Gelatin Films Modified with Acidic and Polyelectrolyte Polymers—Material Selection for Soft Gastroresistant Capsules
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Film Composition and Preparation
2.3. Visual Evaluation and Disintegration Test
2.4. Rheology
2.5. Light Absorbance and Transparency
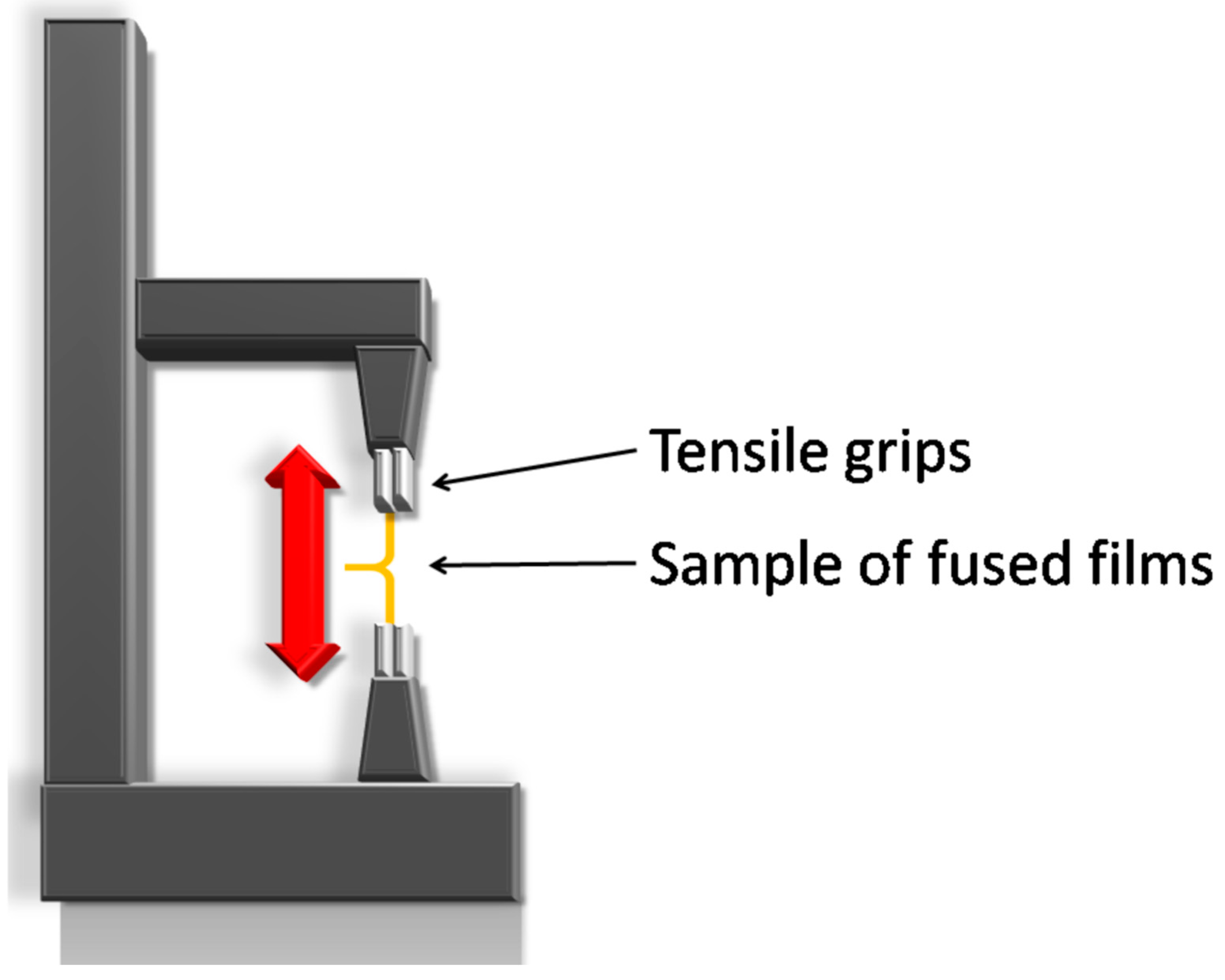
2.6. Texture Analysis
2.7. Self-Adhesiveness Test
2.8. Differential Scanning Calorimetry (DSC)
3. Results
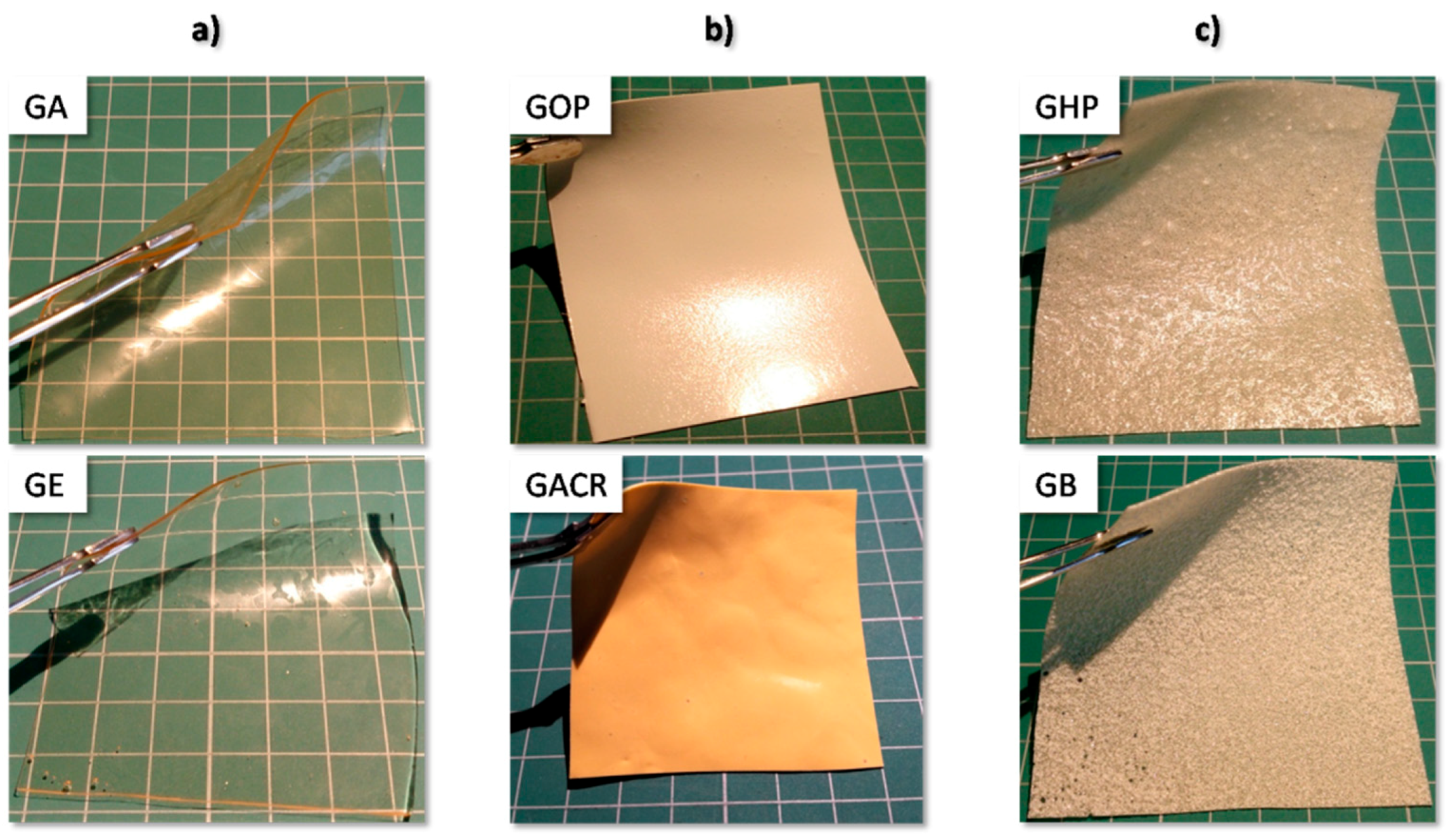
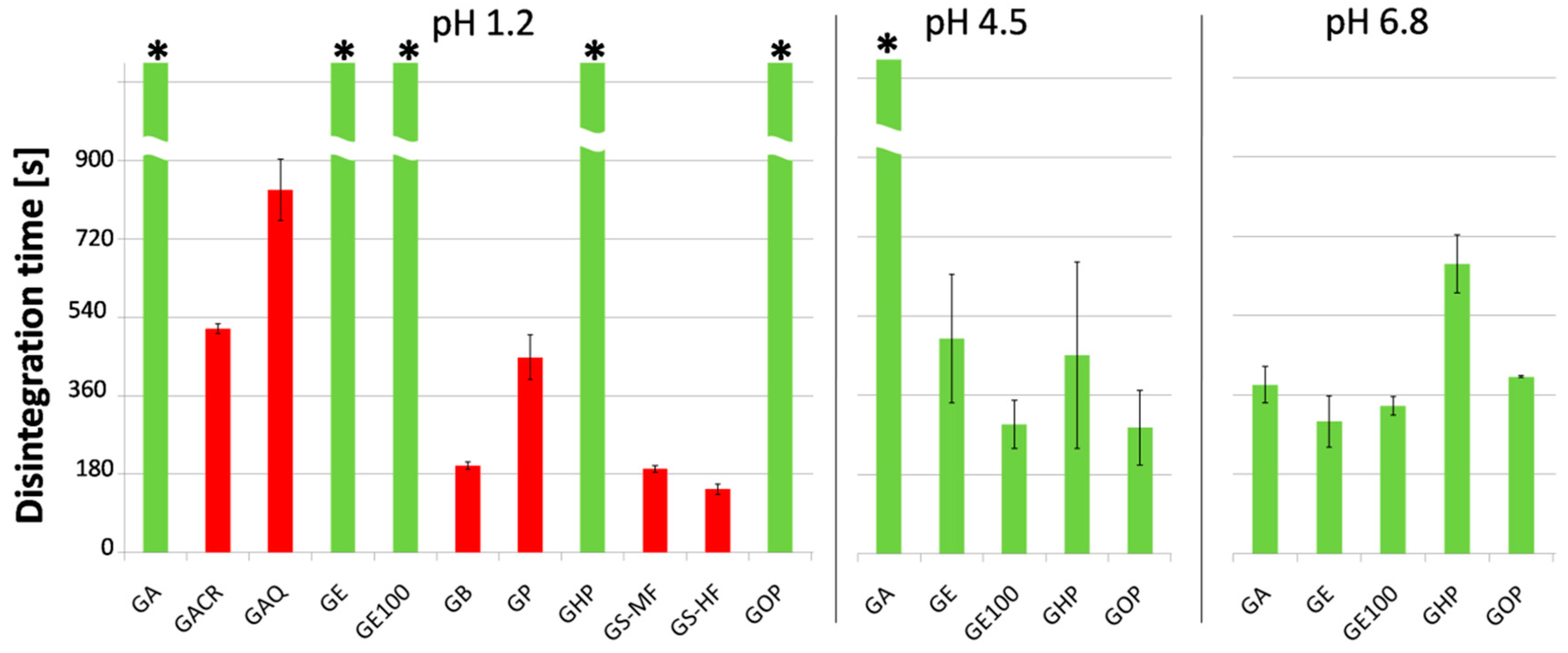
3.1. Visual Evaluation and Disintegration Test
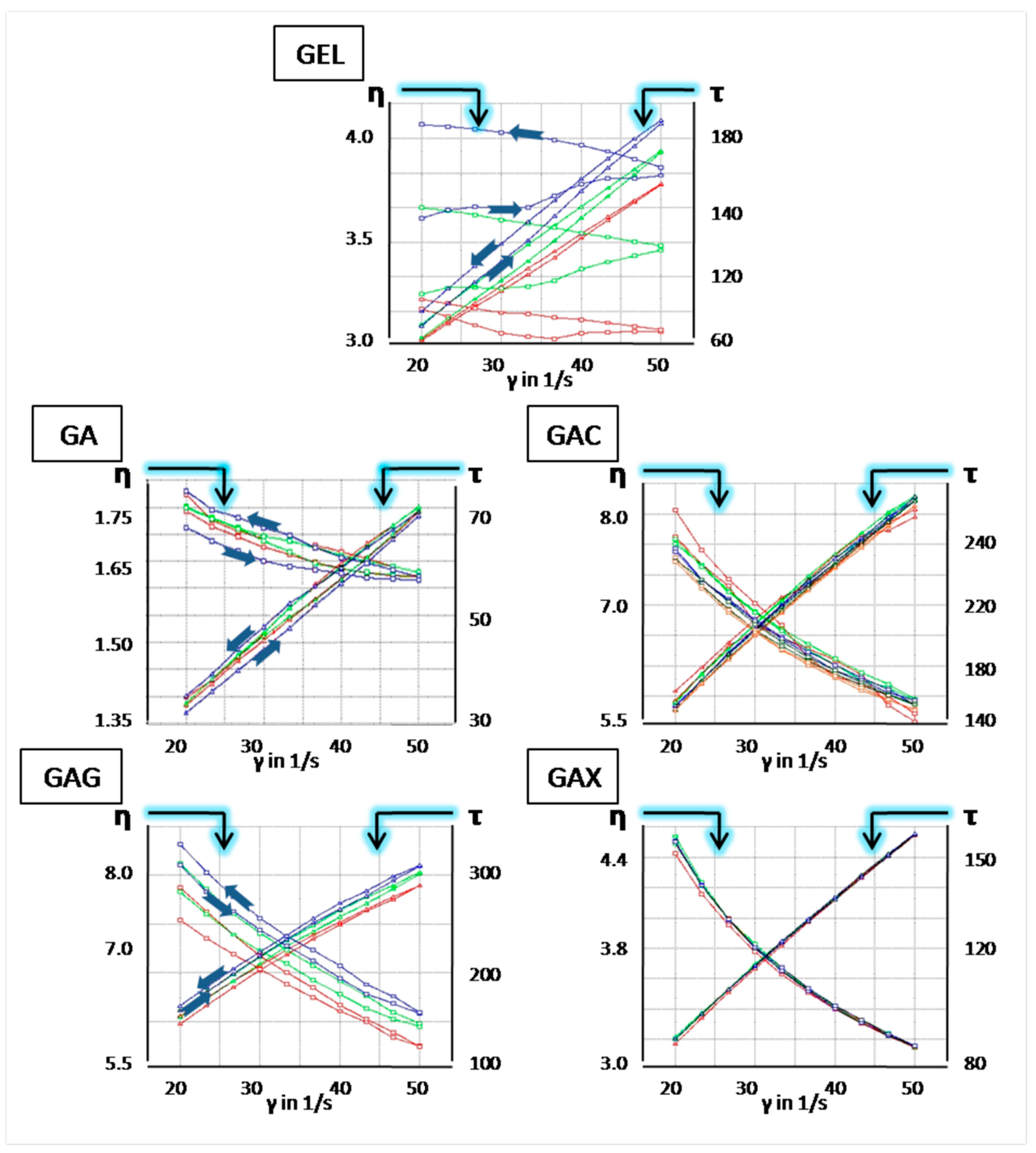
3.2. Rheology
3.3. Differential Scanning Calorimetry
3.4. Physical Properties of Casted Enteric GA Films
3.4.1. Visual, Optic and Mechanical Properties
3.4.2. Self-Adhesiveness Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rowe, R.; Sheskey, P.; Quinn, M. Handbook of Pharmaceutical Excipients, 6th ed.; Pharmaceutical Press: London, UK, 2009; pp. 549–553. [Google Scholar] [CrossRef]
- Dixit, R.P.; Puthli, S.P. Oral strip technology: Overview and future potential. J. Control. Release 2009, 139, 94–107. [Google Scholar] [CrossRef] [PubMed]
- Joshi, N.; Rawat, K.; Bohidar, H.B. pH and ionic strength induced complex coacervation of Pectin and Gelatin A. Food Hydrocoll. 2018, 74, 132–138. [Google Scholar] [CrossRef]
- Biscarat, J.; Galea, B.; Sanchez, J.; Pochat-Bohatier, C. Effect of chemical cross-linking on gelatin membrane solubility with a non-toxic and non-volatile agent: Terephthalaldehyde. Int. J. Biol. Macromol. 2015, 74, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Reich, G. Formulation and physical properties of soft capsules. In Pharmaceutical Capsules; Podczeck, F., Jones, B., Eds.; Pharmaceutical Press: London, UK, 2004; pp. 201–212. [Google Scholar]
- Bigi, A.; Cojazzi, G.; Panzavolta, S.; Roveri, N.; Rubini, K. Stabilization of gelatin films by crosslinking with genipin. Biomaterials 2002, 23, 4827–4832. [Google Scholar] [CrossRef]
- Boanini, E.; Rubini, K.; Panzavolta, S.; Bigi, A. Chemico-physical characterization of gelatin films modified with oxidized alginate. Acta Biomater. 2010, 6, 383–388. [Google Scholar] [CrossRef] [PubMed]
- da Silva, M.A.; Bode, F.; Grillo, I.; Dreiss, C.A. Exploring the kinetics of gelation and final architecture of enzymatically cross-linked chitosan/gelatin gels. Biomacromolecules 2015, 16, 1401–1409. [Google Scholar] [CrossRef] [PubMed]
- Felton, L.A.; Haase, M.M.; Shah, N.H.; Zhang, G.; Infeld, M.H.; Malick, A.W.; Mcginity, J.W. Physical and enteric properties of soft gelatin capsules coated with Eutragit L30 D-55. Int. J. Pharm. 1995, 113, 17–24. [Google Scholar] [CrossRef]
- Hassan, E.M.; Fatmi, A.A.; Chidambaram, N. Enteric composition for the manufacture of soft capsule wall. Patent no. US 8685445, 1 April 2014. [Google Scholar]
- Maciejewski, B.; Weitschies, W.; Schneider, F.; Sznitowska, M. Gastroresistant gelatin films prepared by addition of cellulose acetate phthalate. Pharmazie 2017, 72, 324–328. [Google Scholar]
- Maciejewski, B.; Ström, A.; Larsson, A.; Sznitowska, M. Soft Gelatin Films Modified with Cellulose Acetate Phthalate Pseudolatex Dispersion—Structure and Permeability. Polymers 2018, 10, 981. [Google Scholar] [CrossRef]
- Evans, M.; Ratcliffe, I.; Williams, P.A. Emulsion stabilisation using polysaccharide-protein complexes. Curr. Opin. Colloid Interface Sci. 2013, 18, 272–282. [Google Scholar] [CrossRef]
- Anvari, M.; Pan, C.-H.; Yoon, W.-B.; Chung, D. Characterization of fish gelatin–gum arabic complex coacervates as influenced by phase separation temperature. Int. J. Biol. Macromol. 2015, 79, 894–902. [Google Scholar] [CrossRef] [PubMed]
- Manna, P.J.; Mitra, T.; Pramanik, N.; Kavitha, V.; Gnanamani, A.; Kundu, P.P. Potential use of curcumin loaded carboxymethylated guar gum grafted gelatin film for biomedical applications. Int. J. Biol. Macromol. 2015, 75, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.J.; Tanna, S.; Sahota, T. In vivo study of a polymeric glucose-sensitive insulin delivery system using a rat model. J. Pharm. Sci. 2010, 99, 4215–4227. [Google Scholar] [CrossRef] [PubMed]
- Gautam, S.; Chou, C.F.; Dinda, A.K.; Potdar, P.D.; Mishra, N.C. Surface modification of nanofibrous polycaprolactone/gelatin composite scaffold by collagen type I grafting for skin tissue engineering. Mater. Sci. Eng. C 2014, 34, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Han, J.H.; Floros, J.D. Casting Antimicrobial Packaging Films and Measuring Their Physical Properties and Antimicrobial Activity. J. Plast. Film Sheeting 1997, 13, 287–298. [Google Scholar] [CrossRef]
- Koziolek, M.; Garbacz, G.; Neumann, M.; Weitschies, W. Simulating the postprandial stomach: Physiological considerations for dissolution and release testing. Mol. Pharmaceutics 2013, 10, 1610–1622. [Google Scholar] [CrossRef] [PubMed]
- Lau, M.; Tang, J.; Paulson, A. Texture profile and turbidity of gellan/gelatin mixed gels. Food Res. Int. 2000, 33, 665–671. [Google Scholar] [CrossRef]
- Wang, C.S.; Virgilio, N.; Wood-Adams, P.; Heuzey, M.C. A mechanism for the synergistic gelation properties of gelatin B and xanthan gum aqueous mixtures. Carbohydr. Polym. 2017, 175, 484–492. [Google Scholar] [CrossRef]
- Michon, C.; Cuvelier, G.; Launay, B.; Parker, A. Viscoelastic properties of iota-carrageenan/gelatin mixtures. Carbohydr. Polym. 1996, 31, 161–169. [Google Scholar] [CrossRef]
- Fonkwe, L.G.; Narsimhan, G.; Cha, A.S. Characterization of gelation time and texture of gelatin and gelatin–polysaccharide mixed gels. Food Hydrocoll. 2003, 17, 871–883. [Google Scholar] [CrossRef]
- Pranoto, Y.; Lee, C.M.; Park, H.J. Characterizations of fish gelatin films added with gellan and kappa-carrageenan. LWT Food Sci. Technol. 2007, 40, 766–774. [Google Scholar] [CrossRef]
- Turgeon, S.L.; Beaulieu, M.; Schmitt, C.; Sanchez, C. Protein-polysaccharide interactions: Phase-ordering kinetics, thermodynamic and structural aspects. Curr. Opin. Colloid Interface Sci. 2003, 8, 401–414. [Google Scholar] [CrossRef]
- Schmitt, C.; Turgeon, S.L. Protein/polysaccharide complexes and coacervates in food systems. Adv. Colloid Interface Sci. 2011, 167, 63–70. [Google Scholar] [CrossRef] [PubMed]
- De Kruif, C.G.; Tuinier, R. Polysaccharide protein interactions. Food Hydrocoll. 2001, 15, 555–563. [Google Scholar] [CrossRef]
- Tromp, R.H.; Van de Velde, F.; Van Riel, J.; Paques, M. Confocal scanning light microscopy (CSLM) on mixtures of gelatine and polysaccharides. Food Res. Int. 2001, 34, 931–938. [Google Scholar] [CrossRef]
- Lee, K.Y.; Shim, J.; Lee, H.G. Mechanical properties of gellan and gelatin composite films. Carbohydr. Polym. 2004, 56, 251–254. [Google Scholar] [CrossRef]
- Rando, R.F.; Obara, S.; Osterling, M.C.; Mankowski, M.; Miller, S.R.; Ferguson, M.L.; Krebs, F.C.; Wigdahl, B.; Labib, M.; Kokubo, H. Critical Design Features of Phenyl Carboxylate-Containing Polymer Microbicides. Antimicrob. Agents Chemother. 2006, 50, 3081–3089. [Google Scholar] [CrossRef]

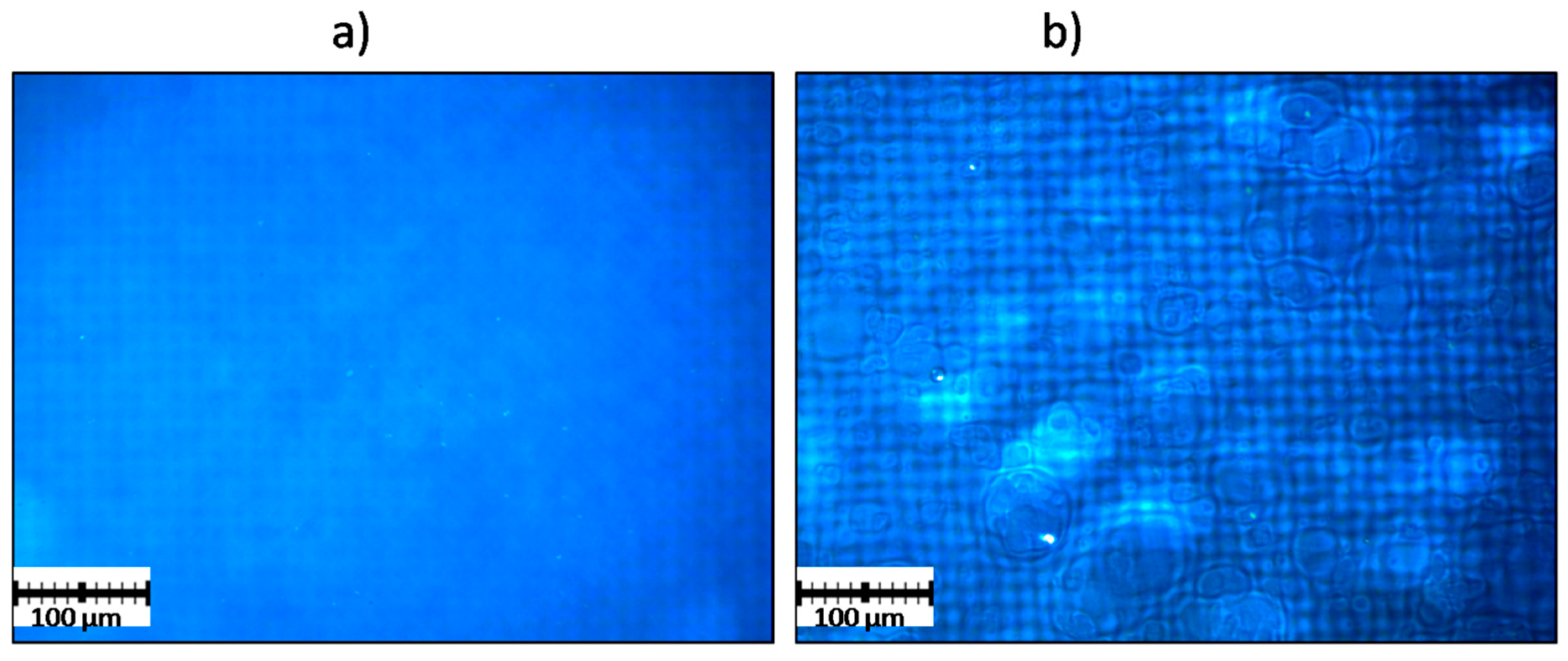
| Symbol | Acid-Resistant Ingredients | Comments | Appearance/Structure | Disintegration (+/-) | ||
|---|---|---|---|---|---|---|
| pH 1.2 | pH 4.5 | pH 6.8 | ||||
| GEL | - | Reference non-modified film | Clear, transparent | + | + | + |
| GA | Aquacoat CPD (Cellulose acetate phthalate (CAP)) | Additives in Aquacoat CPD dispersion:poloxamer | Clear, slightly turbid, transparent | - | - | + |
| GE | Eudragit L30 D-55 (methacrylic acid copolymer) | Additives in Eudragit L30 D-55 dispersion: SLS, Polysorbate 80 | Clear, transparent | - | + | + |
| GE100 | Eudragit L100-55 (methacrylic acid copolymer) | Additives in Eudragit L100 powder: SLS, Polysorbate 80 | Clear, transparent | - | + | + |
| GACR | Acryl Eze II (methacrylic acid copolymer) | Additives in Acryl Eze II powder: SLS, talc, TiO2 poloxamer, calcium silicate, sodium carbonate | Homogeneous, colored, opalescent | + | n/a | n/a |
| GAQ | Aquarius Control ENA (methacrylic acid copolymer) | Additives in Aquarius Control ENA powder: TEC, talc, TiO2, colloidal silica | Homogeneous, colored, opalescent | + | n/a | n/a |
| GOP | Opadry Enteric (Polyvinyl acetate phthalate (PVAP)) | Additives in Opadry Enteric powder: TiO2, TEC, stearic acid | Homogeneous, colored, opalescent | - | + | + |
| GHP | Hypromellose phthalate (HPMCP) | Powder, low solution viscosity, Tg ca. 145 °C | Heterogeneous, opalescent, particles present | - | + | + |
| GB | Cellulose acetate butyrate (CAB) | Powder, medium solution viscosity, Tg ca. 130 °C | Heterogeneous, opalescent, particles present | + | n/a | n/a |
| GP | Cellulose acetate propionate (CP) | Powder, medium solution viscosity, Tg ca. 142 °C | Heterogeneous, opalescent, particles present | + | n/a | n/a |
| GS-MF GS-HF | Hypromellose acetate succinate (HPMCAS) | Powder, two grades tested: medium-acetyl (MF) and high-acetyl (HF), Tg 130–135 °C | Heterogeneous, opalescent, particles present | + | n/a | n/a |
| Symbol | Gelling System | Acid-Resistant Ingredients | Appearance/Structure | Disintegration (+/-) | ||
|---|---|---|---|---|---|---|
| pH 1.2 | pH 4.5 | pH 6.8 | ||||
| GAC | Gelatin/ί-carrageenan | Aquacoat CPD (CAP) | Homogeneous, slightly turbid, transparent | - | - | + |
| GAG | Gelatin/gellan | Aquacoat CPD (CAP) | Slightly turbid, graininess visible in the structure | - | - | + |
| GAX | Gelatin/xanthan | Aquacoat CPD (CAP) | Slightly turbid, homogeneous, transparent | - | - | + |
| Film Composition | Transmittance (600 nm) | Mean Thickness (mm) | Transparency |
|---|---|---|---|
| GEL | 0.8702 | 0.495 | −0.122 |
| GA | 0.8230 | 0.500 | −0.169 |
| GAC | 0.6765 | 0.415 | −0.409 |
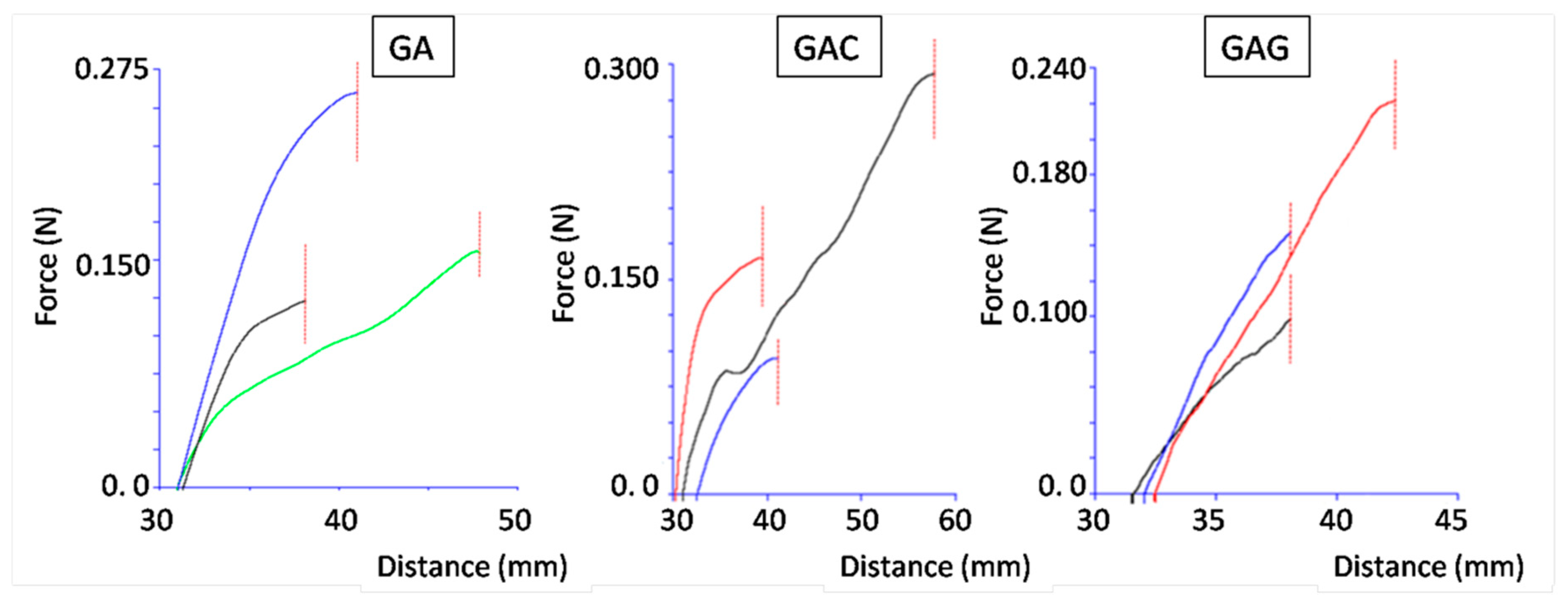
| Film Composition | Moisture Content (% w/w) | Thickness (µm) | Force at Break (N) | Tensile Strength (kPa) | Elongation at Break (%) | Young’s Modulus (MPa) |
|---|---|---|---|---|---|---|
| GEL | 2.3 ± 0.5 | 788 ± 44.6 | >50 * | n/a * | n/a * | 310.7 ± 61.0 |
| GA | 2.8 ± 0.2 | 639 ± 17.7 | 23.5 ± 2.8 | 7.36 ± 0.87 | 200% ± 10% | 98.6 ± 16.5 |
| GAC | 2.8 ± 0.4 | 697 ± 33.0 | 20.2 ± 1.1 | 5.80 ± 0.32 | 226% ± 8% | 51.4 ± 5.2 |
| GAG | 4.9 ± 0.2 | 704 ± 30.4 | 14.7 ± 1.3 | 4.16 ± 0.38 | 211% ± 7% | 45.1 ± 8.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maciejewski, B.; Sznitowska, M. Gelatin Films Modified with Acidic and Polyelectrolyte Polymers—Material Selection for Soft Gastroresistant Capsules. Polymers 2019, 11, 338. https://doi.org/10.3390/polym11020338
Maciejewski B, Sznitowska M. Gelatin Films Modified with Acidic and Polyelectrolyte Polymers—Material Selection for Soft Gastroresistant Capsules. Polymers. 2019; 11(2):338. https://doi.org/10.3390/polym11020338
Chicago/Turabian StyleMaciejewski, Bartosz, and Małgorzata Sznitowska. 2019. "Gelatin Films Modified with Acidic and Polyelectrolyte Polymers—Material Selection for Soft Gastroresistant Capsules" Polymers 11, no. 2: 338. https://doi.org/10.3390/polym11020338
APA StyleMaciejewski, B., & Sznitowska, M. (2019). Gelatin Films Modified with Acidic and Polyelectrolyte Polymers—Material Selection for Soft Gastroresistant Capsules. Polymers, 11(2), 338. https://doi.org/10.3390/polym11020338






