3.2. Cryo-Transmission Electron Microscopy (Cryo-TEM) Results
Cryo-TEM results (
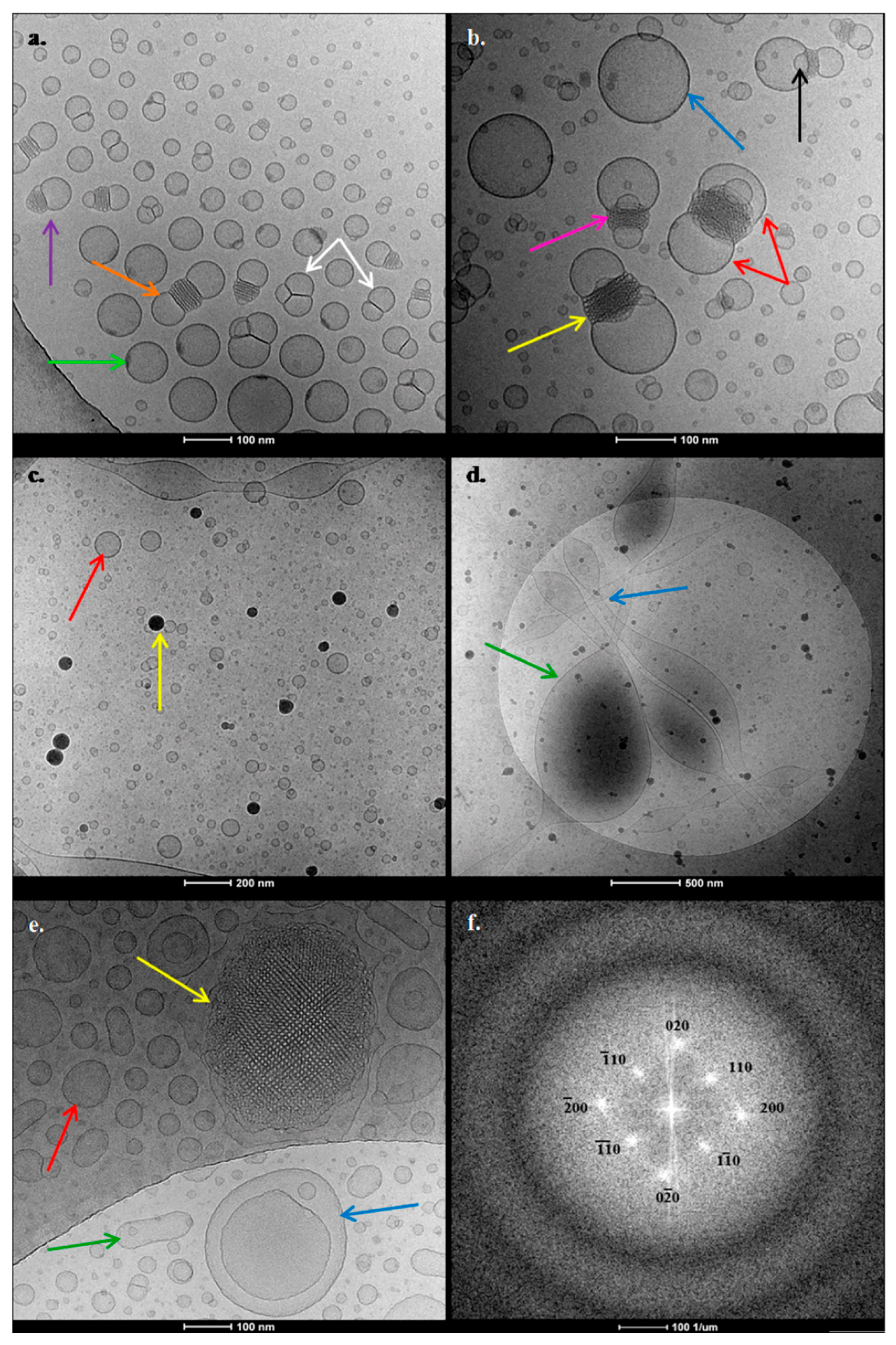
Figure 6,
Table 5) provided information about the morphology and the internal structure of the GMO nanosystems. The three samples were directly applied in the cryo-TEM instrumentation, exhibiting the pH conditions that are described in
Table 1.
Starting from GMO:PDMAEMA-b-PLMA 9:1, two main different categories of objects (
Figure 6a,b) were observed. Their characteristics are summarized in
Table 5. In particular, there was a large population of vesicles (
Figure 6b, blue arrow) exhibiting no internal structure, coexisting with liquid crystalline confined nanoparticles with an ordered structure (
Figure 6b, yellow arrow). The most interesting observation of the GMO:PDMAEMA-b-PLMA 9:1 system is the localization of some organized nanoparticles between two vesicles (
Figure 6b, red arrow). We should note that this “mixed” structure, as well as another observed mixed structure, consisted of one nanoparticle attached with only one vesicle (
Figure 6a, purple arrow); they are both the result of fusion phenomena between ordered nanoparticles and vesicles. The two vesicles are always diametrically attached at each nanoparticle. The two vesicles are either equally sized, providing to the whole structure a “bow-tie”-like shape (
Figure 6a, orange arrow), or not equally sized, providing the whole structure with a “lamp”-like shape (
Figure 6b, pink arrow). In some of these mixed structures, we can also observe smaller characteristic intersecting lamellas surrounding the central confined nanoparticle (
Figure 6b, black arrow). The nanoparticles located in the middle present highly ordered regular inner structures, tending toward a cubosome-like morphology. Moreover, this complex morphology reveals the distribution of the materials. It is possible that the two vesicles contain a greater amount of lipid and lesser amount of polymer, while in the central nanoparticle a larger percentage of the polymer is accumulated, which is able to stabilize the lipids into a more organized structure.
Apart from nanoparticle-vesicles fusion phenomena, there were also many fusion phenomena between vesicles, yielding triplicate or double multi-vesicular structures (
Figure 6a, white arrows). According to the literature, the fusion between unilamellar vesicles is considered to be the precursor of the cubic structure’s creation. The phase transition from lamellar to reverse bicontinuous cubic phase, through intermediate phases, namely stalks and interlamellar attachments (ILAs), has already been stated by Siegel et al. [
64]. The fusion between vesicles (lamellar phases) creates interlamellar attachments via stalk intermediates, which gradually evolve to swollen cubic intermediate phase and eventually to the final highly ordered cubic phase [
65,
66,
67]. Thus, the fusion phenomena between vesicles and nanoparticles, as well as among vesicles that are observed in
Figure 6a,b may represent different stages upon the dynamic formation of cubic ordered structures. Another slight detail that we pointed out is a kind of spot, existing on the surface of some vesicles (
Figure 6a, green arrow). Maybe this spot is the starting point of the fusion phenomena with other vesicles or particles and the evolution from lamellar to organized cubic phases.
Referring to the GMO:PDMAEMA-b-PLMA 9:3 nanosystem, we can observe completely different phenomena. First and foremost, there is an absence of ordered nanoparticles. There are two different size populations of symmetric vesicles (
Figure 6c, red arrow), as described in
Table 5. Moreover, we also observed fusiform and bulgy vesicles (
Figure 6d, green arrow), coexisting with strand objects (
Figure 6d, blue arrow). Last but not least, there were many black objects with strong contrast (
Figure 6c, yellow arrow), possessing sizes of about 15–70 nm, but we were not able to verify their inner structure. The black objects resemble to polymeric or even mixed lipid-polymer micelles with spherical morphology. After all, the PDMAEMA-b-PLMA block copolymer is able to self-assemble into micelles in aqueous solutions, which consisted of the hydrophobic PLMA core and the hydrophilic PDMAEMA corona, as reported by Chrysostomou and Pispas [
21]. The cryo-TEM results of the GMO:PDMAEMA-b-PLMA 9:3 system can be correlated with its different macroscopic appearance. The less milky, but translucent tint of 9:3 can be attributed to the exclusive presence of the vesicles, because the unilamellar vesicles scatter the visible light resulting in the light-blue opalescence which was observed in the dispersion [
42].
We may conclude that the amount of stabilizer plays a key role on the morphology and organization of the mixed systems. According to our results, when the PDMAEMA-b-PLMA percentage increases to 25% w/w (relative to the lipid mass), the ordered structures, which were observed in the 9:1 system, were transformed to vesicular and loose structures. In contrast to GMO-P407-based cubosomes, which can accommodate more than 25 wt.% polymer to lipid [
68], GMO:PDMAEMA-b-PLMA-based cubosomes cannot incorporate such large amounts of polymer. We may assume that the higher concentration of PDMAEMA-b-PLMA yields a higher grade of perturbation into lipid membrane and eventually disrupts it to less organized structures. As the literature describes, the increase of the stabilizer amount increases the percentage of vesicular structures [
68]. Moreover, the high concentration of the stabilizer may lead to formation of mixed micelles that are smaller than cubosomes, as well as mixed GMO/polymer bilayers, which also sterically stabilize the particles against fusion into the cubic phase [
69]. It has also been reported that at high stabilizer concentrations, the lipid can be solubilized into the mixed micelles formed, reducing the percentage of cubic structures [
39]. The literature also reinforces our opinion about the presence of micelles (illustrated by black objects) at the GMO:PDMAEMA-b-PLMA 9:3. Micelles from block copolymers exhibit different type of morphology than the empty vesicles of the
Figure 6 (red arrows). In other cases of PDMAEMA micelles being illustrated with TEM [
21,
46], we can observe spherical, dense morphologies and small sizes. According to Chrysostomou and Pispas [
21], who studied the morphology of the micelles of the same PDMAEMA-b-PLMA block copolymer (that also used in the present study), they observed spherical dense morphologies of dark colour, illustrating most probably only the dense internal core of PLMA and not the more diffuse corona of PDMAEMA chains. Taking into account the above data, we assume that in contrast to the observed empty vesicles, the observed black objects are micellar mixed structures, composed of a dense internal core of PLMA, where are also solubilised some lipid molecules and surrounded by a diffuse corona of PDMAEMA chains.
Concerning the ternary GMO:PDMAEMA-b-PLMA:P407 8:1:1 system, there were also two main categories of objects. We observe vesicles with no internal structure (
Figure 6e, red and green arrows) coexisting with liquid crystalline nanoparticles that exhibited confined, highly ordered, periodical inner structure, resembling cubic phase (
Figure 6e, yellow arrow). These organized nanoparticles were also surrounded by a kind of “shell/coating” that consisted of vesicular-like structures. In order to investigate the internal structure of the organized nanoparticles, we obtained fast Fourier transform (FFT) patterns of the TEM images (
Figure 6f). The patterns show diffuse peaks of brightness, which proves the ordering of internal structure of the particle indicated in
Figure 6e. FFT reflections are likely to correspond to the space group
Im3m [
70,
71]. Controlled tilting of samples or cryo transmission electron tomography is required for precise determination. As far as the vesicles are concerned, they presented three different size populations (
Table 5), having different shapes. We can see spherical vesicles (
Figure 6e, red arrow) accompanied with elongated ones that resemble the shape of bacterial rods (
Figure 6e, green arrow). Many fusion phenomena between vesicles are illustrated, as well as nested vesicular structures, where large vesicles accommodate smaller ones (
Figure 6e, blue arrow), also known as "pregnant" vesicles [
72]. We should note that the presence of P407 in equal ratio to PDMAEMA-b-PLMA provided different conformation at the observed liquid crystalline nanoparticles than the conformation of the respective ones of the 9:1 system. In GMO:PDMAEMA-b-PLMA:P407 8:1:1 system, the nanoparticles were larger than formed by GMO:PDMAEMA-b-PLMA 9:1, exhibiting a larger organized surface, while the vesicles were perimetrically attached, not just in the two edges.
In addition, we should analyze some common points which were presented in all the GMO systems. For example, the vesicular structures, presenting in the all prepared nanosystems may have mixed membranes, composed from both GMO and PDMAEMA-b-PLMA or PDMAEMA-b-PLMA and P407. Taking into account that the PPO block of P407 is capable of inserting within the GMO bilayer [
68], we suppose that the PLMA block also perturbs the lipid bilayer. Furthermore, the different sizes of the illustrated structures can be correlated with the observed values of PDI of the three systems (
Table 2). Moreover, the preparation method also plays a key role in the vesicle formation. We used the TD method with sonication, applying high shear forces in order to achieve homogenous dispersions. As the previous literature describes, during TD method the liquid crystalline nanoparticles may break down into nonequilibrium vesicular structures, which can subsequently fuse to unstable larger ones. However, the increased temperature during the sonication process is able to transform the vesicles into smaller cubic phase nanoparticles. Thus, the coexistence of the different organized structures is well documented, and it is also considered to provide further stabilization [
73] and can explain our results for the 9:1 and 8:1:1 systems. The vesicles that were attached to the organized nanoparticles of 9:1 and 8:1:1 molar ratios are proved to play a critical role upon the stabilization of the cubic phase because they can act as a protecting layer, keeping the cubic nanoparticles dispersed in the aqueous environment [
68]. Demurtas et al. [
67] also confirm the vesicular caps can prevent the exposure of the hydrophobic parts of the stabilizer or the lipids to the water phase.
Although the morphological diversity is considered to be a stabilization factor [
73], the size distribution as presented in
Table 5 should be further improved to one-sized populations with low values of PDI, in order for these nanosystems to be successfully employed as drug delivery nanosystems in the future. We can change the size and size distribution characteristics by modifying the preparation protocol. In our study, where a top down (TD) preparation method along with an intermediate step of thin lipidic film preparation was used, an increase of the input energy can be tried. However, while the energy increase would have the risk of disrupting the observed confined liquid crystalline structure due to an input of excessive energy into the system [
44], it should be accurately applied. Thus, the use of another preparation protocol can also be investigated, such as the bottom up method, where liquid precursor mixtures of lipid–ethanol are diluted with aqueous P407 solutions, providing cubosomes with minimal input of energy [
39,
44,
74]. Spicer and Hayden [
74], who first introduced this method, described how cubosomes prepared by hydrotrope methods have a more homogenous distribution of the stabilizing polymer (e.g., P407) on their surface during the fragmentation process, when compared to cubosomes prepared by mechanical dispersion, resulting eventually in lower values of PDI. The inclusion of ethanol in the formulation increases the solubility of the lipid and thereby significantly reduces its operative viscosity. As Rizwan et al. [
44] described, other hydrotrope solvents apart from ethanol can also be used. Taking into account the already acquired physicochemical and morphological data, additional experiments could be designed, and other preparation protocols could be tried, in order to compare their efficacy, as well as to optimise all the involved parameters, in order to result in the optimal preparation method that is able to provide the desired homogeneity at the presented systems.
3.4. Fluorescence Spectroscopy Results
Fluorescence spectroscopy experiments were carried out in order to investigate some critical parameters of the internal nanostructure and microenvironment of the prepared nanosystems. In particular, we measured the micropolarity and the microfluidity of their membranes depending on the medium pH (i.e., 4.2 and 6.0) and the temperature (25 °C and 45 °C). Pyrene was employed as a hydrophobic probe, able to incorporate itself in the hydrophobic domains that were consisted of lipid and PLMA blocks or lipid, PLMA and PPO blocks. This experimental approach can extract some predictive information upon the potential incorporation of low molecular weight hydrophobic drugs.
Fluorescence spectroscopy results from the GMO and PHYT systems are summarized in
Table 6 and
Table 7, respectively. We observe that the GMO systems presented increased micropolarity (increased values of
I1/I3) and almost the same microfluidity (similar values of
IE/IM) in comparison to PHYT systems. GMO systems were proved to present more polar bilayers/domains, probably due to the different chemical structures of the lipids (GMO presents a double bond in contrast to the PHYT), that result in different microenvironments, as well as different means of pyrene incorporation within the hydrophobic domains of the nanostructures.
We should note that the different membrane micropolarity verifies the different interactions between PDMAEMA-b-PLMA/PDMAEMA-b-PLMA: P407 mixture with GMO, compared to PHYT lipid. For example, in the case of P407 stabilized cubosomes (classic lipid:P407 formulation), the hydrophobic PPO block exists either at the surface of the cubic phase particles or within the GMO lipid bilayer structure, causing a
Pn3m (diamond cubic phase) to
Im3m phase transition (primitive cubic phase), enhancing the water solubilisation capacity of the GMO systems [
68,
75,
76], as well as provoking water swelling into the internal nanostructure [
77]. Contrariwise, the PPO exhibits a lower grade of affinity to the PHYT bilayer, due to the unfavorable branching of PPO methyl groups, provoking a simple absorbance on its surface [
43,
78]. Thus, GMO:P407 bilayers are proved to be more polar that PHYT. We assume that such a different mode of interaction with the lipids may take place in the case of PLMA/PLMA and PPO blocks. The fact that PLMA blocks also have methyl groups, such as the PPO, supports our hypothesis. Moreover, the increased micropolarity of GMO systems may be attributed to the presence of ester groups of the inserted PLMA blocks within the hydrocarbon tail region of the lipid bilayer.
As far as the effect of pH on the microenvironmental parameters is concerned, we observed slight variations up to 0.03 with no specific trend. As PDMAEMA stretches out of the bilayer surface, its pH-induced protonation does affect the inner bilayer. However, there was a small increase (ca. 0.04) of the microfluidity of both groups of systems (with only exception the GMO:PDMAEMA-b-PLMA 9:3 system), at both pH values when the temperature was increased at 45 °C. The temperature-induced PDMAEMA shrinkage and the subsequent contraction of the external polymeric corona may provoke defects at the bilayer, making the membrane more fluid and more permeable to the pyrene. Due to the fact that the PLMA block exhibits high deformability, due to its low glass transition temperature (T
g ca. −53.8 °C) [
21,
63], it is less possible to influence the obtained results. The fluorescence experiments indicate the ability of the nanosystems to accommodate hydrophobic compounds and are useful for the design and development of liquid crystalline nanosystems as future carriers for hydrophobic drugs.