Improved Thermal Insulating Properties of Renewable Polyol Based Polyurethane Foams Reinforced with Chicken Feathers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Biofoam Preparation
2.3. Viscosity of CF/Polyol Mixtures
2.4. Characterization of the Biofoams
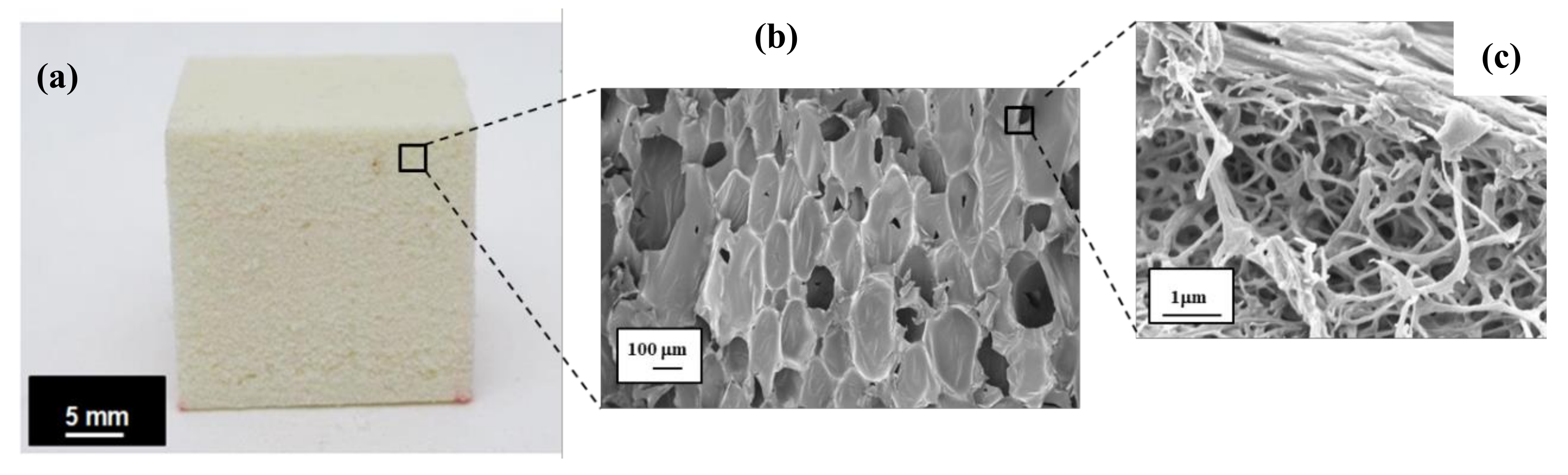
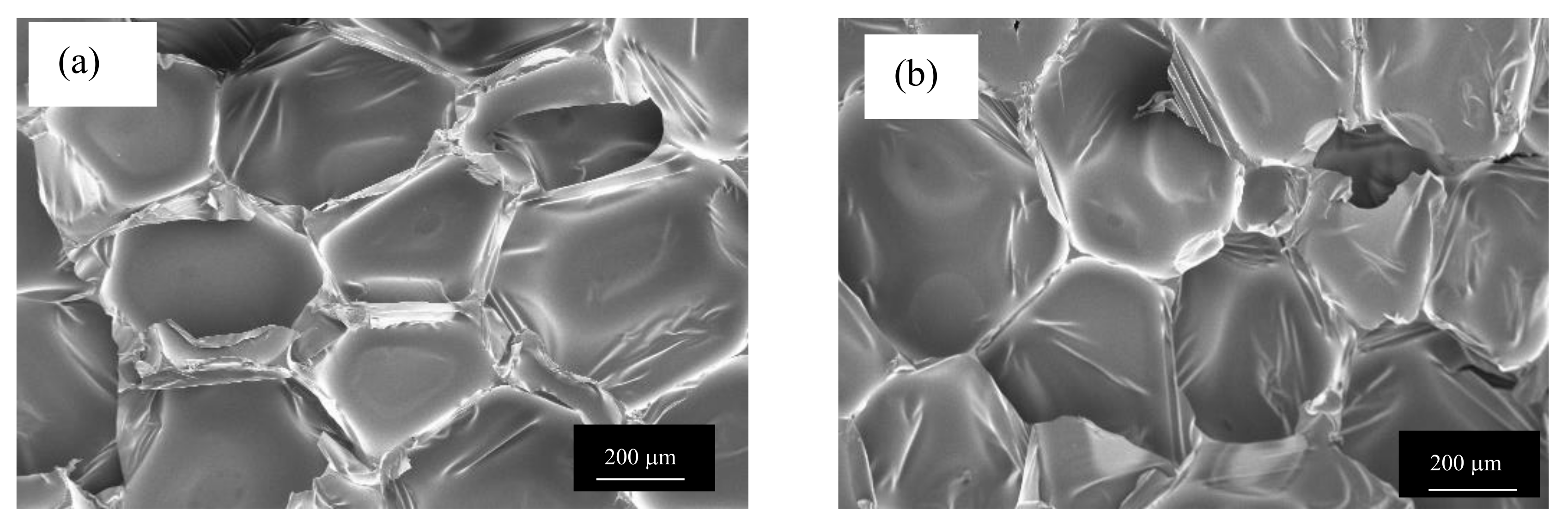
2.4.1. FESEM
2.4.2. Thermal Conductivity
2.4.3. Compression Test
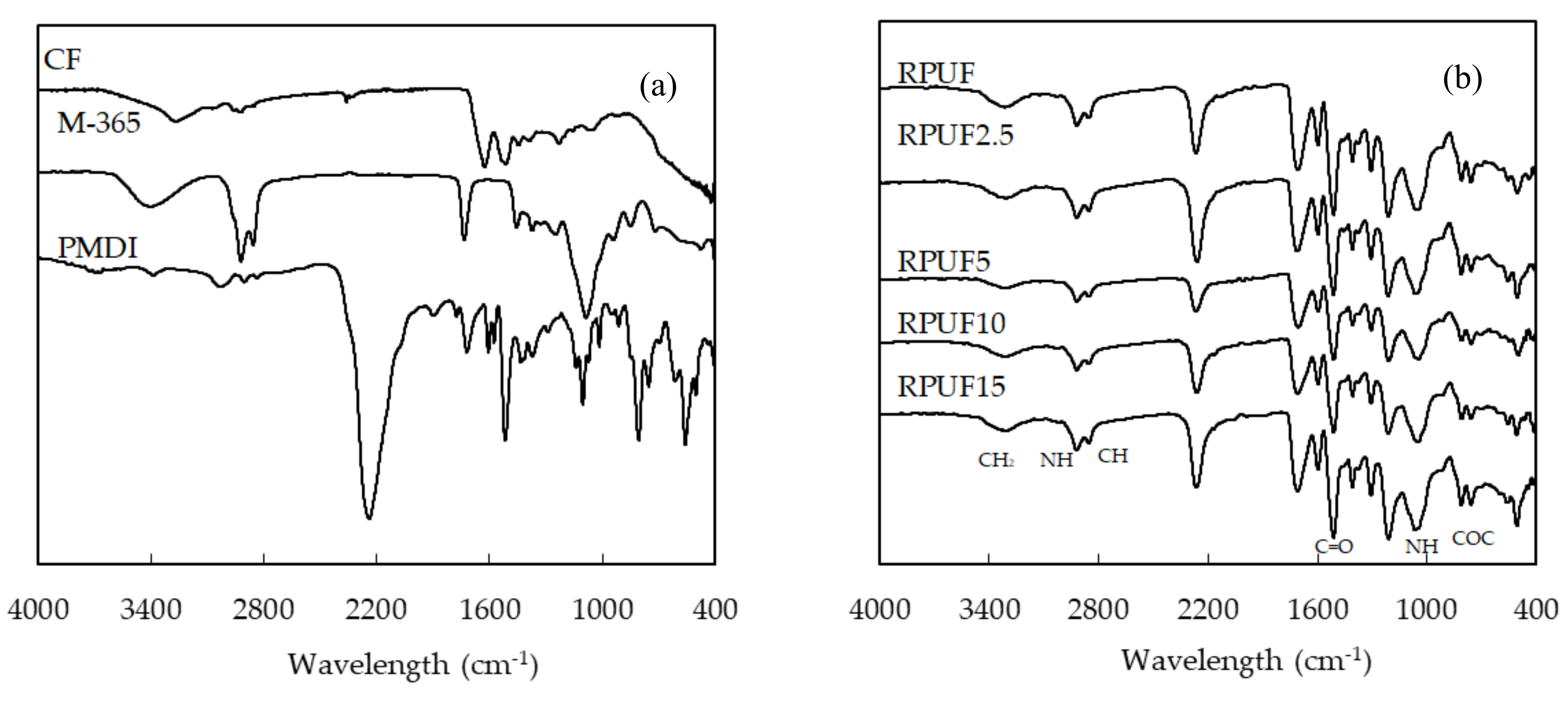
2.4.4. Fourier-Transform Infrared (FTIR) Spectroscopy
2.4.5. Water Absorption
2.4.6. Thermogravimetric Analysis (TGA)
3. Results and Discussions
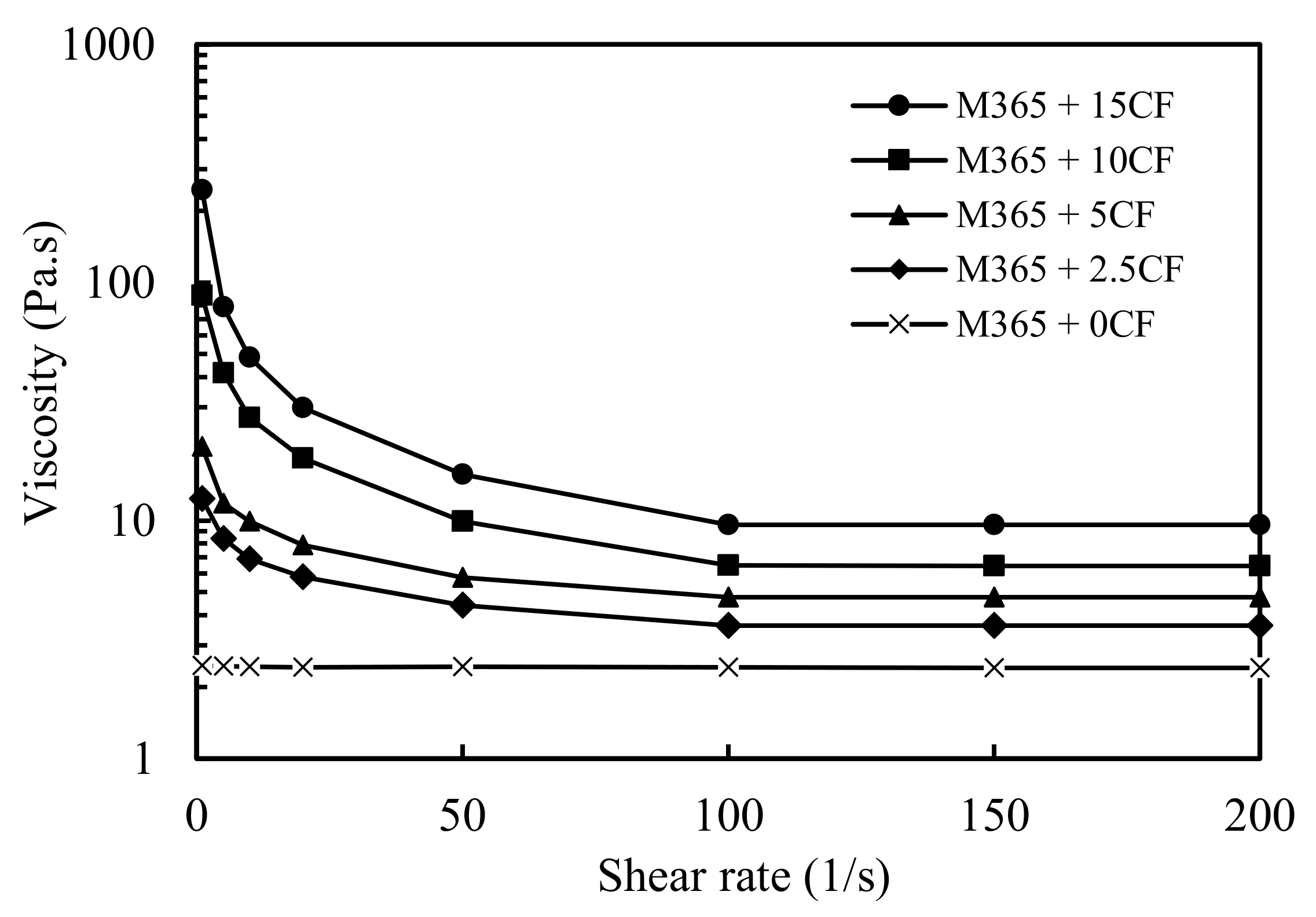
3.1. Viscosity of the CF/Polyol Premixes.
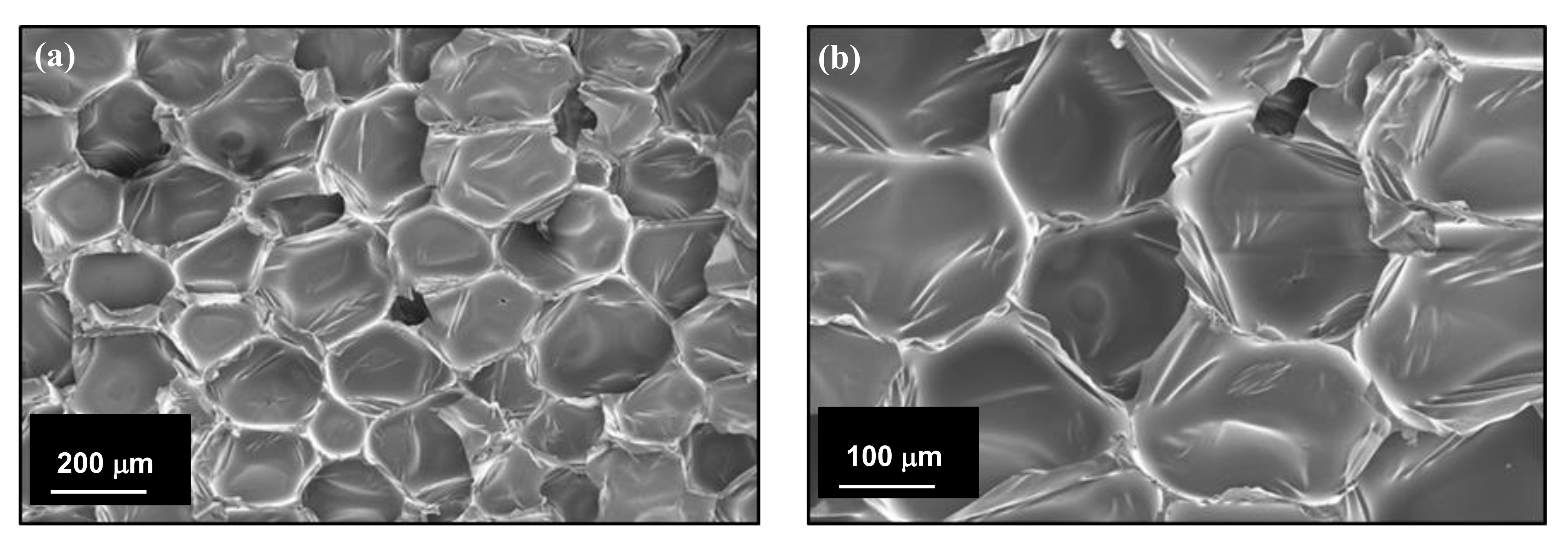
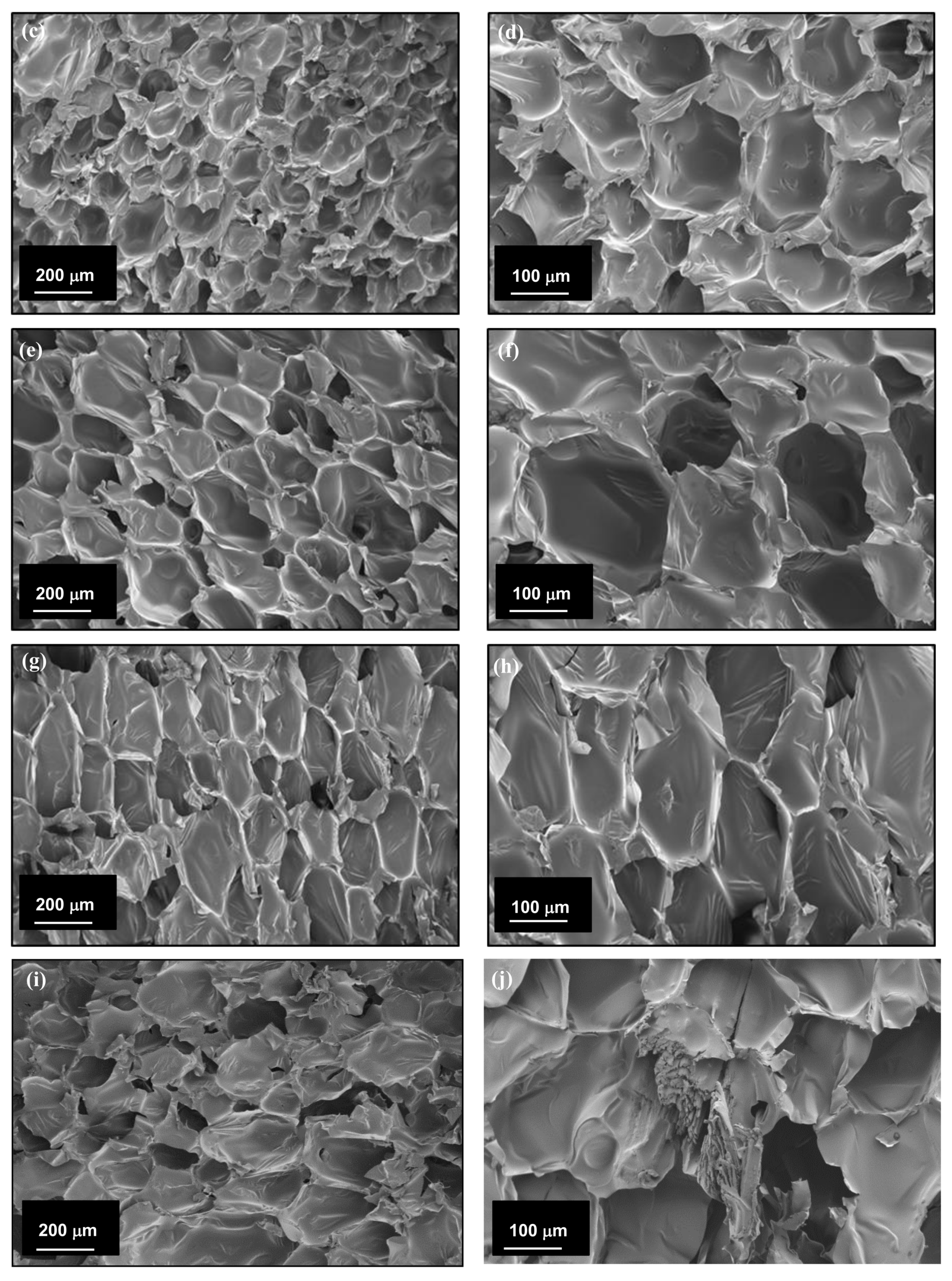
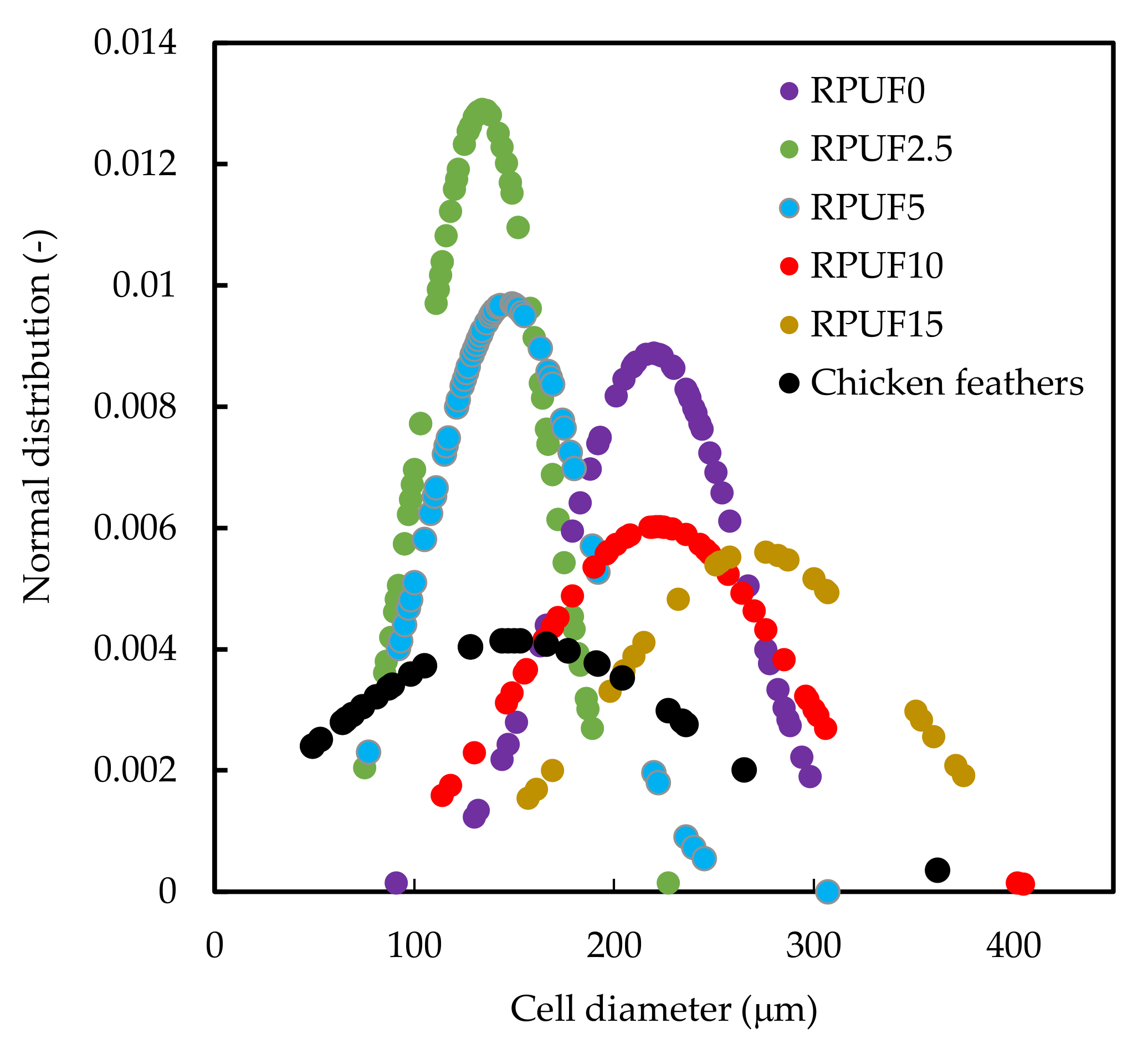
3.2. Morphology of the CF/RPUF Biofoams
3.3. Mechanical Properties of the Biofoams
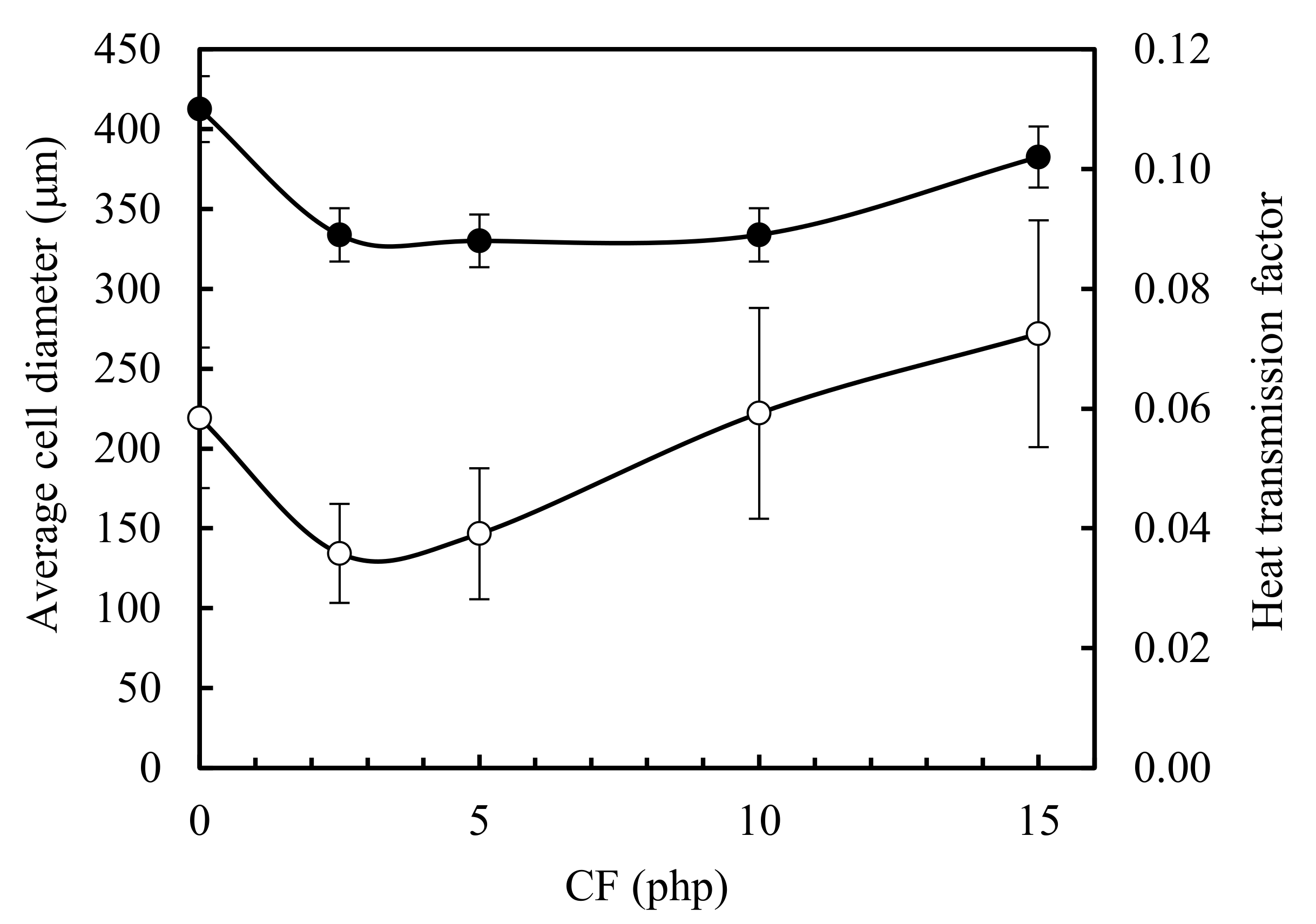
3.4. Thermal Insulation Properties
3.5. FTIR Analysis of the Biofoams
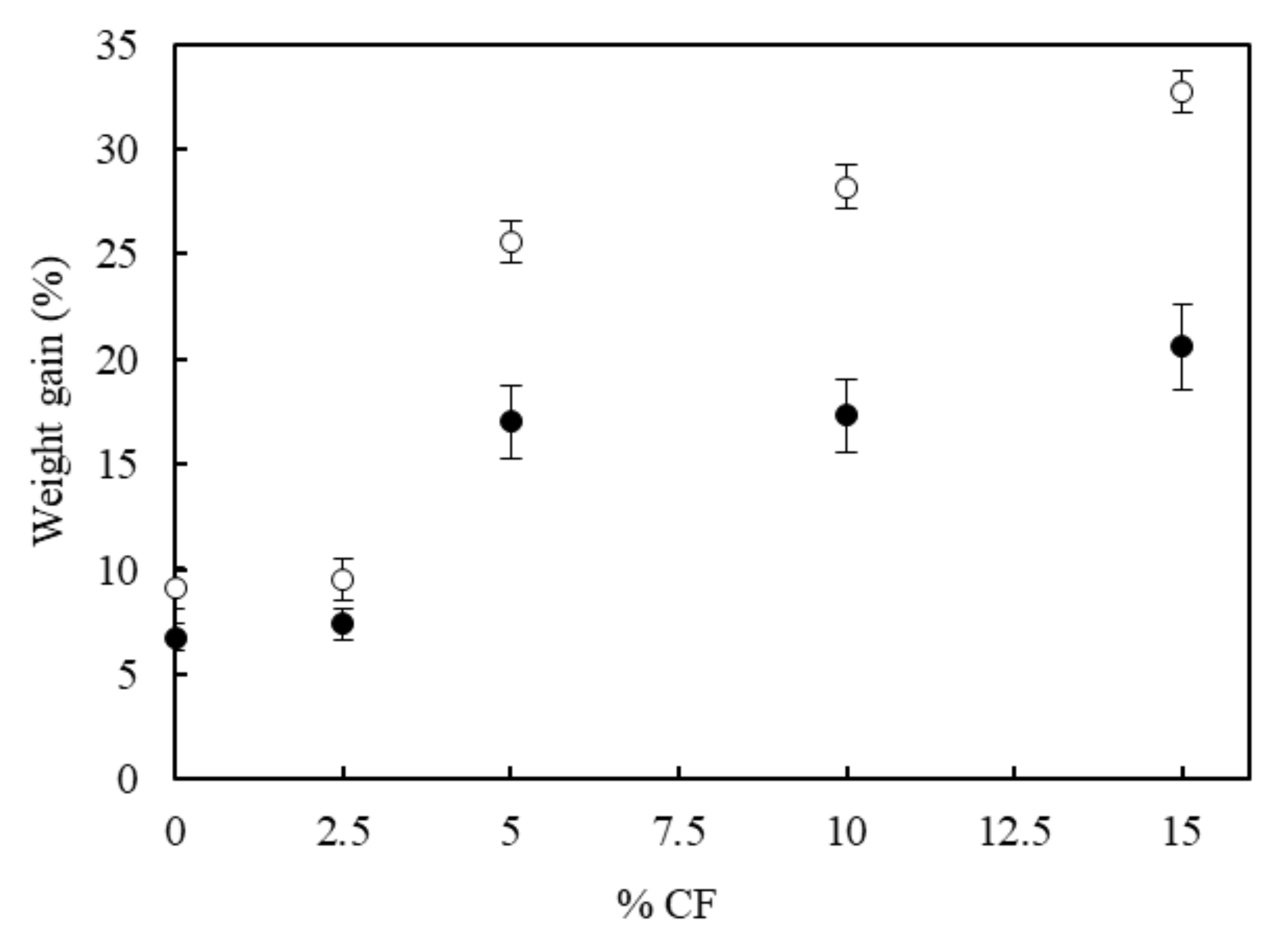
3.6. Water Absorption
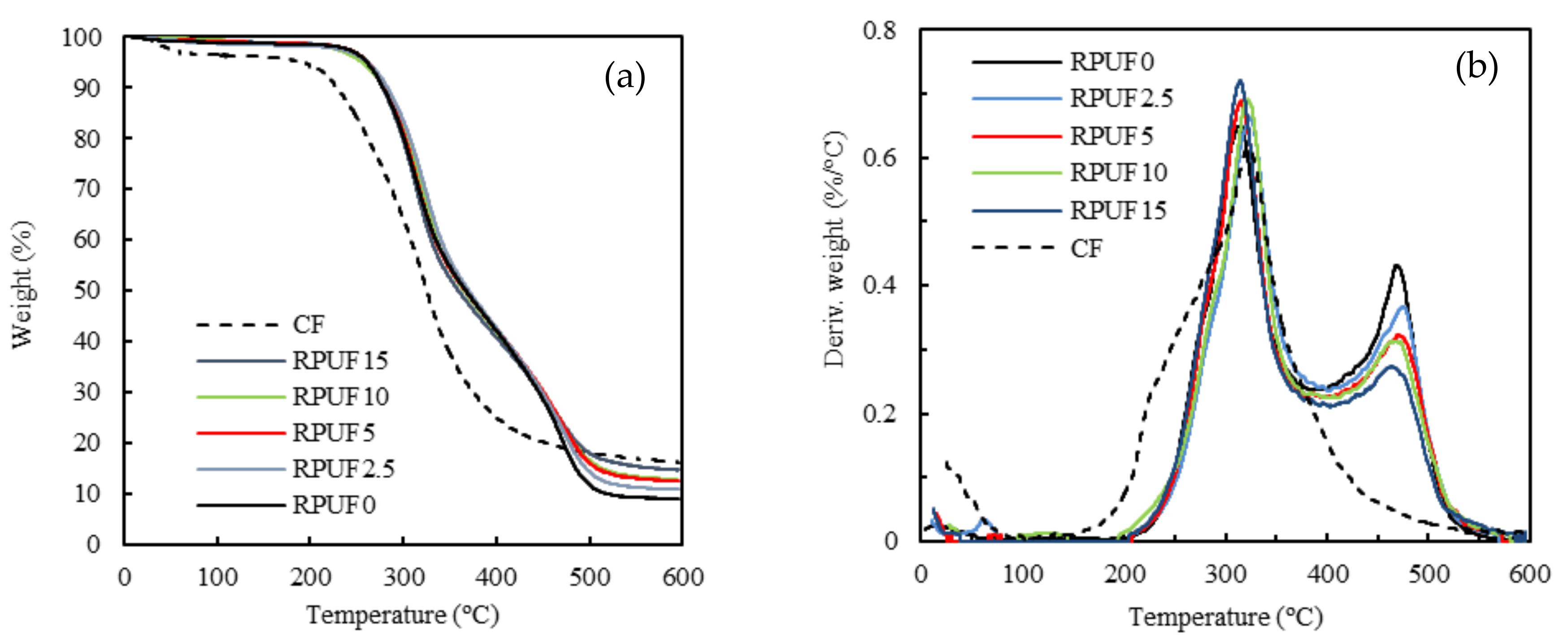
3.7. Thermogravimetric Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Akindoyo, J.O.; Beg, M.D.H.; Ghazali, S.; Islam, M.R.; Jeyaratnama, N.; Yuvaraj, A.R. Polyurethane types, synthesis and applications—A review. RSC Adv. 2016, 6, 114453–114482. [Google Scholar] [CrossRef]
- Antunes, M.; Velasco, J.I. Polymeric Foams. Polymers 2019, 11, 1179. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.W.; Sung, W.F.; Chu, H.S. Thermal conductivity of polyurethane foams. Int. J. Heat Mass Transf. 1999, 42, 2211–2217. [Google Scholar] [CrossRef]
- Tan, S.; Abraham, T.; Ference, D.; Macosko, C.W. Rigid polyurethane foams from a soybean oil-based polyol. Polymer 2011, 52, 467–473. [Google Scholar] [CrossRef]
- Gama, N.V.; Ferreira, A.; Barros-Timmons, A. Polyurethane Foams: Past, Present, and Future. Materials 2018, 11, 1841. [Google Scholar] [CrossRef]
- Mello, V.M.; Martins, G.B.C.; Montenegro, M.A.; Suarez, P.A.Z. Thermal processing of soybean oil to obtain bio-based polymers and bio-oil. Ind. Crops Prod. 2015, 66, 255–261. [Google Scholar] [CrossRef]
- Badri, K.H.; Othman, Z.; Ahmad, S.H. Rigid polyurethane foams from oil palm resources. J. Mater. Sci. 2004, 39, 5541–5542. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, M.; Hu, L.; Zhou, Y. Synthesis of rigid polyurethane foams with castor oil-based flame retardant polyols. Ind. Crops Prod. 2014, 52, 380–388. [Google Scholar] [CrossRef]
- Medeiros, A.M.M.S.; Machado, F.; Rubim, J.C. Synthesis and characterization of a magnetic bio-nanocomposite based on magnetic nanoparticles modified by acrylated fatty acids derived from castor oil. J. Eur. Polym. 2015, 71, 152–163. [Google Scholar] [CrossRef]
- Cangemi, J.M.; dos Santos, M.A.; Neto, S.C.; Chierice, G.O. Biodegradation of polyurethane derived from castor oil. Polímeros Ciência Tecnol. 2008, 18, 201–206. [Google Scholar] [CrossRef]
- Kurańska, M.; Prociak, A. Flax fibers as natural filler for rigid polyurethane-polyisocyanurate foams based on bio-polyol from rapeseed oil. Czas. Tech. Chem. 2015, 112, 47–54. [Google Scholar]
- Prociak, A.; Kurańska, M.; Malewska, E.; Szczepkowski, L.; Zieleniewska, M.; Ryszkowska, J.; Ficoń, J.; Rząsa, A. Biobased polyurethane foams modified with natural fillers. Polimery 2015, 60, 592–599. [Google Scholar] [CrossRef]
- Mosiewicki, M.; DellArciprete, G.A.; Aranguren, M.I.; Marcovich, N.E. Polyurethane Foams Obtained from Castor Oil-based Polyol and Filled with Wood Flour. J. Compos. Mater. 2009, 43, 3057–3072. [Google Scholar] [CrossRef]
- Aranguren, M.I.; Racz, I.; Marcovich, N.E. Microfoams Based on Castor Oil Polyurethanes and Vegetable Fibers. J. Appl. Polym. Sci. 2007, 105, 2791–2800. [Google Scholar] [CrossRef]
- Bertino, M.F.; Strzelec, K. Rigid polyurethane foams reinforced with industrial potato protein. Polym. Test. 2018, 68, 135–145. [Google Scholar] [CrossRef]
- Chen, Y.C.; Tai, W. Castor Oil-Based Polyurethane Resin for Low-Density Composites with Bamboo Charcoal. Polymers 2018, 10, 1100. [Google Scholar] [CrossRef] [PubMed]
- Zieleniewska, M.; Leszczyński, M.K.; Szczepkowski, L.; Bryśkiewicz, A.; Krzyżowska, M.; Bieńd, K.; Ryszkowska, J. Development and applicational evaluation of the rigid polyurethane foam composites with egg shell waste. J. Polym. Degrad. Stab. 2016, 132, 78–86. [Google Scholar] [CrossRef]
- Meat Consumption. Available online: https://data.oecd.org/agroutput/meat-consumption.htm (accessed on 12 November 2019).
- Stingone, J.A.; Wing, S. Poultry litter incineration as a source of energy: Reviewing the potential for impacts on environmental health and justice. New Solut. 2011, 21, 27–42. [Google Scholar] [CrossRef]
- Reddy, N.; Yang, Y. Structure and Properties of Chicken Feather Barbs as Natural Protein Fibers. J. Polym. Environ. 2007, 15, 81–87. [Google Scholar] [CrossRef]
- Bessa, J.; Souza, J.; Lopes, J.B.; Sampaio, J.; Mota, C.; Cunha, F.; Fangueiro, R. Characterization of thermal and acoustic insulation of chicken feather reinforced composites. Procedia Eng. 2007, 200, 472–479. [Google Scholar] [CrossRef]
- Członka, S.; Sienkiewicz, N.; Strąkowska, A.; Strzelec, K. Keratin feathers as a filler for rigid polyurethane foams on the basis of soybean oil polyol. Polym. Test. 2018, 72, 32–45. [Google Scholar] [CrossRef]
- Aranberri, I.; Montes, S.; Azcune, I.; Rekondo, A.; Grande, H.J. Flexible Biocomposites with Enhanced InterfacialCompatibility Based on Keratin Fibers and Sulfur-Containing Poly(urea-urethane)s. Polymers 2018, 10, 1056. [Google Scholar] [CrossRef] [PubMed]
- Aranberri, I.; Montes, S.; Azcune, I.; Rekondo, A.; Grande, H.J. Biodegradable biocomposites with high chicken feather content polymers. Polymers 2017, 9, 593. [Google Scholar] [CrossRef]
- Qin, X. Chicken Feather Fibre Mat/PLA Composites for Thermal Insulation. Master’s Thesis, University of Waikato, Hamilton, New Zealand, 2015. [Google Scholar]
- Dieckmann, E.; Eleftheriou, K.; Audic, T.; Lee, K.Y.; Sheldrick, L.; Cheeseman, C. New sustainable materials from waste feathers: Properties of hotpressed feather/cotton/bi-component fibre boards. Sustain. Mater. Technol. 2019, 19, 30011–30019. [Google Scholar] [CrossRef]
- Thermal Conductivity of Selected Materials and Gases. Available online: http://www.engineeringtoolbox.com/thermal-conductivity-d_429.html (accessed on 12 November 2019).
- International Organization for Standardization. ISO 845:2006. Cellular Plastics and Rubbers—Determination of Apparent Density; International Organization for Standardization: Geneva, Switzerland, 2006; Available online: https://www.iso.org/standard/39408.html (accessed on 12 November 2019).
- International Organization for Standardization. ISO 6942:2002. Protective Clothing—Protection against Heat and Fire—Method of Test: Evaluation of Materials and Material Assemblies When Exposed to a Source of Radiant Heat; International Organization for Standardization: Geneva, Switzerland, 2002. [Google Scholar]
- International Organization for Standardization. ISO 844. Rigid Cellular Plastics—Determination of Compression Properties; International Organization for Standardization: Geneva, Switzerland, 2014. [Google Scholar]
- ASTM International. ASTM D570-98(2018). Standard Test Method for Water Absorption of Plastics; ASTM International: West Conshohocken, PA, USA, 2018; Available online: https://www.astm.org/Standards/D570.htm (accessed on 12 November 2019).
- Silva, M.C.; Takahashi, J.A.; Chaussy, D.; Belgacem, M.N. Composites of rigid polyurethane foam and cellulose fiber residue. J. Appl. Polym. Sci. 2010, 117, 3665–3672. [Google Scholar] [CrossRef]
- Kang, S.M.; Kim, M.J.; Kwon, S.H.; Park, H.; Jeong, H.M.; Kim, B.K. Polyurethane foam/silica chemical hybrids for shape memory effects. J. Mater. Sci. 2012, 27, 2837–2843. [Google Scholar] [CrossRef]
- Hamilton, A.R.; Thomsen, O.T.; Madaleno, L.A.O.; Jensen, L.R.; Rauhe, J.C.M.; Pyrz, R. Evaluation of the anisotropic mechanical properties of reinforced polyurethane. Compos. Sci. Technol. 2013, 87, 210–217. [Google Scholar] [CrossRef]
- Goto, A.; Yamaguchi, K.; Hamada, H. Effect of deformational behavior of cell structure on mechanical properties of polyurethane foam under compressive loading. In Proceedings of the Annual Technical Conference ANTEC, Chicago, IL, USA, 16–20 May 2004. [Google Scholar]
- Gu, R.; Konar, S.; Sain, M. Preparation and Characterization of Sustainable Polyurethane Foams from Soybean Oils. J. Am. Oil Chem. Soc. 2012, 89, 2103–2111. [Google Scholar] [CrossRef]
- Fan, H.; Tekeei, A.; Suppes, G.J.; Hsich, F.H. Properties of biobased rigid polyurethane foams reinforced with fillers: Microspheres and nanoclays. Int. J. Polym. Sci. 2012, 2012, 1–8. [Google Scholar] [CrossRef]
- Mrajji, O.; Wazna, M.E.; Boussoualem, Y.; Bouari, A.E.; Cherkaoui, O. Feather waste as a thermal insulation solution: Treatment, elaboration and characterization. J. Ind. Text. 2019, 1528083719869393. [Google Scholar] [CrossRef]
- Khosa, M.A.; Wu, J.; Ullah, A. Chemical modification, characterization, and application of chicken feathers as novel biosorbents. RSC Adv. 2013, 3, 20800–20810. [Google Scholar] [CrossRef]
- Ibrahim, S.; Ahmad, A.; Mohamed, N.S. Synthesis and characterization of castor oil-based polyurethane for potential application as host in polymer electrolytes. Mater. Sci. 2015, 38, 1155–1161. [Google Scholar] [CrossRef][Green Version]
- Pourjavaheri, F.; Mohades, F.; Jones, O.; Sherkat, F.; Kong, I.; Gupta, A.; Shanks, R.A. Avian Keratin Fibre Based Bio-composites. World J. Eng. 2017, 14, 183–187. [Google Scholar] [CrossRef]
- Starón, P.; Banach, M.; Kowalski, Z. Keratin—Origins, properties, application. Chemik 2011, 65, 1019–1026. [Google Scholar]
- Sair, A.; Oushabi, A.; Kammouni, A.; Tanane, O.; Abboud, Y.; El Bouari, A. Mechanical and thermal conductivity properties of hemp fiber reinforced polyurethane composites. Case Stud. Constr. Mater. 2012, 8, 203–212. [Google Scholar] [CrossRef]
- Martinez-Hernandez, A.L.; Velasco-Santos, C.; De Icaza, M.; Castano, V.M. Microstructural characterisation of keratin fibres from chicken feathers. Int. J. Environ. Pollut. 2005, 23, 162–178. [Google Scholar] [CrossRef]
- Amieva, E.J.; Velasco-Santos, C.; Martínez-Hernández, A.L.; Rivera-Armenta, J.L.; Mendoza-Martínez, A.M.; Castaño, V.M. Composites from chicken feathers quill and recycled polypropylene. J. Compos. Mater. 2015, 49, 275–283. [Google Scholar] [CrossRef]
- Jiao, L.; Xiao, H.; Wang, Q.; Sun, J. Thermal degradation characteristics of rigid polyurethane foam and the volatile products analysis with TG-FTIR-MS. Polym. Degard. Stab. 2013, 98, 2687–2696. [Google Scholar] [CrossRef]
- Cheng, S.; Lau, K.T.; Liu, T.; Zhao, Y.; Lam, P.M.; Yin, Y. Mechanical and thermal properties of chicken feather fiber/PLA green composites. Compos. Part B 2009, 40, 650–654. [Google Scholar] [CrossRef]


| Compound | Property | Value |
|---|---|---|
| Polyol | Density (25 °C) | 1.02 g/cm3 |
| Viscosity (25 °C) | 2.0 Pa·s | |
| Acid Value | 1 | |
| Equivalent weight | 154 | |
| Functionality | 4 | |
| Hydroxyl value | 365 | |
| PMDI | Density (25 °C) | 1.23 g/cm3 |
| Viscosity (25 °C) | 0.17–0.23 Pa·s | |
| NCO content | 30–32 | |
| Functionality | 2.6–2.7 |
| Component | RPUF0 | RPUF2.5 | RPUF5 | RPUF10 | RPUF15 | |
|---|---|---|---|---|---|---|
| Part A | Polyol M-365 | 100 | 100 | 100 | 100 | 100 |
| Surfactant (Tegostab B 8404) | 3 | 3 | 3 | 3 | 3 | |
| Amine (Tegoamin B75) | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | |
| Blowing agent (distilled water) | 3 | 3 | 3 | 3 | 3 | |
| Catalyst (Kosmos 29) | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | |
| Chicken feathers | 0 | 2.5 | 5 | 10 | 15 | |
| Part B | PMDI (Ongronat® 2100) | 135 | 135 | 135 | 135 | 135 |
| Sample | Cell Size (μm) |
|---|---|
| RPUF0 | 219 ± 44 |
| RPUF2.5 | 134 ± 31 |
| RPUF5 | 146 ± 41 |
| RPUF10 | 222 ± 66 |
| RPUF15 | 271 ± 71 |
| Sample | Young’s Modulus (MPa) | Compressive Strength (10% def; KPa) | Compressive Strength (25% def; KPa) |
|---|---|---|---|
| RPUF0 | 3.2 ± 0.4 | 230.2 ± 13.5 | 348.2 ± 11.6 |
| RPUF2.5 | 2.2 ± 0.2 | 177.1 ± 3.7 | 271.1 ± 7.6 |
| RPUF5 | 1.7 ± 0.5 | 129.8 ± 26.6 | 234.5 ± 27.1 |
| RPUF10 | 1.8 ± 0.4 | 131.1 ± 34.2 | 229.5 ± 40.6 |
| RPUF15 | 2.2 ± 0.3 | 150.5 ± 13.4 | 215.5 ± 21.4 |
| Tested RPUF | Heat Transmission Factors (HTF) |
|---|---|
| RPUF0 | 0.110 |
| RPUF2.5 | 0.089 |
| RPUF5 | 0.088 |
| RPUF10 | 0.089 |
| RPUF15 | 0.102 |
| Sample | T (5%; °C) | T (25%; °C) | T (50%; °C) | Residual Mass after 600 °C (%) |
|---|---|---|---|---|
| RPUF0 | 261 | 308 | 367 | 9.08 |
| RPUF2.5 | 262 | 315 | 370 | 10.84 |
| RPUF5 | 262 | 310 | 366 | 12.52 |
| RPUF10 | 257 | 313 | 365 | 12.58 |
| RPUF15 | 259 | 307 | 359 | 14.73 |
| CF | 196 | 271 | 325 | 16.24 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aranberri, I.; Montes, S.; Wesołowska, E.; Rekondo, A.; Wrześniewska-Tosik, K.; Grande, H.-J. Improved Thermal Insulating Properties of Renewable Polyol Based Polyurethane Foams Reinforced with Chicken Feathers. Polymers 2019, 11, 2002. https://doi.org/10.3390/polym11122002
Aranberri I, Montes S, Wesołowska E, Rekondo A, Wrześniewska-Tosik K, Grande H-J. Improved Thermal Insulating Properties of Renewable Polyol Based Polyurethane Foams Reinforced with Chicken Feathers. Polymers. 2019; 11(12):2002. https://doi.org/10.3390/polym11122002
Chicago/Turabian StyleAranberri, Ibon, Sarah Montes, Ewa Wesołowska, Alaitz Rekondo, Krystyna Wrześniewska-Tosik, and Hans-Jürgen Grande. 2019. "Improved Thermal Insulating Properties of Renewable Polyol Based Polyurethane Foams Reinforced with Chicken Feathers" Polymers 11, no. 12: 2002. https://doi.org/10.3390/polym11122002
APA StyleAranberri, I., Montes, S., Wesołowska, E., Rekondo, A., Wrześniewska-Tosik, K., & Grande, H.-J. (2019). Improved Thermal Insulating Properties of Renewable Polyol Based Polyurethane Foams Reinforced with Chicken Feathers. Polymers, 11(12), 2002. https://doi.org/10.3390/polym11122002






