Large and Giant Unilamellar Vesicle(s) Obtained by Self-Assembly of Poly(dimethylsiloxane)-b-poly(ethylene oxide) Diblock Copolymers, Membrane Properties and Preliminary Investigation of Their Ability to Form Hybrid Polymer/Lipid Vesicles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
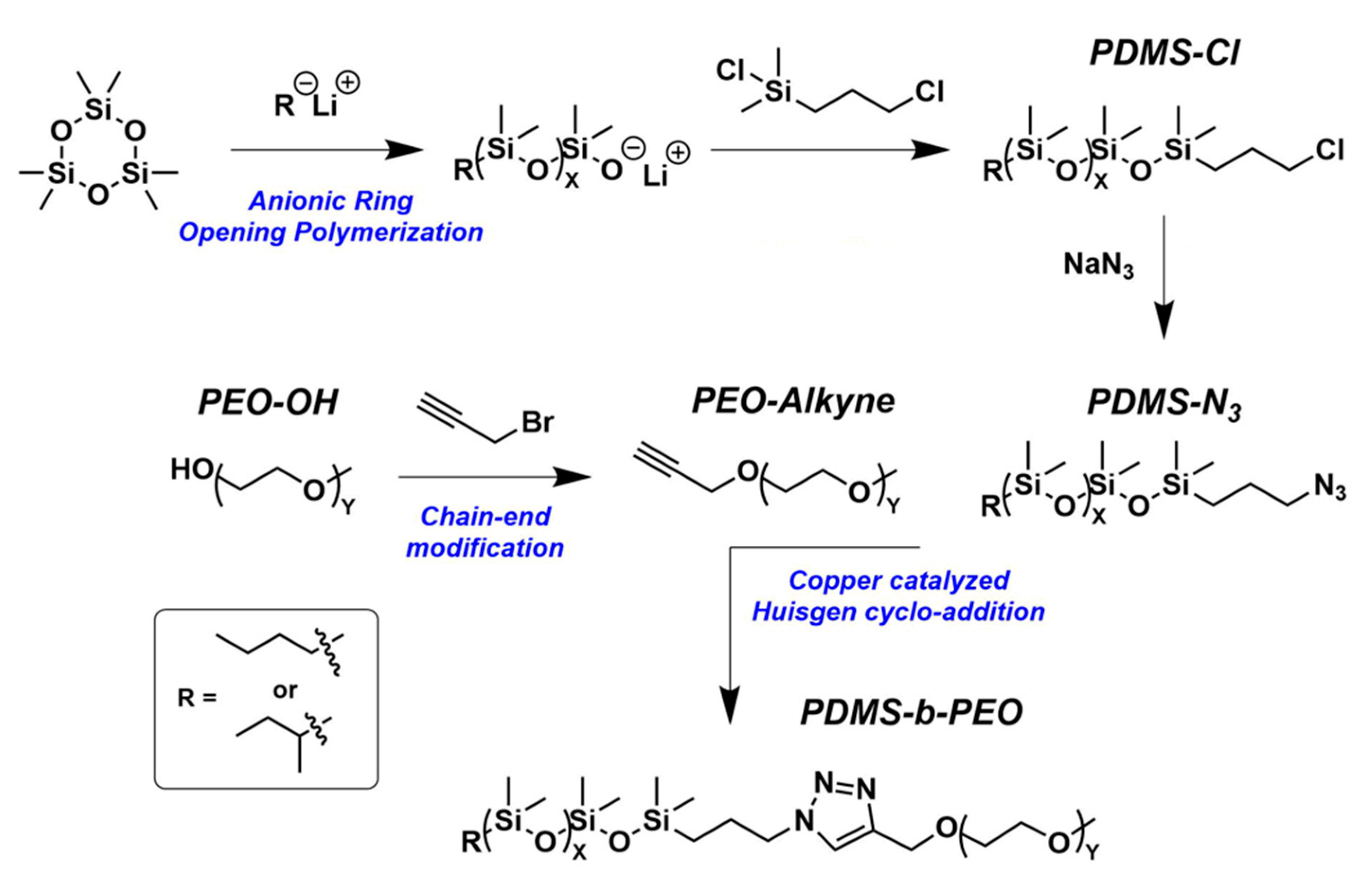
2.2. ω-chloro-PDMS Synthesis: Example of PDMS27-Cl
2.3. ω-azido-PDMS Synthesis: Example of PDMS27-N3
2.4. α-alkyne-PEO Synthesis: Example of PEO17-Alkyne
2.5. PDMS-b-PEO Synthesis: Example of PDMS27-b-PEO17
2.6. 1H Nuclear Magnetic Resonance Characterization
2.7. Size Exclusion Chromatography
2.8. Process to Obtain Self-assembled Nano-structures
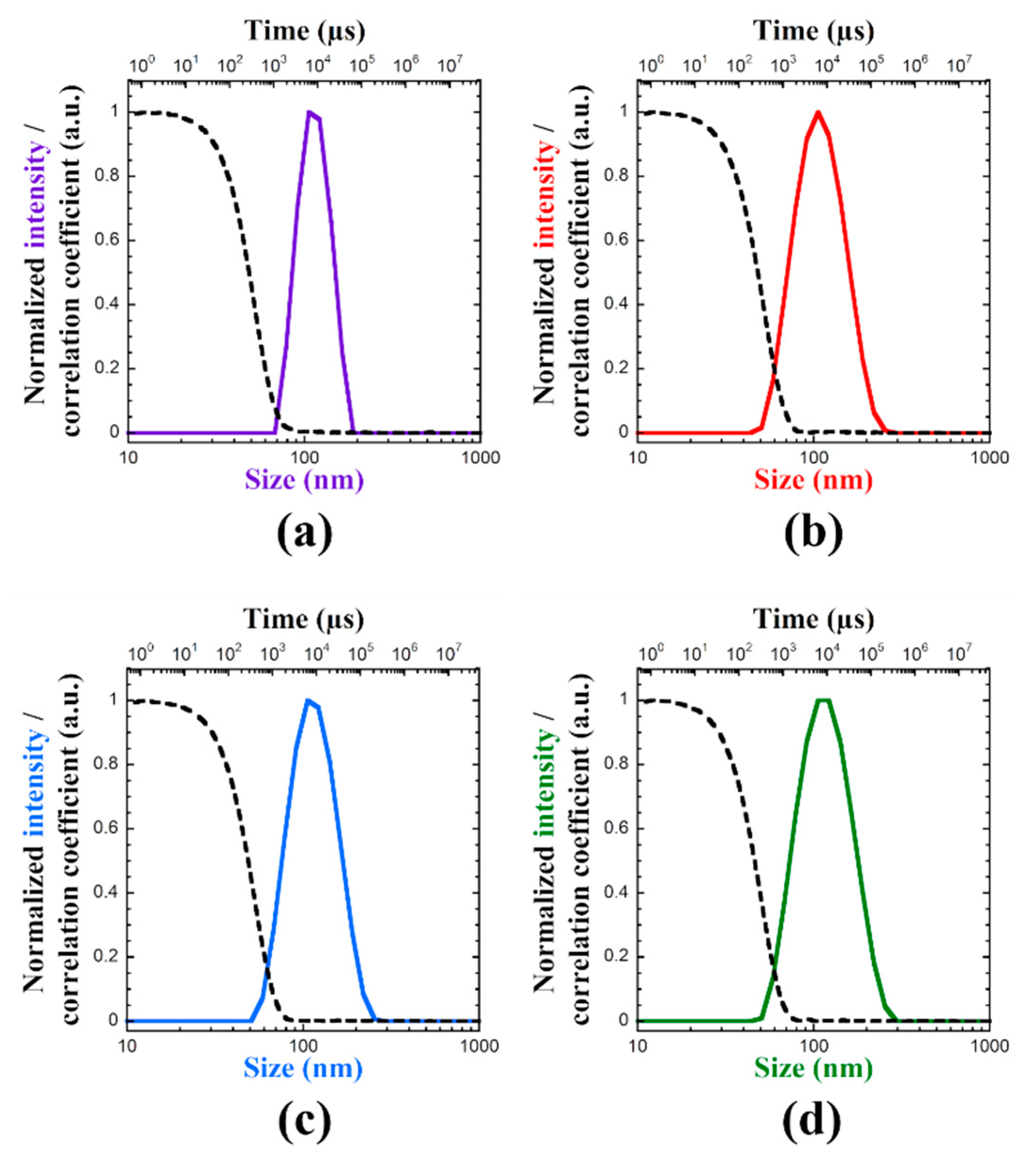
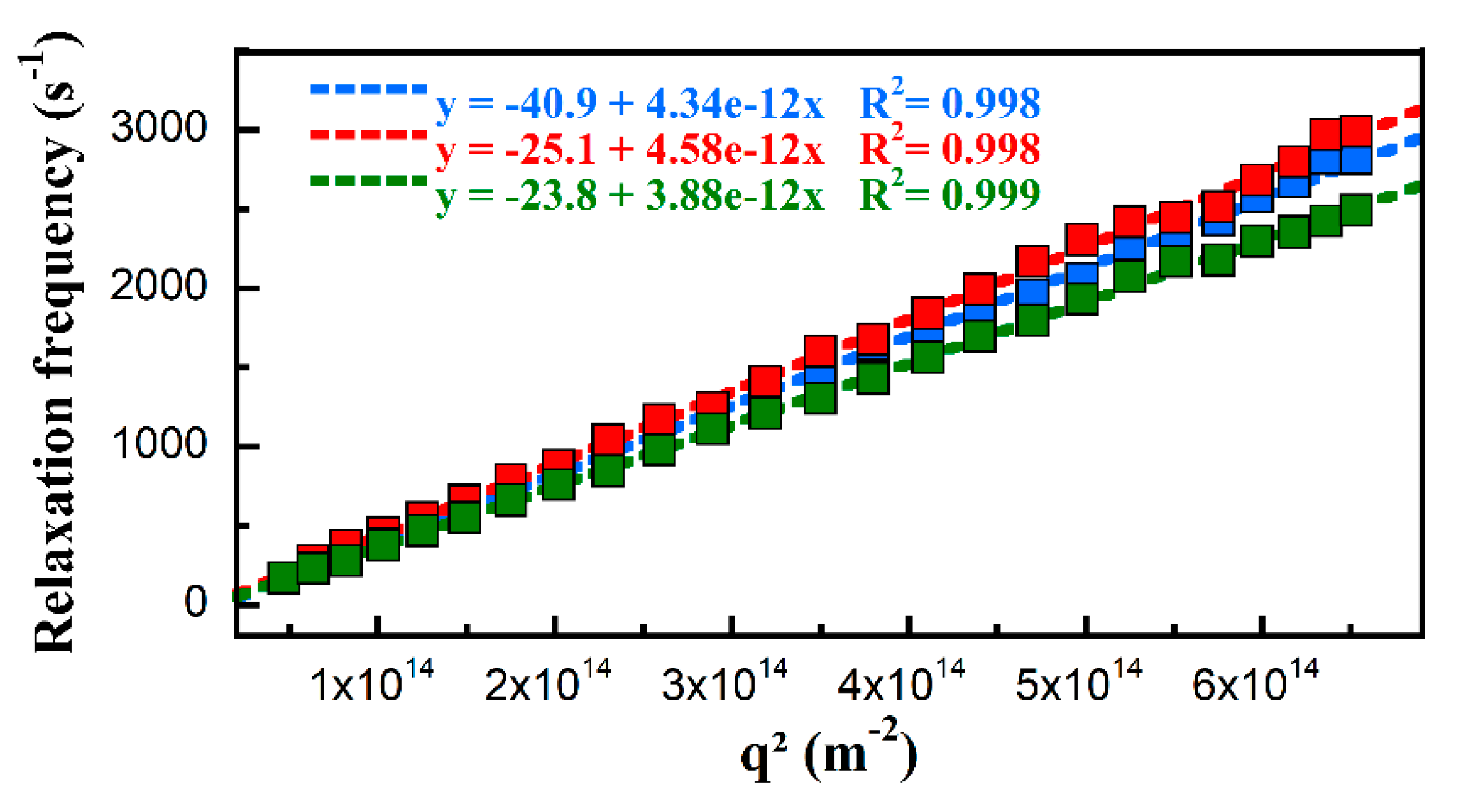
2.9. Dynamic and Static Light Scattering
2.10. Small Angle Neutron Scattering
2.11. Cryo Transmission Electron Microscopy
2.12. Giant Unilamellar Vesicle Preparation
2.13. Micropipette Experiments
3. Results and Discussion
3.1. Block Copolymer Synthesis and Characterization
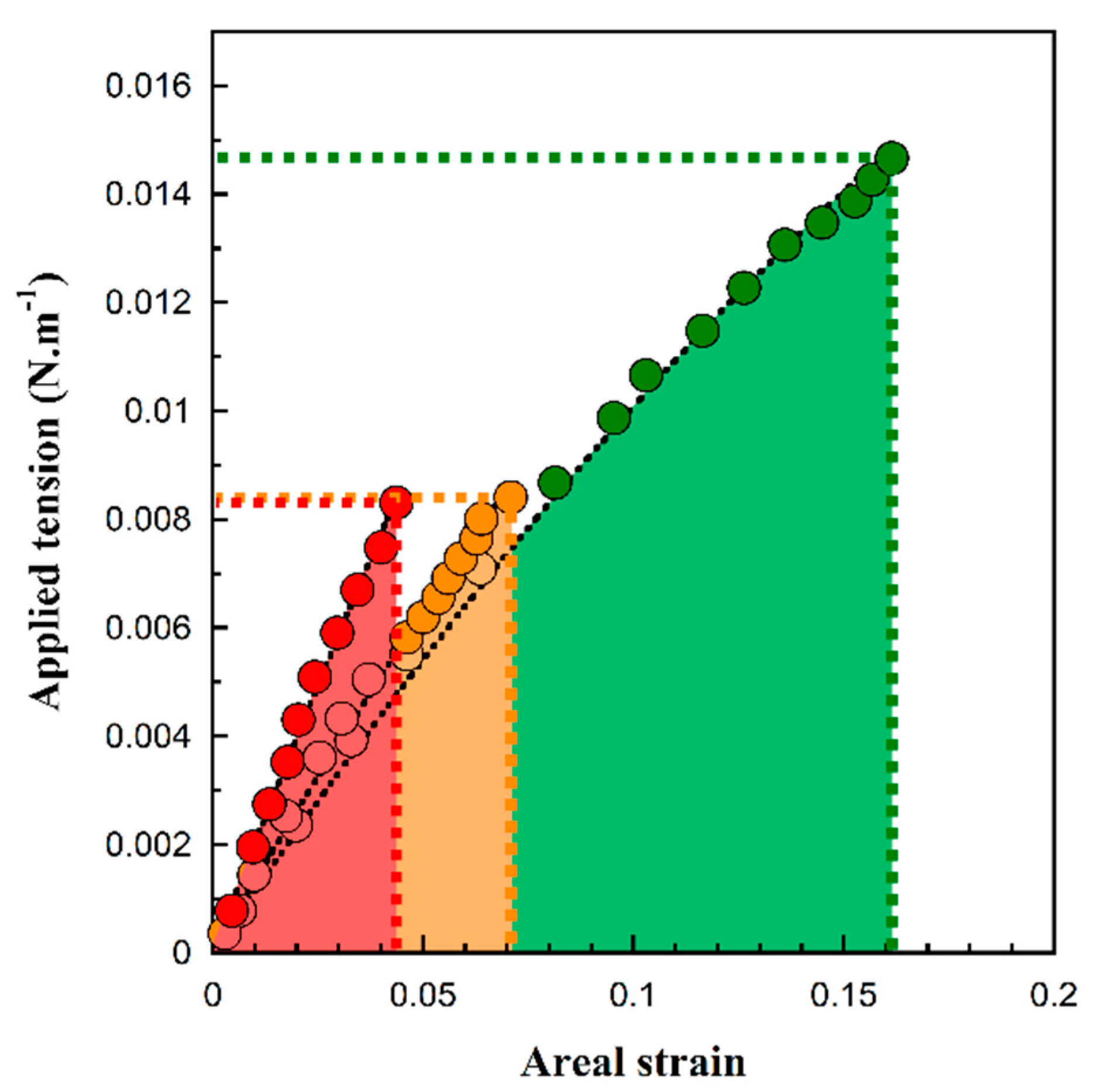
3.2. Giant Hybrid Unilamellar Vesicles Formation and their Mechanical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Schulz, M.; Binder, W.H. Mixed Hybrid Lipid/Polymer Vesicles as a Novel Membrane Platform. Macromol. Rapid Commun. 2015, 36, 2031–2041. [Google Scholar] [CrossRef] [PubMed]
- Le Meins, J.F.; Schatz, C.; Lecommandoux, S.; Sandre, O. Hybrid polymer/lipid vesicles: State of the art and future perspectives. Mater. Today 2013, 16, 397–402. [Google Scholar] [CrossRef]
- Lim, S.; de Hoog, H.-P.; Parikh, A.; Nallani, M.; Liedberg, B. Hybrid, Nanoscale Phospholipid/Block Copolymer Vesicles. Polymers 2013, 5, 1102–1114. [Google Scholar] [CrossRef]
- Nam, J.; Vanderlick, T.K.; Beales, P.A. Formation and dissolution of phospholipid domains with varying textures in hybrid lipo-polymersomes. Soft Matter 2012, 8, 7982–7988. [Google Scholar] [CrossRef]
- Gettel, D.L.; Sanborn, J.; Patel, M.A.; de Hoog, H.-P.; Liedberg, B.; Nallani, M.; Parikh, A.N. Mixing, Diffusion, and Percolation in Binary Supported Membranes Containing Mixtures of Lipids and Amphiphilic Block Copolymers. J. Am. Chem.Soc. 2014, 136, 10186–10189. [Google Scholar] [CrossRef] [PubMed]
- Paxton, W.F.; McAninch, P.T.; Achyuthan, K.E.; Shin, S.H.R.; Monteith, H.L. Monitoring and modulating ion traffic in hybrid lipid/polymer vesicles. Colloids Surf. B Biointerfaces 2017, 159, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Mumtaz Virk, M.; Reimhult, E. Phospholipase A2-Induced Degradation and Release from Lipid-Containing Polymersomes. Langmuir 2018, 34, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Magnani, C.; Montis, C.; Mangiapia, G.; Mingotaud, A.F.; Mingotaud, C.; Roux, C.; Joseph, P.; Berti, D.; Lonetti, B. Hybrid vesicles from lipids and block copolymers: Phase behavior from the micro-to the nano-scale. Colloids Surf. B Biointerfaces 2018, 168, 18–28. [Google Scholar] [CrossRef]
- Paxton, W.F.; McAninch, P.T.; Shin, S.H.R.; Brumbach, M.T. Adsorption and fusion of hybrid lipid/polymer vesicles onto 2D and 3D surfaces. Soft Matter 2018, 14, 8112–8118. [Google Scholar] [CrossRef]
- Kang, M.; Lee, B.; Leal, C. Three-Dimensional Microphase Separation and Synergistic Permeability in Stacked Lipid–Polymer Hybrid Membranes. Chem. Mater. 2017, 29, 9120–9132. [Google Scholar] [CrossRef]
- Bixner, O.; Bello, G.; Virk, M.; Kurzhals, S.; Scheberl, A.; Gal, N.; Matysik, A.; Kraut, R.; Reimhult, E. Magneto-Thermal Release from Nanoscale Unilamellar Hybrid Vesicles. ChemNanoMat 2016, 2, 1111–1121. [Google Scholar] [CrossRef]
- Khan, S.; Li, M.; Muench, S.P.; Jeuken, L.J.C.; Beales, P.A. Durable proteo-hybrid vesicles for the extended functional lifetime of membrane proteins in bionanotechnology. Chem. Commun. 2016, 52, 11020–11023. [Google Scholar] [CrossRef] [PubMed]
- Winzen, S.; Bernhardt, M.; Schaeffel, D.; Koch, A.; Kappl, M.; Koynov, K.; Landfester, K.; Kroeger, A. Submicron hybrid vesicles consisting of polymer-lipid and polymer-cholesterol blends. Soft Matter 2013, 9, 5883–5890. [Google Scholar] [CrossRef]
- Kowal, J.; Wu, D.; Mikhalevich, V.; Palivan, C.G.; Meier, W. Hybrid Polymer–Lipid Films as Platforms for Directed Membrane Protein Insertion. Langmuir 2015, 31, 4868–4877. [Google Scholar] [CrossRef] [PubMed]
- Dao, T.P.T.; Fernandes, F.; Fauquignon, M.; Ibarboure, E.; Prieto, M.; Le Meins, J.F. The combination of block copolymers and phospholipids to form giant hybrid unilamellar vesicles (GHUVs) does not systematically lead to intermediate’ membrane properties. Soft Matter 2018, 14, 6476–6484. [Google Scholar] [CrossRef] [PubMed]
- Dao, T.P.; Fernandes, F.; Ibarboure, E.; Ferji, K.; Prieto, M.; Sandre, O.; Le Meins, J.F. Modulation of phase separation at the micron scale and nanoscale in giant polymer/lipid hybrid unilamellar vesicles (GHUVs). Soft Matter 2017, 13, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Dao, T.P.T.; Brûlet, A.; Fernandes, F.; Er-Rafik, M.; Ferji, K.; Schweins, R.; Chapel, J.P.; Fedorov, A.; Schmutz, M.; Prieto, M.; et al. Le Meins, Mixing Block Copolymers with Phospholipids at the Nanoscale: From Hybrid Polymer/Lipid Wormlike Micelles to Vesicles Presenting Lipid Nanodomains. Langmuir 2017, 33, 1705–1715. [Google Scholar] [CrossRef] [PubMed]
- Dao, T.P.T.; Fernandes, F.; Er-Rafik, M.; Salva, R.; Schmutz, M.; Brulet, A.; Prieto, M.; Sandre, O.; Le Meins, J.F. Phase Separation and Nanodomain Formation in Hybrid Polymer/Lipid Vesicles. ACS Macro Lett. 2015, 4, 182–186. [Google Scholar] [CrossRef]
- Chemin, M.; Brun, P.M.; Lecommandoux, S.; Sandre, O.; Le Meins, J.F. Hybrid polymer/lipid vesicles: Fine control of the lipid and polymer distribution in the binary membrane. Soft Matter 2012, 8, 2867–2874. [Google Scholar] [CrossRef]
- Ruysschaert, T.; Sonnen, A.F.P.; Haefele, T.; Meier, W.; Winterhalter, M.; Fournier, D. Hybrid nanocapsules: Interactions of ABA block copolymers with liposomes. J. Am. Chem. Soc. 2005, 127, 6242–6247. [Google Scholar] [CrossRef]
- Schulz, M.; Olubummo, A.; Bacia, K.; Binder, W.H. Lateral surface engineering of hybrid lipid-BCP vesicles and selective nanoparticle embedding. Soft Matter 2014, 10, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Olubummo, A.; Schulz, M.; Schöps, R.; Kressler, J.; Binder, W.H. Phase Changes in Mixed Lipid/Polymer Membranes by Multivalent Nanoparticle Recognition. Langmuir 2014, 30, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Schulz, M.; Werner, S.; Bacia, K.; Binder, W.H. Controlling molecular recognition with lipid/polymer domains in vesicle membranes. Angew. Chem. Int. Ed. 2013, 52, 1829–1833. [Google Scholar] [CrossRef] [PubMed]
- Pippa, N.; Deli, E.; Mentzali, E.; Pispas, S.; Demetzos, C. PEO-b-PCL grafted DPPC liposomes: Physicochemical characterization and stability studies of novel bio-inspired advanced Drug Delivery nano Systems (aDDnSs). J. Nanosci. Nanotechnol. 2014, 14, 5676–5681. [Google Scholar] [CrossRef]
- Pippa, N.; Kaditi, E.; Pispas, S.; Demetzos, C. PEO-b-PCL-DPPC chimeric nanocarriers: Self-assembly aspects in aqueous and biological media and drug incorporation. Soft Matter 2013, 9, 4073–4082. [Google Scholar] [CrossRef]
- Bieligmeyer, M.; Artukovic, F.; Nussberger, S.; Hirth, T.; Schiestel, T.; Müller, M. Reconstitution of the membrane protein OmpF into biomimetic block copolymer—phospholipid hybrid membranes. Beilstein J. Nanotechnol. 2016, 7, 881–892. [Google Scholar] [CrossRef]
- Pippa, N.; Stellas, D.; Skandalis, A.; Pispas, S.; Demetzos, C.; Libera, M.; Marcinkowski, A.; Trzebicka, B. Chimeric lipid/block copolymer nanovesicles: Physico-chemical and bio-compatibility evaluation. Eur. J. Pharm. Biopharm. 2016, 107, 295–309. [Google Scholar] [CrossRef]
- Sivanantham, M.; Feng, H.; Winnik, F. Formation of novel thermo-responsive hybrid vesicles: Influence of molar ratio of lipids and heating. J. Polym. Res. 2018, 25, 251. [Google Scholar] [CrossRef]
- Nam, J.; Beales, P.A.; Vanderlick, T.K. Giant Phospholipid/Block Copolymer Hybrid Vesicles: Mixing Behavior and Domain Formation. Langmuir 2011, 27, 1–6. [Google Scholar] [CrossRef]
- Chen, D.; Santore, M.M. Hybrid copolymer-phospholipid vesicles: Phase separation resembling mixed phospholipid lamellae, but with mechanical stability and control. Soft Matter 2015, 11, 2617–2626. [Google Scholar] [CrossRef]
- Discher, D.E.; Eisenberg, A. Polymer vesicles. Science 2002, 297, 967–973. [Google Scholar] [CrossRef]
- Kamat, N.P.; Lee, M.H.; Lee, D.; Hammer, D.A. Micropipette aspiration of double emulsion-templated polymersomes. Soft Matter 2011, 7, 9863–9866. [Google Scholar] [CrossRef]
- Dimova, R.; Seifert, U.; Pouligny, B.; Förster, S.; Döbereiner, H.G. Hyperviscous diblock copolymer vesicles. Eur. Phys. J. E 2002, 7, 241–250. [Google Scholar] [CrossRef]
- Angelova, M.I.; Dimitrov, D.S. Liposome electroformation. Faraday Discuss. Chem. Soc. 1986, 81, 303–311. [Google Scholar] [CrossRef]
- Evans, E.; Rawicz, W. Entropy-driven tension and bending elasticity in condensed-fluid membranes. Phys. Rev. Lett. 1990, 64, 2094–2097. [Google Scholar] [CrossRef]
- Kickelbick, G.; Bauer, J.; Hüsing, N.; Andersson, M.; Palmqvist, A. Spontaneous Vesicle Formation of Short-Chain Amphiphilic Polysiloxane-b-Poly(ethylene oxide) Block Copolymers. Langmuir 2003, 19, 3198–3201. [Google Scholar] [CrossRef]
- Li, D.; Li, C.; Wan, G.; Hou, W. Self-assembled vesicles of amphiphilic poly(dimethylsiloxane)-b-poly(ethylene glycol) copolymers as nanotanks for hydrophobic drugs. Colloids Surf. A Physicochem. Eng. Asp. 2010, 372, 1–8. [Google Scholar] [CrossRef]
- Bermudez, H.; Brannan, A.K.; Hammer, D.A.; Bates, F.S.; Discher, D.E. Molecular weight dependence of polymersome membrane structure, elasticity, and stability. Macromolecules 2002, 35, 8203–8208. [Google Scholar] [CrossRef]
- Srinivas, G.; Discher, D.E.; Klein, M.L. Self-assembly and properties of diblock copolymers by coarse-grain molecular dynamics. Nat. Mater. 2004, 3, 638–644. [Google Scholar] [CrossRef]
- Itel, F.; Chami, M.; Najer, A.; Loercher, S.; Wu, D.; Dinu, I.A.; Meier, W. Molecular Organization and Dynamics in Polymersome Membranes: A Lateral Diffusion Study. Macromolecules 2014, 47, 7588–7596. [Google Scholar]
- Nam, J.; Santore, M.M. Adhesion Plaque Formation Dynamics between Polymer Vesicles in the Limit of Highly Concentrated Binding Sites. Langmuir 2007, 23, 7216–7224. [Google Scholar] [CrossRef] [PubMed]
- Discher, B.M.; Won, Y.Y.; Ege, D.S.; Lee, J.C.M.; Bates, F.S.; Discher, D.E.; Hammer, D.A. Polymersomes: Tough vesicles made from diblock copolymers. Science 1999, 284, 1143–1146. [Google Scholar] [CrossRef] [PubMed]
- Dao, T.P.T.; Fauquignon, M.; Fernandes, F.; Ibarboure, E.; Vax, A.; Prieto, M.; Le Meins, J.F. Membrane properties of giant polymer and lipid vesicles obtained by electroformation and pva gel-assisted hydration methods. Colloids Surf. A Physicochem. Eng. Asp. 2017, 533, 347–353. [Google Scholar] [CrossRef]

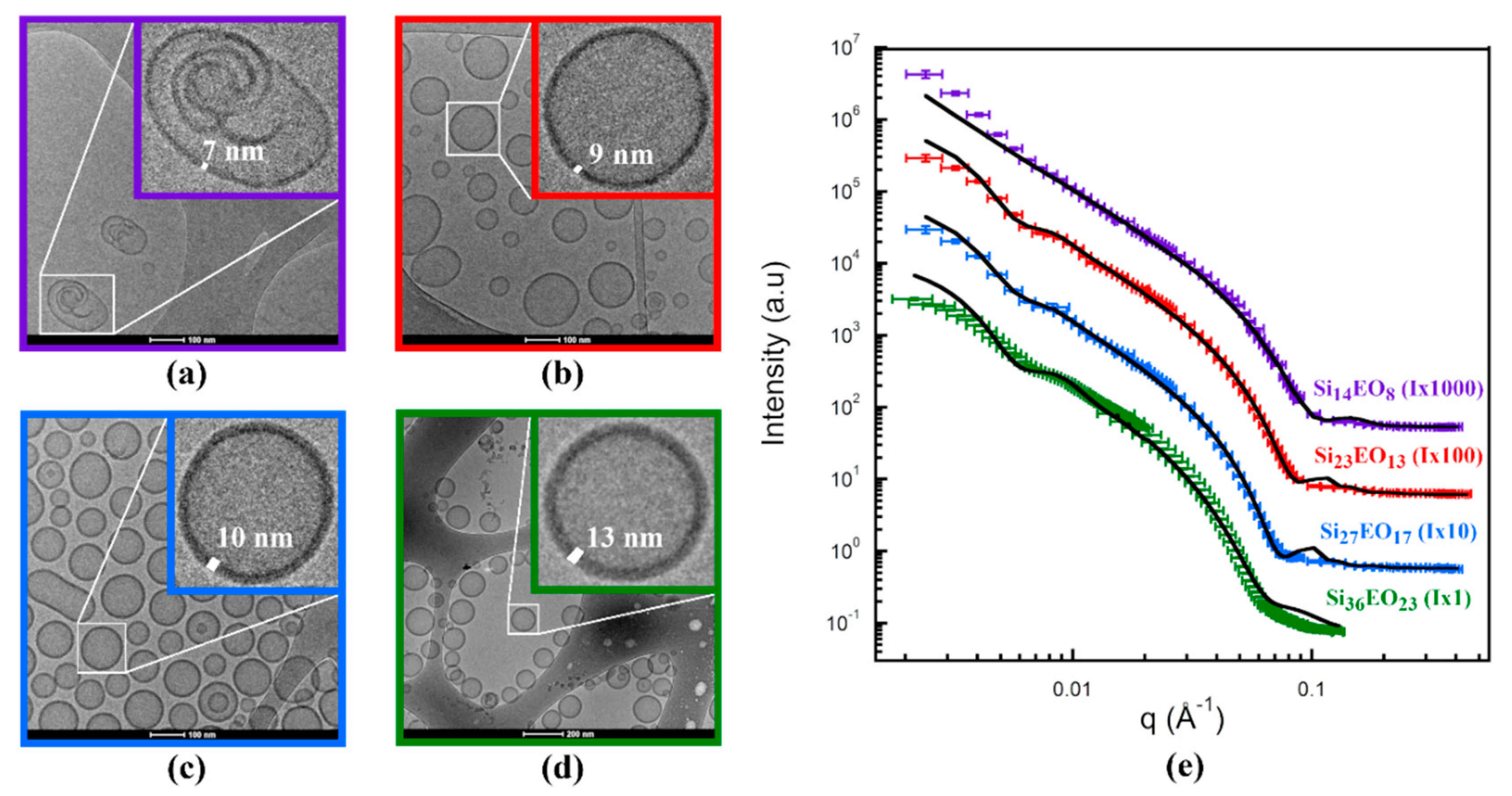
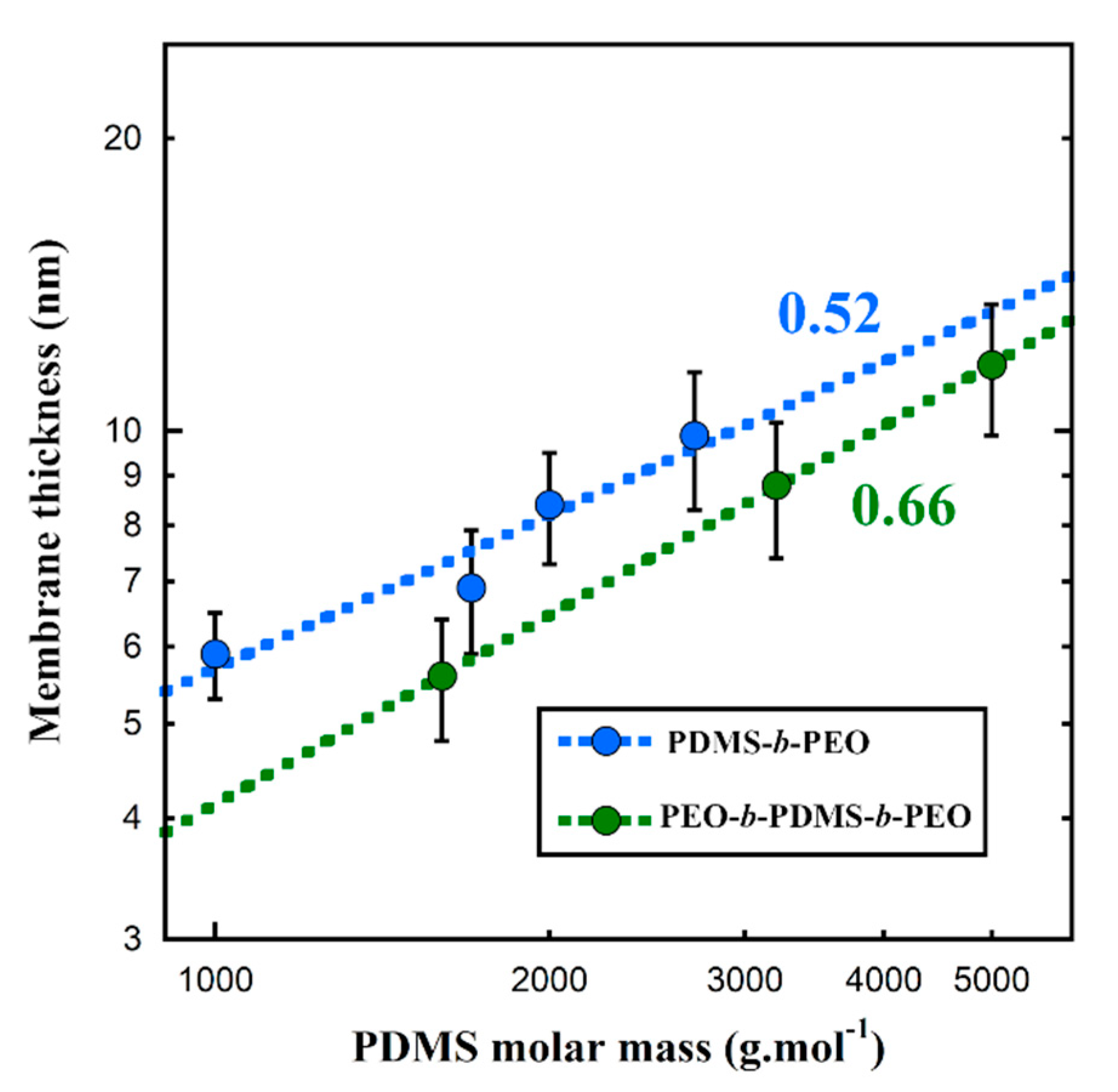
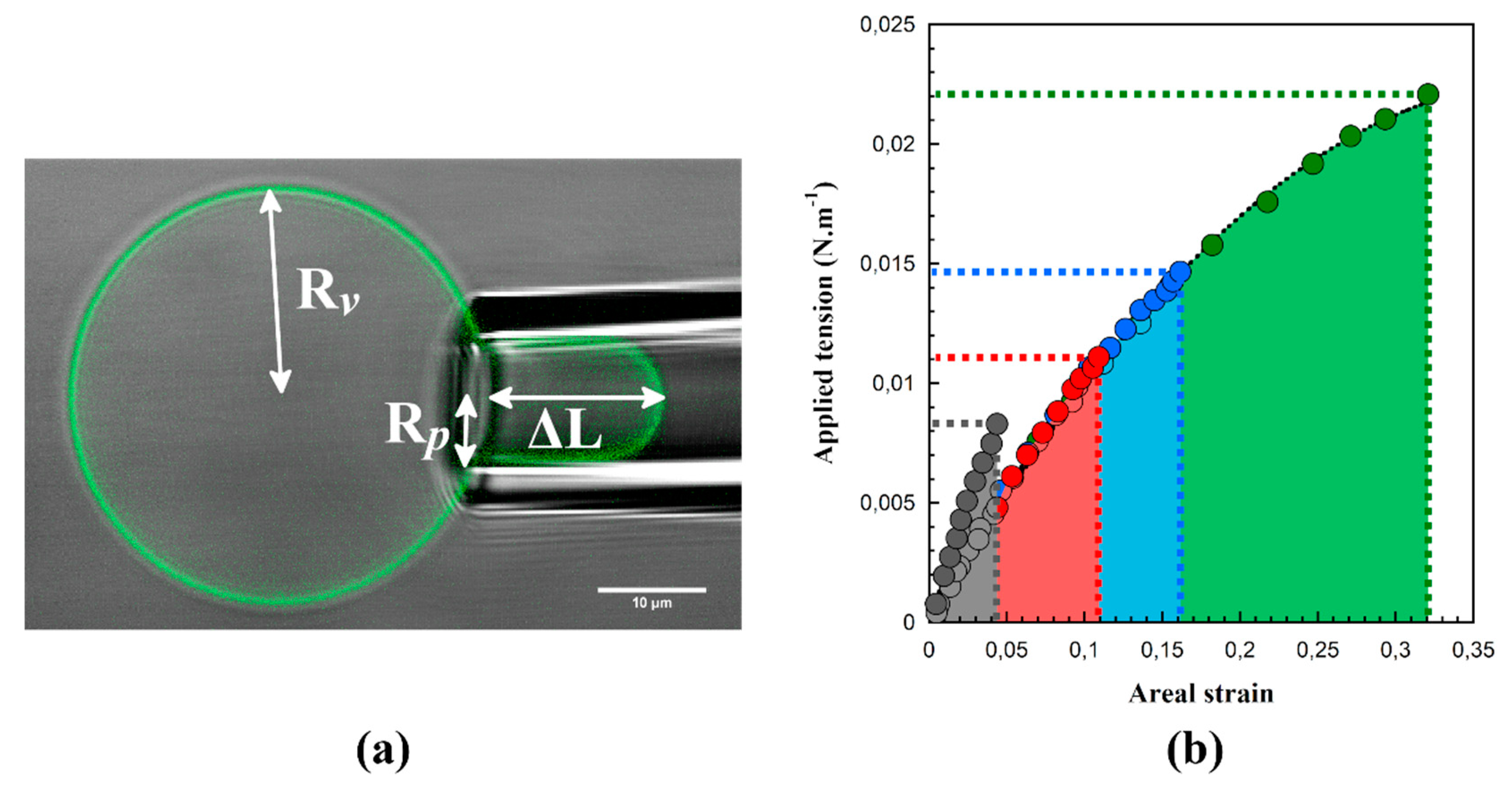
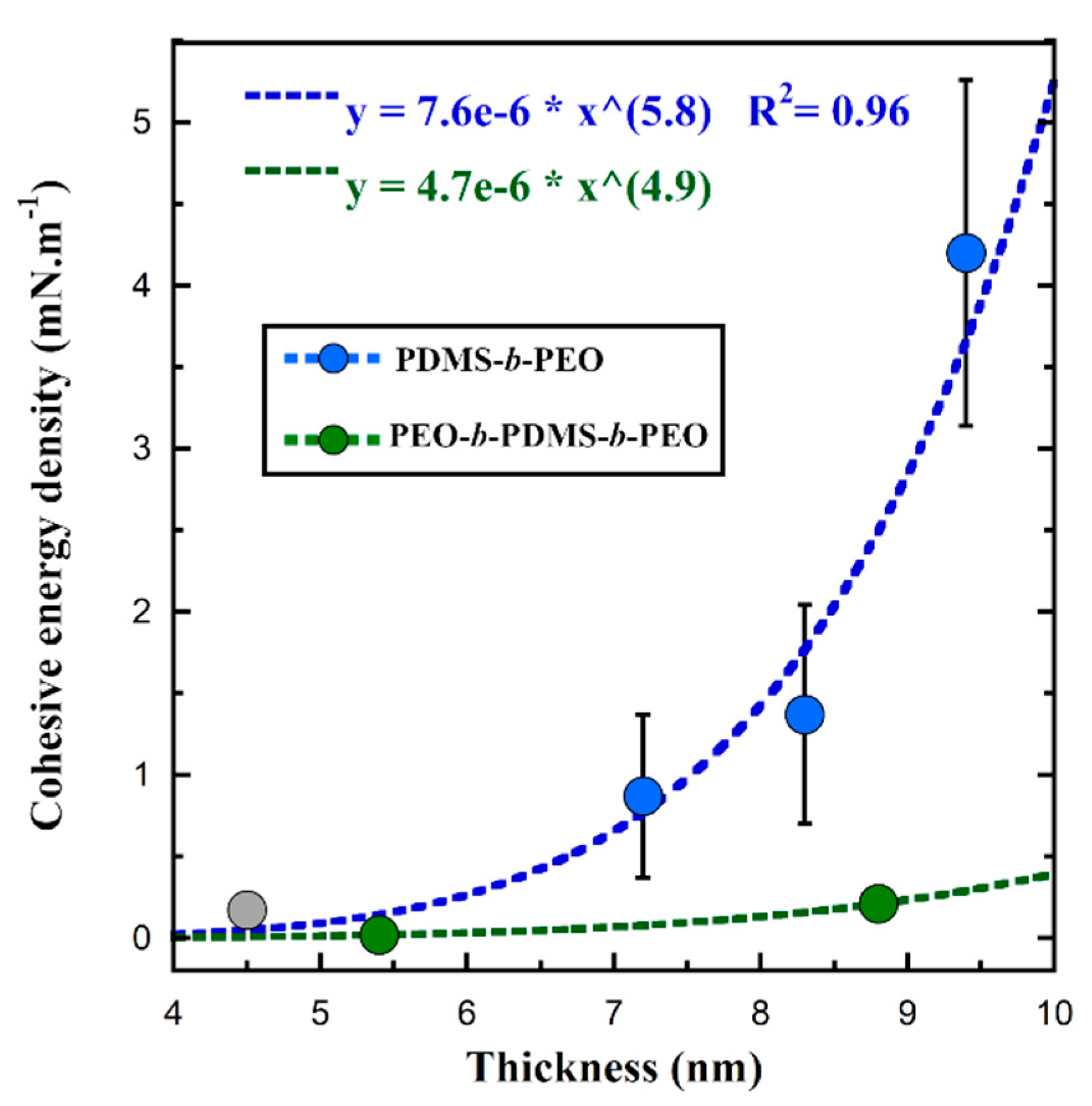

| Si36EO23 | Si27EO17 | Si23EO13 | Si14EO8 | ||
|---|---|---|---|---|---|
| PDMS36-b-PEO23 | PDMS27-b-PEO17 | PDMS23-b-PEO13 | PDMS14-b-PEO8 | ||
| 1H NMR | PDMS (g·mol−1) | 2700 | 2000 | 1700 | 1000 |
| PEO (g·mol−1) | 1000 | 700 | 600 | 400 | |
| copolymer (g·mol−1) | 4000 | 2900 | 2500 | 1600 | |
| Hydrophilic weight fraction (%) | 27 | 26 | 26 | 29 | |
| SEC | PDMS (g·mol−1) | 2700 | 2000 | 1700 | 1000 |
| Ð PDMS | 1.09 | 1.18 | 1.26 | 1.12 | |
| PEO (g·mol−1) | 1300 | 900 | 600 | 400 | |
| Ð PEO | 1.06 | 1.04 | 1.11 | 1.09 | |
| Mn Copolymer (g·mol−1) | 5000 | 3100 | 2500 | 1900 | |
| Ð copolymer | 1.04 | 1.11 | 1.15 | 1.13 | |
| Hydrophilic weight fraction (%) | 33 | 31 | 26 | 29 |
| Si36EO23 | Si27EO17 | Si23EO13 | Si14EO8 | |||
|---|---|---|---|---|---|---|
| dn/dc | g·mL−1 | 0.094 | 0.100 | 0.100 | - | |
| (c) | g·mol−1 | Guinier plot | 2.0 × 108 | 1.4 × 108 | 1.3 × 108 | - |
| Rg | nm | 62 | 50 | 50 | - | |
| Nagg | - | 49,150 | 45,900 | 52,600 | - | |
| Rh | nm | MALS | 62 | 56 | 53 | - |
| PDI | - | 0.069 | 0.060 | 0.057 | - | |
| Rg/Rh | - | 0.98 | 0.90 | 0.95 | - | |
| Membrane thickness ± Standard deviation | nm | Cryo-TEM | 13.1 ± 1.5 | 10.0 ± 1.0 | 8.6 ± 0.9 | 7.1 ± 1.0 |
| SANS Fit | 9.9 ± 1.6 | 8.4 ± 1.1 | 6.9 ± 1.0 | 5.9 ± 0.6 |
| Si36EO23 | Si27EO17 | Si23EO13 | POPC | |
|---|---|---|---|---|
| Membrane thickness d (nm) (from SANS) | 9.9 ± 1.6 | 8.4 ± 1.1 | 6.9 ± 1.0 | 4.5 ± 1.1 |
| Stretching modulus Ka (mN·m−1) | 113 ± 3 | 121 ± 8 | 118 ± 10 | 204 ± 13 |
| Lysis strain (%) | 32 ± 5 | 16 ± 4 | 12 ± 4 | 4 ± 1 |
| Lysis stress (mN·m−1) | 22 ± 2 | 15 ± 3 | 12 ± 3 | 8 ± 2 |
| Cohesive energy density (mN·m−1) | 4.20 ± 1.06 | 1.37 ± 0.67 | 0.87 ± 0.5 | 0.17 ± 0.09 |
| Si27EO17 | Si27EO17 + 10 wt.% POPC | POPC | |
|---|---|---|---|
| Stretching modulus Ka (mN·m−1) | 121 ± 8 | 125 ± 12 | 204 ± 13 |
| Lysis strain (%) | 16 ± 4 | 7 ± 2 | 4 ± 1 |
| Lysis stress (mN·m−1) | 15 ± 3 | 8 ± 2 | 8 ± 2 |
| Cohesive energy density (mN·m−1) | 1.37 ± 0.67 | 0.32 ± 0.15 | 0.17 ± 0.09 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fauquignon, M.; Ibarboure, E.; Carlotti, S.; Brûlet, A.; Schmutz, M.; Le Meins, J.-F. Large and Giant Unilamellar Vesicle(s) Obtained by Self-Assembly of Poly(dimethylsiloxane)-b-poly(ethylene oxide) Diblock Copolymers, Membrane Properties and Preliminary Investigation of Their Ability to Form Hybrid Polymer/Lipid Vesicles. Polymers 2019, 11, 2013. https://doi.org/10.3390/polym11122013
Fauquignon M, Ibarboure E, Carlotti S, Brûlet A, Schmutz M, Le Meins J-F. Large and Giant Unilamellar Vesicle(s) Obtained by Self-Assembly of Poly(dimethylsiloxane)-b-poly(ethylene oxide) Diblock Copolymers, Membrane Properties and Preliminary Investigation of Their Ability to Form Hybrid Polymer/Lipid Vesicles. Polymers. 2019; 11(12):2013. https://doi.org/10.3390/polym11122013
Chicago/Turabian StyleFauquignon, Martin, Emmanuel Ibarboure, Stéphane Carlotti, Annie Brûlet, Marc Schmutz, and Jean-François Le Meins. 2019. "Large and Giant Unilamellar Vesicle(s) Obtained by Self-Assembly of Poly(dimethylsiloxane)-b-poly(ethylene oxide) Diblock Copolymers, Membrane Properties and Preliminary Investigation of Their Ability to Form Hybrid Polymer/Lipid Vesicles" Polymers 11, no. 12: 2013. https://doi.org/10.3390/polym11122013
APA StyleFauquignon, M., Ibarboure, E., Carlotti, S., Brûlet, A., Schmutz, M., & Le Meins, J.-F. (2019). Large and Giant Unilamellar Vesicle(s) Obtained by Self-Assembly of Poly(dimethylsiloxane)-b-poly(ethylene oxide) Diblock Copolymers, Membrane Properties and Preliminary Investigation of Their Ability to Form Hybrid Polymer/Lipid Vesicles. Polymers, 11(12), 2013. https://doi.org/10.3390/polym11122013