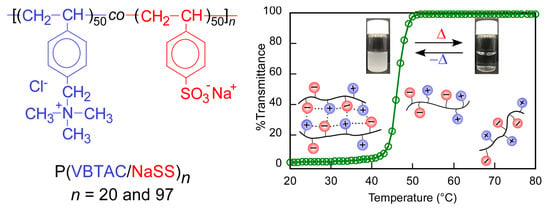
Upper Critical Solution Temperature (UCST) Behavior of Polystyrene-Based Polyampholytes in Aqueous Solution
Abstract
:1. Introduction
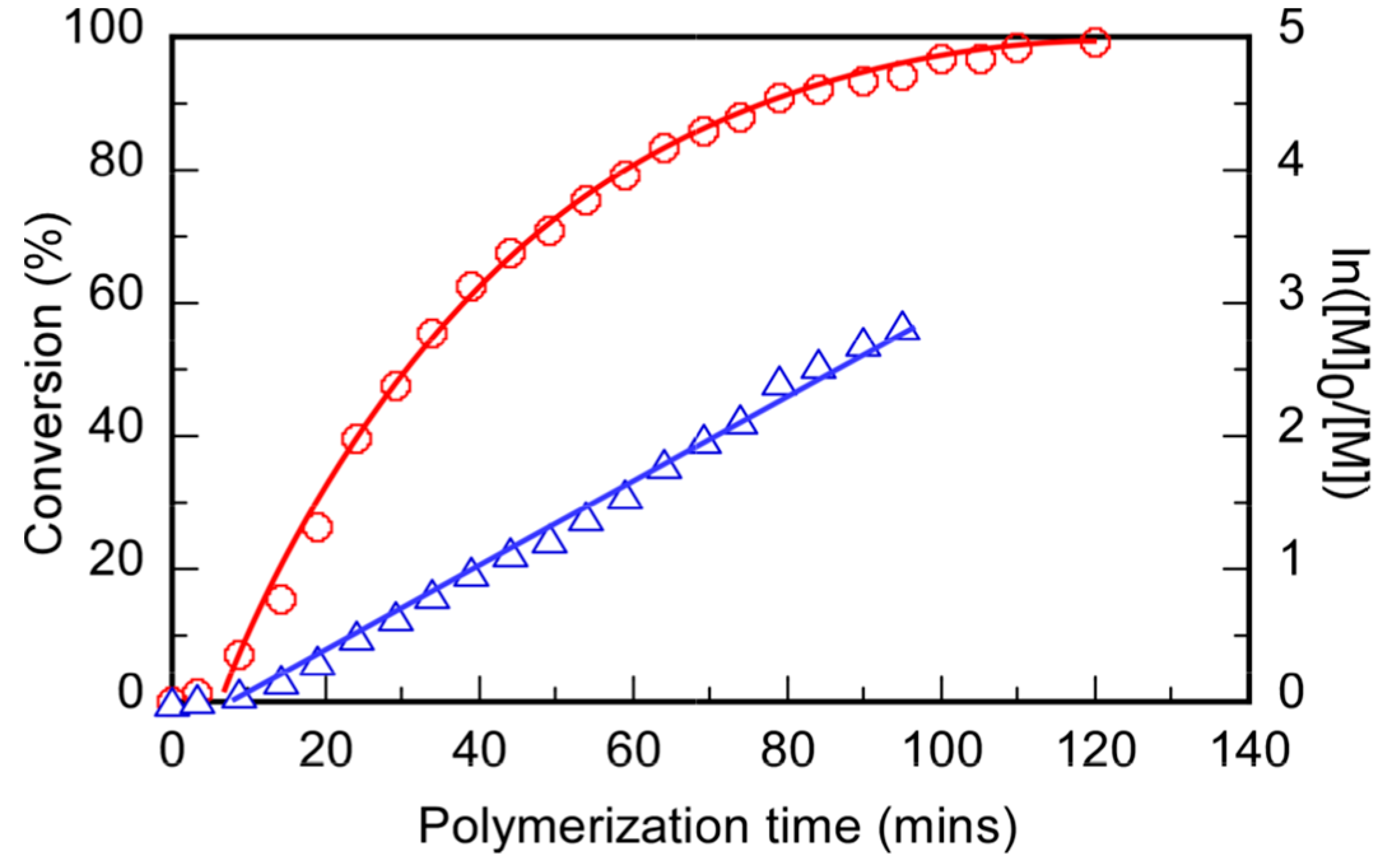
2. Experimental
2.1. Materials
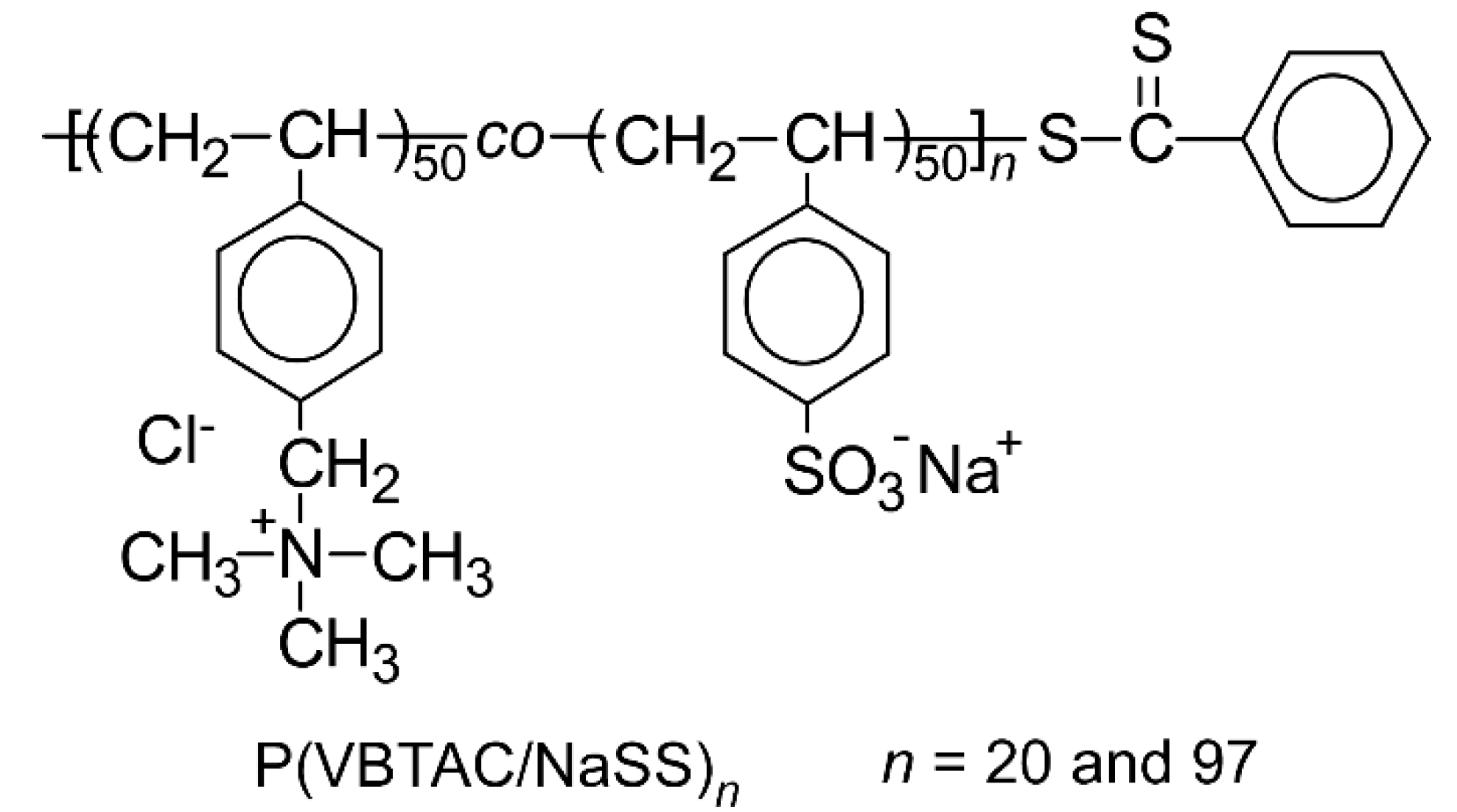
2.2. Preparation of Polyampholytes (P(VBTAC/NaSS)n)
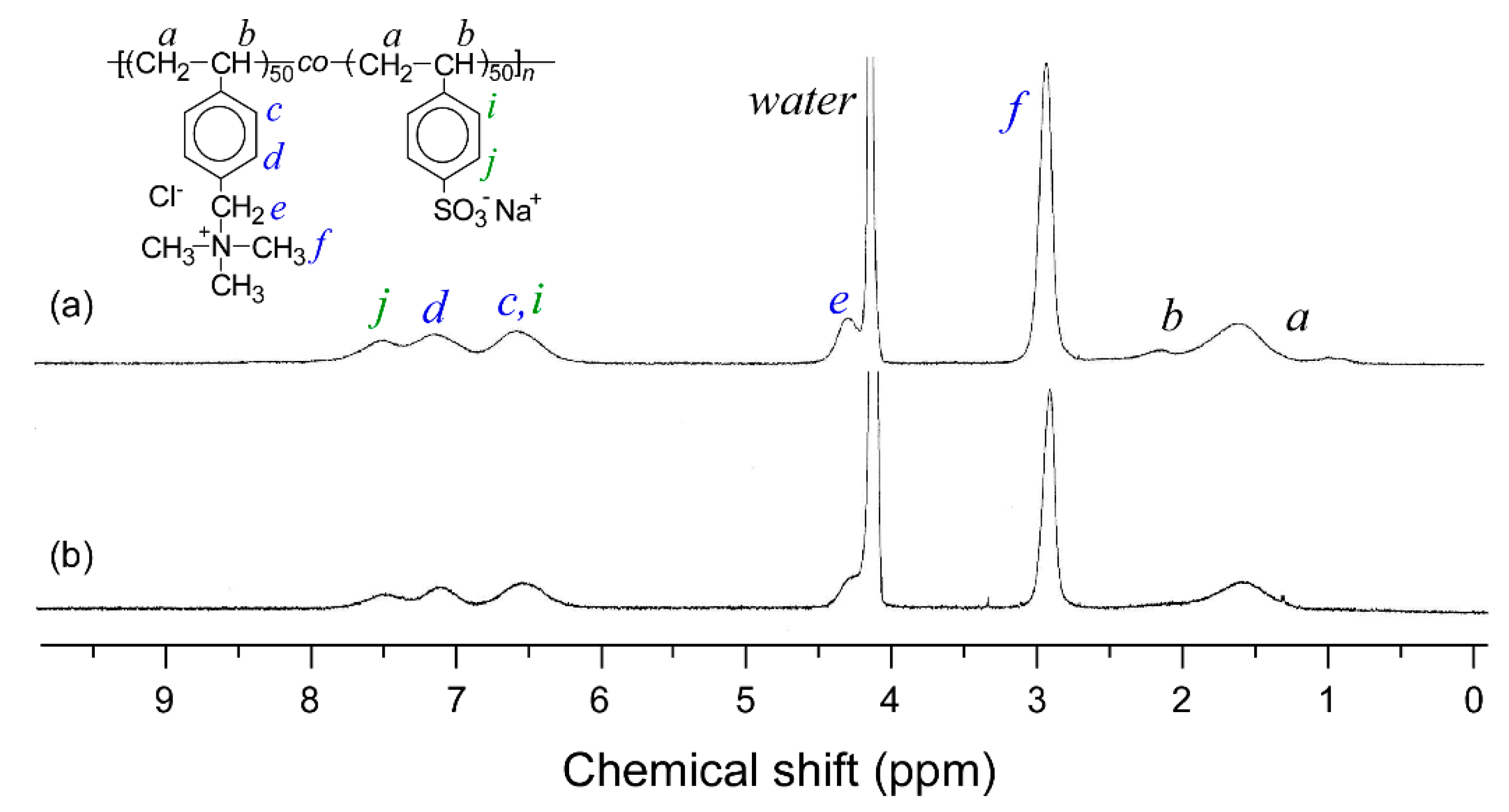
2.3. Measurements
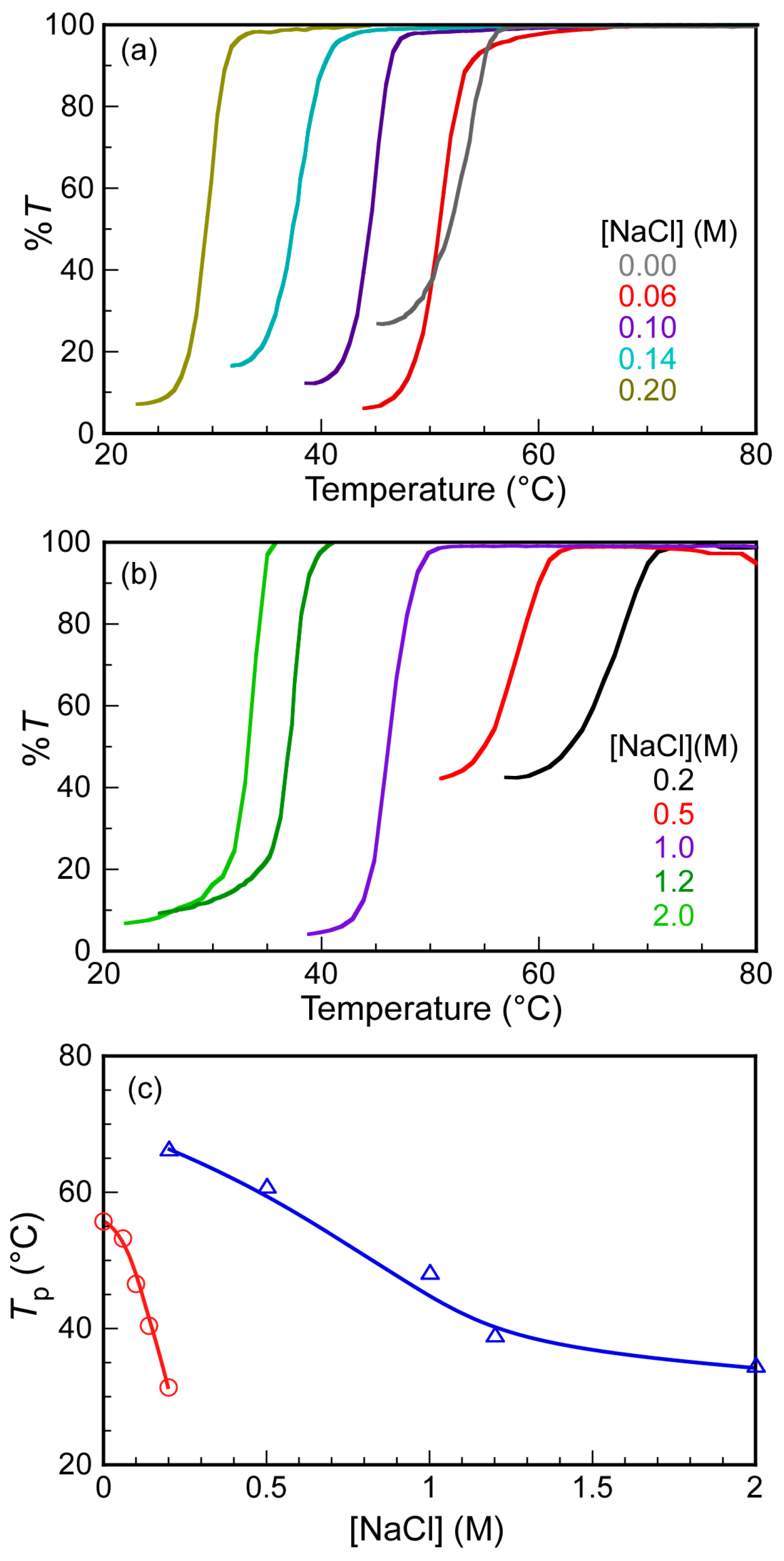
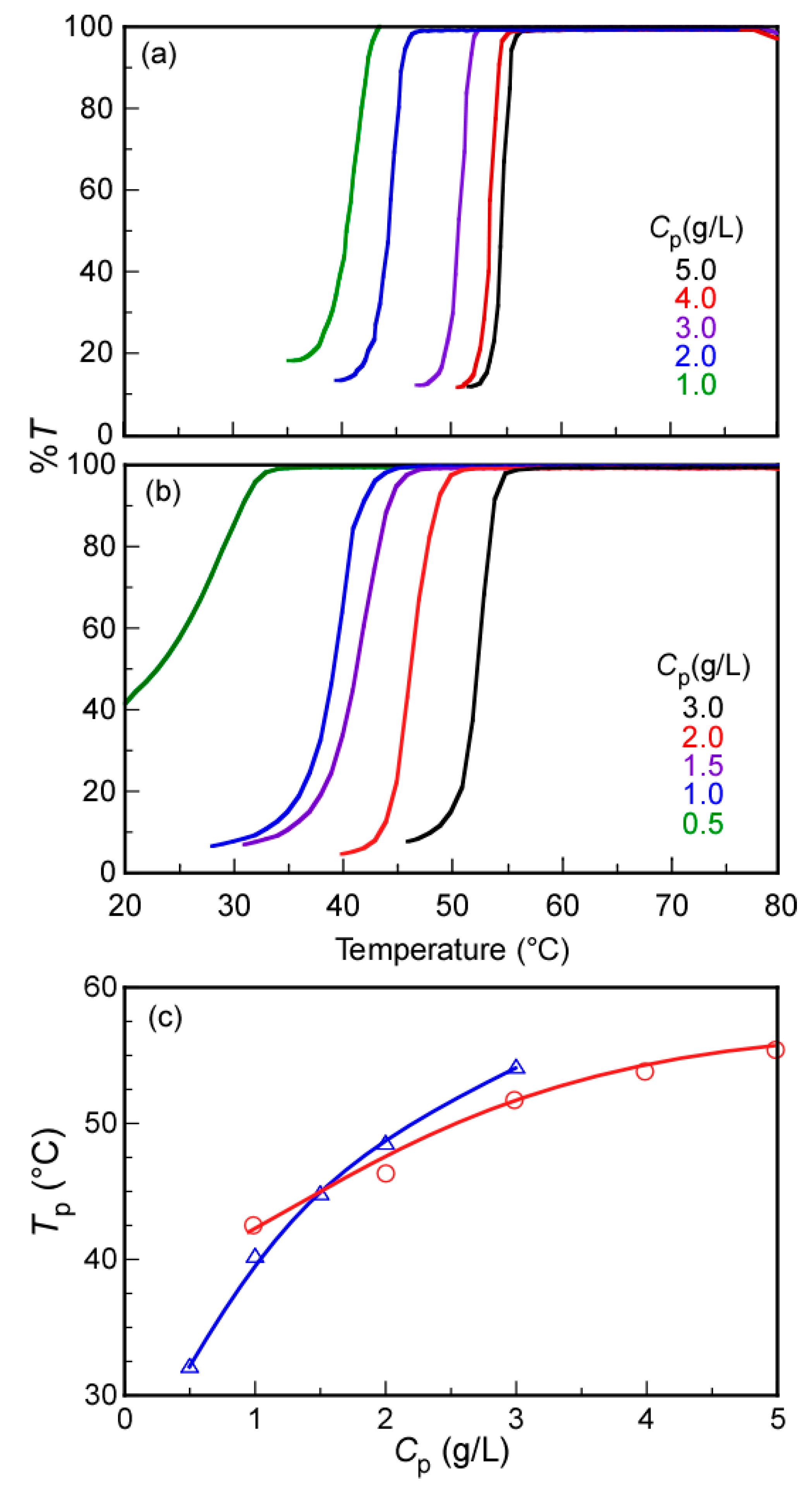
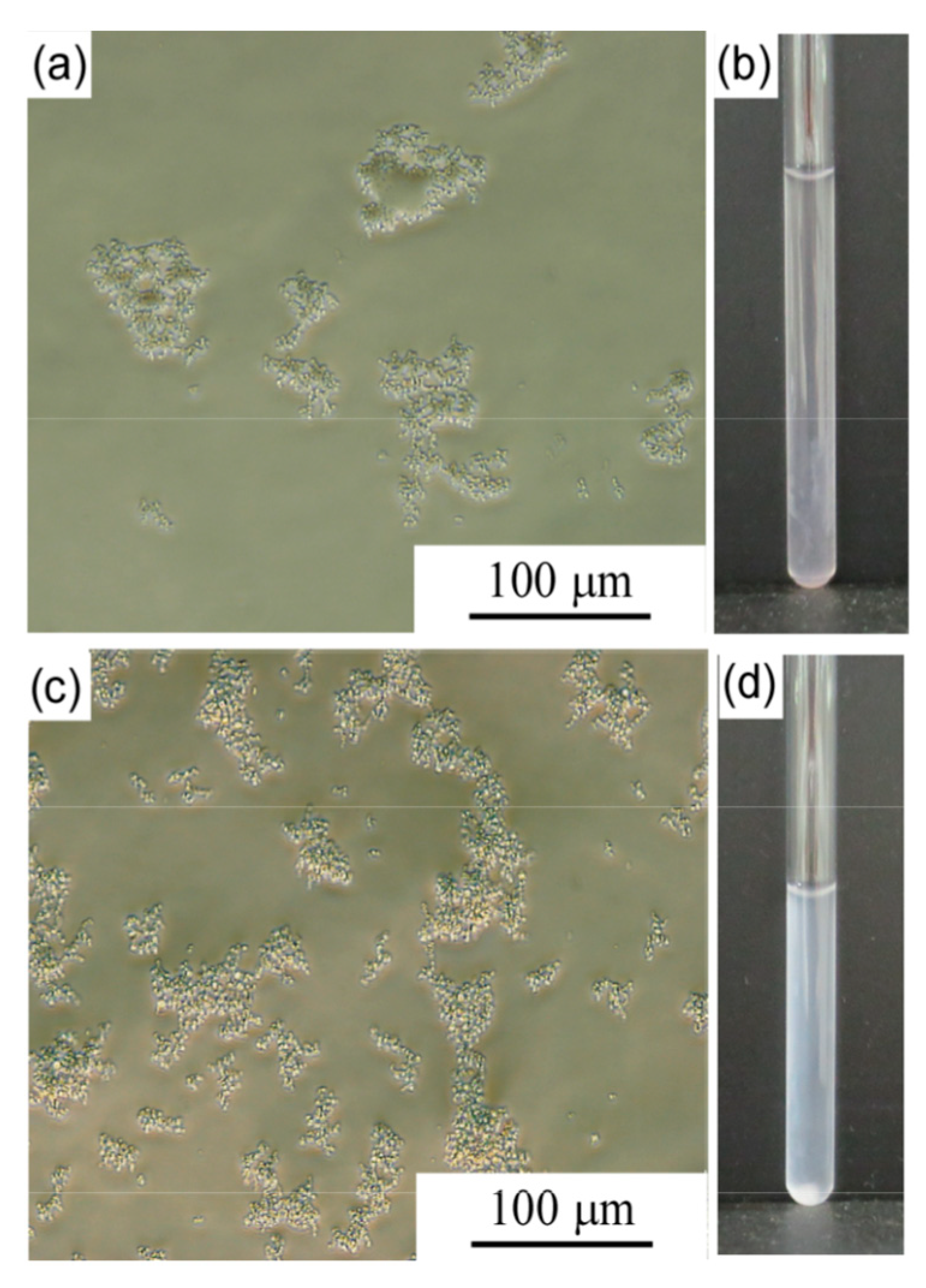
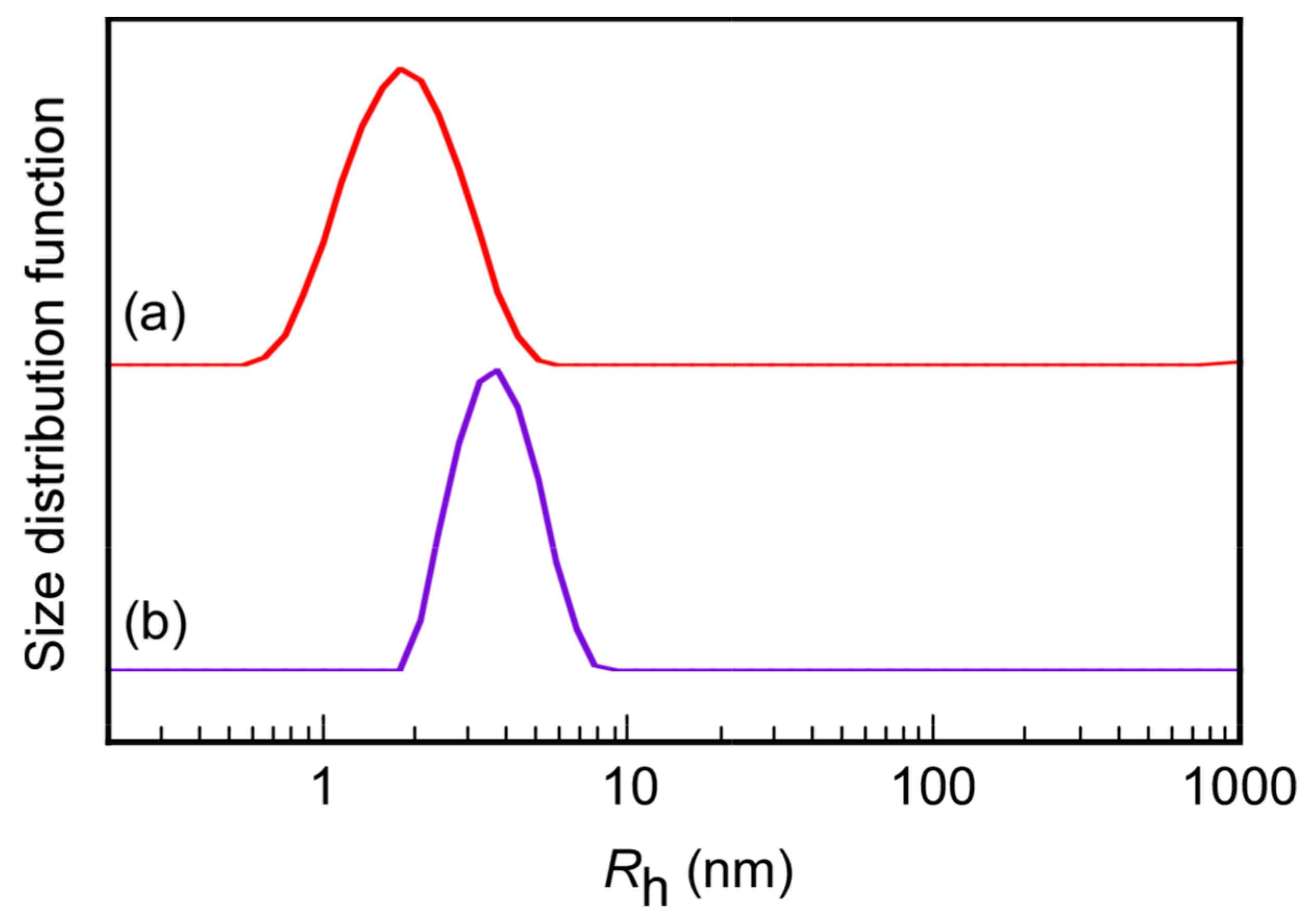
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Afrassiabi, A.; Hoffman, A.S.; Cadwell, L.A. Effect of temperature on the release rate of biomolecules from thermally reversible hydrogels. J. Membr. Sci. 1987, 33, 191–200. [Google Scholar] [CrossRef]
- Wei, H.; Cheng, S.X.; Zhang, X.Z.; Zhuo, R.X. Thermo-sensitive polymeric micelles based on poly(N-isopropylacrylamide) as drug carriers. Prog. Polym. Sci. 2009, 34, 893–910. [Google Scholar] [CrossRef]
- Kurisawa, M.; Yokoyama, M.; Okano, T. Gene expression control by temperature with thermo-responsive polymeric gene carriers. J. Controlled Release 2000, 69, 127–137. [Google Scholar] [CrossRef]
- Abdelaty, M.S.A. Environmental functional photo-cross-linked hydrogel bilayer thin films from vanillin (part 2): Temperature-responsive layer A, functional, temperature and pH layer B. Polym. Bull. 2018, 75, 4837–4858. [Google Scholar] [CrossRef]
- Reese, C.E.; Mikhonin, A.V.; Kamenjicki, M.; Tikhonov, A.; Asher, S.A. Nanogel nanosecond photonic crystal optical switching. J. Am. Chem. Soc. 2004, 126, 1493–1496. [Google Scholar] [CrossRef] [PubMed]
- Jańczewski, D.; Tomczak, N.; Han, M.Y.; Vancso, G.J. Stimulus responsive PNIPAM/QD hybrid microspheres by copolymerization with surface engineered QDs. Macromolecules 2009, 42, 1801–1804. [Google Scholar]
- Pan, J.; Zhang, L.; Bai, L.; Zhang, Z.; Chen, H.; Cheng, Z.; Zhu, X. Atom transfer radical polymerization of methyl methacrylate with a thermo-responsive ligand: Construction of thermoregulated phase-transfer catalysis in an aqueous-organic biphasic system. Polym. Chem. 2013, 4, 2876–2883. [Google Scholar] [CrossRef]
- Aseyev, V.; Tenhu, H.; Winnik, F.M. Non-ionic thermoresponsive polymers in water. Adv. Polym. Sci. 2011, 242, 29–89. [Google Scholar]
- Seuring, J.; Agarwal, S. Polymers with upper critical solution temperature in aqueous solution. Macromol. Rapid Commun. 2012, 33, 1898–1920. [Google Scholar] [CrossRef]
- Heskins, M.; Guillet, J.E. Solution properties of poly(N-isopropylacrylamide). J. Polym. Sci. Polym. Chem. 1968, 8, 1441–1455. [Google Scholar] [CrossRef]
- Halperin, A.; Kroeger, M.; Winnik, F.M. Poly(N-isopropylacrylamide) phase diagrams: Fifty years of research. Angew. Chem. Int. Ed. 2015, 54, 15342–15367. [Google Scholar] [CrossRef] [PubMed]
- Meeussen, F.; Nies, E.; Berghmans, H.; Verbrugghe, S.; Goethals, E.; Du Prez, F. Phase behavior of poly(N-vinyl caprolactam) in water. Polymer 2000, 41, 8597–8602. [Google Scholar] [CrossRef]
- Vancoillie, G.; Frank, D.; Hoogenboom, R. Thermoresponsive poly(oligo ethylene glycol acrylates). Prog. Polym. Sci. 2014, 39, 1074–1095. [Google Scholar] [CrossRef]
- Seuring, J.; Agarwal, S. First example of a universal and costeffective approach: Polymers with tunable upper critical solution temperature in water and wlectrolyte solution. Macromolecules 2012, 45, 3910–3918. [Google Scholar] [CrossRef]
- Shimada, N.; Ino, H.; Maie, K.; Nakayama, M.; Kano, A.; Maruyama, A. Ureido-derivatized polymers based on both poly(allylurea) and poly(L-citrulline) exhibit UCST-type phase transition behavior under physiologically relevant conditions. Biomacromolecules 2011, 12, 3418–3422. [Google Scholar] [CrossRef] [PubMed]
- Glatzel, S.; Laschewsky, A.; Lutz, J. Well-defined uncharged polymers with a sharp UCST in water and in physiological milieu. Macromolecules 2011, 44, 413–415. [Google Scholar] [CrossRef]
- Seuring, J.; Bayer, F.M.; Huber, K.; Agarwal, S. Upper critical solution temperature of poly(N-acryloyl glycinamide) in water: A concealed property. Macromolecules 2012, 45, 374–384. [Google Scholar] [CrossRef]
- Schulz, D.N.; Peiffer, D.G.; Agarwal, P.K.; Larabee, J.; Kaladas, J.J.; Soni, L.; Handwerker, B.; Garner, R.T. Phase behaviour and solution properties of sulphobetaine polymers. Polymer 1986, 27, 1734–1742. [Google Scholar] [CrossRef]
- Monroy Soto, V.M.; Galin, J.C. Poly(sulphopropylbetaines): 2. Dilute solution properties. Polymer 1984, 25, 254–262. [Google Scholar] [CrossRef]
- Katchalsky, A.; Shavit, N.; Eisenberg, H. Dissociation of weak polymeric acids and bases. J. Polym. Sci. 1954, 3, 69–84. [Google Scholar] [CrossRef]
- McCormick, C.L.; Johnson, C.B. Water-soluble copolymers. 29. Ampholytic copolymers of sodium 2-acrylamido-2-methylpropanesulfonate with (2-acrylamido-2-methylpropyl)dimethylammonium chloride: Solution properties. Macromolecules 1988, 21, 694–699. [Google Scholar] [CrossRef]
- Corpart, J.M.; Candan, F. Aqueous solution properties of ampholytic copolymers prepared in microemulsions. Macromolecules 1993, 26, 1333–1343. [Google Scholar] [CrossRef]
- Yang, J.H.; John, M.S. The conformation and dynamics study of amphoteric copolymers, P(sodium 2-methacryloyloxyethanesulfonate-co-2-methacryloyloxyethyltrimethylammonium iodide), using viscometry, 14N-, and 23Na-NMR. J. Polym. Sci. A 1995, 33, 2613–2621. [Google Scholar] [CrossRef]
- Takeoka, Y.; Berker, A.N.; Du, R.; Enoki, T.; Grosberg, A.; Kardar, M.; Oya, T.; Tanaka, K.; Wang, G.; Yu, X.; et al. First Order Phase Transition and Evidence for Frustrations in Polyampholytic Gels. Phys. Rev. Lett. 1999, 82, 4863–4865. [Google Scholar] [CrossRef]
- Sun, T.L.; CuiJian, K.; Gong, P. Tough, self-recovery and self-healing polyampholyte hydrogels. Polym. Sci. Ser. C 2017, 59, 11–17. [Google Scholar] [CrossRef]
- Pafiti, K.S.; Elladiou, M.; Patrickios, C.S. “Inverse polyampholyte” hydrogels from double-cationic hydrogels: Synthesis by RAFT polymerization and characterization. Macromolecules 2014, 47, 1819–1827. [Google Scholar] [CrossRef]
- Wang, R.; Lowe, A.B. RAFT polymerization of styrenic-based phosphonium monomers and a new family of well-defined statistical and block polyampholytes. J. Polym. Sci. A 2007, 45, 2468–2483. [Google Scholar] [CrossRef]
- Zhang, Q.; Hoogenboom, R. UCST behavior of polyampholytes based on stoichiometric RAFT copolymerization of cationic and anionic monomers. Chem. Commun. 2015, 51, 70–73. [Google Scholar] [CrossRef]
- Morisada, S.; Suzuki, H.; Emura, S.; Hirokawa, Y.; Nakano, Y. Temperature-swing adsorption of aromatic compounds in water using polyampholyte gel. Adsorption 2008, 14, 621–628. [Google Scholar] [CrossRef]
- Pineda, B.A.; Liu, F.; Agarwal, S. Importance of compositional homogeneity of macromolecular chains for UCST-type transitions in water: Controlled versus conventional radical polymerization. J. Polym. Sci. A Polym. Chem. 2014, 52, 1878–1884. [Google Scholar] [CrossRef]
- Mitsukami, Y.; Donovan, M.S.; Lowe, A.B.; McCormick, C.L. Water-soluble polymers. 81. Direct synthesis of hydrophilic styrenic-based homopolymers and block copolymers in aqueous solution via RAFT. Macromolecules 2001, 34, 2248–2256. [Google Scholar] [CrossRef]
- Zhang, X.; Giani, O.; Monge, S.; Robin, J.J. RAFT polymerization of N,N-diethylacrylamide: Influence of chain transfer agent and solvent on kinetics and induction period. Polymer 2010, 51, 2947–2953. [Google Scholar] [CrossRef]
- Barner-Kowollik, C.; Buback, M.; Charleux, B.; Coote, M.L.; Drache, M.; Fukuda, T.; Goto, A.; Klumperman, B.; Lowe, A.B.; Mcleary, J.B.; et al. Mechanism and kinetics of dithiobenzoate-mediated RAFT polymerization. I. The current situation. J. Polym. Sci. A 2006, 44, 5809–5831. [Google Scholar] [CrossRef]
- Salamone, J.C.; Tsai, C.C.; Olson, A.P.; Watterson, A.C. Ampholytic polystyrene ionomers from cationic–anionic monomer pairs. J. Polym. Sci. A 1980, 18, 2983–2992. [Google Scholar] [CrossRef]
- Salamone, J.C.; Raheja, M.K.; Anwaruddin, Q.; Watterson, A.C. Polymerization of vinylpyridinium salts. XIII. Preparation of 4-vinyl-N-methylpyridinium p-styrenesulfonate charge transfer ion-pair comonomer. J. Polym. Sci. Polym. Lett. Ed. 1985, 23, 655–659. [Google Scholar] [CrossRef]
- Salamone, J.C.; Ahmed, I.; Rodriguez, E.L.; Quach, L.; Watterson, A.C. Synthesis and solution properties of ampholytic acrylamide ionomers. J. Macromol. Sci. Chem. 1988, A25, 811–837. [Google Scholar] [CrossRef]
- McCormick, C.L.; Johnson, C.B. Water-soluble polymers. 28. Ampholytic copolymers of sodium 2-acrylamido-2-methylpropanesulfonate with (2-acrylamido-2-methylpropyl)dimethylammonium chloride: Synthesis and characterization. Macromolecules 1988, 21, 686–693. [Google Scholar] [CrossRef]
- Corpart, J.M.; Selb, J.; Candau, F. Characterization of high charge density ampholytic copolymers prepared by microemulsion polymerization. Polymer 1993, 34, 3873–3886. [Google Scholar] [CrossRef]
- Fujihara, A.; Shimada, N.; Maruyama, A.; Ishihara, K.; Nakai, K.; Yusa, S. Preparation of upper critical solution temperature (UCST) responsive diblock copolymers bearing pendant ureido groups and their micelle formation behavior in water. Soft Matter 2015, 11, 5204–5213. [Google Scholar] [CrossRef]
- Mäntsälä, P.; Lang, M. 1-Anilino-8-naphthalene sulfonate and N-phenyl-1-naphthylamine as the indicators of bacterial thermosensitivity. FEBS Lett. 1973, 36, 265–267. [Google Scholar] [CrossRef]
- Hildebrand, V.; Heydenreich, M.; Laschewsky, A.; Moller, H.M.; Müller-Buschbaum, P.; Papadakis, C.M.; Schanzenbach, D.; Wischerhoff, E. “Schizophrenic” self-assembly of dual thermoresponsive block copolymers bearing a zwitterionic and a non-ionic hydrophilic block. Polymer 2017, 122, 347–357. [Google Scholar] [CrossRef]
- Nizardo, N.M.; Schanzenbach, D.; Schönemann, E.; Laschewsky, A. Exploring poly(ethylene glycol)-polyzwitterion diblock copolymers as biocompatible smart macrosurfactants featuring UCST-phase behavior in normal saline solution. Polymers 2018, 10, 325. [Google Scholar] [CrossRef]
- Arotçaréna, M.; Heise, B.; Ishaya, S.; Laschewsky, A. Switching the inside and the outside of aggregates of water-soluble block copolymers with double thermoresponsivity. J. Am. Chem. Soc. 2002, 124, 3787–3793. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Li, C.; Zhang, Y.; Furyk, S.; Cremer, P.S.; Bergbreiter, D.E. High-throughput studies of the effects of polymer structure and solution components on the phase separation of thermoresponsive polymers. Macromolecueles 2004, 37, 1031–1036. [Google Scholar] [CrossRef]
- Zhang, Y.; Furyk, S.; Bergbreiter, D.E.; Cremer, P.S. Specific ion effects on the water solubility of macromolecules: PNIPAM and the Hofmeister series. J. Am. Chem. Soc. 2005, 127, 14505–14510. [Google Scholar] [CrossRef]
- Hou, L.; Wu, P. Understanding the UCST-type transition of P(AAm-co-AN) in H2O and D2O: Dramatic effects of solvent isotopes. Soft Matter 2015, 11, 7059–7065. [Google Scholar] [CrossRef]
- Huff, A.; Patton, K.; Odhner, H.; Jacobs, D.T.; Clover, B.C.; Greer, S.C. Micellization and phase separation for triblock copolymer 17R4 in H2O and in D2O. Langmuir 2011, 27, 1707–1712. [Google Scholar] [CrossRef]
- Luan, C.H.; Wry, D.W. Solvent deuteration enhancement of hydrophobicity: DSC study of the inverse temperature transition of elastin-based polypeptides. J. Phys. Chem. 1991, 95, 7896–7900. [Google Scholar] [CrossRef]
| Samples | p a (%) | VBTAC Content b (mol%) | DP (Theory) c | Mn (Theory) d g/mol | Tp (°C) e | ||
|---|---|---|---|---|---|---|---|
| %T f | Rhg | SI h | |||||
| P(VBTAC/NaSS)20 | 99.2 | 48 | 20 | 4.18 × 103 | 46.5 | 46.1 | 46.0 |
| P(VBTAC/NaSS)97 | 97.1 | 52 | 97 | 2.03 × 104 | 48.7 | 48.3 | 48.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanta Sharker, K.; Ohara, Y.; Shigeta, Y.; Ozoe, S.; Yusa, S.-i. Upper Critical Solution Temperature (UCST) Behavior of Polystyrene-Based Polyampholytes in Aqueous Solution. Polymers 2019, 11, 265. https://doi.org/10.3390/polym11020265
Kanta Sharker K, Ohara Y, Shigeta Y, Ozoe S, Yusa S-i. Upper Critical Solution Temperature (UCST) Behavior of Polystyrene-Based Polyampholytes in Aqueous Solution. Polymers. 2019; 11(2):265. https://doi.org/10.3390/polym11020265
Chicago/Turabian StyleKanta Sharker, Komol, Yuki Ohara, Yusuke Shigeta, Shinji Ozoe, and Shin-ichi Yusa. 2019. "Upper Critical Solution Temperature (UCST) Behavior of Polystyrene-Based Polyampholytes in Aqueous Solution" Polymers 11, no. 2: 265. https://doi.org/10.3390/polym11020265
APA StyleKanta Sharker, K., Ohara, Y., Shigeta, Y., Ozoe, S., & Yusa, S.-i. (2019). Upper Critical Solution Temperature (UCST) Behavior of Polystyrene-Based Polyampholytes in Aqueous Solution. Polymers, 11(2), 265. https://doi.org/10.3390/polym11020265