Modification of Polyurethane Sponge Based on the Thiol–Ene Click Reaction and Its Application for Oil/Water Separation
Abstract
1. Introduction
2. Experimental
2.1. Materials
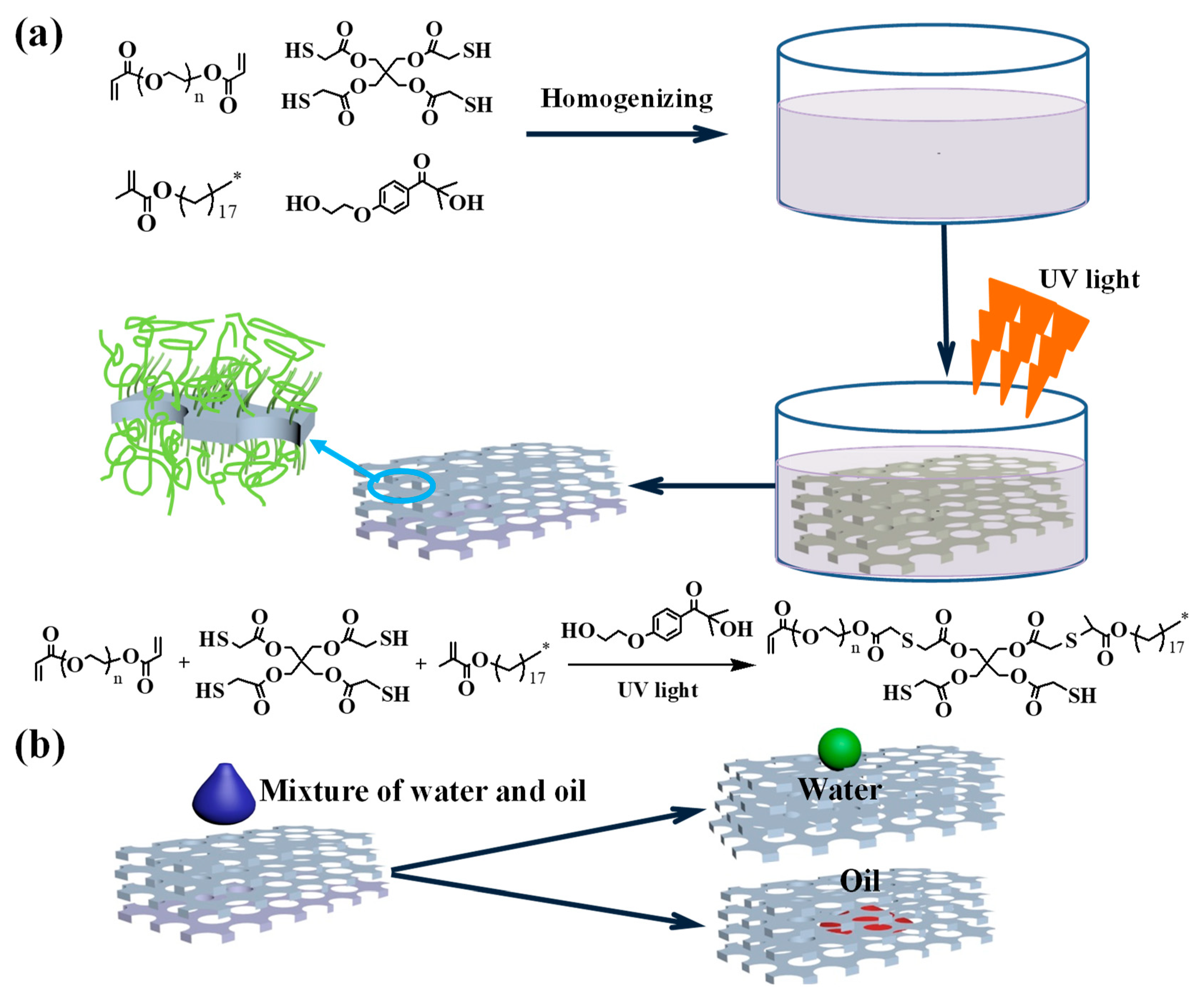
2.2. Modification of Polyurethane Sponge Based on the Thiol–Ene Click Reaction
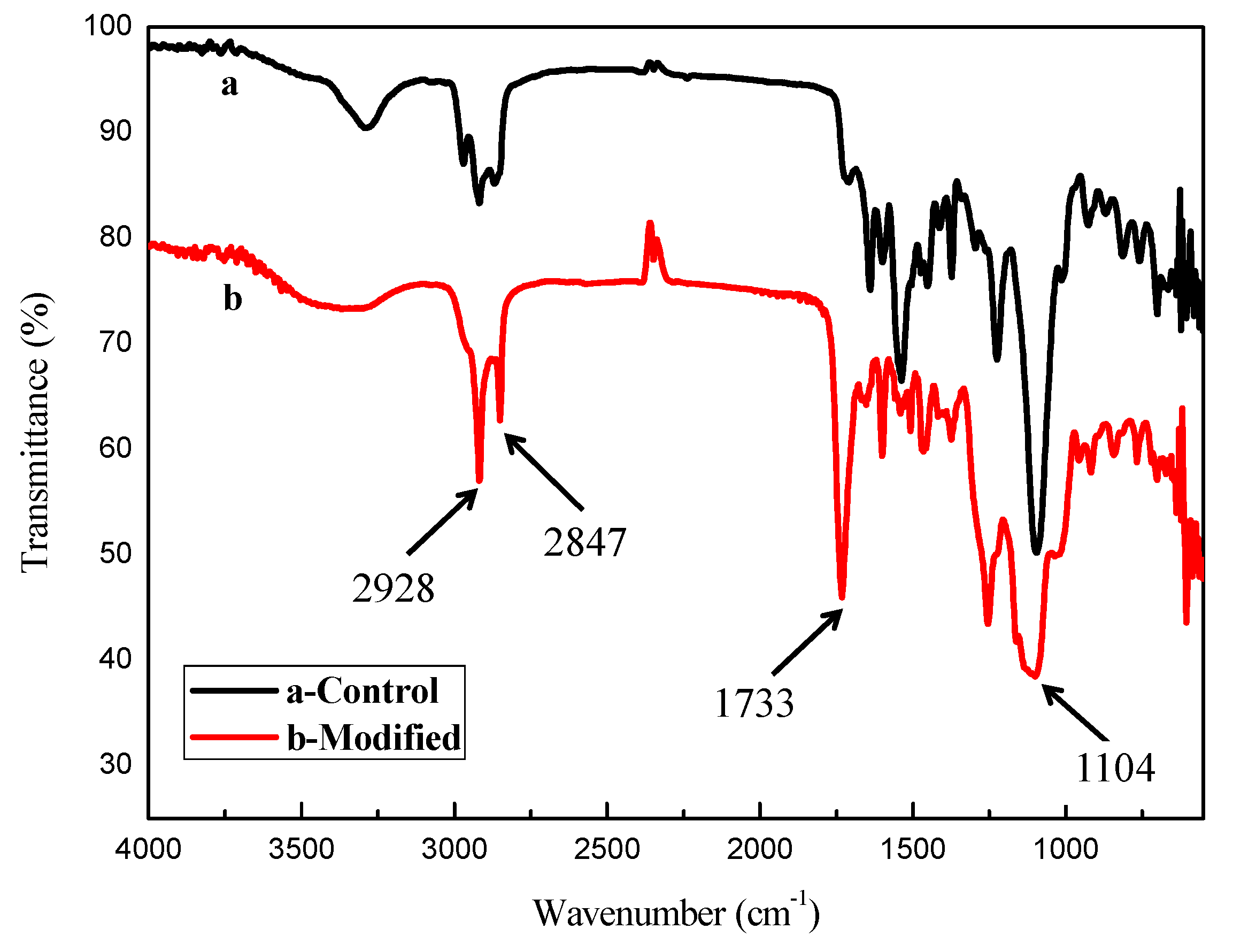
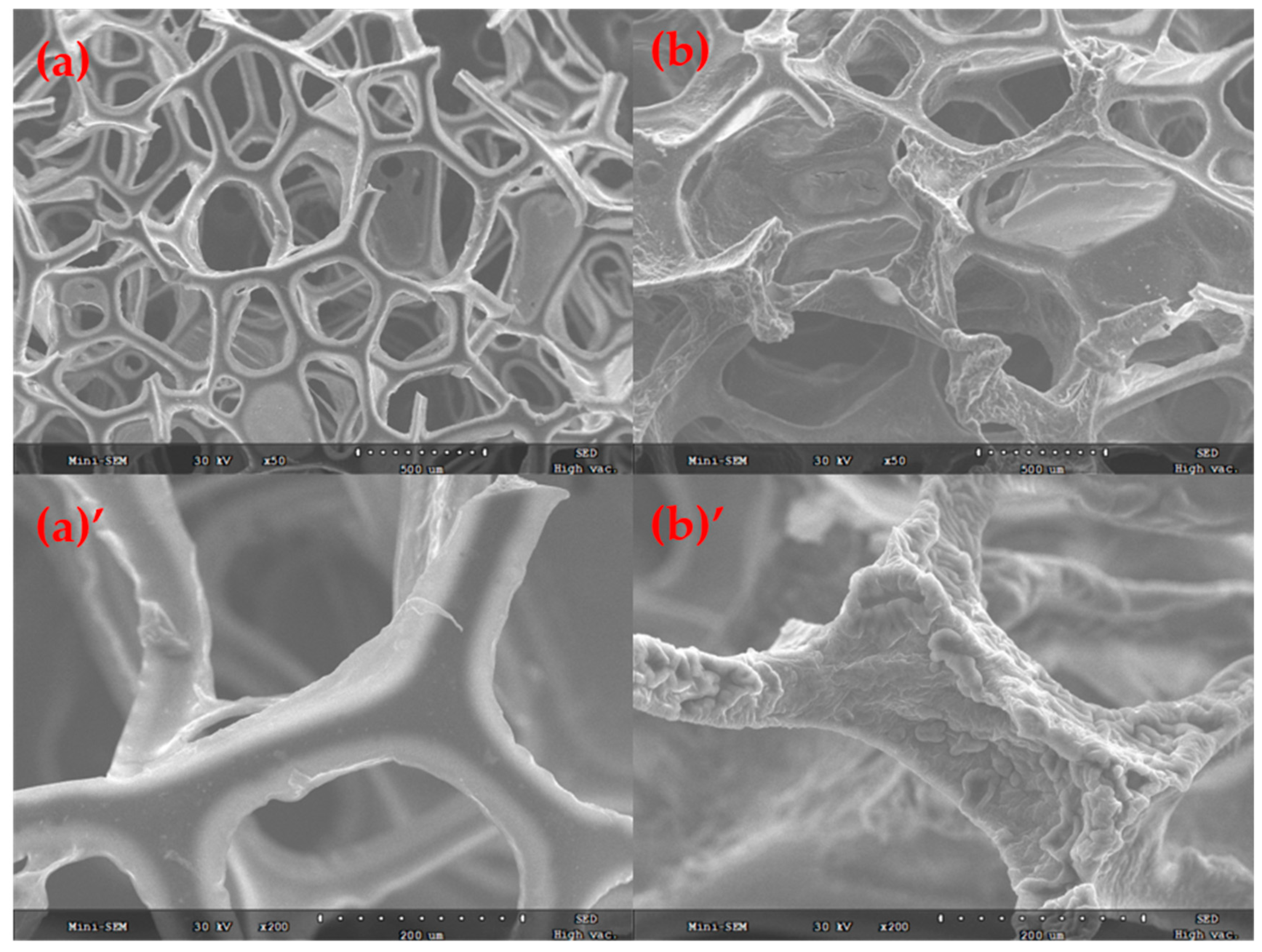
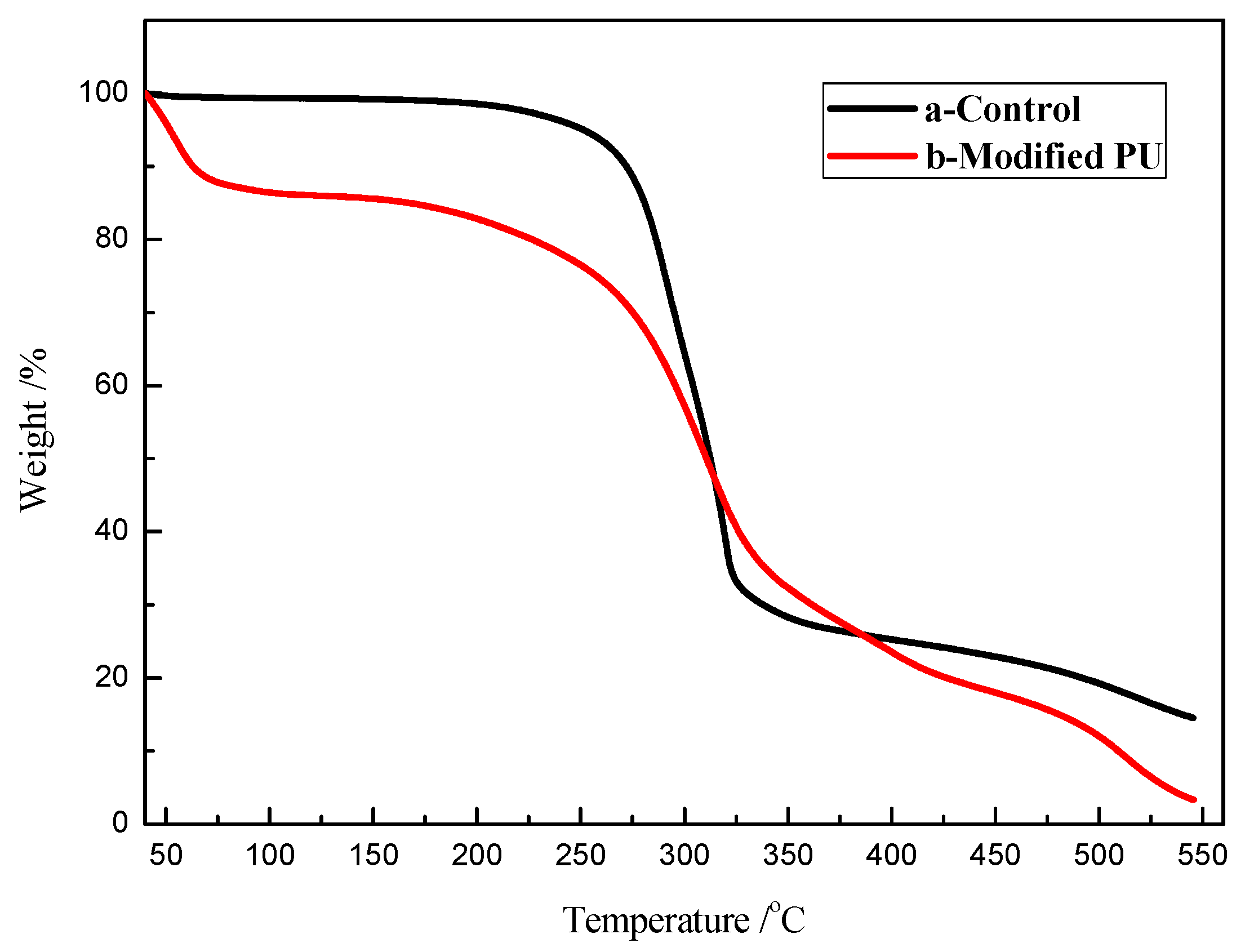
2.3. Characterizations
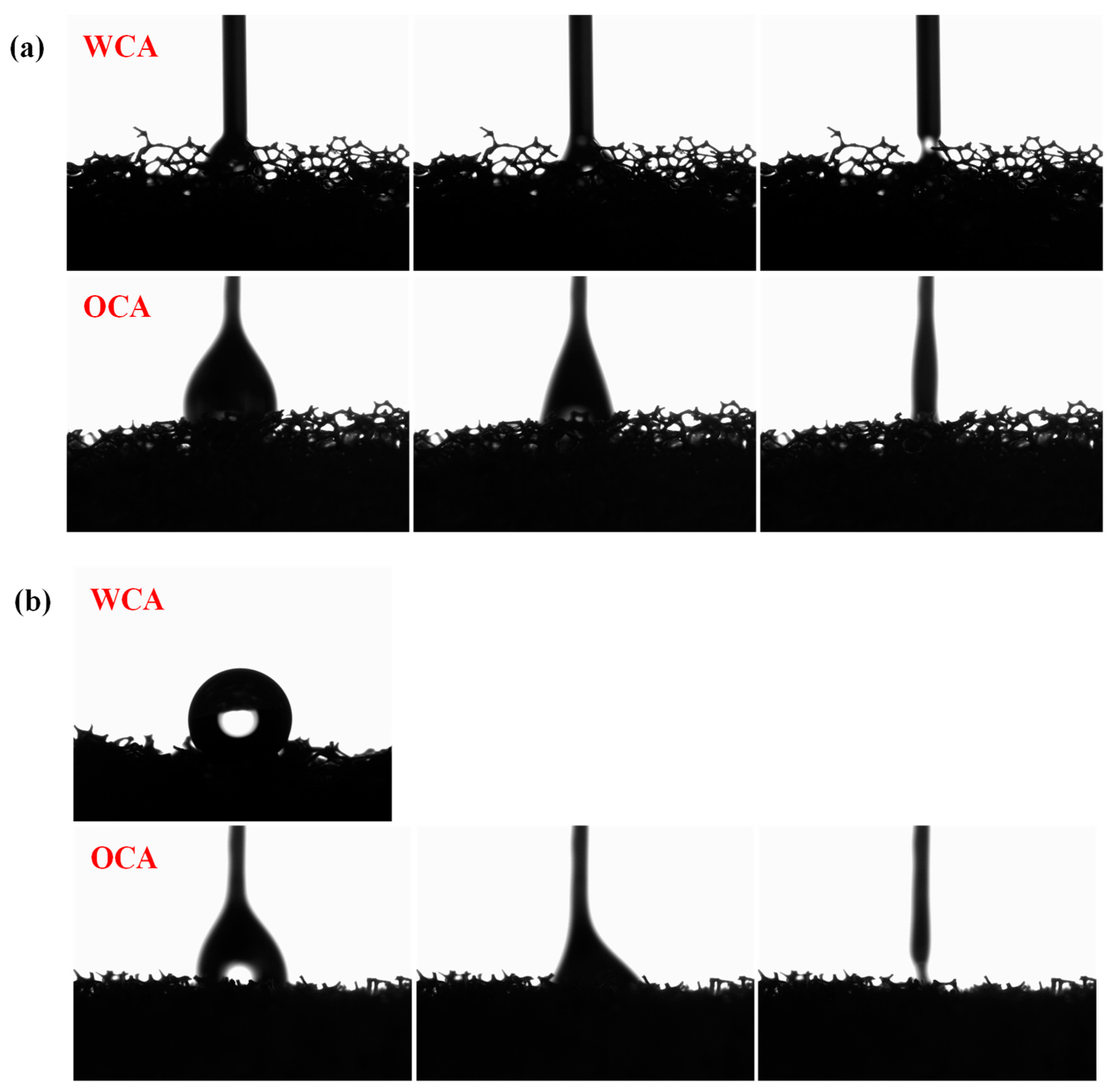
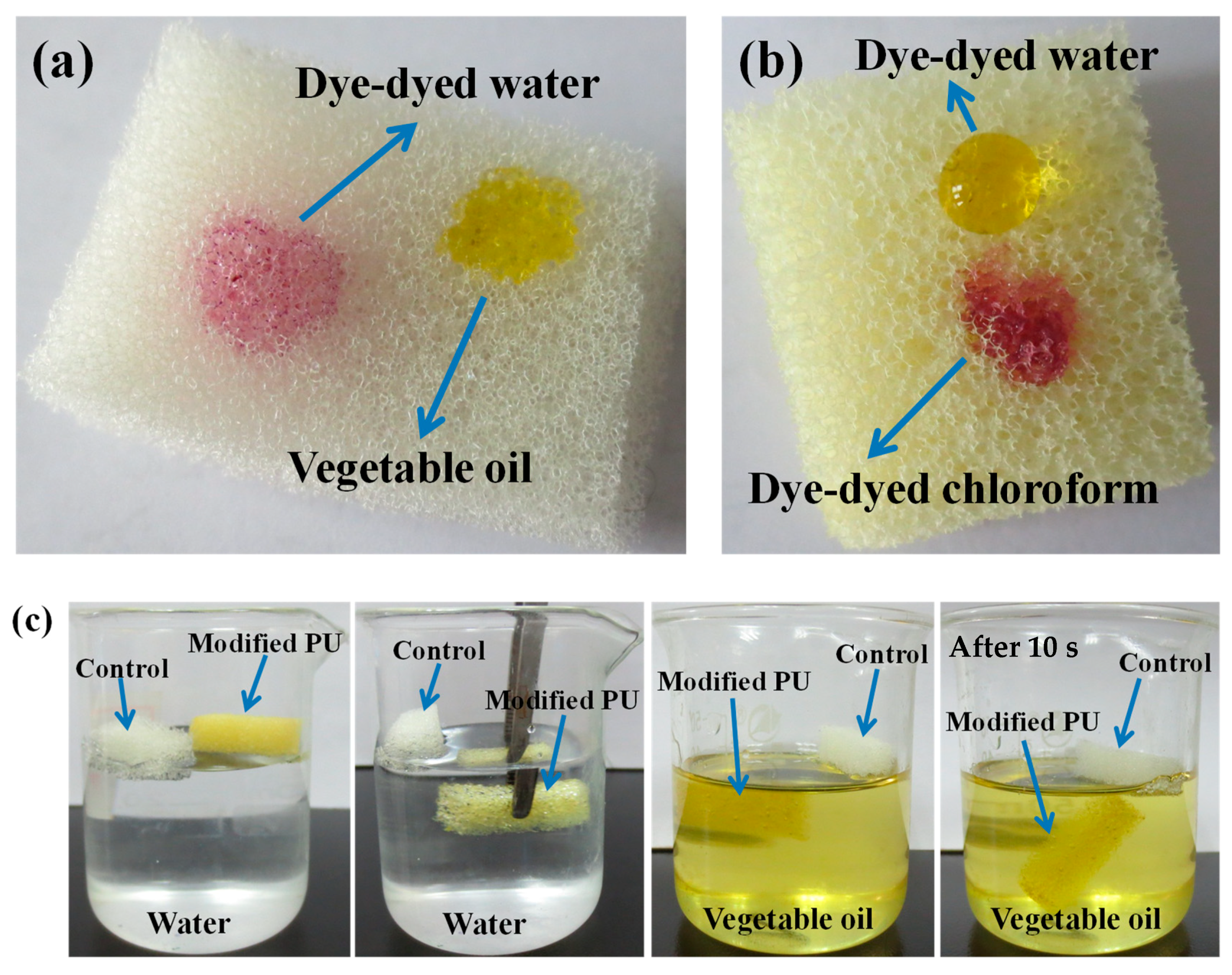
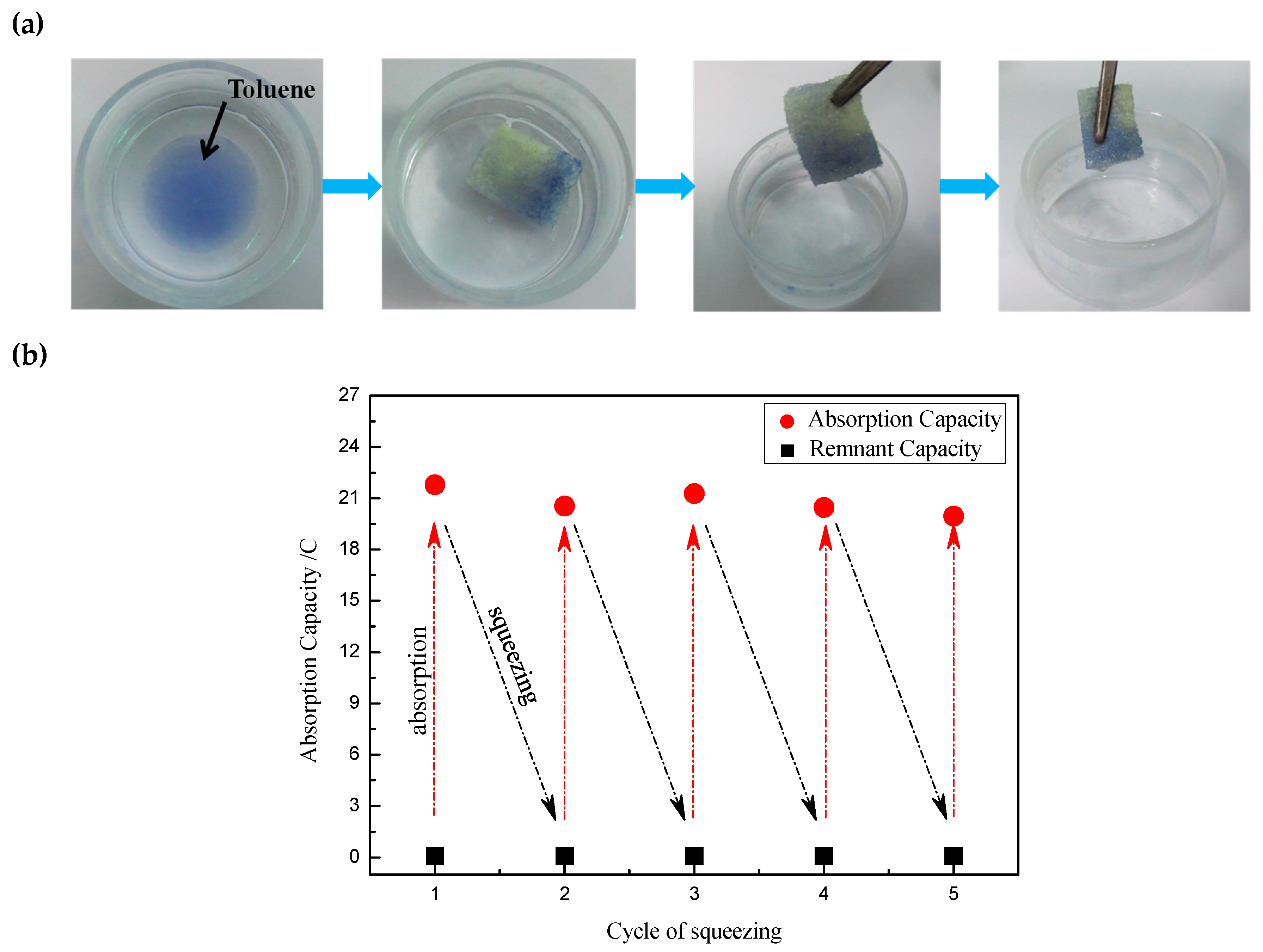
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yagoub, H.; Zhu, L.; Shibraen, M.H.M.A.; Altam, A.A.; Babiker, D.M.D.; Rehan, K.; Mukwaya, V.; Xu, J.; Yang, S. Manipulating the surface wettability of polysaccharide based complex membrane for oil/water separation. Carbohydr. Polym. 2019, 225, 115231. [Google Scholar] [CrossRef] [PubMed]
- Latthe, S.S.; Sutar, R.S.; Kodag, V.S.; Bhosale, A.K.; Madhan Kumar, A.; Sadasivuni, K.K.; Xing, R.M.; Liu, S.H. Self–cleaning superhydrophobic coatings: Potential industrial applications. Prog. Org. Coat. 2019, 128, 52–58. [Google Scholar] [CrossRef]
- Spier, C.; Stringfellow, W.T.; Hazen, T.C.; Conrad, M. Distribution of hydrocarbons released during the 2010 MC252 oil spill in deep offshore waters. Environ. Pollut. 2013, 173, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Vásquez, L.; Campagnolo, L.; Athanassiou, A.; Fragouli, D. Expanded Graphite-Polyurethane Foams for Water–Oil Filtration. ACS Appl. Mater. Interfaces 2019, 11, 30207–30217. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. A smart membrane with antifouling capability and switchable oil wettability for high-efficiency oil/water emulsions separation. J. Membr. Sci. 2018, 555, 69–77. [Google Scholar] [CrossRef]
- Bayat, A.; Aghamiri, S.F.; Moheb, A.; Vakili-Nezhaad, G.R. Oil Spill Cleanup from Sea Water by Sorbent Materials. Chem. Eng. Technol. 2005, 28, 1525–1528. [Google Scholar] [CrossRef]
- Chen, L.; Wu, S.; Lu, H.; Huang, K.; Zhao, L. Numerical Simulation and Structural Optimization of the Inclined Oil/Water Separator. PLoS ONE 2015, 10, e0124095. [Google Scholar] [CrossRef][Green Version]
- Baig, N.; Alghunaimi, F.I.; Dossary, H.S.; Saleh, T.A. Superhydrophobic and superoleophilic carbon nanofiber grafted polyurethane for oil-water separation. Process Saf. Environ. Prot. 2019, 123, 327–334. [Google Scholar] [CrossRef]
- Sultanov, F.R.; Daulbayev, C.; Bakbolat, B.; Mansurov, Z.A.; Urazgaliyeva, A.A.; Ebrahim, R.; Pei, S.S.; Huang, K.-P. Microwave-enhanced chemical vapor deposition graphene nanoplatelets-derived 3D porous materials for oil/water separation. Carbon Lett. 2019, 1–12. [Google Scholar]
- Saththasivam, J.; Wubulikasimu, Y.; Ogunbiyi, O.; Liu, Z. Fast and efficient separation of oil/saltwater emulsions with anti-fouling ZnO microsphere/carbon nanotube membranes. J. Water Process Eng. 2019, 32, 100901. [Google Scholar] [CrossRef]
- Nguyen, D.D.; Tai, N.-H.; Lee, S.-B.; Kuo, W.-S. Superhydrophobic and superoleophilic properties of graphene-based sponges fabricated using a facile dip coating method. Energy Environ. Sci. 2012, 5, 7908–7912. [Google Scholar] [CrossRef]
- Wang, C.-F.; Lin, S.-J. Robust Superhydrophobic/Superoleophilic Sponge for Effective Continuous Absorption and Expulsion of Oil Pollutants from Water. ACS Appl. Mater. Interfaces 2013, 5, 8861–8864. [Google Scholar] [CrossRef] [PubMed]
- Beshkar, F.; Khojasteh, H.; Salavati-Niasari, M. Recyclable magnetic superhydrophobic straw soot sponge for highly efficient oil/water separation. J. Colloid Interface Sci. 2017, 497, 57–65. [Google Scholar] [CrossRef]
- Gupta, R.K.; Dunderdale, G.J.; England, M.W.; Hozumi, A. Oil/water separation techniques: A review of recent progresses and future directions. J. Mater. Chem. A 2017, 5, 16025–16058. [Google Scholar] [CrossRef]
- Cho, E.-C.; Chang-Jian, C.-W.; Hsiao, Y.-S.; Lee, K.-C.; Huang, J.-H. Interfacial engineering of melamine sponges using hydrophobic TiO2 nanoparticles for effective oil/water separation. J. Taiwan Inst. Chem. Eng. 2016, 67, 476–483. [Google Scholar] [CrossRef]
- Stolz, A.; Le Floch, S.; Reinert, L.; Ramos, S.M.M.; Tuaillon-Combes, J.; Soneda, Y.; Chaudet, P.; Baillis, D.; Blanchard, N.; Duclaux, L.; et al. Melamine-derived carbon sponges for oil-water separation. Carbon 2016, 107, 198–208. [Google Scholar] [CrossRef]
- Sun, S.; Tang, S.; Chang, X.; Wang, N.; Wang, D.; Liu, T.; Lei, Y.; Zhu, Y. A bifunctional melamine sponge decorated with silver-reduced graphene oxide nanocomposite for oil-water separation and antibacterial applications. Appl. Surf. Sci. 2019, 473, 1049–1061. [Google Scholar] [CrossRef]
- Chen, J.; You, H.; Xu, L.; Li, T.; Jiang, X.; Li, C.M. Facile synthesis of a two-tier hierarchical structured superhydrophobic-superoleophilic melamine sponge for rapid and efficient oil/water separation. J. Colloid Interface Sci. 2017, 506, 659–668. [Google Scholar] [CrossRef]
- Pham, V.H.; Dickerson, J.H. Superhydrophobic Silanized Melamine Sponges as High Efficiency Oil Absorbent Materials. ACS Appl. Mater. Interfaces 2014, 6, 14181–14188. [Google Scholar] [CrossRef]
- Meng, X.; Zhan, H.; Xi, B.; Li, X.; Zhou, X.; Deng, Q.; Liang, L. Fabrication of superhydrophobic sponge with hierarchical structure and application for oil/water separation. J. Macromol. Sci. Part A 2017, 54, 877–884. [Google Scholar] [CrossRef]
- He, L.; Szopinski, D.; Wu, Y.; Luinstra, G.A.; Theato, P. Toward Self-Healing Hydrogels Using One-Pot Thiol−Ene Click and Borax-Diol Chemistry. ACS Macro Lett. 2015, 4, 673–678. [Google Scholar] [CrossRef]
- Huang, S.; Li, X.; Jiao, Y.; Shi, J. Fabrication of a Superhydrophobic, Fire-Resistant, and Mechanical Robust Sponge upon Polyphenol Chemistry for Efficiently Absorbing Oils/Organic Solvents. Ind. Eng. Chem. Res. 2015, 54, 1842–1848. [Google Scholar] [CrossRef]
- Lei, Z.; Zhang, G.; Deng, Y.; Wang, C. Thermo-Responsive Melamine Sponges with Switchable Wettability by Interface-Initiated Atom Transfer Radical Polymerization for Oil/Water Separation. ACS Appl. Mater. Interfaces 2017, 9, 8967–8974. [Google Scholar] [CrossRef] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, L.; Dong, Y.; Liu, Y.; Meng, X. Modification of Polyurethane Sponge Based on the Thiol–Ene Click Reaction and Its Application for Oil/Water Separation. Polymers 2019, 11, 2072. https://doi.org/10.3390/polym11122072
Liang L, Dong Y, Liu Y, Meng X. Modification of Polyurethane Sponge Based on the Thiol–Ene Click Reaction and Its Application for Oil/Water Separation. Polymers. 2019; 11(12):2072. https://doi.org/10.3390/polym11122072
Chicago/Turabian StyleLiang, Liping, Yanyan Dong, Yuqing Liu, and Xu Meng. 2019. "Modification of Polyurethane Sponge Based on the Thiol–Ene Click Reaction and Its Application for Oil/Water Separation" Polymers 11, no. 12: 2072. https://doi.org/10.3390/polym11122072
APA StyleLiang, L., Dong, Y., Liu, Y., & Meng, X. (2019). Modification of Polyurethane Sponge Based on the Thiol–Ene Click Reaction and Its Application for Oil/Water Separation. Polymers, 11(12), 2072. https://doi.org/10.3390/polym11122072





