Advances in Conjugated Polymer Lasers
Abstract
:1. Introduction
2. Laser Process of Conjugated Polymers
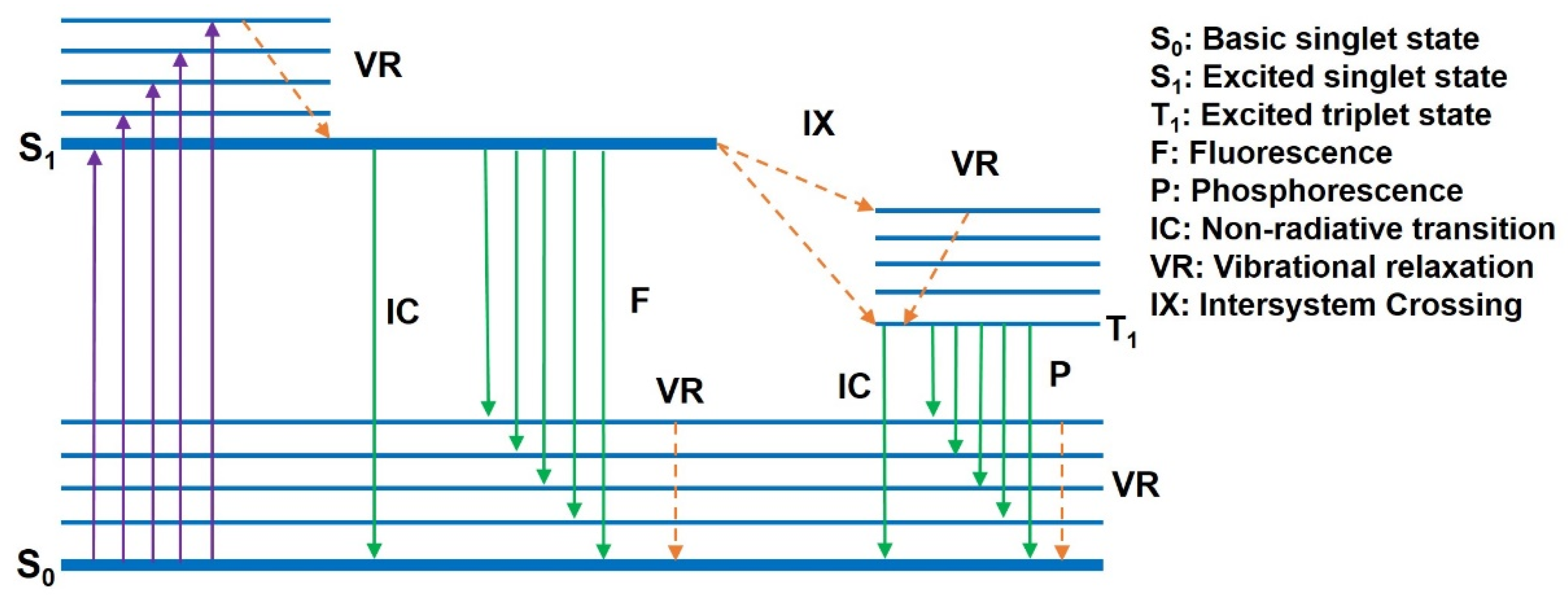
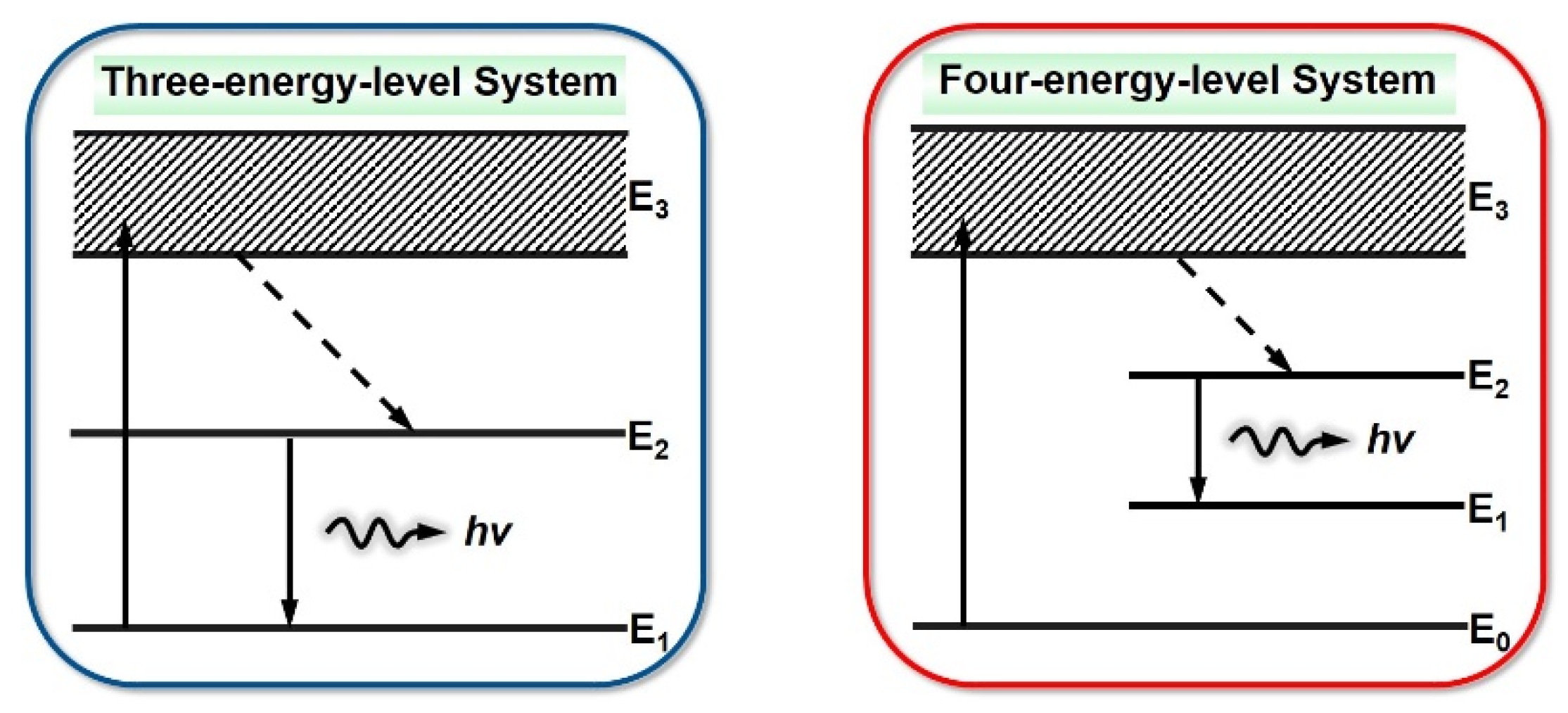
2.1. Photoluminescence Principle of Conjugated Polymers
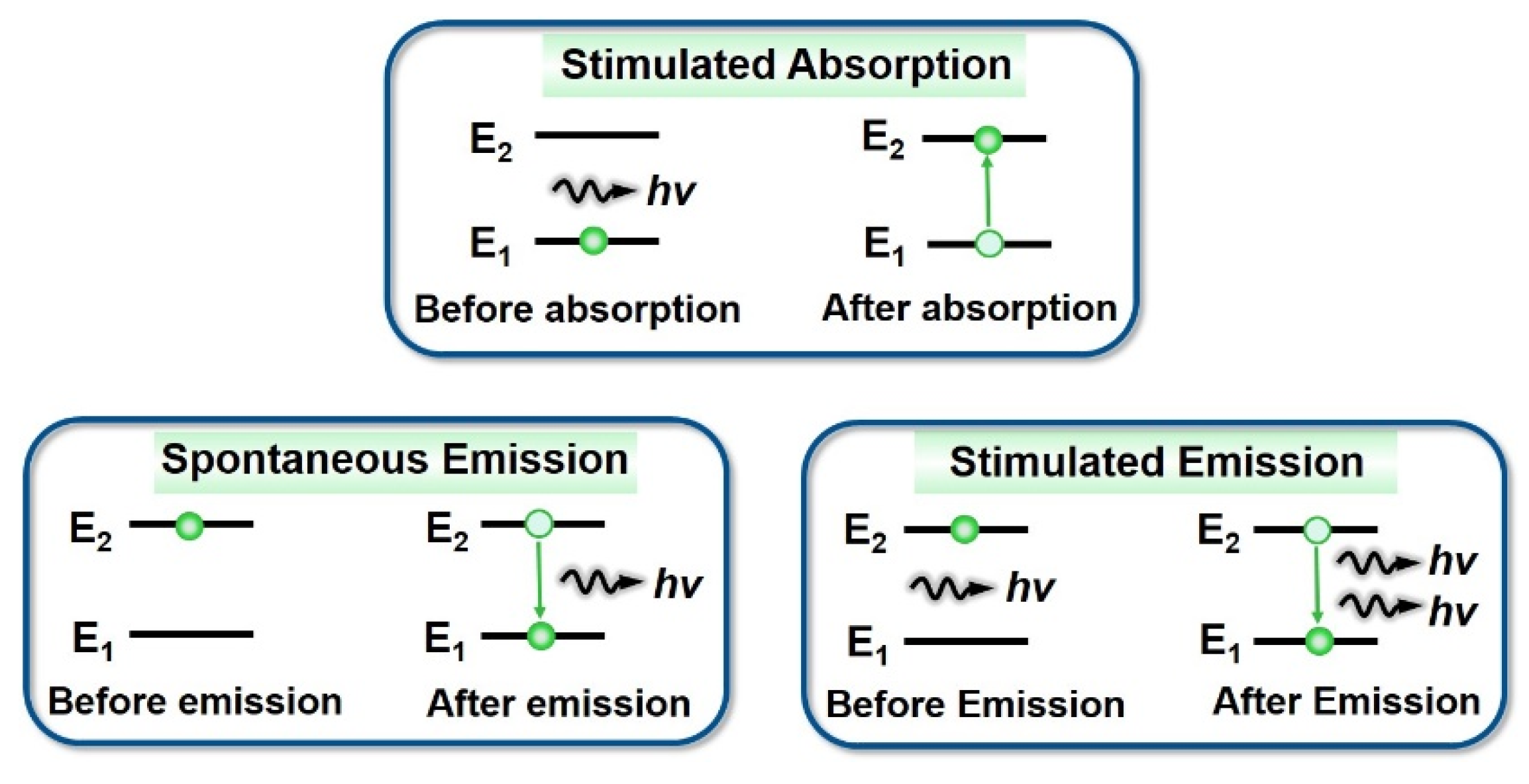
2.2. Stimulated Radiation Mechanism of Conjugated Polymers
- (1)
- High laser emission efficiency. The absorption coefficient is high (αmax > 105 cm−1) and the stimulated emission cross-section is large (about 10−15 cm2) [42]. This is due to the large Stokes shift between the absorption spectra and the fluorescence emission spectra of the conjugated polymers. The self-absorption coefficient of the molecule is small, but the absorption coefficient of the excited light is high, and the stimulated radiation has a large advantage relative to spontaneous radiation. For example, a 150 nm film can absorb more than 90% of pump photons, which helps to lower the operating threshold. Also as mentioned above, the laser efficiency of conjugated polymers is not severely affected by the concentration quenching effect, so the conjugated polymer materials still have high luminescence efficiency in the solid state.
- (2)
- Easy to achieve particle number inversion. The molecule has a conjugated π–π* bond structure. Moreover, direct transitions between the bonds have a high density of intersections, so they undergo particle number inversion at very low pump intensity (<1000 W/cm2). The binding between the molecules relies on van der Waals forces that make the overlap of the electron clouds very small. Furthermore, the carriers are highly localized.
- (3)
- Sources of materials are abundant. Different wavelengths of emitted light can be obtained by adjusting the chain length of the conjugated polymer and modifying the groups of the main chain. The spectra range covers the whole visible light region. The wide spectrum also means that the wavelength of conjugated polymer lasers can be modulated in a wide range, allowing different application requirements.
2.3. Effect of Conjugated Polymer Structures on the Laser Threshold
- (a)
- Effect of the conjugate chain length. The bandgap in conjugated polymers is determined by the degree of π-delocalization along the backbone—the so-called effective conjugation length. The different numbers of unsaturated double bonds and aromatic rings in the molecule lead to different conjugate degrees and molecular plane degrees, which result in different luminescence efficiencies and different luminescence wavelengths. Generally, the luminescence intensity of conjugated polymers which contain aromatic rings or aromatic complex rings is large. This is because a larger conjugate system will cause delocalized electrons to be excited more easily, resulting in a higher quantum efficiency.
- (b)
- Effect of substituents. If a molecule contains some groups that increase the luminescence efficiency, these groups called chromophores. Chromophores are generally electron donors (e.g., -NH2, -NHR, -NR2, -OH, -OR, -CN, etc.). Polymers containing chromophores have unbonded lone pair electrons (known as n electrons), and n electrons’ clouds can be almost parallel to the π orbital on the aromatic ring, so they actually share the electron structure of the π conjugated electrons. Furthermore, they expand the conjugated system, so the luminescence efficiency of these conjugated polymers increases.
- (c)
- Effect of space. A large number of studies have found that conjugated polymers with a relatively rigid planar structure have a more stable homogeneous conjugated system and a higher luminescence efficiency. This is mainly due to the reduction of internal conversion probability caused by vibration dissipation [1,15].
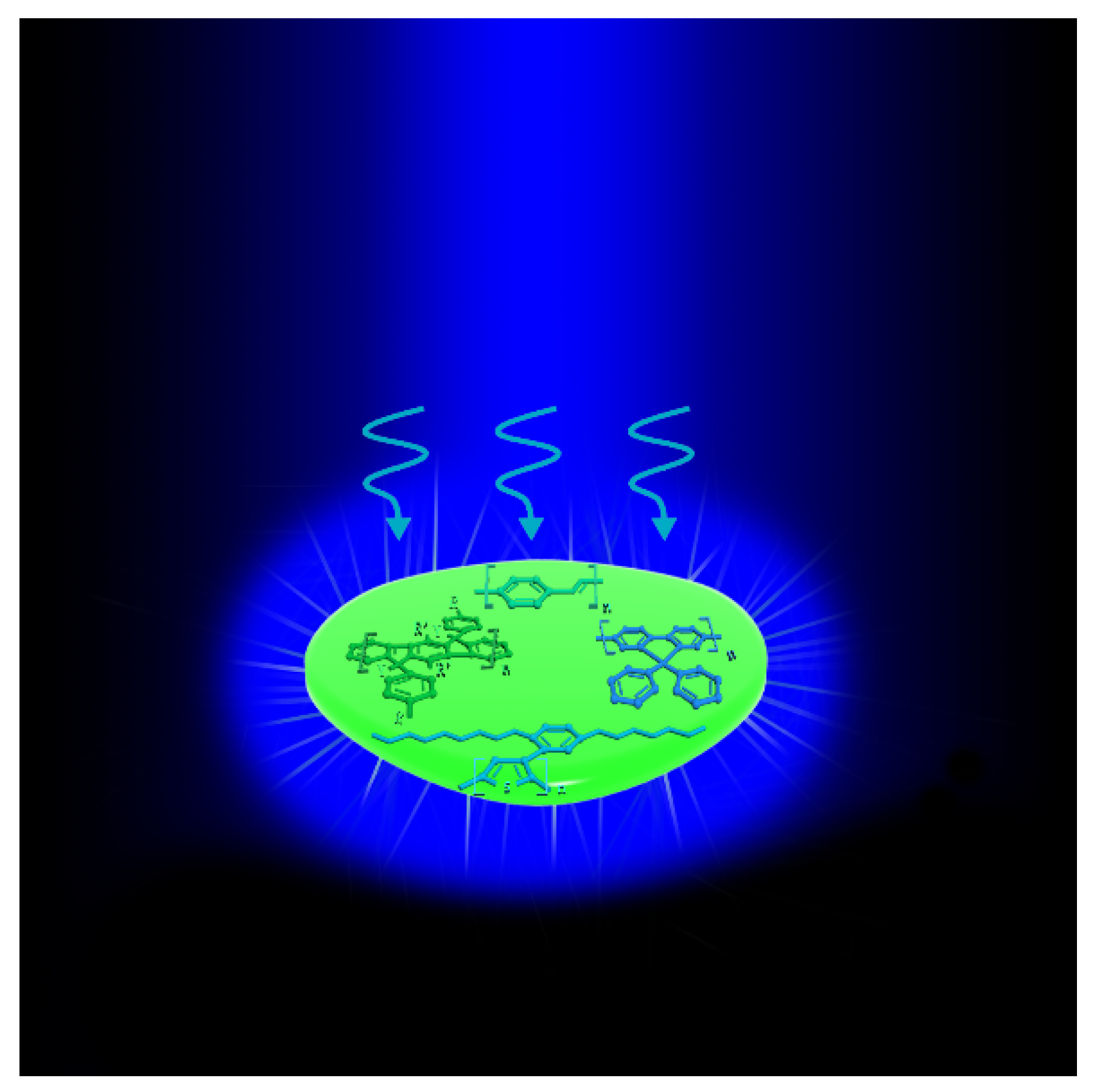
3. Types of Conjugated Polymer Laser Materials
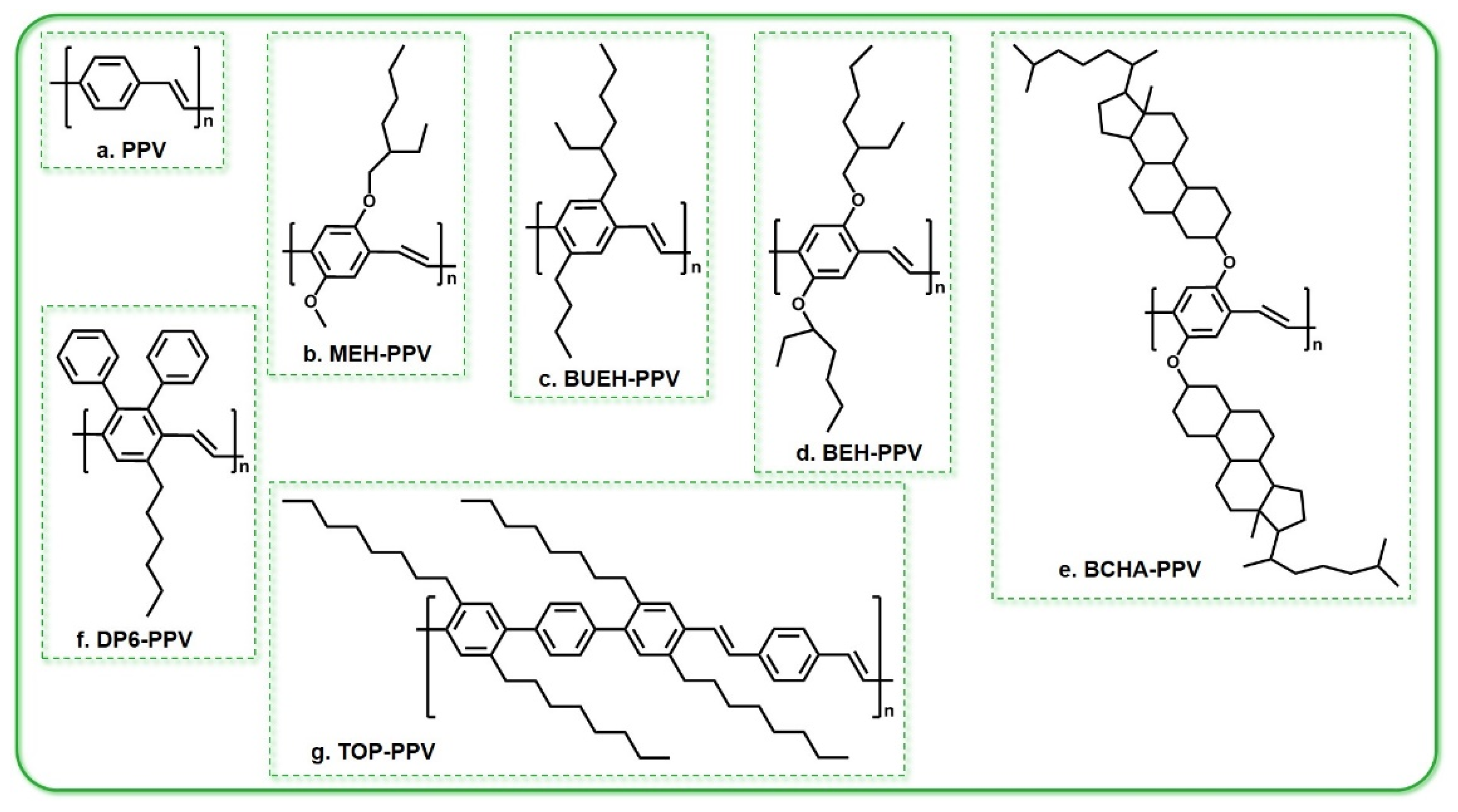
3.1. PPV and PPV Derivatives
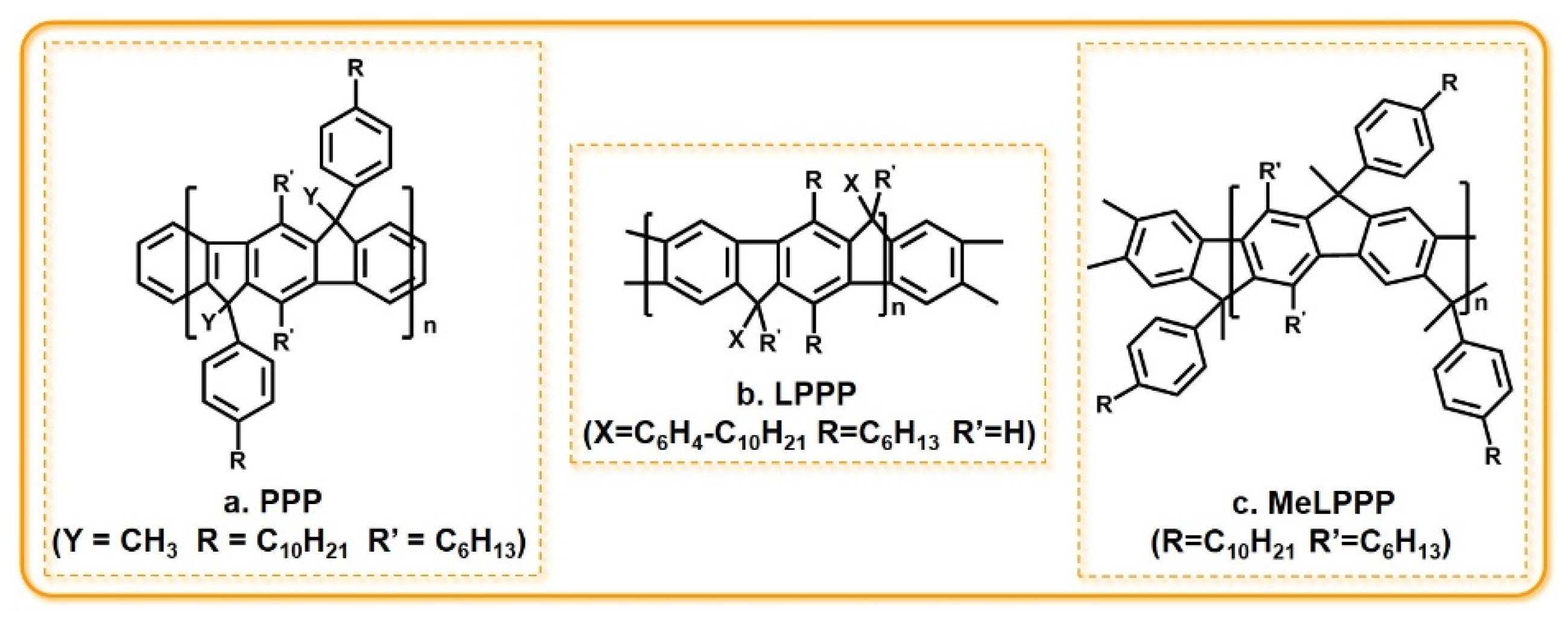
3.2. PPP and PPP Derivatives
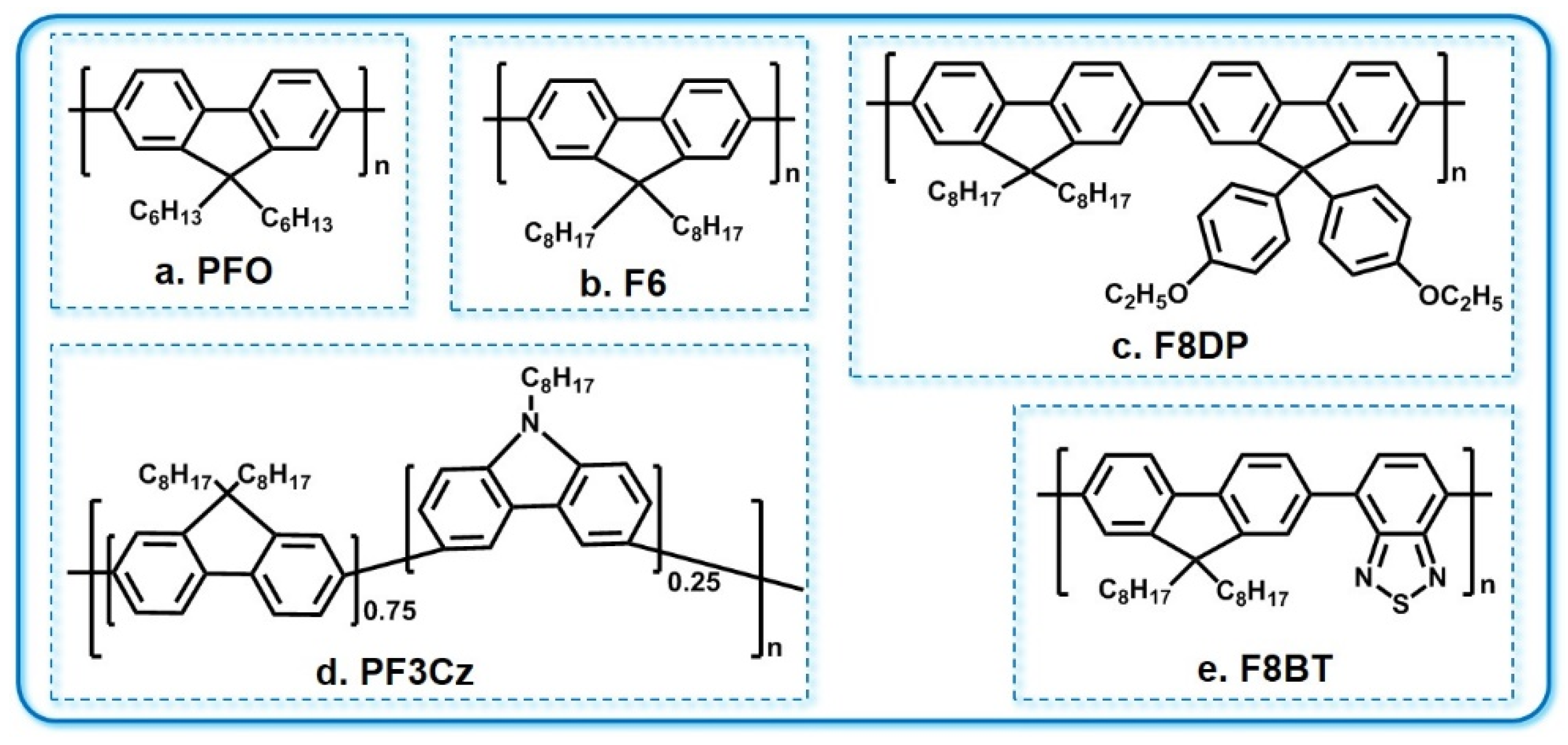
3.3. PF and PF Derivatives
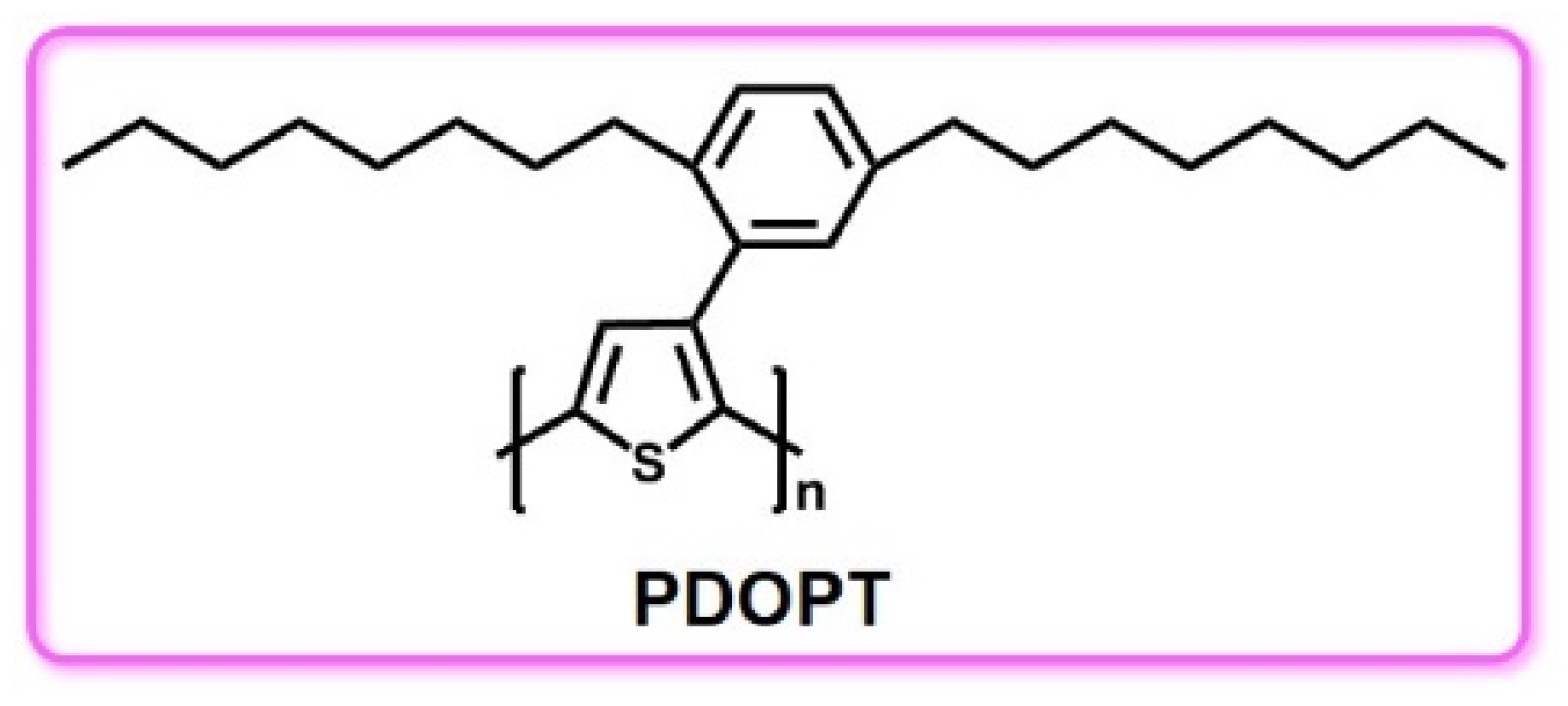
3.4. PT and PT Derivatives
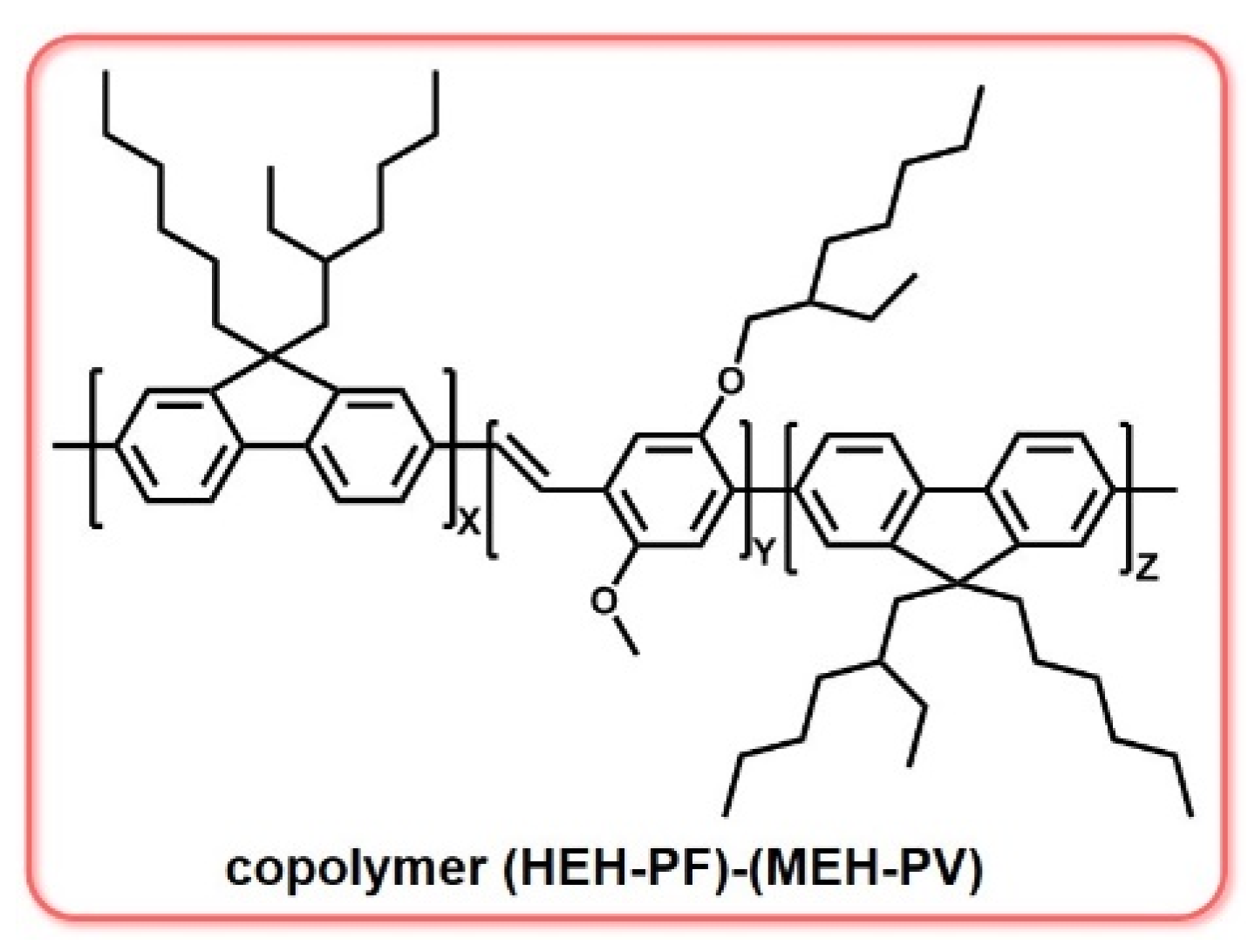
3.5. Copolymer
4. Conclusions and Future Developments
- (1)
- To reach the pump emission threshold, the working current density with electromechanical luminescence is usually at about 105 A/cm2, while the current density in the conjugated polymers is about at 102 A/cm2, with a difference of more than three orders of magnitude [63]. To achieve such a high current density, the carrier mobility and thermal stability of conjugated polymer materials must be greatly improved, otherwise the joule heat generated by high voltage will cause damage to the organic materials and result in the invalidation of the device.
- (2)
- In an electric pump structure, the conjugated polymer materials must be embedded between a pair of positive and negative poles. On the one hand, the electrode will produce a great deal of light loss [64]. An even more important aspect is that organic polymer materials must use a grating laser resonant structure (a structure of several hundred nanometers in size) which will largely crack the electrodes deposited on them, reduce the ohm characteristics of electrical contact, and affect carrier injection. Therefore, the effect of the resonant cavity structure on the electrode mass must be considered when designing a conjugated polymer laser structure.
Author Contributions
Funding
Conflicts of Interest
References
- Samuel, I.D.W.; Turnbull, G.A. Organic Semiconductor Lasers. Chem. Rev. 2007, 107, 1272–1295. [Google Scholar] [CrossRef] [PubMed]
- Tessler, N. Lasers Based on Semiconducting Organic Materials. Adv. Mater. 1999, 11, 363–370. [Google Scholar] [CrossRef]
- McGehee, M.D.; Heeger, A.J. Semiconducting (Conjugated) Polymers as Materials for Solid-State Lasers. Adv. Mater. 2000, 12, 1655–1668. [Google Scholar] [CrossRef]
- Kozlov, V.G.; Forrest, S.R. Lasing action in organic semiconductor thin films. Curr. Opin. Solid State Mater. Sci. 1999, 4, 203–208. [Google Scholar] [CrossRef]
- Frolov, S.V.; Ozaki, M.; Gellermann, W.; Vardeny, Z.V.; Yoshino, K. Mirrorless Lasing in Conducting Polymer poly(2,5-dioctyloxy-p-phenylenevinylene) Films. Jpn. J. Appl. Phys. 1996, 35, 1371–1373. [Google Scholar] [CrossRef]
- Turnbull, G.A.; Andrew, P.; Barnes, W.L.; Samuel, I.D.W. Photonic mode dispersion of a two-dimensional distributed feedback polymer laser. Phys. Rev. B 2003, 67, 165107. [Google Scholar] [CrossRef]
- Berggren, M.; Dodabalapur, A.; Slusher, R.E. Stimulated emission and lasing in dye-doped organic thin films with Forster transfer. Appl. Phys. Lett. 1997, 71, 2229–2232. [Google Scholar] [CrossRef]
- Schneider, D.; Rabe, T.; Riedl, T.; Dobbertin, T.; Kröger, M.; Becker, E.; Johannes, H.H.; Kowalsky, W.; Weimann, T.; Wang, J.; et al. An Ultraviolet Organic Thin-Film Solid-State Laser for Biomarker Applications. Adv. Mater. 2005, 17, 31–34. [Google Scholar] [CrossRef]
- McGehee, M.D.; Gupta, R.; Veenstra, S.; Miller, E.K.; Díaz-García, M.A.; Heeger, A.J. Amplified spontaneous emission from photopumped films of a co njugated polymer. Phys. Rev. B 1998, 58, 7035–7039. [Google Scholar] [CrossRef]
- Schülzgen, A.; Spiegelberg, C.; Morrell, M.M.; Mendes, S.B.; Kippelen, B.; Peyghambarian, N.; Nabor, M.F.; Mash, E.A. Near diffraction-limited laser emission from a polymer in a high finesse planar cavity. Appl. Phys. Lett. 1998, 72, 269–271. [Google Scholar] [CrossRef]
- Burroughes, J.H.; Bradley, D.D.C.; Brown, A.R.; Marks, R.N.; Mackay, K.; Friend, R.H.; Burns, P.L.; Holmes, A.B. Light-emitting diodes based on conjugated polymers. Nature 1990, 347, 539–541. [Google Scholar] [CrossRef]
- Moses, D. High quantum efficiency luminescence from a conducting polymer in solution: A novel polymer laser dye. Appl. Phys. Lett. 1992, 60, 3215–3216. [Google Scholar] [CrossRef]
- Brouwer, H.J.; Krasnikov, V.V.; Hilberer, A.; Wildeman, J.; Hadziioannou, G. Novel high efficiency copolymer laser dye in the blue wavelength region. Appl. Phys. Lett. 1995, 66, 3404–3406. [Google Scholar] [CrossRef]
- Hide, F.; Schwartz, B.J.; Díaz-García, M.A.; Heeger, A.J. Laser emission from solutions and films containing semiconducting polymer and titanium dioxide nanocrystals. Chem. Phys. Lett. 1996, 256, 424–430. [Google Scholar] [CrossRef]
- Hide, F.; Díaz-García, M.A.; Schwartz, B.J.; Andersson, M.R.; Pei, Q.B.; Heeger, A.J. Semiconducting Polymers: A New Class of Solid-State Laser Materials. Science 1996, 273, 1833–1836. [Google Scholar] [CrossRef]
- Kozlov, V.G.; Bulović, V.; Burrows, P.E.; Forrest, S.R. Laser action in organic semiconductor waveguide and double-heterostructure devices. Nature 1997, 389, 362–364. [Google Scholar] [CrossRef]
- Friend, R.H.; Gymer, R.W.; Holmes, A.B.; Burroughes, J.H.; Marks, R.N.; Taliani, C.; Bradley, D.D.C.; Dos Santos, D.A.; Brédas, J.L.; Lögdlund, M.; et al. Electroluminescence in conjugated polymers. Nature 1999, 397, 121–128. [Google Scholar] [CrossRef]
- Tessler, N.; Denton, G.J.; Friend, R.H. Lasing from conjugated-polymer microcavities. Nature 1996, 382, 695–697. [Google Scholar] [CrossRef]
- Turnbull, G.A.; Andrew, P.; Barnes, W.L.; Samuel, I.D.W. Operating characteristics of a semiconducting polymer laser pumped by a microchip laser. Appl. Phys. Lett. 2003, 82, 313–315. [Google Scholar] [CrossRef]
- O’Carroll, D.; Lieberwirth, I.; Redmond, G. Microcavity effects and optically pumped lasing in single conjugated polymer nanowires. Nat. Nanotechnol. 2007, 2, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Herrnsdorf, J.; Wang, Y.; McKendry, J.J.D.; Gong, Z.; Massoubre, D.; Guilhabert, B.; Tsiminis, G.; Turnbull, G.A.; Samuel, I.D.W.; Laurand, N.; et al. Micro-LED pumped polymer laser: A discussion of future pump sources for organic lasers. Laser Photonics Rev. 2013, 7, 1065–1078. [Google Scholar] [CrossRef]
- Zhai, T.R.; Wang, Y.L.; Chen, L.; Zhang, X.P. Direct writing of tunable multi-wavelength polymer lasers on a flexible substrate. Nanoscale 2015, 7, 12312–12317. [Google Scholar] [CrossRef] [PubMed]
- Zhai, T.R.; Wang, Y.R.; Chen, L.; Wu, X.F.; Lia, S.T.; Zhang, X.P. Red–green–blue laser emission from cascaded polymer membranes. Nanoscale 2015, 7, 19935–19939. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.J.; Liu, Z.H.; Qian, Y.J.; Shi, K.B.; Sun, Y.J.; Wu, C.F.; Gong, Q.H.; Xiao, Y.F. A Tunable Optofluidic Microlaser in a Photostable Conjugated Polymer. Adv. Mater. 2018, 30, 1804556. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Ibnaouf, K.H.; AlSalhi, M.S.; Masilamani, V. Laser from the dimer state of a conjugated polymer (PFO) in solution. Polymer 2014, 55, 727–732. [Google Scholar] [CrossRef]
- Alfahd, S.A.; Rajendra, S.P.; Al-Mujammi, W.; Devaraj, D.; Masilamani, V.; AlSalhi, M.S. An Efficient Violet Amplified Spontaneous Emission (ASE) from a Conjugated Polymer (PFO-co-pX) in Solution. Materials 2017, 10, 265. [Google Scholar] [CrossRef] [PubMed]
- Mujamammi, W.M.; Prasad, S.; AlSalhi, M.S.; Masilamani, V. Relaxation Oscillation with Picosecond Spikes in a Conjugated Polymer Laser. Polymers 2016, 8, 364. [Google Scholar] [CrossRef]
- Mujamammi, W.M.; Prasad, S.; AlSalhi, M.S.; Masilamani, V. Time Evolution of the Excimer State of a Conjugated Polymer Laser. Polymers 2017, 9, 648. [Google Scholar] [CrossRef]
- AlSalhi, M.S.; Almotiri, A.R.; Prasad, S.; Aljaafreh, M.J.; Othman, A.H.S.; Masilamai, V. A Temperature-Tunable Thiophene Polymer Laser. Polymers 2018, 10, 470. [Google Scholar] [CrossRef]
- Hassan, M.U.; Liu, Y.C.; Butt, H.; Hasan, K.U.; Chang, J.F.; Olawoyin, A.A.; Friend, R.H. Low Thresholds for a Nonconventional Polymer Blend—Amplified Spontaneous Emission and Lasing in F81−x:SYx System. J. Polym. Sci. Part B Polym. Phys. 2016, 54, 15–21. [Google Scholar] [CrossRef]
- Oki, O.; Kushida, S.; Mikosch, A.; Hatanaka, K.; Takeda, Y.; Minakata, S.; Kuwabara, J.; Kanbara, T.; Dao, T.D.; Ishii, S.; et al. FRET-mediated near infrared whispering gallery. modes: Studies on the relevance of intracavity energy transfer with Q-factors. Mater. Chem. Front. 2018, 2, 270–274. [Google Scholar] [CrossRef]
- Bai, L.B.; Liu, B.; Han, Y.M.; Yu, M.N.; Wang, J.; Zhang, X.W.; Ou, C.J.; Lin, J.Y.; Zhu, W.S.; Xie, L.H.; et al. Steric-Hindrance-Functionalized Polydiarylfluorenes: Conformational Behavior, Stabilized Blue Electroluminescence, and Efficient Amplified Spontaneous Emission. ACS Appl. Mater. Interfaces 2017, 9, 37856–37863. [Google Scholar] [CrossRef] [PubMed]
- Karl, M.; Glackin, J.M.E.; Schubert, M.; Kronenberg, N.M.; Turnbull, G.A.; Samuel, I.D.W.; Gather, M.C. Flexible and ultra-lightweight polymer membrane lasers. Nat. Commun. 2018, 9, 1525. [Google Scholar] [CrossRef] [PubMed]
- Lampert, Z.E.; Papanikolas, J.M.; Lappi, S.E.; Reynolds, C.L. Intrinsic gain and gain degradation modulated by excitation pulse width in a semiconducting conjugated polymer. Opt. Laser Technol. 2017, 94, 77–85. [Google Scholar] [CrossRef]
- Steppert, A.K.; Mikosch, A.; Haraszti, T.; Göstl, R.; Kuehne, A.J.C. Reversible Laser Threshold Modulation in Dithienylethene Conjugated Polymer Blends: A Concept for q-Switching in Organic DFB Lasers. ACS Photonics 2019, 6, 558–564. [Google Scholar] [CrossRef]
- Blom, P.W.M.; Vissenberg, M.C.J.M. Dispersive Hole Transport in Poly(p-Phenylene Vinylene). Phys. Rev. Lett. 1998, 80, 3819–3822. [Google Scholar] [CrossRef]
- Davids, P.S.; Kogan, S.M.; Parker, I.D.; Smith, D.L. Charge injection in organic light-emitting diodes: Tunneling into low mobility materials. Appl. Phys. Lett. 1996, 69, 2270–2272. [Google Scholar] [CrossRef]
- Schweitzer, B.; Wegmann, G.; Giessen, H.; Hertel, D.; Bässler, H.; Mahrt, R.F.; Scherf, U.; Müllen, K. The optical gain mechanism in solid conjugated polymers. Appl. Phys. Lett. 1998, 72, 2933–2935. [Google Scholar] [CrossRef]
- Rudenko, A.I.; Bässler, H. Spectral diffusion in random media: A master equation approach. Chem. Phys. Lett. 1991, 128, 581–587. [Google Scholar] [CrossRef]
- Kersting, R.; Lemmer, U.; Mahrt, R.F.; Leo, K.; Kurz, H.; Bässler, H.; Göbel, E.O. Femtosecond energy relaxation in π-conjugated polymers. Phys. Rev. Lett. 1993, 70, 3820–3823. [Google Scholar] [CrossRef] [PubMed]
- Rabe, T.; Hoping, M.; Schneider, D.; Becker, E.; Johannes, H.H.; Kowalsky, W.; Weimann, T.; Wang, J.; Hinze, P.; Nehls, B.S.; et al. Threshold Reduction in Polymer Lasers Based on Poly(9,9-dioctylfluorene) with Statistical Binaphthyl Units. Adv. Funct. Mater. 2005, 15, 1188–1192. [Google Scholar] [CrossRef]
- Frolov, S.; Gellermann, W.; Ozaki, M.; Vardeny, Z.; Ozaki, M. Cooperative Emission in π-Conjugated Polymer Thin Films. Phys. Rev. Lett. 1997, 78, 729–732. [Google Scholar] [CrossRef]
- Fasano, V.; Moffa, M.; Camposeo, A.; Persano, L.; Pisignano, D. Controlled Atmosphere Electrospinning of Organic Nanofibers with Improved Light Emission and Waveguiding Properties. Macromolecules 2015, 48, 7803–7809. [Google Scholar] [CrossRef] [PubMed]
- Denton, G.J.; Tessler, N.; Stevens, M.A.; Friend, R.H. Spectral Narrowing in Optically Pumped Polyb-phenylenevinylene) Films. Adv. Mater. 1997, 9, 547–551. [Google Scholar]
- Bauer, C.; Giessen, H.; Schnabel, B.; Kley, E.B.; Schmitt, C.; Scherf, U.; Mahrt, R.F. A Surface-Emitting Circular Grating Polymer Laser. Adv. Mater. 2001, 13, 1161–1164. [Google Scholar] [CrossRef]
- Stehr, J.; Crewett, J.; Schindler, F.; Sperling, R.; Plessen, G.V.; Lemmer, U.; Lupton, J.M.; Klar, T.A.; Feldmann, J.; Holleitner, A.W.; et al. A Low Threshold Polymer Laser Based on Metallic Nanoparticle Gratings. Adv. Mater. 2003, 15, 1726–1729. [Google Scholar] [CrossRef]
- Heliotis, G.; Xia, R.; Bradley, D.D.C.; Turnbull, G.A.; Samuel, I.D.W.; Andrew, P.; Barnes, W.L. Blue, surface-emitting, distributed feedback polyfluorene lasers. Appl. Phys. Lett. 2003, 83, 2118–2120. [Google Scholar] [CrossRef]
- Heliotis, G.; Xia, R.D.; Turnbull, G.A.; Andrew, P.; Barnes, W.L.; Samuel, I.D.W.; Bradley, D.D.C. Emission Characteristics and Performance Comparison of Polyfluorene Lasers with One- and Two-Dimensional Distributed Feedback. Adv. Funct. Mater. 2004, 14, 91–97. [Google Scholar] [CrossRef]
- McGehee, M.D.; Díaz-García, M.A.; Hide, F.; Gupta, R.; Miller, E.K.; Moses, D.; Heeger, A.J. Semiconducting polymer distributed feedback lasers. Appl. Phys. Lett. 1998, 72, 1536–1538. [Google Scholar] [CrossRef]
- Gupta, R.; Stevenson, M.; Dogariu, A.; McGehee, M.D.; Park, J.Y.; Srdanov, V.; Heeger, A.J.; Wang, H. Low-threshold amplified spontaneous emission in blends of conjugated polymers. Appl. Phys. Lett. 1998, 73, 3492–3494. [Google Scholar] [CrossRef]
- Holzer, W.; Penzkofer, A.; Gong, S.H.; Bleyer, A.; Bradley, D.D.C. Laser Action in Poly(m-phenylenevinylene-co-2,5-dioctoxy-p-phenylenevinylene). Adv. Mater. 1996, 8, 974–978. [Google Scholar] [CrossRef]
- Stagira, S.; Zavelani-Rossi, M.; Nisoli, M.; DeSilvestri, S.; Lanzani, G.; Zenz, C.; Mataloni, P.; Leising, G. Single-mode picosecond blue laser emission from a solid conjugated polymer. Appl. Phys. Lett. 1998, 73, 2860–2862. [Google Scholar] [CrossRef]
- Haugeneder, A.; Hilmer, M.; Kallinger, C.; Perner, M.; Spirkl, W.; Lemmer, U.; Feldmann, J.; Scherf, U. Mechanism of gain narrowing in conjugated polymer thin films. Appl. Phys. B 1998, 66, 389–392. [Google Scholar] [CrossRef]
- Kallinger, C.; Hilmer, M.; Haugeneder, A.; Perner, M.; Spirkl, W.; Lemmer, U.; Feldmann, J.; Scherf, U.; Müllen, K.; Gombert, A.; et al. A Flexible Conjugated Polymer Laser. Adv. Mater. 1998, 10, 920–923. [Google Scholar] [CrossRef]
- Xia, R.; Heliotis, G.; Stavrinou, P.N.; Bradley, D.D.C. Polyfluorene distributed feedback lasers operating in the green-yellow spectral region. Appl. Phys. Lett. 2005, 87, 031104. [Google Scholar] [CrossRef]
- Campoy-Quiles, M.; Heliotis, G.; Xia, R.D.; Ariu, M.; Pintani, M.; Etchegoin, P.; Bradley, D.D.C. Ellipsometric Characterization of the Optical Constants of Polyfluorene Gain Media. Adv. Funct. Mater. 2005, 15, 925–933. [Google Scholar] [CrossRef]
- Xia, R.D.; Heliotis, G.; Hou, Y.B.; Bradley, D.D.C. Fluorene-based conjugated polymer optical gain media. Org. Electron. 2003, 4, 165–177. [Google Scholar] [CrossRef]
- Xia, R.D.; Heliotis, G.; Bradley, D.D.C. Fluorene-based polymer gain media for solid-state laser emission across the full visible spectrum. Appl. Phys. Lett. 2003, 82, 3599–3601. [Google Scholar] [CrossRef]
- Müller, G. Experimental analysis and modeling of melt growth processes. J. Cryst. Growth 2002, 237, 1628–1637. [Google Scholar] [CrossRef]
- Lan, C.W. Effects of ampoule rotation on flows and segregation in vertical Bridgman crystal growth. J. Cryst. Growth 1999, 197, 983–991. [Google Scholar] [CrossRef]
- Granlund, T.; Theander, M.; Berggren, M.; Andersson, M.; Ruzeckas, A.; Sundström, V.; Björk, G.; Granström, M.; Inganäs, O. A polythiophene microcavity laser. Chem. Phys. Lett. 1998, 288, 879–884. [Google Scholar] [CrossRef]
- Theander, M.; Granlund, T.; Johanson, D.M.; Ruseckas, A.; Sundström, V.; Andersson, M.R.; Inganäs, O. Lasing in a Microcavity with an Oriented Liquid-Crystalline Polyfluorene Copolymer as Active Layer. Adv. Mater. 2001, 13, 323–327. [Google Scholar] [CrossRef]
- Scherf, U.; Riechel, S.; Lemmer, U.; Mahrt, R.F. Conjugated polymers: Lasing and stimulated emission. Curr. Opin. Solid State Mater. Sci. 2001, 5, 143–154. [Google Scholar] [CrossRef]
- Yang, Y.; Turnbull, G.A.; Samuel, I.D.W. Hybrid optoelectronics: A polymer laser pumped by a nitride light-emitting diode. Appl. Phys. Lett. 2008, 92, 163306. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, H.; Hu, C.; Chen, T.; Hu, D.; Zhang, M.; Xie, K. Advances in Conjugated Polymer Lasers. Polymers 2019, 11, 443. https://doi.org/10.3390/polym11030443
Xia H, Hu C, Chen T, Hu D, Zhang M, Xie K. Advances in Conjugated Polymer Lasers. Polymers. 2019; 11(3):443. https://doi.org/10.3390/polym11030443
Chicago/Turabian StyleXia, Hongyan, Chang Hu, Tingkuo Chen, Dan Hu, Muru Zhang, and Kang Xie. 2019. "Advances in Conjugated Polymer Lasers" Polymers 11, no. 3: 443. https://doi.org/10.3390/polym11030443
APA StyleXia, H., Hu, C., Chen, T., Hu, D., Zhang, M., & Xie, K. (2019). Advances in Conjugated Polymer Lasers. Polymers, 11(3), 443. https://doi.org/10.3390/polym11030443



