Effect of Chemical Structure and Salt Concentration on the Crystallization and Ionic Conductivity of Aliphatic Polyethers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
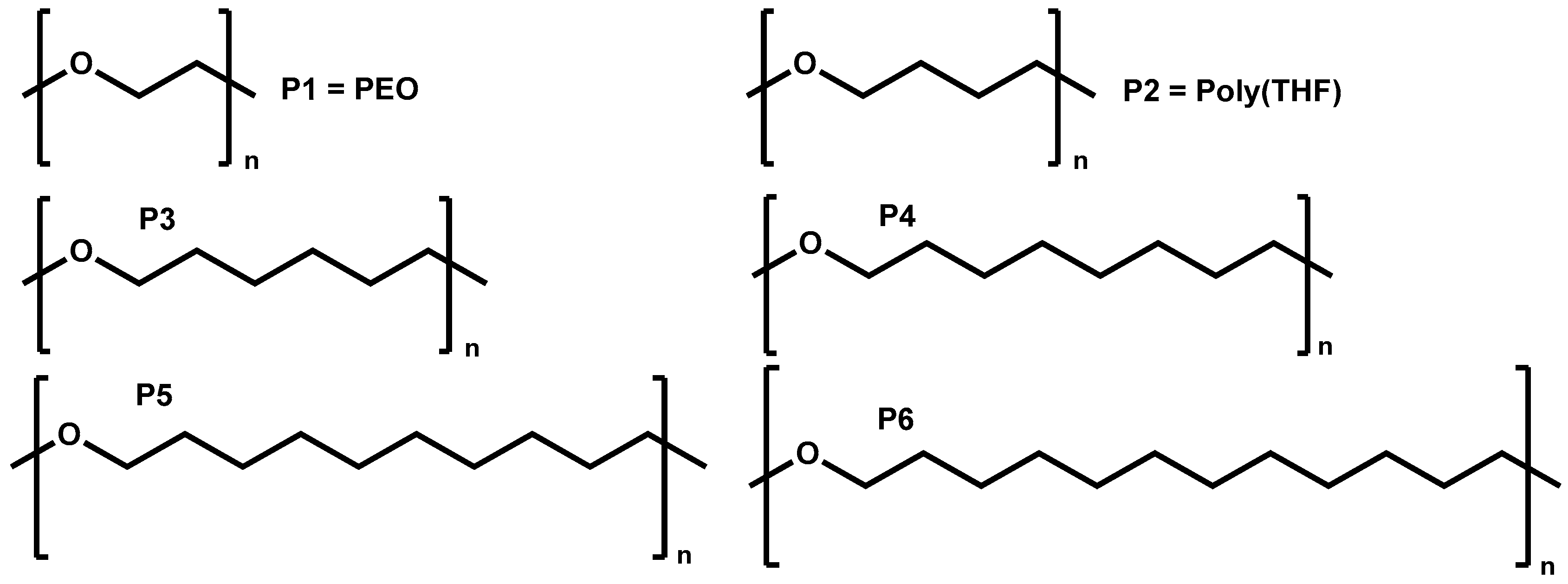
2.2. Synthesis of the Linear Polyethers
2.3. Elaboration of SPE Solid Polymer Electrolytes
3. Characterization Methods
4. Results and Discussion
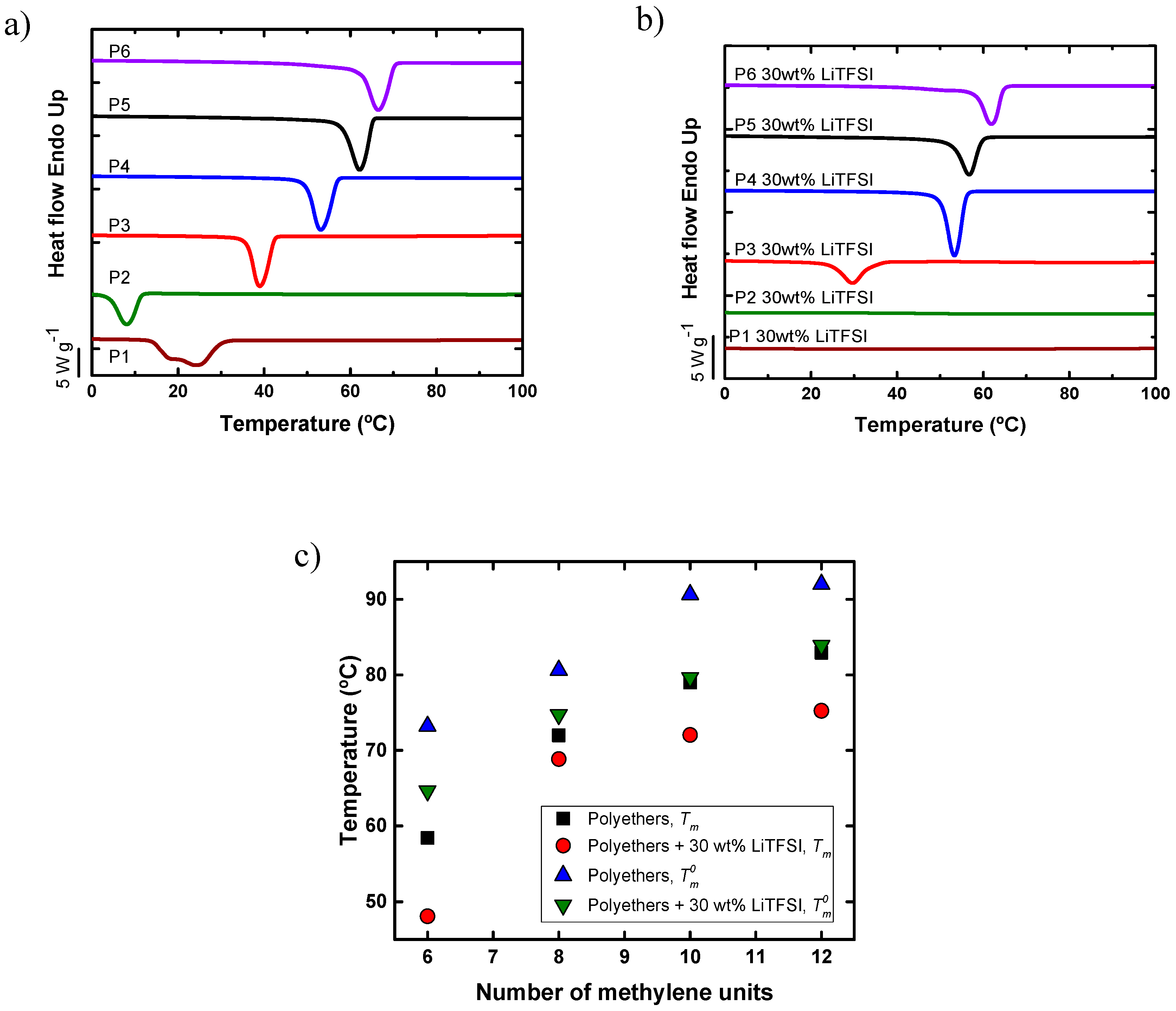
4.1. Non-Isothermal Crystallization of Aliphatic Polyethers in the Presence of LiTFSI
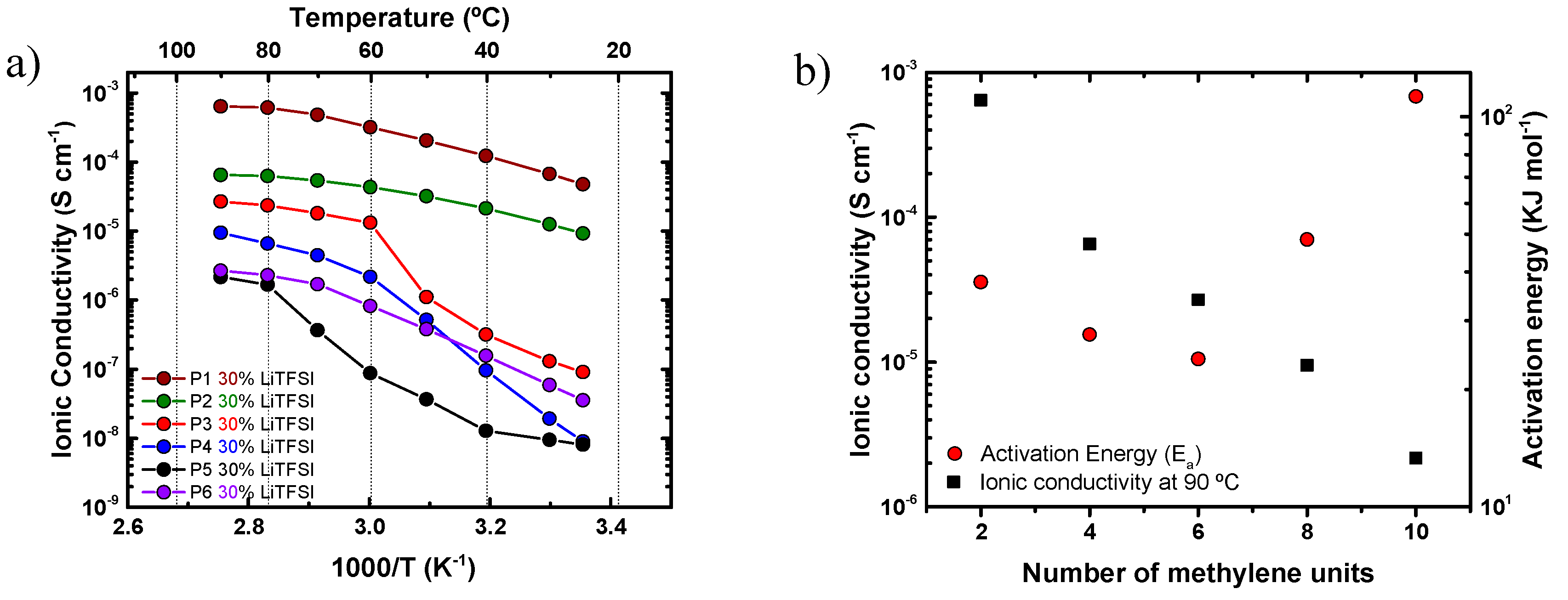
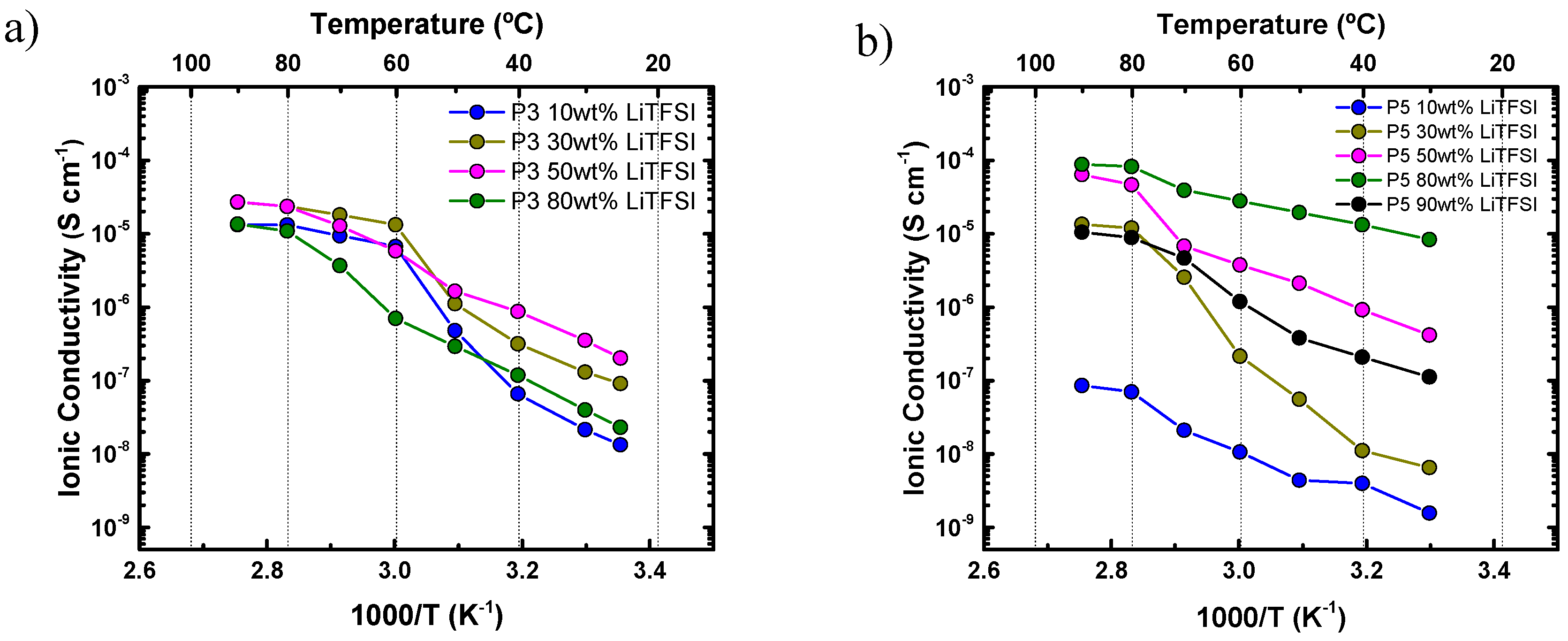
4.2. Ionic Conductivity of Aliphatic Polyethers in the Presence of LiTFSI
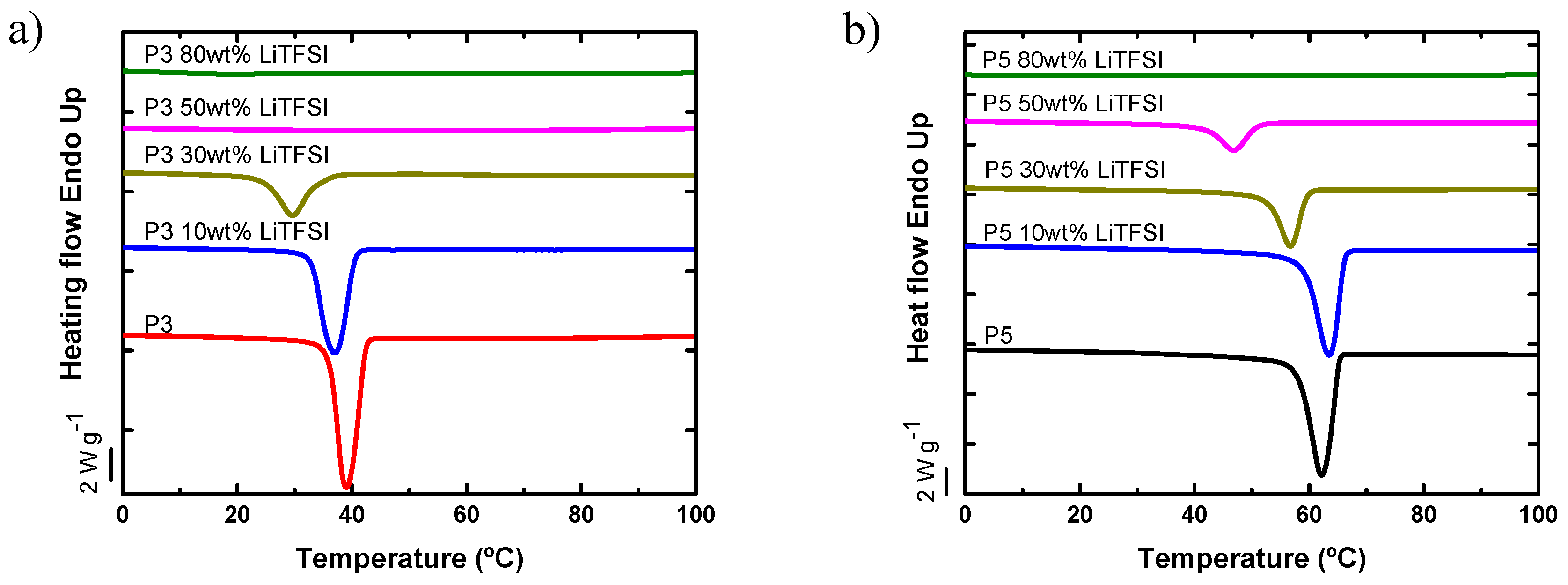
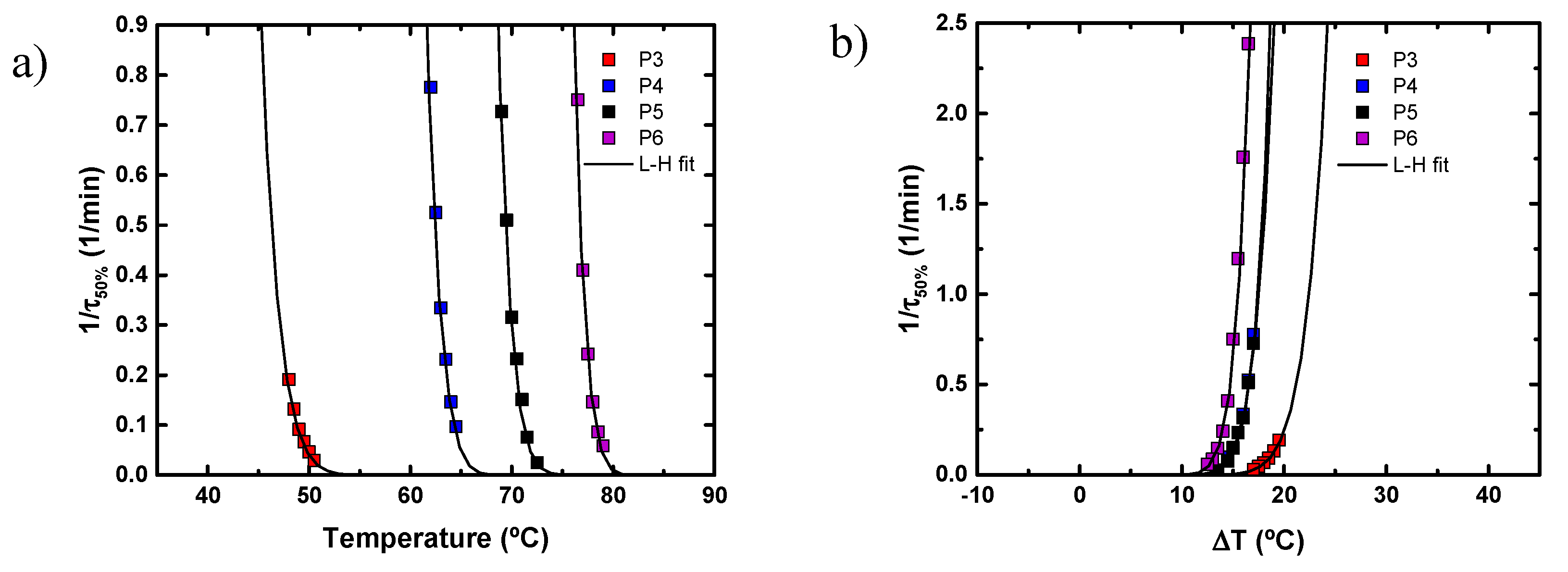
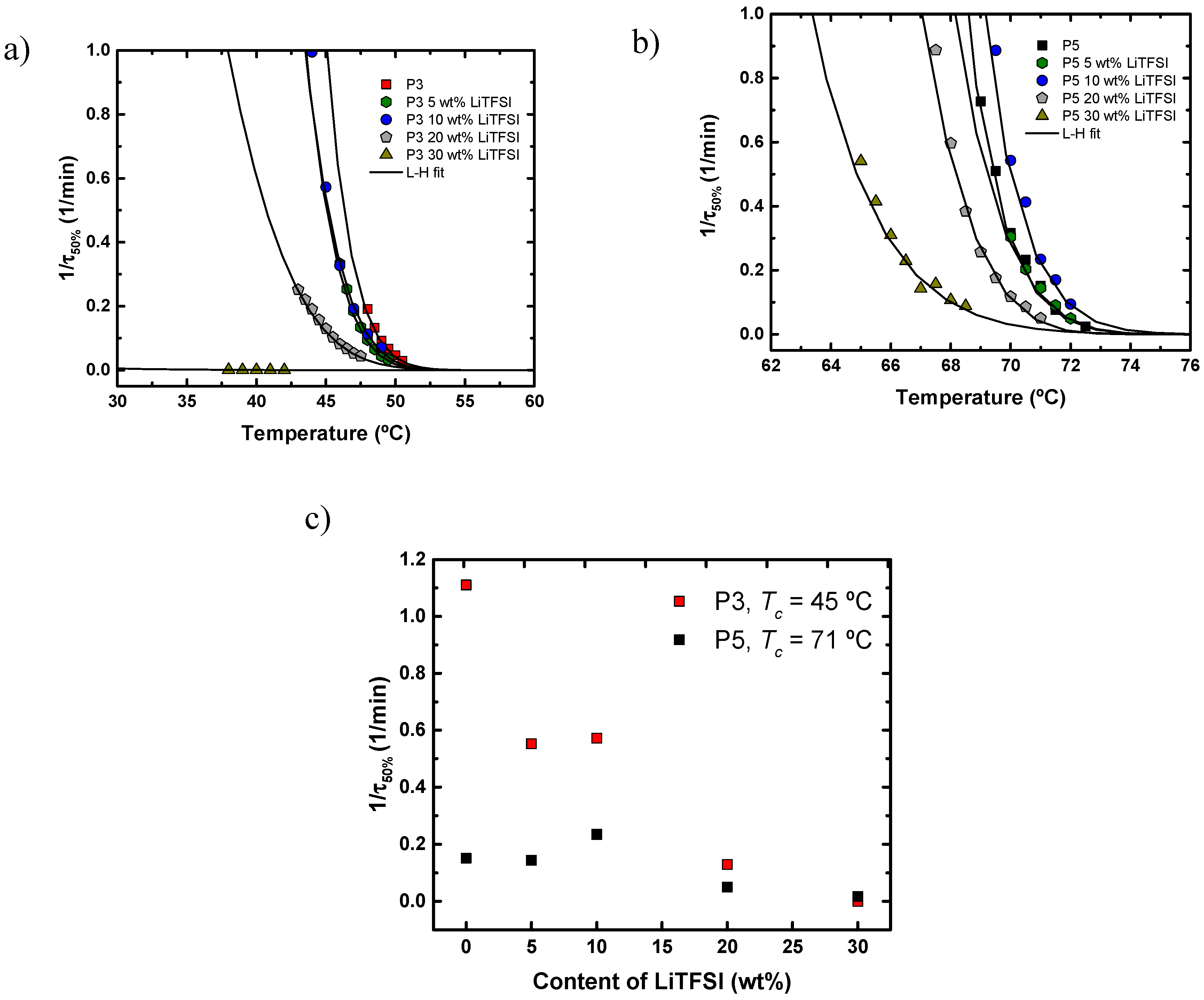
4.3. Isothermal Crystallization of Aliphatic Polyethers in the Presence of LiTFSI
4.4. Diluent Effect of LiTFSI
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Porcarelli, L.; Gerbaldi, C.; Bella, F.; Nair, J.R. Super Soft All-Ethylene Oxide Polymer Electrolyte for Safe All-Solid Lithium Batteries. Sci. Rep. 2016, 6, 19892. [Google Scholar] [CrossRef] [PubMed]
- Paillard, E.; Alloin, F.; Cointeaux, L.; Iojoiu, C.; Sanchez, J.Y. Poly(oxyethylene) electrolytes based on lithium nitrophenyl sulfonamide and hexanitrodiphenylamide. Electrochim. Acta 2011, 57, 20–26. [Google Scholar] [CrossRef]
- Thiam, A.; Antonelli, C.; Iojoiu, C.; Alloin, F.; Sanchez, J.Y. Optimizing ionic conduction of poly(oxyethylene) electrolytes through controlling the cross-link density. Electrochim. Acta 2017, 240, 307–315. [Google Scholar] [CrossRef]
- Aziz, S.B.; Woo, T.J.; Kadir, M.F.Z.; Ahmed, H.M. A conceptual review on polymer electrolytes and ion transport models. J. Sci. Adv. Mater. Devices 2018, 3, 1–17. [Google Scholar] [CrossRef]
- Meabe, L.; Huynh, T.V.; Lago, N.; Sardon, H.; Li, C.; O’Dell, L.A.; Armand, M.; Forsyth, M.; Mecerreyes, D. Poly(ethylene oxide carbonates) solid polymer electrolytes for lithium batteries. Electrochim. Acta 2018. [Google Scholar] [CrossRef]
- Mindemark, J.; Lacey, M.J.; Bowden, T.; Brandell, D. Progress in Polymer Science Beyond PEO—Alternative host materials for Li + -conducting solid polymer electrolytes. Prog. Polym. Sci. 2018, 81, 114–143. [Google Scholar] [CrossRef]
- Xue, Z.; He, D.; Xie, X. Poly(ethylene oxide)-based electrolytes for lithium-ion batteries. J. Mater. Chem. A 2015, 3, 19218–19253. [Google Scholar] [CrossRef]
- Geiculescu, O.E.; Yang, J.; Zhou, S.; Shafer, G.; Xie, Y.; Albright, J.; Creager, S.E.; Pennington, W.T.; DesMarteau, D.D. Solid Polymer Electrolytes from Polyanionic Lithium Salts Based on the LiTFSI Anion Structure. J. Electrochem. Soc. 2004, 151, A1363. [Google Scholar] [CrossRef]
- Zhao, Y.; Tao, R.; Fujinami, T. Enhancement of ionic conductivity of PEO-LiTFSI electrolyte upon incorporation of plasticizing lithium borate. Electrochim. Acta 2006, 51, 6451–6455. [Google Scholar] [CrossRef]
- Choi, B.K.; Kim, Y.W. Thermal history effects on the ionic conductivity of PEO-salt electrolytes. Mater. Sci. Eng. B 2004, 107, 244–250. [Google Scholar] [CrossRef]
- Quartarone, E.; Mustarelli, P.; Magistris, A. PEO-based composite polymer electrolytes. Solid State Ion. 1998, 110, 1–14. [Google Scholar] [CrossRef]
- Marzantowicz, M.; Dygas, J.R.; Krok, F.; Łasińska, A.; Florjańczyk, Z.; Zygadło-Monikowska, E.; Affek, A. Crystallization and melting of PEO:LiTFSI polymer electrolytes investigated simultaneously by impedance spectroscopy and polarizing microscopy. Electrochim. Acta 2005, 50, 3969–3977. [Google Scholar] [CrossRef]
- Park, S.J.; Han, A.R.; Shin, J.S.; Kim, S. Influence of crystallinity on ion conductivity of PEO-based solid electrolytes for lithium batteries. Macromol. Res. 2010, 18, 336–340. [Google Scholar] [CrossRef]
- Sadoway, D.R. Block and graft copolymer electrolytes for high-performance, solid-state, lithium batteries. J. Power Sources 2004, 129, 1–3. [Google Scholar] [CrossRef]
- Niitani, T.; Shimada, M.; Kawamura, K.; Kanamura, K. Characteristics of new-type solid polymer electrolyte controlling nano-structure. J. Power Sources 2005, 146, 386–390. [Google Scholar] [CrossRef]
- Snyder, J.F.; Carter, R.H.; Wetzel, E.D. Electrochemical and mechanical behavior in mechanically robust solid polymer electrolytes for use in multifunctional structural batteries. Chem. Mater. 2007, 19, 3793–3801. [Google Scholar] [CrossRef]
- Borgohain, M.M.; Joykumar, T.; Bhat, S.V. Studies on a nanocomposite solid polymer electrolyte with hydrotalcite as a filler. Solid State Ion. 2010, 181, 964–970. [Google Scholar] [CrossRef]
- Sim, L.H.; Chan, C.H.; Nasir, N.H.A. The effect of molecular mass of PEO and the salt content on its isothermal crystallization behaviour for PEO:LiClO4electrolyte. Aip Conf. Proc. 2010, 1250, 201–204. [Google Scholar]
- Zhang, Y.; Huo, H.; Li, J.; Shang, Y.; Chen, Y.; Funari, S.S.; Jiang, S. Crystallization behavior of poly(ε-caprolactone) and poly (ε-caprolactone)/LiClO4complexes from the melt. CrystEngComm 2012, 14, 7972–7980. [Google Scholar] [CrossRef]
- Basterretxea, A.; Gabirondo, E.; Jehanno, C.; Zhu, H.; Flores, I.; Müller, A.J.; Etxeberria, A.; Mecerreyes, D.; Coulembier, O.; Sardon, H. Polyether Synthesis by Bulk Self-Condensation of Diols Catalyzed by Non-Eutectic Acid–Base Organocatalysts. Acs Sustain. Chem. Eng. 2019. [Google Scholar] [CrossRef]
- Lorenzo, A.T.; Arnal, M.L.; Albuerne, J.; Müller, A.J. DSC isothermal polymer crystallization kinetics measurements and the use of the Avrami equation to fit the data: Guidelines to avoid common problems. Polym. Test. 2007, 26, 222–231. [Google Scholar] [CrossRef]
- Das, A.; Thakur, A.K.; Kumar, K. Exploring low temperature Li+ion conducting plastic battery electrolyte. Ionics 2013, 19, 1811–1823. [Google Scholar] [CrossRef]
- Wang, Z.S.; Kawauchi, H.; Kashima, T.; Arakawa, H. Significant influence of TiO2 photoelectrode morphology on the energy converion efficiency of N719 dye-sensitized solar cell. Coord. Chem. Rev. 2004, 248, 1381–1389. [Google Scholar] [CrossRef]
- Tao, C.; Gao, M.H.; Yin, B.H.; Li, B.; Huang, Y.P.; Xu, G.; Bao, J.J. A promising TPU/PEO blend polymer electrolyte for all-solid-state lithium ion batteries. Electrochim. Acta 2017, 257, 31–39. [Google Scholar] [CrossRef]
- Luo, J.; Jensen, A.H.; Brooks, N.R.; Sniekers, J.; Knipper, M.; Aili, D.; Li, Q.; Vanroy, B.; Wübbenhorst, M.; Yan, F.; et al. 1,2,4-Triazolium perfluorobutanesulfonate as an archetypal pure protic organic ionic plastic crystal electrolyte for all-solid-state fuel cells. Energy Environ. Sci. 2015, 8, 1276–1291. [Google Scholar] [CrossRef]
- Müller, A.J.; Michell, R.M.; Lorenzo, A.T. Isothermal Crystallization Kinetics of Polymers. In Polymer Morphology: Principles, Characterization, and Processing; Guo, Q., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 181–203. [Google Scholar]
- Gumede, T.P.; Luyt, A.S.; Pérez-Camargo, R.A.; Müller, A.J. The influence of paraffin wax addition on the isothermal crystallization of LLDPE. J. Appl. Polym. Sci. 2017, 134, 1–7. [Google Scholar] [CrossRef]
- Flory, P.J. Principles of POLYMER Chemistry; Cornell University Press: New York, NY, USA, 1953; ISBN 0801401348. [Google Scholar]
- Mandelkern, L. Crystallization of Polymers, 2nd ed.; Cambridge University Press: Cambridge, UK, 2002. [Google Scholar]
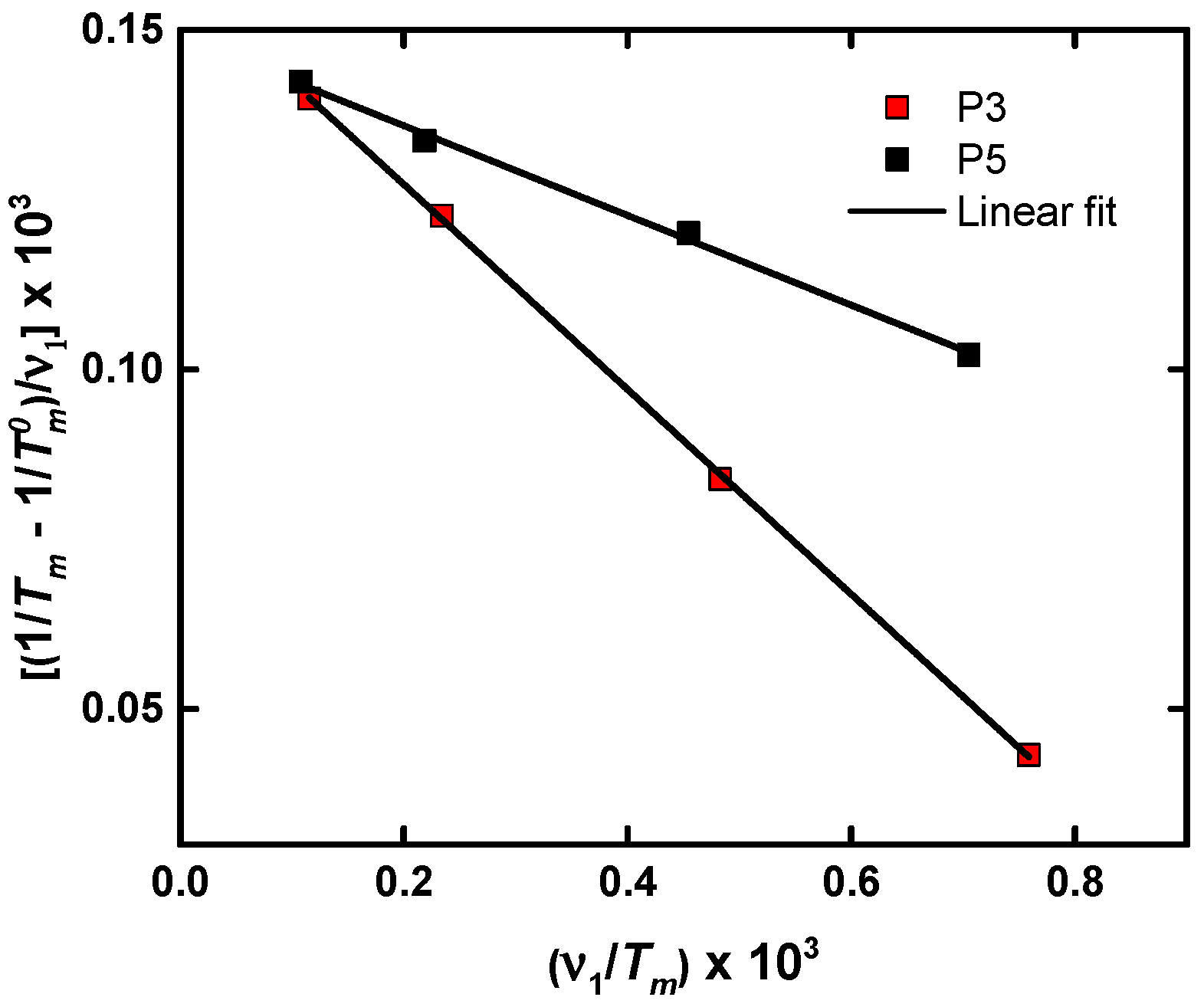
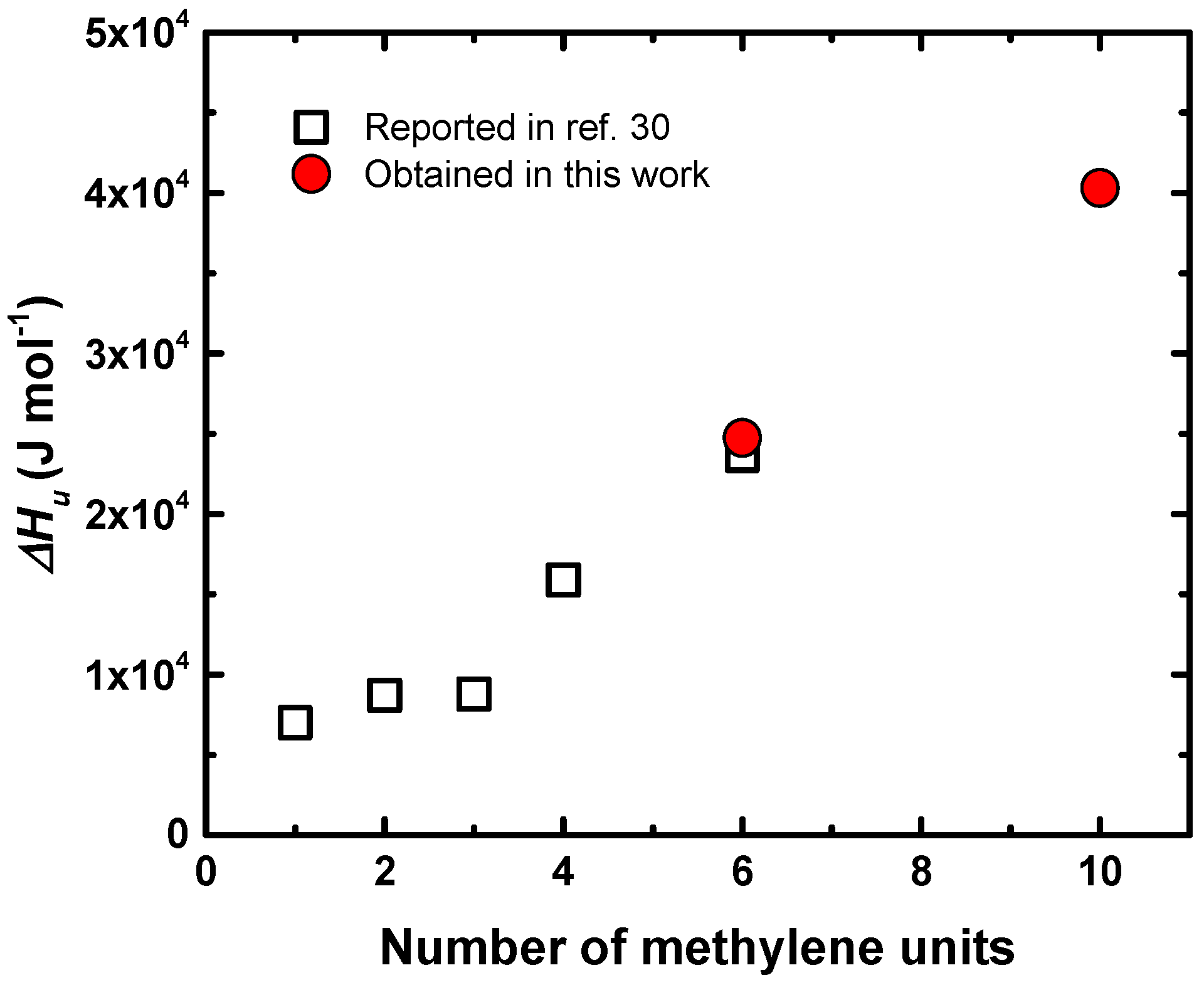
| Sample | ΔHm (J g−1) | Crystallinity (%) |
|---|---|---|
| P1 | 149 | 69 |
| P2 | 85 | 36 |
| P3 | 127 | 51 |
| P3 10 wt % LiTFSI | 99 | 45 |
| P3 30 wt % LiTFSI | 49 | 29 |
| P3 50 wt % LiTFSI | 0 | 0 |
| P4 | 145 | 56 |
| P5 | 135 | 52 |
| P5 10 wt % LiTFSI | 119 | 51 |
| P5 30 wt % LiTFSI | 80 | 44 |
| P5 50 wt % LiTFSI | 47 | 36 |
| P5 80 wt % LiTFSI | 0 | 0 |
| P6 | 142 | 53 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olmedo-Martínez, J.L.; Meabe, L.; Basterretxea, A.; Mecerreyes, D.; Müller, A.J. Effect of Chemical Structure and Salt Concentration on the Crystallization and Ionic Conductivity of Aliphatic Polyethers. Polymers 2019, 11, 452. https://doi.org/10.3390/polym11030452
Olmedo-Martínez JL, Meabe L, Basterretxea A, Mecerreyes D, Müller AJ. Effect of Chemical Structure and Salt Concentration on the Crystallization and Ionic Conductivity of Aliphatic Polyethers. Polymers. 2019; 11(3):452. https://doi.org/10.3390/polym11030452
Chicago/Turabian StyleOlmedo-Martínez, Jorge L., Leire Meabe, Andere Basterretxea, David Mecerreyes, and Alejandro J. Müller. 2019. "Effect of Chemical Structure and Salt Concentration on the Crystallization and Ionic Conductivity of Aliphatic Polyethers" Polymers 11, no. 3: 452. https://doi.org/10.3390/polym11030452
APA StyleOlmedo-Martínez, J. L., Meabe, L., Basterretxea, A., Mecerreyes, D., & Müller, A. J. (2019). Effect of Chemical Structure and Salt Concentration on the Crystallization and Ionic Conductivity of Aliphatic Polyethers. Polymers, 11(3), 452. https://doi.org/10.3390/polym11030452