Macro- and Microphase Separated Protein-Polyelectrolyte Complexes: Design Parameters and Current Progress
Abstract
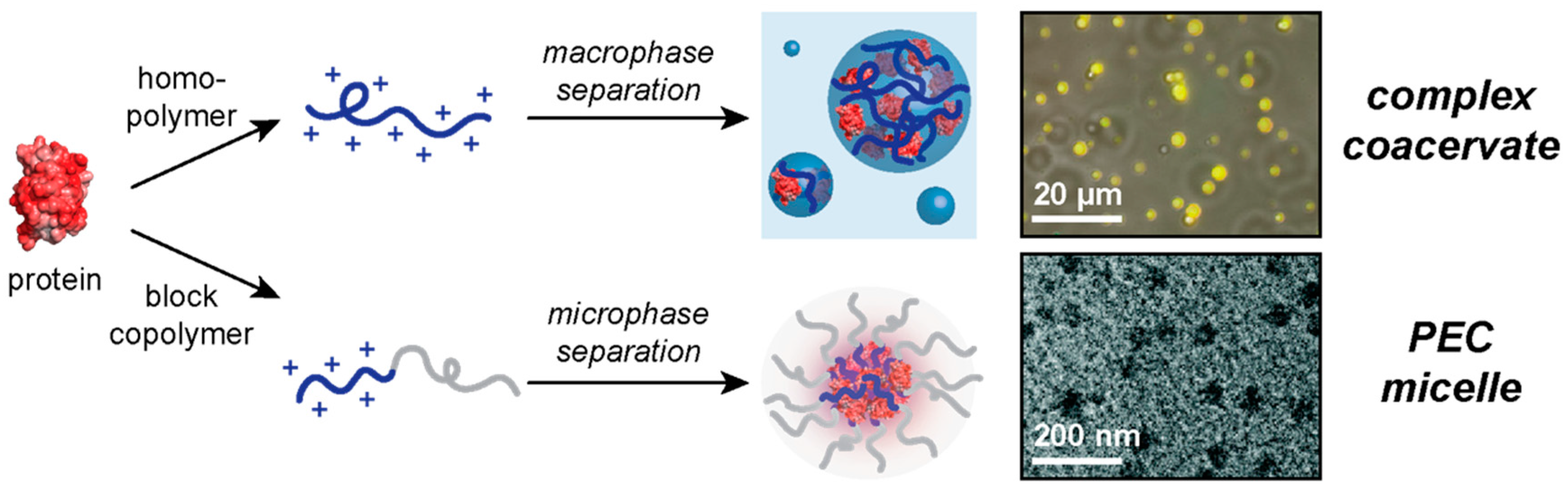
1. Introduction
2. Macrophase Separation of Protein–Polyelectrolyte Complexes
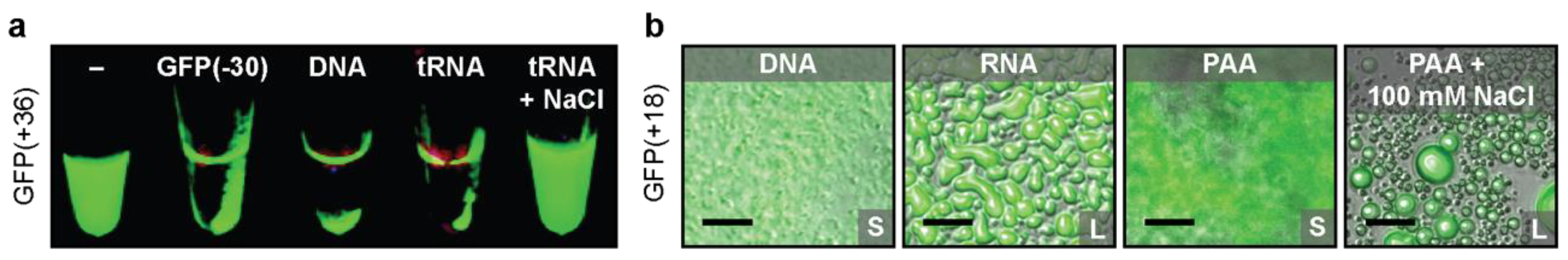
2.1. Protein Design Parameters
2.2. Polymer Design Parameters
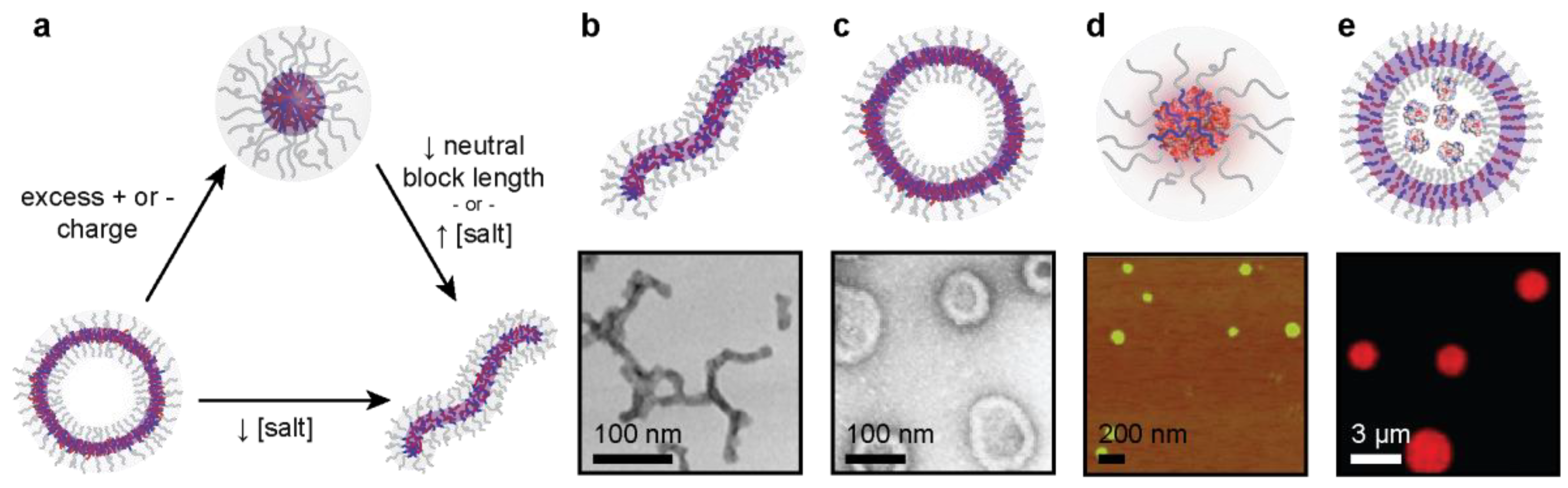
3. Microphase Separation of Protein–Polyelectrolyte Complexes
3.1. Polymer Design Parameters
3.2. Protein Design Parameters
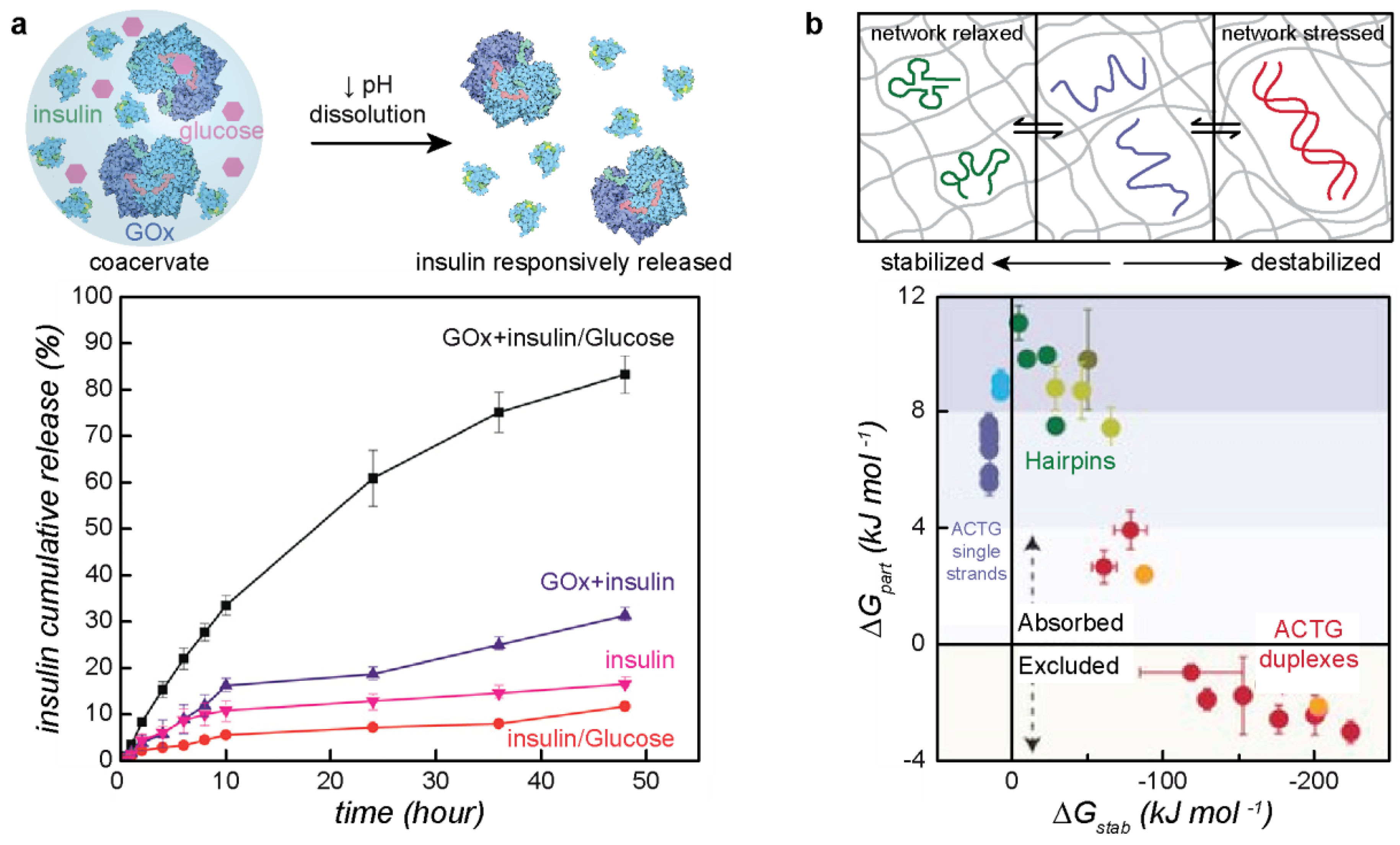
4. Recent Development of Applications and Characterization Techniques
4.1. Applications of Protein–Polyelectrolyte Complexes
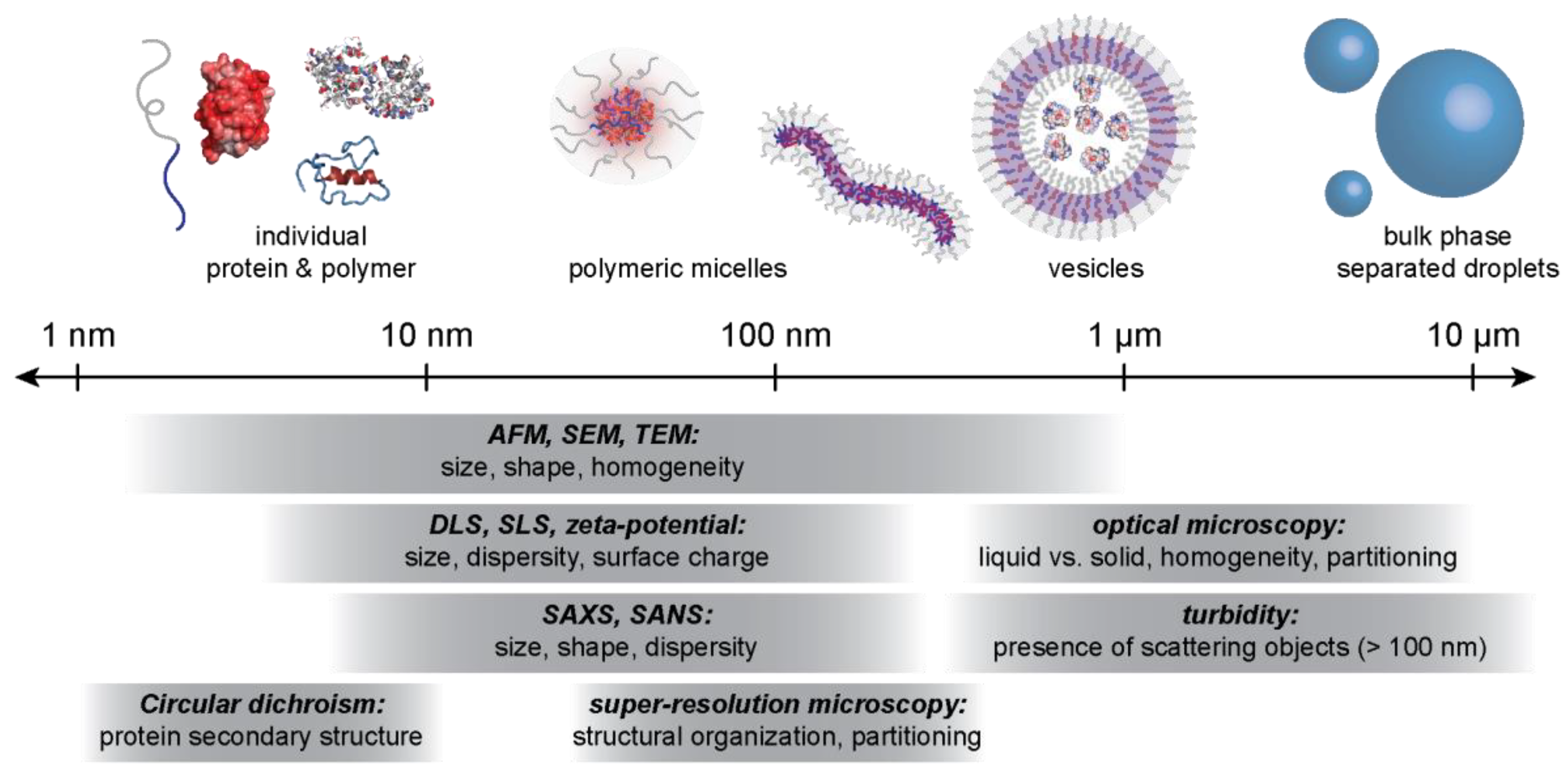
4.2. Characterization Techniques for Macro- and Microphase Separated Protein–Polyelectrolyte Complexes
5. Conclusions and Outlook
Funding
Conflicts of Interest
References
- de Jong Bungenberg, H.G.; Kruyt, H.R. Chemistry—Coacervation (Partial miscibility in colloid systems). Proc. K. Ned. Akad. Wet. 1929, 32, 849–856. [Google Scholar]
- Overbeek, J.T.G.; Voorn, M.J. Phase separation in polyelectrolyte solutions. Theory of complex coacervation. J. Cell. Comp. Physiol. 1957, 49, 7–26. [Google Scholar] [CrossRef]
- Armstrong, J.P.K.; Olof, S.N.; Jakimowicz, M.D.; Hollander, A.P.; Mann, S.; Davis, S.A.; Miles, M.J.; Patil, A.J.; Perriman, A.W. Cell paintballing using optically targeted coacervate microdroplets. Chem. Sci. 2015, 6, 6106–6111. [Google Scholar] [CrossRef] [PubMed]
- Santiago, L.G.; Castro, G.R. Novel technologies for the encapsulation of bioactive food compounds. Curr. Opin. Food Sci. 2016, 7, 78–85. [Google Scholar] [CrossRef]
- Eghbal, N.; Choudhary, R. Complex coacervation: Encapsulation and controlled release of active agents in food systems. LWT 2018, 90, 254–264. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Pei, Y.; Xiong, W.; Zhang, C.; Xu, W.; Liu, S.; Li, B. Curcumin encapsulated in the complex of lysozyme/carboxymethylcellulose and implications for the antioxidant activity of curcumin. Food Res. Int. 2015, 75, 98–105. [Google Scholar] [CrossRef]
- Anvari, M.; Chung, D. Dynamic rheological and structural characterization of fish gelatin – Gum arabic coacervate gels cross-linked by tannic acid. Food Hydrocoll. 2016, 60, 516–524. [Google Scholar] [CrossRef]
- Huang, G.-Q.; Du, Y.-L.; Xiao, J.-X.; Wang, G.-Y. Effect of coacervation conditions on the viscoelastic properties of N,O-carboxymethyl chitosan—Gum Arabic coacervates. Food Chem. 2017, 228, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Aumiller, W.M.; Keating, C.D. Experimental models for dynamic compartmentalization of biomolecules in liquid organelles: Reversible formation and partitioning in aqueous biphasic systems. Adv. Colloid Interface Sci. 2017, 239, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Niu, L.; Zhu, X.; Zhao, M.; Zhang, Z.; Mann, S.; Liang, D. Non-equilibrium behaviour in coacervate-based protocells under electric-field-induced excitation. Nat. Commun. 2016, 7, 10658. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, S.I.; Collins, C.M.; Khaledi, M.G. Perfluorinated Alcohols Induce Complex Coacervation in Mixed Surfactants. Langmuir 2016, 32, 2321–2330. [Google Scholar] [CrossRef]
- Valetti, N.W.; Brassesco, M.E.; Picó, G.A. Polyelectrolytes–protein complexes: A viable platform in the downstream processes of industrial enzymes at scaling up level. J. Chem. Technol. Biotechnol. 2016, 91, 2921–2928. [Google Scholar] [CrossRef]
- Kaushik, P.; Dowling, K.; Barrow, C.J.; Adhikari, B. Complex coacervation between flaxseed protein isolate and flaxseed gum. Food Res. Int. 2015, 72, 91–97. [Google Scholar] [CrossRef]
- Huang, G.-Q.; Liu, L.-N.; Han, X.-N.; Xiao, J.-X. Intestine-targeted delivery potency of the O-carboxymethyl chitosan–gum Arabic coacervate: Effects of coacervation acidity and possible mechanism. Mater. Sci. Eng. C 2017, 79, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Min, K.A.; Cho, J.-H.; Song, Y.-K.; Kim, C.-K. Iron casein succinylate-chitosan coacervate for the liquid oral delivery of iron with bioavailability and stability enhancement. Arch. Pharm. Res. 2016, 39, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Pathak, J.; Rawat, K.; Aswal, V.K.; Bohidar, H.B. Interactions in globular proteins with polyampholyte: Coacervation route for protein separation. RSC Adv. 2015, 5, 13579–13589. [Google Scholar] [CrossRef]
- Harada, A.; Kataoka, K. Formation of Polyion Complex Micelles in an Aqueous Milieu from a Pair of Oppositely-Charged Block Copolymers with Poly(ethylene glycol) Segments. Macromolecules 1995, 28, 5294–5299. [Google Scholar] [CrossRef]
- Spruijt, E.; Sprakel, J.; Stuart, M.A.C.; Gucht, J. van der Interfacial tension between a complex coacervate phase and its coexisting aqueous phase. Soft Matter 2010, 6, 172–178. [Google Scholar] [CrossRef]
- Qin, J.; Priftis, D.; Farina, R.; Perry, S.L.; Leon, L.; Whitmer, J.; Hoffmann, K.; Tirrell, M.; de Pablo, J.J. Interfacial Tension of Polyelectrolyte Complex Coacervate Phases. ACS Macro Lett. 2014, 3, 565–568. [Google Scholar] [CrossRef]
- Priftis, D.; Farina, R.; Tirrell, M. Interfacial Energy of Polypeptide Complex Coacervates Measured via Capillary Adhesion. Langmuir 2012, 28, 8721–8729. [Google Scholar] [CrossRef]
- Huang, K.-Y.; Yoo, H.Y.; Jho, Y.; Han, S.; Hwang, D.S. Bicontinuous Fluid Structure with Low Cohesive Energy: Molecular Basis for Exceptionally Low Interfacial Tension of Complex Coacervate Fluids. ACS Nano 2016, 10, 5051–5062. [Google Scholar] [CrossRef]
- Li, L.; Srivastava, S.; Andreev, M.; Marciel, A.B.; de Pablo, J.J.; Tirrell, M.V. Phase Behavior and Salt Partitioning in Polyelectrolyte Complex Coacervates. Macromolecules 2018, 51, 2988–2995. [Google Scholar] [CrossRef]
- Priftis, D.; Tirrell, M. Phase behaviour and complex coacervation of aqueous polypeptide solutions. Soft Matter 2012, 8, 9396–9405. [Google Scholar] [CrossRef]
- Harada, A.; Kataoka, K. Novel Polyion Complex Micelles Entrapping Enzyme Molecules in the Core: Preparation of Narrowly-Distributed Micelles from Lysozyme and Poly(ethylene glycol)−Poly(aspartic acid) Block Copolymer in Aqueous Medium. Macromolecules 1998, 31, 288–294. [Google Scholar] [CrossRef]
- Harada, A.; Kataoka, K. Novel Polyion Complex Micelles Entrapping Enzyme Molecules in the Core. 2. Characterization of the Micelles Prepared at Nonstoichiometric Mixing Ratios. Langmuir 1999, 15, 4208–4212. [Google Scholar] [CrossRef]
- Lee, Y.; Fukushima, S.; Bae, Y.; Hiki, S.; Ishii, T.; Kataoka, K. A Protein Nanocarrier from Charge-Conversion Polymer in Response to Endosomal pH. J. Am. Chem. Soc. 2007, 129, 5362–5363. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Ishii, T.; Cabral, H.; Kim, H.J.; Seo, J.-H.; Nishiyama, N.; Oshima, H.; Osada, K.; Kataoka, K. Charge-Conversional Polyionic Complex Micelles—Efficient Nanocarriers for Protein Delivery into Cytoplasm. Angew. Chem. Int. Ed. 2009, 48, 5309–5312. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.H.; Park, M.J.; Li, Y.; Im, G.H.; Kim, J.-H.; Kim, H.N.; Lee, J.W.; Jeon, P.; Bang, O.Y.; Lee, J.H.; et al. The use of pH-sensitive positively charged polymeric micelles for protein delivery. Biomaterials 2012, 33, 9157–9164. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.-H.; Leon, L.; Chung, E.J.; Huang, R.-T.; Sontag, T.J.; Reardon, C.A.; Getz, G.S.; Tirrell, M.; Fang, Y. Inhibition of atherosclerosis-promoting microRNAs via targeted polyelectrolyte complex micelles. J. Mater. Chem. B Mater. Biol. Med. 2014, 2, 8142–8153. [Google Scholar] [CrossRef]
- Kim, A.; Miura, Y.; Ishii, T.; Mutaf, O.F.; Nishiyama, N.; Cabral, H.; Kataoka, K. Intracellular Delivery of Charge-Converted Monoclonal Antibodies by Combinatorial Design of Block/Homo Polyion Complex Micelles. Biomacromolecules 2016, 17, 446–453. [Google Scholar] [CrossRef]
- Min, H.S.; Kim, H.J.; Ahn, J.; Naito, M.; Hayashi, K.; Toh, K.; Kim, B.S.; Matsumura, Y.; Kwon, I.C.; Miyata, K.; et al. Tuned Density of Anti-Tissue Factor Antibody Fragment onto siRNA-Loaded Polyion Complex Micelles for Optimizing Targetability into Pancreatic Cancer Cells. Biomacromolecules 2018, 19, 2320–2329. [Google Scholar] [CrossRef]
- Chuanoi, S.; Anraku, Y.; Hori, M.; Kishimura, A.; Kataoka, K. Fabrication of Polyion Complex Vesicles with Enhanced Salt and Temperature Resistance and Their Potential Applications as Enzymatic Nanoreactors. Biomacromolecules 2014, 15, 2389–2397. [Google Scholar] [CrossRef] [PubMed]
- Jaturanpinyo, M.; Harada, A.; Yuan, X.; Kataoka, K. Preparation of Bionanoreactor Based on Core−Shell Structured Polyion Complex Micelles Entrapping Trypsin in the Core Cross-Linked with Glutaraldehyde. Bioconj. Chem. 2004, 15, 344–348. [Google Scholar] [CrossRef]
- Lim, Z.W.; Ping, Y.; Miserez, A. Glucose-Responsive Peptide Coacervates with High Encapsulation Efficiency for Controlled Release of Insulin. Bioconj. Chem. 2018, 29, 2176–2180. [Google Scholar] [CrossRef] [PubMed]
- Obermeyer, A.C.; Mills, C.E.; Dong, X.-H.; Flores, R.J.; Olsen, B.D. Complex coacervation of supercharged proteins with polyelectrolytes. Soft Matter 2016, 12, 3570–3581. [Google Scholar] [CrossRef]
- Lindhoud, S.; Claessens, M.M.A.E. Accumulation of small protein molecules in a macroscopic complex coacervate. Soft Matter 2015, 12, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Zacharia, N.S. Protein encapsulation via polyelectrolyte complex coacervation: Protection against protein denaturation. J. Chem. Phys. 2018, 149, 163326. [Google Scholar] [CrossRef]
- Black, K.A.; Priftis, D.; Perry, S.L.; Yip, J.; Byun, W.Y.; Tirrell, M. Protein Encapsulation via Polypeptide Complex Coacervation. ACS Macro Lett. 2014, 3, 1088–1091. [Google Scholar] [CrossRef]
- Marciel, A.B.; Chung, E.J.; Brettmann, B.K.; Leon, L. Bulk and nanoscale polypeptide based polyelectrolyte complexes. Adv. Colloid Interface Sci. 2017, 239, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Comert, F.; Malanowski, A.J.; Azarikia, F.; Dubin, P.L. Coacervation and precipitation in polysaccharide–protein systems. Soft Matter 2016, 12, 4154–4161. [Google Scholar] [CrossRef] [PubMed]
- Cummings, C.S.; Obermeyer, A.C. Phase Separation Behavior of Supercharged Proteins and Polyelectrolytes. Biochemistry 2018, 57, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, M.S.; Phillips, K.J.; Liu, D.R. Supercharging Proteins Can Impart Unusual Resilience. J. Am. Chem. Soc. 2007, 129, 10110–10112. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.-W.; Lytle, T.K.; Radhakrishna, M.; Madinya, J.J.; Vélez, J.; Sing, C.E.; Perry, S.L. Sequence and entropy-based control of complex coacervates. Nat. Commun. 2017, 8, 1273. [Google Scholar] [CrossRef]
- Xu, Y.; Mazzawi, M.; Chen, K.; Sun, L.; Dubin, P.L. Protein Purification by Polyelectrolyte Coacervation: Influence of Protein Charge Anisotropy on Selectivity. Biomacromolecules 2011, 12, 1512–1522. [Google Scholar] [CrossRef] [PubMed]
- Comert, F.; Xu, A.Y.; Madro, S.P.; Liadinskaia, V.; Dubin, P.L. The so-called critical condition for polyelectrolyte-colloid complex formation. J. Chem. Phys. 2018, 149, 163321. [Google Scholar] [CrossRef]
- Flanagan, S.E.; Malanowski, A.J.; Kizilay, E.; Seeman, D.; Dubin, P.L.; Donato-Capel, L.; Bovetto, L.; Schmitt, C. Complex Equilibria, Speciation, and Heteroprotein Coacervation of Lactoferrin and β-Lactoglobulin. Langmuir 2015, 31, 1776–1783. [Google Scholar] [CrossRef]
- Krishnan, Y.; Rees, H.A.; Rossitto, C.P.; Kim, S.-E.; Hung, H.-H.K.; Frank, E.H.; Olsen, B.D.; Liu, D.R.; Hammond, P.T.; Grodzinsky, A.J. Green fluorescent proteins engineered for cartilage-targeted drug delivery: Insights for transport into highly charged avascular tissues. Biomaterials 2018, 183, 218–233. [Google Scholar] [CrossRef]
- Pathak, J.; Rawat, K.; Bohidar, H.B. Charge heterogeneity induced binding and phase stability in β-lacto-globulin–gelatin B gels and coacervates at their common pI. RSC Adv. 2015, 5, 67066–67076. [Google Scholar] [CrossRef]
- Kapelner, R.A.; Obermeyer, A.C. Ionic polypeptide tags for protein phase separation. Chem. Sci. 2019, 10, 2700–2707. [Google Scholar] [CrossRef]
- Kim, H.J.; Yang, B.; Park, T.Y.; Lim, S.; Cha, H.J. Complex coacervates based on recombinant mussel adhesive proteins: Their characterization and applications. Soft Matter 2017, 13, 7704–7716. [Google Scholar] [CrossRef] [PubMed]
- Zai-Rose, V.; West, S.J.; Kramer, W.H.; Bishop, G.R.; Lewis, E.A.; Correia, J.J. Effects of Doxorubicin on the Liquid-Liquid Phase Change Properties of Elastin-Like Polypeptides. Biophys. J. 2018, 115, 1431–1444. [Google Scholar] [CrossRef]
- Miller, D.R.; Das, S.; Huang, K.-Y.; Han, S.; Israelachvili, J.N.; Waite, J.H. Mussel Coating Protein-Derived Complex Coacervates Mitigate Frictional Surface Damage. ACS Biomater. Sci. Eng. 2015, 1, 1121–1128. [Google Scholar] [CrossRef]
- Wei, W.; Petrone, L.; Tan, Y.; Cai, H.; Israelachvili, J.N.; Miserez, A.; Waite, J.H. An Underwater Surface-Drying Peptide Inspired by a Mussel Adhesive Protein. Adv. Funct. Mater. 2016, 26, 3496–3507. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Cabello, J.C.; González de Torre, I.; Ibañez-Fonseca, A.; Alonso, M. Bioactive scaffolds based on elastin-like materials for wound healing. Adv. Drug Deliv. Rev. 2018, 129, 118–133. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, K.; Elbert, K.C.; Balog, E.R.M.; Martinez, J.S.; Rocha, R.C. A metallo-biopolymer conjugate of elastin-like polypeptide: Photoluminescence enhancement in the coacervate microenvironment. J. Biol. Inorg. Chem. 2018, 23, 1153–1157. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Huang, J.; Lee, Y.; Dutta, S.; Yoo, H.Y.; Jung, Y.M.; Jho, Y.; Zeng, H.; Hwang, D.S. Complexation and coacervation of like-charged polyelectrolytes inspired by mussels. Proc. Natl. Acad. Sci. USA 2016, 113, E847–E853. [Google Scholar] [CrossRef] [PubMed]
- Uversky, V.N.; Kuznetsova, I.M.; Turoverov, K.K.; Zaslavsky, B. Intrinsically disordered proteins as crucial constituents of cellular aqueous two phase systems and coacervates. FEBS Lett. 2015, 589, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Elbaum-Garfinkle, S.; Kim, Y.; Szczepaniak, K.; Chen, C.C.-H.; Eckmann, C.R.; Myong, S.; Brangwynne, C.P. The disordered P granule protein LAF-1 drives phase separation into droplets with tunable viscosity and dynamics. Proc. Natl. Acad. Sci. USA 2015, 112, 7189–7194. [Google Scholar] [CrossRef]
- Zhang, H.; Elbaum-Garfinkle, S.; Langdon, E.M.; Taylor, N.; Occhipinti, P.; Bridges, A.A.; Brangwynne, C.P.; Gladfelter, A.S. RNA Controls PolyQ Protein Phase Transitions. Mol. Cell 2015, 60, 220–230. [Google Scholar] [CrossRef]
- Aumiller, W.M.; Pir Cakmak, F.; Davis, B.W.; Keating, C.D. RNA-Based Coacervates as a Model for Membraneless Organelles: Formation, Properties, and Interfacial Liposome Assembly. Langmuir 2016, 32, 10042–10053. [Google Scholar] [CrossRef]
- Uversky, V.N. Protein intrinsic disorder-based liquid–liquid phase transitions in biological systems: Complex coacervates and membrane-less organelles. Adv. Colloid Interface Sci. 2017, 239, 97–114. [Google Scholar] [CrossRef]
- Protter, D.S.W.; Rao, B.S.; Van Treeck, B.; Lin, Y.; Mizoue, L.; Rosen, M.K.; Parker, R. Intrinsically Disordered Regions Can Contribute Promiscuous Interactions to RNP Granule Assembly. Cell Rep. 2018, 22, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.L.; Watson, M.; Wilkins, O.G.; Cato, L.; Travers, A.; Thomas, J.O.; Stott, K. Highly disordered histone H1−DNA model complexes and their condensates. Proc. Natl. Acad. Sci. USA 2018, 115, 11964–11969. [Google Scholar] [CrossRef]
- Croguennec, T.; Tavares, G.M.; Bouhallab, S. Heteroprotein complex coacervation: A generic process. Adv. Colloid Interface Sci. 2017, 239, 115–126. [Google Scholar] [CrossRef]
- Chapeau, A.-L.; Bertrand, N.; Briard-Bion, V.; Hamon, P.; Poncelet, D.; Bouhallab, S. Coacervates of whey proteins to protect and improve the oral delivery of a bioactive molecule. J. Funct. Foods 2017, 38, 197–204. [Google Scholar] [CrossRef]
- Peixoto, P.D.S.; Tavares, G.M.; Croguennec, T.; Nicolas, A.; Hamon, P.; Roiland, C.; Bouhallab, S. Structure and Dynamics of Heteroprotein Coacervates. Langmuir 2016, 32, 7821–7828. [Google Scholar] [CrossRef]
- Li, P.; Banjade, S.; Cheng, H.-C.; Kim, S.; Chen, B.; Guo, L.; Llaguno, M.; Hollingsworth, J.V.; King, D.S.; Banani, S.F.; et al. Phase transitions in the assembly of multivalent signalling proteins. Nature 2012, 483, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Banani, S.F.; Rice, A.M.; Peeples, W.B.; Lin, Y.; Jain, S.; Parker, R.; Rosen, M.K. Compositional Control of Phase-Separated Cellular Bodies. Cell 2016, 166, 651–663. [Google Scholar] [CrossRef]
- McCall, P.M.; Srivastava, S.; Perry, S.L.; Kovar, D.R.; Gardel, M.L.; Tirrell, M.V. Partitioning and Enhanced Self-Assembly of Actin in Polypeptide Coacervates. Biophys. J. 2018, 114, 1636–1645. [Google Scholar] [CrossRef]
- Xu, A.Y.; Melton, L.D.; Ryan, T.M.; Mata, J.P.; Rekas, A.; Williams, M.A.K.; McGillivray, D.J. Effects of polysaccharide charge pattern on the microstructures of β-lactoglobulin-pectin complex coacervates, studied by SAXS and SANS. Food Hydrocoll. 2018, 77, 952–963. [Google Scholar] [CrossRef]
- Maldonado, L.; Sadeghi, R.; Kokini, J. Nanoparticulation of bovine serum albumin and poly-d-lysine through complex coacervation and encapsulation of curcumin. Colloids Surf. B Biointerfaces 2017, 159, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Pathak, J.; Rawat, K.; Aswal, V.K.; Bohidar, H.B. Hierarchical Surface Charge Dependent Phase States of Gelatin–Bovine Serum Albumin Dispersions Close to Their Common pI. J. Phys. Chem. B 2014, 118, 11161–11171. [Google Scholar] [CrossRef] [PubMed]
- Pathak, J.; Rawat, K.; Aswal, V.K.; Bohidar, H.B. Hierarchical Internal Structures in Gelatin–Bovine Serum Albumin/β-Lactoglobulin Gels and Coacervates. J. Phys. Chem. B 2016, 120, 9506–9512. [Google Scholar] [CrossRef]
- Tian, L.; Kang, H.C.; Bae, Y.H. Endosomolytic Reducible Polymeric Electrolytes for Cytosolic Protein Delivery. Biomacromolecules 2013, 14, 2570–2581. [Google Scholar] [CrossRef] [PubMed]
- Shakya, A.; King, J.T. Non-Fickian Molecular Transport in Protein–DNA Droplets. ACS Macro Lett. 2018, 7, 1220–1225. [Google Scholar] [CrossRef]
- Iwashita, K.; Handa, A.; Shiraki, K. Coacervates and coaggregates: Liquid–liquid and liquid–solid phase transitions by native and unfolded protein complexes. Int. J. Biol. Macromol. 2018, 120, 10–18. [Google Scholar] [CrossRef]
- Souza, C.J.F.; da Costa, A.R.; Souza, C.F.; Tosin, F.F.S.; Garcia-Rojas, E.E. Complex coacervation between lysozyme and pectin: Effect of pH, salt, and biopolymer ratio. Int. J. Biol. Macromol. 2018, 107, 1253–1260. [Google Scholar] [CrossRef] [PubMed]
- Schuurmans, C.C.L.; Abbadessa, A.; Bengtson, M.A.; Pletikapic, G.; Eral, H.B.; Koenderink, G.; Masereeuw, R.; Hennink, W.E.; Vermonden, T. Complex coacervation-based loading and tunable release of a cationic protein from monodisperse glycosaminoglycan microgels. Soft Matter 2018, 14, 6327–6341. [Google Scholar] [CrossRef]
- Souza, C.J.F.; Garcia-Rojas, E.E. Effects of salt and protein concentrations on the association and dissociation of ovalbumin-pectin complexes. Food Hydrocoll. 2015, 47, 124–129. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, Y.; Eschmann, N.A.; Zhou, H.; Rauch, J.N.; Hernandez, I.; Guzman, E.; Kosik, K.S.; Han, S. RNA stores tau reversibly in complex coacervates. PLoS Biol. 2017, 15, e2002183. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.K.; Kaushik, P.; Rawat, K.; Aswal, V.K.; Bohidar, H.B. Solvent hydrophobicity induced complex coacervation of dsDNA and in situ formed zein nanoparticles. Soft Matter 2017, 13, 6784–6791. [Google Scholar] [CrossRef]
- Kaushik, P.; Rawat, K.; Aswal, V.K.; Kohlbrecher, J.; Bohidar, H.B. Mixing ratio dependent complex coacervation versus bicontinuous gelation of pectin with in situ formed zein nanoparticles. Soft Matter 2018, 14, 6463–6475. [Google Scholar] [CrossRef] [PubMed]
- Pack, D.W.; Hoffman, A.S.; Pun, S.; Stayton, P.S. Design and development of polymers for gene delivery. Nat. Rev. Drug Discov. 2005, 4, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Hwang, M.P.; Ding, X.; Gao, J.; Acharya, A.P.; Little, S.R.; Wang, Y. A biocompatible betaine-functionalized polycation for coacervation. Soft Matter 2018, 14, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Zern, B.J.; Chu, H.; Osunkoya, A.O.; Gao, J.; Wang, Y. A Biocompatible Arginine-based Polycation. Adv. Funct. Mater. 2011, 21, 434–440. [Google Scholar] [CrossRef]
- Chu, H.; Gao, J.; Wang, Y. Design, synthesis, and biocompatibility of an arginine-based polyester. Biotechnol. Prog. 2012, 28, 257–264. [Google Scholar] [CrossRef]
- van de Weert, M.; Andersen, M.B.; Frokjaer, S. Complex Coacervation of Lysozyme and Heparin: Complex Characterization and Protein Stability. Pharm. Res. 2004, 21, 2354–2359. [Google Scholar] [CrossRef]
- Semenova, M. Protein–polysaccharide associative interactions in the design of tailor-made colloidal particles. Curr. Opin. Colloid Interface Sci. 2017, 28, 15–21. [Google Scholar] [CrossRef]
- Hoffmann, K.Q.; Perry, S.L.; Leon, L.; Priftis, D.; Tirrell, M.; Pablo, J.J. de A molecular view of the role of chirality in charge-driven polypeptide complexation. Soft Matter 2015, 11, 1525–1538. [Google Scholar] [CrossRef] [PubMed]
- Vieregg, J.R.; Lueckheide, M.; Marciel, A.B.; Leon, L.; Bologna, A.J.; Rivera, J.R.; Tirrell, M.V. Oligonucleotide–Peptide Complexes: Phase Control by Hybridization. J. Am. Chem. Soc. 2018, 140, 1632–1638. [Google Scholar] [CrossRef] [PubMed]
- Vieregg, J.R.; Tang, T.-Y.D. Polynucleotides in cellular mimics: Coacervates and lipid vesicles. Curr. Opin. Colloid Interface Sci. 2016, 26, 50–57. [Google Scholar] [CrossRef]
- Aumiller, W.M.; Keating, C.D. Phosphorylation-mediated RNA/peptide complex coacervation as a model for intracellular liquid organelles. Nat. Chem. 2016, 8, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.R.; Milin, A.N.; Moosa, M.M.; Onuchic, P.L.; Deniz, A.A. Reentrant Phase Transition Drives Dynamic Substructure Formation in Ribonucleoprotein Droplets. Angew. Chem. Int. Ed. 2017, 56, 11354–11359. [Google Scholar] [CrossRef]
- Zeeb, B.; Mi-Yeon, L.; Gibis, M.; Weiss, J. Growth phenomena in biopolymer complexes composed of heated WPI and pectin. Food Hydrocoll. 2018, 74, 53–61. [Google Scholar] [CrossRef]
- Warnakulasuriya, S.; Pillai, P.K.S.; Stone, A.K.; Nickerson, M.T. Effect of the degree of esterification and blockiness on the complex coacervation of pea protein isolate and commercial pectic polysaccharides. Food Chem. 2018, 264, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Comert, F.; Dubin, P.L. Liquid-liquid and liquid-solid phase separation in protein-polyelectrolyte systems. Adv. Colloid Interface Sci. 2017, 239, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Reiche, K.; Hartl, J.; Blume, A.; Garidel, P. Liquid-liquid phase separation of a monoclonal antibody at low ionic strength: Influence of anion charge and concentration. Biophys. Chem. 2017, 220, 7–19. [Google Scholar] [CrossRef]
- Pacalin, N.M.; Leon, L.; Tirrell, M. Directing the phase behavior of polyelectrolyte complexes using chiral patterned peptides. Eur. Phys. J. Spec. Top. 2016, 225, 1805–1815. [Google Scholar] [CrossRef]
- Perry, S.L.; Leon, L.; Hoffmann, K.Q.; Kade, M.J.; Priftis, D.; Black, K.A.; Wong, D.; Klein, R.A.; Pierce Iii, C.F.; Margossian, K.O.; et al. Chirality-selected phase behaviour in ionic polypeptide complexes. Nat. Commun. 2015, 6, 6052. [Google Scholar] [CrossRef]
- Johnston, B.M.; Johnston, C.W.; Letteri, R.A.; Lytle, T.K.; Sing, C.E.; Emrick, T.; Perry, S.L. The effect of comb architecture on complex coacervation. Org. Biomol. Chem. 2017, 15, 7630–7642. [Google Scholar] [CrossRef]
- Voets, I.K.; de Keizer, A.; Cohen Stuart, M.A. Complex coacervate core micelles. Adv. Colloid Interface Sci. 2009, 147–148, 300–318. [Google Scholar] [CrossRef] [PubMed]
- Laaser, J.E.; Jiang, Y.; Petersen, S.R.; Reineke, T.M.; Lodge, T.P. Interpolyelectrolyte Complexes of Polycationic Micelles and Linear Polyanions: Structural Stability and Temporal Evolution. J. Phys. Chem. B 2015, 119, 15919–15928. [Google Scholar] [CrossRef]
- Haladjova, E.; Rangelov, S.; Tsvetanov, C.B.; Pispas, S. DNA encapsulation via nanotemplates from cationic block copolymer micelles. Soft Matter 2012, 8, 2884–2889. [Google Scholar] [CrossRef]
- Guerrero-Cázares, H.; Tzeng, S.Y.; Young, N.P.; Abutaleb, A.O.; Quiñones-Hinojosa, A.; Green, J.J. Biodegradable Polymeric Nanoparticles Show High Efficacy and Specificity at DNA Delivery to Human Glioblastoma in Vitro and in Vivo. ACS Nano 2014, 8, 5141–5153. [Google Scholar] [CrossRef]
- Pippa, N.; Karayianni, M.; Pispas, S.; Demetzos, C. Complexation of cationic-neutral block polyelectrolyte with insulin and in vitro release studies. Int. J. Pharm. 2015, 491, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Rumyantsev, A.M.; Zhulina, E.B.; Borisov, O.V. Scaling Theory of Complex Coacervate Core Micelles. ACS Macro Lett. 2018, 7, 811–816. [Google Scholar] [CrossRef]
- Takahashi, R.; Sato, T.; Terao, K.; Yusa, S. Reversible Vesicle–Spherical Micelle Transition in a Polyion Complex Micellar System Induced by Changing the Mixing Ratio of Copolymer Components. Macromolecules 2016, 49, 3091–3099. [Google Scholar] [CrossRef]
- Borisov, O.V.; Zhulina, E.B. Effect of Salt on Self-Assembly in Charged Block Copolymer Micelles. Macromolecules 2002, 35, 4472–4480. [Google Scholar] [CrossRef]
- van der Burgh, S.; de Keizer, A.; Cohen Stuart, M.A. Complex Coacervation Core Micelles. Colloidal Stability and Aggregation Mechanism. Langmuir 2004, 20, 1073–1084. [Google Scholar] [CrossRef]
- Takahashi, R.; Narayanan, T.; Yusa, S.; Sato, T. Kinetics of Morphological Transition between Cylindrical and Spherical Micelles in a Mixture of Anionic–Neutral and Cationic–Neutral Block Copolymers Studied by Time-Resolved SAXS and USAXS. Macromolecules 2018, 51, 3654–3662. [Google Scholar] [CrossRef]
- Aloi, A.; Guibert, C.; Olijve, L.L.C.; Voets, I.K. Morphological evolution of complex coacervate core micelles revealed by iPAINT microscopy. Polymer 2016, 107, 450–455. [Google Scholar] [CrossRef]
- van der Kooij, H.M.; Spruijt, E.; Voets, I.K.; Fokkink, R.; Cohen Stuart, M.A.; van der Gucht, J. On the Stability and Morphology of Complex Coacervate Core Micelles: From Spherical to Wormlike Micelles. Langmuir 2012, 28, 14180–14191. [Google Scholar] [CrossRef] [PubMed]
- Schrage, S.; Sigel, R.; Schlaad, H. Formation of Amphiphilic Polyion Complex Vesicles from Mixtures of Oppositely Charged Block Ionomers. Macromolecules 2003, 36, 1417–1420. [Google Scholar] [CrossRef]
- Anraku, Y.; Kishimura, A.; Oba, M.; Yamasaki, Y.; Kataoka, K. Spontaneous Formation of Nanosized Unilamellar Polyion Complex Vesicles with Tunable Size and Properties. J. Am. Chem. Soc. 2010, 132, 1631–1636. [Google Scholar] [CrossRef] [PubMed]
- Hori, M.; Cabral, H.; Toh, K.; Kishimura, A.; Kataoka, K. Robust Polyion Complex Vesicles (PICsomes) under Physiological Conditions Reinforced by Multiple Hydrogen Bond Formation Derived by Guanidinium Groups. Biomacromolecules 2018, 19, 4113–4121. [Google Scholar] [CrossRef] [PubMed]
- Mutaf, O.F.; Anraku, Y.; Kishimura, A.; Kataoka, K. Unilamellar polyion complex vesicles (PICsomes) with tunable permeabilities for macromolecular solutes with different shapes and sizes. Polymer 2017, 133, 1–7. [Google Scholar] [CrossRef]
- Kwolek, U.; Nakai, K.; Pluta, A.; Zatorska, M.; Wnuk, D.; Lasota, S.; Bednar, J.; Michalik, M.; Yusa, S.; Kepczynski, M. Polyion complex vesicles (PICsomes) from strong copolyelectrolytes. Stability and in vitro studies. Colloids Surf. B Biointerfaces 2017, 158, 658–666. [Google Scholar] [CrossRef]
- Totland, C.; Martinez-Santiago, J.; Ananthapadmanabhan, K.P.; Somasundaran, P. Composition and Structural Transitions of Polyelectrolyte–Surfactant Complexes in the Presence of Fatty Acid Studied by NMR and Cryo-SEM. Langmuir 2015, 31, 1623–1631. [Google Scholar] [CrossRef]
- Wibowo, A.; Osada, K.; Matsuda, H.; Anraku, Y.; Hirose, H.; Kishimura, A.; Kataoka, K. Morphology Control in Water of Polyion Complex Nanoarchitectures of Double-Hydrophilic Charged Block Copolymers through Composition Tuning and Thermal Treatment. Macromolecules 2014, 47, 3086–3092. [Google Scholar] [CrossRef]
- Fan, Y.; Tang, S.; Thomas, E.L.; Olsen, B.D. Responsive Block Copolymer Photonics Triggered by Protein–Polyelectrolyte Coacervation. ACS Nano 2014, 8, 11467–11473. [Google Scholar] [CrossRef]
- MacKnight, W.J.; Ponomarenko, E.A.; Tirrell, D.A. Self-Assembled Polyelectrolyte−Surfactant Complexes in Nonaqueous Solvents and in the Solid State. Acc. Chem. Res. 1998, 31, 781–788. [Google Scholar] [CrossRef]
- Dähling, C.; Houston, J.E.; Radulescu, A.; Drechsler, M.; Brugnoni, M.; Mori, H.; Pergushov, D.V.; Plamper, F.A. Self-Templated Generation of Triggerable and Restorable Nonequilibrium Micelles. ACS Macro Lett. 2018, 7, 341–346. [Google Scholar] [CrossRef]
- Kishimura, A.; Koide, A.; Osada, K.; Yamasaki, Y.; Kataoka, K. Encapsulation of Myoglobin in PEGylated Polyion Complex Vesicles Made from a Pair of Oppositely Charged Block Ionomers: A Physiologically Available Oxygen Carrier. Angew. Chem. Int. Ed. 2007, 46, 6085–6088. [Google Scholar] [CrossRef]
- Takahashi, R.; Sato, T.; Terao, K.; Yusa, S. Intermolecular Interactions and Self-Assembly in Aqueous Solution of a Mixture of Anionic–Neutral and Cationic–Neutral Block Copolymers. Macromolecules 2015, 48, 7222–7229. [Google Scholar] [CrossRef]
- Israelachvili, J.N.; Mitchell, D.J.; Ninham, B.W. Theory of self-assembly of hydrocarbon amphiphiles into micelles and bilayers. J. Chem. Soc. Faraday Trans. 1976, 72, 1525–1568. [Google Scholar] [CrossRef]
- Lindhoud, S.; de Vries, R.; Norde, W.; Stuart, M.A.C. Structure and Stability of Complex Coacervate Core Micelles with Lysozyme. Biomacromolecules 2007, 8, 2219–2227. [Google Scholar] [CrossRef]
- Lindhoud, S.; Voorhaar, L.; de Vries, R.; Schweins, R.; Cohen Stuart, M.A.; Norde, W. Salt-Induced Disintegration of Lysozyme-Containing Polyelectrolyte Complex Micelles. Langmuir 2009, 25, 11425–11430. [Google Scholar] [CrossRef]
- Ren, J.; Zhang, Y.; Zhang, J.; Gao, H.; Liu, G.; Ma, R.; An, Y.; Kong, D.; Shi, L. pH/Sugar Dual Responsive Core-Cross-Linked PIC Micelles for Enhanced Intracellular Protein Delivery. Biomacromolecules 2013, 14, 3434–3443. [Google Scholar] [CrossRef] [PubMed]
- Lindhoud, S.; de Vries, R.; Schweins, R.; Stuart, M.A.C.; Norde, W. Salt-induced release of lipase from polyelectrolyte complex micelles. Soft Matter 2008, 5, 242–250. [Google Scholar] [CrossRef]
- Jiang, Y.; Lu, H.; Chen, F.; Callari, M.; Pourgholami, M.; Morris, D.L.; Stenzel, M.H. PEGylated Albumin-Based Polyion Complex Micelles for Protein Delivery. Biomacromolecules 2016, 17, 808–817. [Google Scholar] [CrossRef] [PubMed]
- Harada, A.; Kataoka, K. Chain Length Recognition: Core-Shell Supramolecular Assembly from Oppositely Charged Block Copolymers. Science 1999, 283, 65–67. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Yamasaki, Y.; Harada, A.; Kataoka, K. Characterization of stable lysozyme-entrapped polyion complex (PIC) micelles with crosslinked core by glutaraldehyde. Polymer 2005, 46, 7749–7758. [Google Scholar] [CrossRef]
- Wu, F.-G.; Jiang, Y.-W.; Chen, Z.; Yu, Z.-W. Folding Behaviors of Protein (Lysozyme) Confined in Polyelectrolyte Complex Micelle. Langmuir 2016, 32, 3655–3664. [Google Scholar] [CrossRef]
- Yuan, X.; Harada, A.; Yamasaki, Y.; Kataoka, K. Stabilization of Lysozyme-Incorporated Polyion Complex Micelles by the ω-End Derivatization of Poly(ethylene glycol)−Poly(α,β-aspartic acid) Block Copolymers with Hydrophobic Groups. Langmuir 2005, 21, 2668–2674. [Google Scholar] [CrossRef] [PubMed]
- Nolles, A.; Hooiveld, E.; Westphal, A.H.; van Berkel, W.J.H.; Kleijn, J.M.; Borst, J.W. FRET Reveals the Formation and Exchange Dynamics of Protein-Containing Complex Coacervate Core Micelles. Langmuir 2018, 34, 12083–12092. [Google Scholar] [CrossRef] [PubMed]
- Nolles, A.; Westphal, A.H.; Kleijn, J.M.; van Berkel, W.J.H.; Borst, J.W. Colorful Packages: Encapsulation of Fluorescent Proteins in Complex Coacervate Core Micelles. Int. J. Mol. Sci. 2017, 18, 1557. [Google Scholar] [CrossRef]
- Nolles, A.; van Dongen, N.J.E.; Westphal, A.H.; Visser, A.J.W.G.; Kleijn, J.M.; van Berkel, W.J.H.; Borst, J.W. Encapsulation into complex coacervate core micelles promotes EGFP dimerization. Phys. Chem. Chem. Phys. 2017, 19, 11380–11389. [Google Scholar] [CrossRef] [PubMed]
- Nolles, A.; Westphal, A.H.; de Hoop, J.A.; Fokkink, R.G.; Kleijn, J.M.; van Berkel, W.J.H.; Borst, J.W. Encapsulation of GFP in Complex Coacervate Core Micelles. Biomacromolecules 2015, 16, 1542–1549. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, A.; Kojima, C.; Iijima, M.; Harada, A.; Kono, K. Polyion complex micelles formed from glucose oxidase and comb-type polyelectrolyte with poly(ethylene glycol) grafts. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 3842–3852. [Google Scholar] [CrossRef]
- Mills, C.E.; Obermeyer, A.; Dong, X.; Walker, J.; Olsen, B.D. Complex Coacervate Core Micelles for the Dispersion and Stabilization of Organophosphate Hydrolase in Organic Solvents. Langmuir 2016, 32, 13367–13376. [Google Scholar] [CrossRef]
- Lee, Y.; Ishii, T.; Kim, H.J.; Nishiyama, N.; Hayakawa, Y.; Itaka, K.; Kataoka, K. Efficient delivery of bioactive antibodies into the cytoplasm of living cells by charge-conversional polyion complex micelles. Angew. Chem. Int. Ed. Engl. 2010, 49, 2552–2555. [Google Scholar] [CrossRef]
- Lindhoud, S.; Norde, W.; Cohen Stuart, M.A. Effects of Polyelectrolyte Complex Micelles and Their Components on the Enzymatic Activity of Lipase. Langmuir 2010, 26, 9802–9808. [Google Scholar] [CrossRef] [PubMed]
- Eratte, D.; Dowling, K.; Barrow, C.J.; Adhikari, B.P. In-vitro digestion of probiotic bacteria and omega-3 oil co-microencapsulated in whey protein isolate-gum Arabic complex coacervates. Food Chem. 2017, 227, 129–136. [Google Scholar] [CrossRef] [PubMed]
- McCord, J.P.; Muddiman, D.C.; Khaledi, M.G. Perfluorinated alcohol induced coacervates as extraction media for proteomic analysis. J. Chromatogr. A 2017, 1523, 293–299. [Google Scholar] [CrossRef]
- Nott, T.J.; Craggs, T.D.; Baldwin, A.J. Membraneless organelles can melt nucleic acid duplexes and act as biomolecular filters. Nat. Chem. 2016, 8, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Agut, W.; Brûlet, A.; Schatz, C.; Taton, D.; Lecommandoux, S. pH and Temperature Responsive Polymeric Micelles and Polymersomes by Self-Assembly of Poly[2-(dimethylamino)ethyl methacrylate]-b-Poly(glutamic acid) Double Hydrophilic Block Copolymers. Langmuir 2010, 26, 10546–10554. [Google Scholar] [CrossRef]
- Harada, A.; Kataoka, K. Pronounced activity of enzymes through the incorporation into the core of polyion complex micelles made from charged block copolymers. J. Control. Release 2001, 72, 85–91. [Google Scholar] [CrossRef]
- Danial, M.; Klok, H.-A.; Norde, W.; Cohen Stuart, M.A. Complex Coacervate Core Micelles with a Lysozyme-Modified Corona. Langmuir 2007, 23, 8003–8009. [Google Scholar] [CrossRef]
- da S. Gulão, E.; de Souza, C.J.F.; Andrade, C.T.; Garcia-Rojas, E.E. Complex coacervates obtained from peptide leucine and gum arabic: Formation and characterization. Food Chem. 2016, 194, 680–686. [Google Scholar] [CrossRef]
- Chabba, S.; Vashishat, R.; Mahajan, R.K. Characterization of interactions between β-lactoglobulin with surface active ionic liquids in aqueous medium. J. Mol. Liq. 2018, 259, 134–143. [Google Scholar] [CrossRef]
- Lueckheide, M.; Vieregg, J.R.; Bologna, A.J.; Leon, L.; Tirrell, M.V. Structure–Property Relationships of Oligonucleotide Polyelectrolyte Complex Micelles. Nano Lett. 2018, 18, 7111–7117. [Google Scholar] [CrossRef]
- Boudier, A.; Aubert-Pouëssel, A.; Louis-Plence, P.; Gérardin, C.; Jorgensen, C.; Devoisselle, J.-M.; Bégu, S. The control of dendritic cell maturation by pH-sensitive polyion complex micelles. Biomaterials 2009, 30, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Miles, A.J.; Wallace, B.A. Circular dichroism spectroscopy of membrane proteins. Chem. Soc. Rev. 2016, 45, 4859–4872. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef]
- Stetefeld, J.; McKenna, S.A.; Patel, T.R. Dynamic light scattering: A practical guide and applications in biomedical sciences. Biophys. Rev. 2016, 8, 409–427. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lee, J.-S. Staring at protein-surfactant interactions: Fundamental approaches and comparative evaluation of their combinations—A review. Anal. Chim. Acta 2019. [Google Scholar] [CrossRef]
- Anvari, M.; Pan, C.-H.; Yoon, W.-B.; Chung, D. Characterization of fish gelatin–gum arabic complex coacervates as influenced by phase separation temperature. Int. J. Biol. Macromol. 2015, 79, 894–902. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Winter, H.H.; Perry, S.L. Linear viscoelasticity of complex coacervates. Adv. Colloid Interface Sci. 2017, 239, 46–60. [Google Scholar] [CrossRef]
- Kudaibergenov, S.E.; Nuraje, N. Intra- and Interpolyelectrolyte Complexes of Polyampholytes. Polymers 2018, 10, 1146. [Google Scholar] [CrossRef]
- Panganiban, B.; Qiao, B.; Jiang, T.; DelRe, C.; Obadia, M.M.; Nguyen, T.D.; Smith, A.A.A.; Hall, A.; Sit, I.; Crosby, M.G.; et al. Random heteropolymers preserve protein function in foreign environments. Science 2018, 359, 1239–1243. [Google Scholar] [CrossRef]
- Mier, P.; Andrade-Navarro, M.A. Toward completion of the Earth’s proteome: An update a decade later. Brief Bioinform. 2017. [Google Scholar] [CrossRef] [PubMed]
- Mitrea, D.M.; Chandra, B.; Ferrolino, M.C.; Gibbs, E.B.; Tolbert, M.; White, M.R.; Kriwacki, R.W. Methods for Physical Characterization of Phase-Separated Bodies and Membrane-less Organelles. J. Mol. Biol. 2018, 430, 4773–4805. [Google Scholar] [CrossRef] [PubMed]



| Protein | Molecular Weight (kDa) | pI | Appx. Charge 1 | Polyelectrolyte Partners 2 |
|---|---|---|---|---|
| α-Chymotrypsinogen 3 | 25.6 | 8.7 | 4 | PDMAEMA [35]; qP4VP [35] |
| Actin | 42.1 | 5.4 | −13 | pLK and pRE [69] |
| β-lactoglobulin | 19.9 | 5.0 | −7 | hyaluronic acid [40];tragacanthin [40]; lactoferrin [46]; gelatin B [48]; pectin [70] |
| BSA | 69.3 | 6.2 | −12 | PAA and PAH [37]; pLK and pRE [38]; hyaluronic acid [45]; pDK [71]; gelatin B [72]; gelatin B and β-lactoglobulin [16,73]; RPC-bPEI [74] |
| C-terminal histone 1 | 11.2 | 11.2 | 45 | dsDNA [63] |
| GFP | ~27.8 | 6.6 | −7 | PDDA and ATP [3]; qP4VP [49]; PEI [49]; PLL [49] |
| supercharged GFP | ~28 | - | +36 to −30 | RNA [41,42]; DNA [41,42]; PAA [41]; PSS [41]; supercharged GFP [42]; qP4VP [49]; PEI [49]; PLL [49] |
| glucose oxidase | 63.3 | 5.1 | −28 | insulin and DgHBP-2 [34] |
| Histone | 30–70 | N/A | N/A | ssDNA [75] |
| LAF-1 | 76.3 | 7.1 | −2 | RNA [58] |
| lysozyme | 14.3 | 7.3 | 8 | PDMAEMA [35] 3; qP4VP [35] 3; PAA and PDMAEMA [36]; RPA-bPEI [74]; ovalbumin [76]; pectin [77]; HAMA and CSMA [78] |
| mfp-1 | 108 | 10.0 | 189 | hyaluronic acid [52]; MADQUAT [56] |
| monoclonal antibody | 145 | 8.3 | N/A | hyaluronic acid [40] |
| myoglobin 3 | 17.1 | 7.8 | 0 | PDMAEMA [35]; qP4VP [35] |
| Ovalbumin | 42.7 | 5.3 | −12 | Pectin [79]; lysozyme [76] |
| RNase 3 | 16.5 | 8.6 | 6 | PDMAEMA [35]; qP4VP [35] |
| tau | 78.9 | 6.7 | −7 | RNA [80] |
| Whi 3 | 71.3 | 8.4 | 4 | RNA [59] |
| zein | 26.5 | 6.2 | 2 | dsDNA [81]; Pectin [82] |
| Protein | Molecular Weight (kDa) | pI | Appx. Charge 1 | Polyelectrolyte Partners 2 |
|---|---|---|---|---|
| α-Chymotrypsinogen | 25.6 | 8.7 | 4 | PEG-b-p(Asp) [33]; POEGMA-b-q4VP and PAA [140] |
| equine heart cytochrome 3 | 11.8 | 9.6 | 9 | PEG-b-p(Asp-DET) [27]; PEG-b-p(Asp-EDA-Suc) [27] |
| glucose oxidase | 63.3 | 5.1 | −28 | PEG-g-PAA [139] |
| human serum albumin | 66.5 | 6.2 | −11 | PEG-b-PAE-c-API [28] |
| immunoglobulin g (IgG) 3 | 170 | 7.4 | Natively 0.89 | PEG-b-p(Asp-DET) [141] |
| lipase (Humicola lanuginosa) | 31.8 | 5.6 | −7 | P2MVP-b-PEO and PAA [129,142] |
| lysozyme | 14.3 | 7.3 | 8 | PEG-b-p(Asp-EDA-Cit) [26]; POEGMA-b-qP4VP [35] 3; PAA-b-PAAm and PDMAEMA [126,127]; P2MVP-b-PEO and PAA [129]; POEGMA-g-BSA [130]; mPEG-b-p(L-Asp) [133]; PEG-b-p(Asp) [25,132,134] |
| myoglobin 3 | 17.1 | 7.8 | 0 | POEGMA-b-q4VP [35] |
| organophosphate hydrolase | 39.0 | 8.9 | 4 | POEGMA-b-qP4VP and PAA [140] |
| RNase 3 | 16.5 | 8.6 | 6 | POEGMA-b-qP4VP [35] |
| Sprouty 1 | 35 | 8.7 | 8 | POEGMA-g-BSA [130] |
| Fluorescent Proteins | ||||
| EGFP | 26.9 | 6.0 | −7 | P2MVP-b-PEO [137,138] |
| mEGFP | 27.0 | 6.1 | −7 | P2MVP-b-PEO [136] |
| mCherry | 26.7 | 6.5 | −6 | P2MVP-b-PEO [136] |
| mKO2 | 24.5 | 5.9 | −9 | P2MVP-b-PEO [136] |
| mTurquoise2 | 26.9 | 5.8 | −8 | P2MVP-b-PEO, SYFP2, SBFP2, and mTurquoise2 [135]; P2MVP-b-PEO [136] |
| SBFP2 | 26.8 | 6.2 | −7 | P2MVP-b-PEO, SYFP2, SBFP2, and mTurquoise2 [135]; P2MVP-b-PEO [136] |
| SYFP2 | 26.9 | 6.1 | −7 | P2MVP-b-PEO, SYFP2, SBFP2, and mTurquoise2 [135]; P2MVP-b-PEO [136] |
| TagRFP | 26.1 | 7.5 | 0 | P2MVP-b-PEO [136] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horn, J.M.; Kapelner, R.A.; Obermeyer, A.C. Macro- and Microphase Separated Protein-Polyelectrolyte Complexes: Design Parameters and Current Progress. Polymers 2019, 11, 578. https://doi.org/10.3390/polym11040578
Horn JM, Kapelner RA, Obermeyer AC. Macro- and Microphase Separated Protein-Polyelectrolyte Complexes: Design Parameters and Current Progress. Polymers. 2019; 11(4):578. https://doi.org/10.3390/polym11040578
Chicago/Turabian StyleHorn, Justin M., Rachel A. Kapelner, and Allie C. Obermeyer. 2019. "Macro- and Microphase Separated Protein-Polyelectrolyte Complexes: Design Parameters and Current Progress" Polymers 11, no. 4: 578. https://doi.org/10.3390/polym11040578
APA StyleHorn, J. M., Kapelner, R. A., & Obermeyer, A. C. (2019). Macro- and Microphase Separated Protein-Polyelectrolyte Complexes: Design Parameters and Current Progress. Polymers, 11(4), 578. https://doi.org/10.3390/polym11040578





