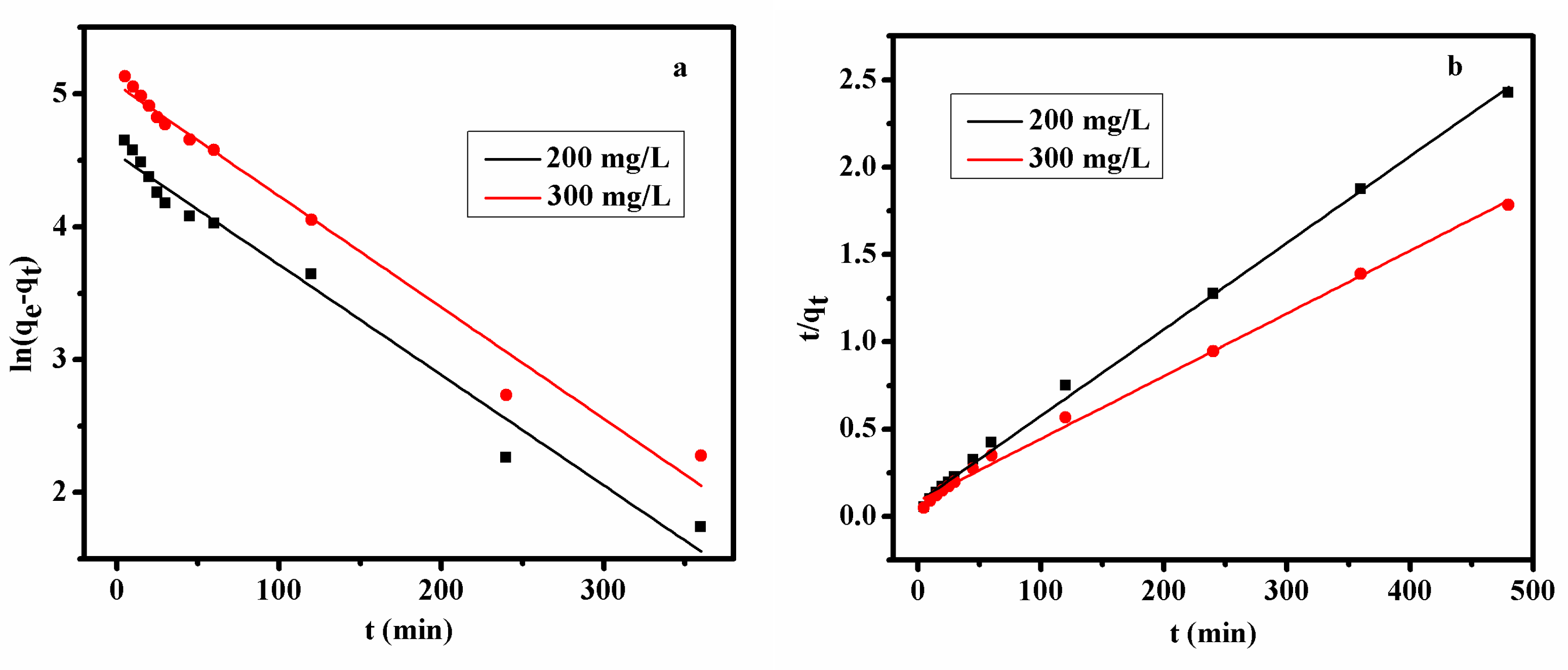
3.2.6. Adsorption Kinetics
3.2.7. Regeneration and Reusability
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rammel, R.S.; Zatiti, S.A.; Jamal, M.M.E. Biosorption of crystal violet by Chaetophora elegans alga. J. Chem. Technol. Metall. 2012, 46, 283–292. [Google Scholar]
- Liang, Z.; Yan, C.-F.; Rtimi, S.; Bandara, J. Piezoelectric materials for catalytic/photocatalytic removal of pollutants: Recent advances and outlook. Appl. Catal. B-Environ. 2019, 241, 256–260. [Google Scholar] [CrossRef]
- Sadegh, H.; Zare, K.; Maazinejad, B.; Shahryari-ghoshekandi, R.; Tyagi, I.; Agarwal, S.; Gupta, V.K. Synthesis of MWCNT-COOH-Cysteamine composite and its application for dye removal. J. Mol. Liq. 2016, 215, 221–228. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, P.; Wang, J.; Huang, R. Adsorption of Amido Black 10B from aqueous solutions onto Zr (IV) surface-immobilized cross-linked chitosan/bentonite composite. Appl. Surf. Sci. 2016, 369, 558–566. [Google Scholar] [CrossRef]
- Wang, Y.; Xia, G.; Wu, C.; Sun, J.; Song, R.; Huang, W. Porous chitosan doped with graphene oxide as highly effective adsorbent for methyl orange and amido black 10B. Carbohydr. Polym. 2015, 115, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hu, P.; Wang, J.; Huang, R. Crosslinked quaternized chitosan/bentonite composite for the removal of Amino black 10B from aqueous solutions. Int. J. Biol. Macromol. 2016, 93, 217–225. [Google Scholar] [CrossRef]
- Hu, P.; Wang, J.; Huang, R. Simultaneous removal of Cr(VI) and Amido black 10B (AB10B) from aqueous solutions using quaternized chitosan coated bentonite. Int. J. Biol. Macromol. 2016, 92, 694–701. [Google Scholar] [CrossRef]
- Gao, Q.; Xu, J.; Bu, X.-H. Recent advances about metal–organic frameworks in the removal of pollutants from wastewater. Coord. Chem. Rev. 2019, 378, 17–31. [Google Scholar] [CrossRef]
- Mo, J.; Yang, Q.; Zhang, N.; Zhang, W.; Zheng, Y.; Zhang, Z. A review on agro-industrial waste (AIW) derived adsorbents for water and wastewater treatment. J. Environ. Manag. 2018, 227, 395–405. [Google Scholar] [CrossRef]
- Sadat, S.A.; Ghaedi, A.M.; Panahimehr, M.; Baneshi, M.M.; Vafaei, A.; Ansarizadeh, M. Rapid room-temperature synthesis of cadmium zeolitic imidazolate framework nanoparticles based on 1,1′-carbonyldiimidazole as ultra-high-efficiency adsorbent for ultrasound-assisted removal of malachite green dye. Appl. Surf. Sci. 2019, 467–468, 1204–1212. [Google Scholar] [CrossRef]
- Tian, S.-Y.; Guo, J.-H.; Zhao, C.; Peng, Z.; Gong, C.-H.; Yu, L.-G.; Liu, X.-H.; Zhang, J.-W. Preparation of Cellulose/Graphene Oxide Composite Membranes and Their Application in Removing Organic Contaminants in Wastewater. J. Nanosci. Nanotechnol. 2019, 19, 2147–2153. [Google Scholar] [CrossRef]
- Zou, Q.; Li, H.; Yang, Y.; Miao, Y.; Huo, Y. Bi2O3/TiO2 photocatalytic film coated on floated glass balls for efficient removal of organic pollutant. Appl. Surf. Sci. 2019, 467–468, 354–360. [Google Scholar] [CrossRef]
- Sajid, M.M.; Shad, N.A.; Khan, S.B.; Zhang, Z.; Amin, N. Facile synthesis of Zinc vanadate Zn3(VO4)2 for highly efficient visible light assisted photocatalytic activity. J. Alloy. Compd. 2019, 775, 281–289. [Google Scholar] [CrossRef]
- Yao, N.; Khusid, B.; Sirkar, K.K.; Dehn, D.J. Nanoparticle filtration through microporous ECTFE membrane in an alcoholic solution. Sep. Purif. Technol. 2019, 210, 754–763. [Google Scholar] [CrossRef]
- Zhu, M.; Xiong, R.; Huang, C. Bio-based and photocrosslinked electrospun antibacterial nanofibrous membranes for air filtration. Carbohydr. Polym. 2019, 205, 55–62. [Google Scholar] [CrossRef]
- Fu, W.; Zhang, W. Microwave-enhanced membrane filtration for water treatment. J. Membr. Sci. 2018, 568, 97–104. [Google Scholar] [CrossRef]
- Harouna, B.M.; Benkortbi, O.; Hanini, S.; Amrane, A. Modeling of transitional pore blockage to cake filtration and modified fouling index-Dynamical surface phenomena in membrane filtration. Chem. Eng. Sci. 2019, 193, 298–311. [Google Scholar] [CrossRef]
- Yang, R.; Li, H.; Huang, M.; Yang, H.; Li, A. A review on chitosan-based flocculants and their applications in water treatment. Water Res. 2016, 95, 59–89. [Google Scholar] [CrossRef]
- Li, K.; Li, P.; Cai, J.; Xiao, S.; Yang, H.; Li, A. Efficient adsorption of both methyl orange and chromium from their aqueous mixtures using a quaternary ammonium salt modified chitosan magnetic composite adsorbent. Chemosphere 2016, 154, 310–318. [Google Scholar] [CrossRef]
- Angelova, R.; Baldikova, E.; Pospiskova, K.; Safarikova, M.; Safarik, I. Magnetically modified sheaths of Leptothrix sp. as an adsorbent for Amido black 10B removal. J. Magn. Magn. Mater. 2017, 427, 314–319. [Google Scholar] [CrossRef]
- Yang, R.; Li, D.; Li, A.; Yang, H. Adsorption properties and mechanisms of palygorskite for removal of various ionic dyes from water. Appl. Clay Sci. 2018, 151, 20–28. [Google Scholar] [CrossRef]
- Wang, W.; Wang, X.; Song, C.; Wei, X.; Ding, J.; Xiao, J. Sulfuric Acid Modified Bentonite as the Support of Tetraethylenepentamine for CO2 Capture. Energy Fuels 2013, 27, 1538–1546. [Google Scholar] [CrossRef]
- Liu, Y.; Cai, Y.; Yang, G.; Pan, Y.; Zhou, Y.; Zeng, G.; Tang, L. Chapter 2: Mesoporous carbon based composites for removal of recalcitrant pollutants from water. In Nanohybrid and Nanoporous Materials for Aquatic Pollution Control; Elsevier: Amsterdam, The Netherlands, 2019; pp. 31–61. [Google Scholar]
- Abdukhalikov, K. Bent functions and line ovals. Finite Fields Appl. 2017, 47, 94–124. [Google Scholar] [CrossRef]
- Chen, C.W.; Wu, W.J.; Zeng, X.S.; Jiang, Z.H.; Shi, L. Study on several mesoporous materials catalysts applied to the removal of trace olefins from aromatics and commercial sidestream tests. Ind. Eng. Chem. Res. 2009, 48, 10359–10363. [Google Scholar] [CrossRef]
- Pan, J.; Wang, C.; Guo, S.; Li, J.; Yang, Z. Cu supported over Al-pillared interlayer clays catalysts for direct hydroxylation of benzene to phenol. Catal. Commun. 2008, 9, 176–181. [Google Scholar] [CrossRef]
- Wolters, F.; Emmerich, K. Thermal reactions of smectites-relation of dehydroxylation temperature to octahedral structure. Thermochim. Acta 2007, 462, 80–88. [Google Scholar] [CrossRef]
- Öztürk, N.; Tabak, A.; Akgöl, S.; Denizli, A. Reversible immobilization of catalase by using a novel bentonite–cysteine (Bent–Cys) microcomposite affinity sorbents. Colloids Surf. A Physicochem. Eng. Asp. 2008, 322, 148–154. [Google Scholar] [CrossRef]
- Monvisade, P.; Siriphannon, P. Chitosan intercalated montmorillonite: Preparation, characterization and cationic dye adsorption. Appl. Clay Sci. 2009, 42, 427–431. [Google Scholar] [CrossRef]

| Adsorbent | t (min) | pH | c0 (mg L−1) | ma (g) | Vs (mL) | T (°C) | S (rpm) |
|---|---|---|---|---|---|---|---|
| Bent-PAA | 480 | 2–9 | 200 | 0.03 | 30 | 25 | 200 |
| 10–480 | 2 | 100–200 | 0.03 | 30 | 25 | 200 | |
| 480 | 2 | 50–400 | 0.03 | 30 | 25–45 | 200 |
| T (°C) | qexp (mg/g) | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|---|
| R2 | qm (mg/g) | KL (L/mg) | R2 | KF (L/g) | n | ||
| 25 | 117.28 | 0.998 | 128 | 0.222 | 0.782 | 6.3 | 10.21 |
| 35 | 141.6 | 0.998 | 142.86 | 0.269 | 0.929 | 6.46 | 7.97 |
| 45 | 144.08 | 0.999 | 166.67 | 0.3 | 0.788 | 6.41 | 7.17 |
| Pseudo-First-Order Model | Pseudo-Second-Order Model | |||
|---|---|---|---|---|
| C (mg/L) | R2 | k1 (mg−1) | R2 | k2 (g·mg−1·min−1) |
| 200 | 0.976 | 0.0083 | 0.998 | 0.00495 |
| 300 | 0.979 | 0.0084 | 0.997 | 0.00359 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, W.; Xia, T.; Pei, M.; Du, Y.; Wang, L. Bentonite Modified by Allylamine Polymer for Adsorption of Amido Black 10B. Polymers 2019, 11, 502. https://doi.org/10.3390/polym11030502
Guo W, Xia T, Pei M, Du Y, Wang L. Bentonite Modified by Allylamine Polymer for Adsorption of Amido Black 10B. Polymers. 2019; 11(3):502. https://doi.org/10.3390/polym11030502
Chicago/Turabian StyleGuo, Wenjuan, Tingcheng Xia, Meishan Pei, Yankai Du, and Luyan Wang. 2019. "Bentonite Modified by Allylamine Polymer for Adsorption of Amido Black 10B" Polymers 11, no. 3: 502. https://doi.org/10.3390/polym11030502
APA StyleGuo, W., Xia, T., Pei, M., Du, Y., & Wang, L. (2019). Bentonite Modified by Allylamine Polymer for Adsorption of Amido Black 10B. Polymers, 11(3), 502. https://doi.org/10.3390/polym11030502





