Durable Antimicrobial Behaviour from Silver-Graphene Coated Medical Textile Composites
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Substrates
2.2. Hummer’s Method for Reduced Graphene Oxide (rGO) Synthesis
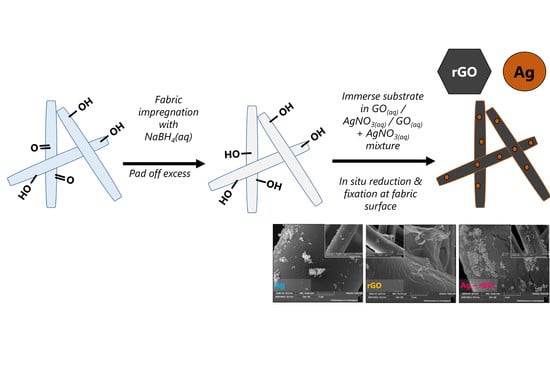
2.3. Wet Chemical Experimental Impregnation Method
2.4. Antimicrobial Testing (AATCC TM100; ISO20743)
2.5. Physico-Chemical Nanoparticle (NP) Characterisation
2.6. Wash Fastness of Composite Textiles (AATCC TM61-2001; ISO105-C10:2006)
2.7. NP Leachant Testing Using Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES)
3. Results and Discussion
3.1. Nanoparticle-Impregnated Polyviscose Substrate Synthesis
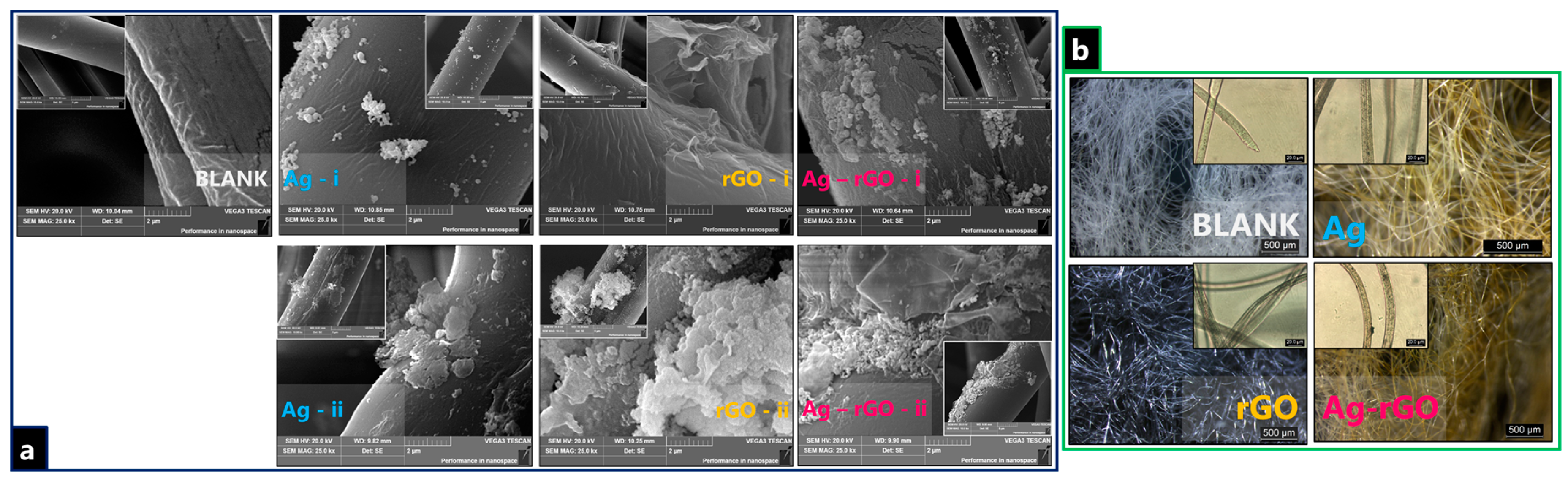
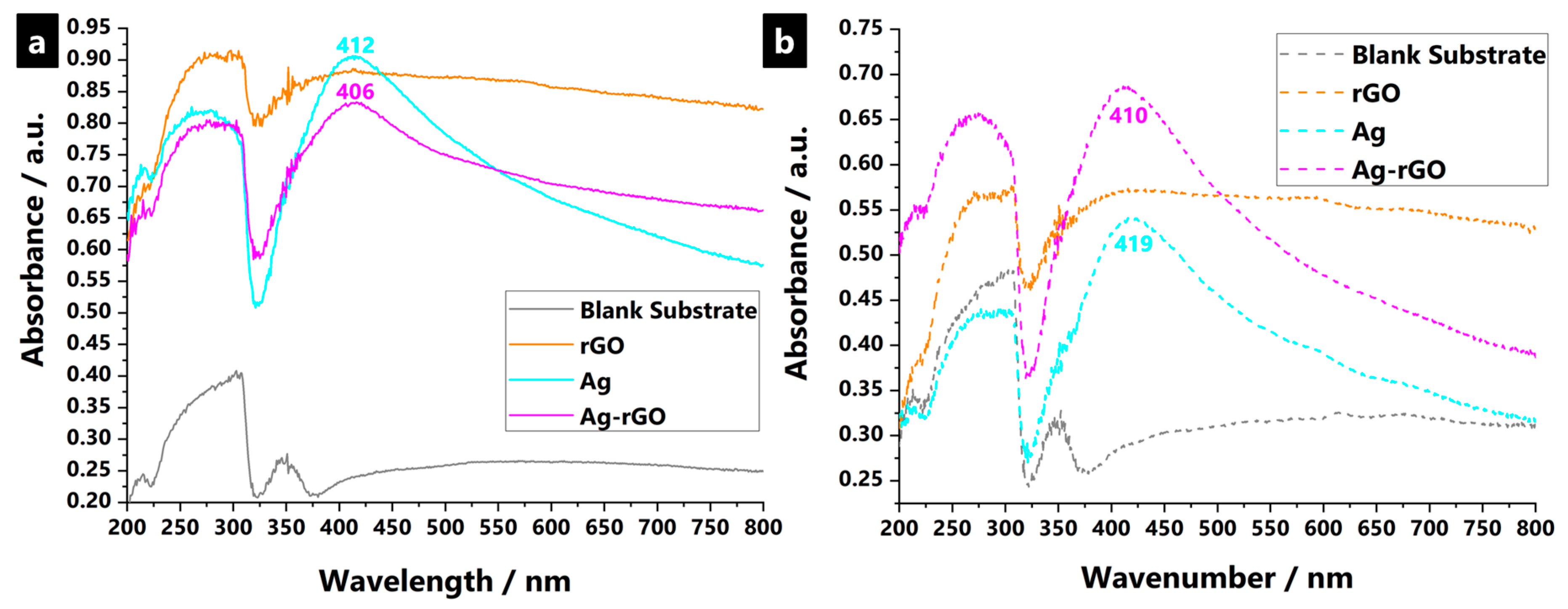
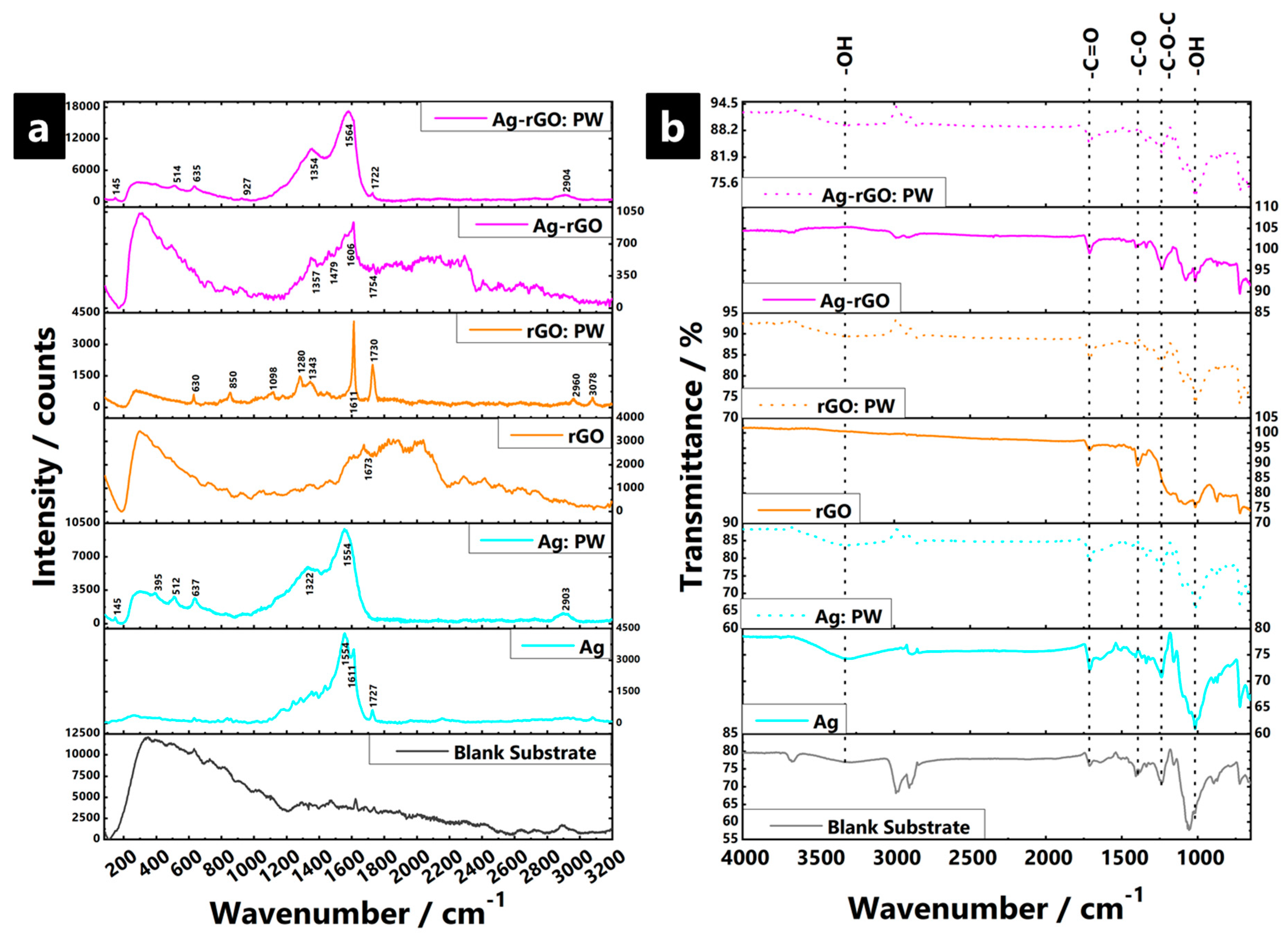
3.2. Nanoparticle-Impregnated Composite Bonding Interactions, Materials Characterisation, and Durability Testing
3.3. Antimicrobial Properties Testing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Akbar, R.; WHO. World Health Organization: Ten Threats to Global Health in 2019. Available online: https://www.who.int/emergencies/ten-threats-to-global-health-in-2019 (accessed on 25 March 2019).
- World Economics Forum. The Global Risks Report; World Economics Forum: Cologne, Switzerland, 2018; Volume 13, pp. 52–53. [Google Scholar]
- An, J.; Guo, G.; Yin, R.; Luo, Q.; Li, X.; Liu, F.; Wang, D. Facile preparation of silver/reduced graphene oxide/chitosan colloid and application of the nanocomposite in antibacterial and catalytic activity. Polym. Int. 2018, 67, 515–527. [Google Scholar] [CrossRef]
- Yip, J.; Liu, L.; Wong, K.H.; Leung, P.H.M.; Yuen, C.W.M.; Cheung, M.C. Investigation of antifungal & antibacterial effects of fabric padded with highly stable selenium nanoparticles. J. Appl. Polym. Sci. 2014, 131, 8886–8893. [Google Scholar]
- Tan, F.; Leung, P.H.M.; Liu, Z.; Zhang, Y.; Xiao, L.; Ye, W.; Zhang, X.; Yi, L.; Yang, M. A PDMS microfluidic impedance immunosensor for E. coli O157:H7 and Staphylococcus aureus detection via antibody-immobilized nanoporous membrane. Sens. Actuators B Chem. 2011, 159, 328–335. [Google Scholar] [CrossRef]
- Li, Y.; Leung, P.; Yao, L.; Song, Q.W.; Newton, E. Antimicrobial effect of surgical masks coated with nanoparticles. J. Hosp. Infect. 2006, 62, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Petkova, P.; Francesko, A.; Fernandes, M.M.; Mendoza, E.; Perelshtein, I.; Gedanken, A.; Tzanov, T. Sonochemical coating of textiles with hybrid ZnO/chitosan antimicrobial nanoparticles. ACS Appl. Mater. Interfaces 2014, 6, 1164–1172. [Google Scholar] [CrossRef]
- Abramov, O.V.; Gedanken, A.; Koltypin, Y.; Perkas, N.; Perelshtein, I.; Joyce, E.; Mason, T.J. Pilot scale sonochemical coating of nanoparticles onto textiles to produce biocidal fabrics. Surf. Coat. Technol. 2009, 204, 718–722. [Google Scholar] [CrossRef]
- Ashour, M.; El-Nakhal, K. Nosocomial infection in patients admitted to an intensive care unit at Al-Shifa Hospital in the Gaza Strip, occupied Palestinian territory: A retrospective assessment. Lancet 2012, 380, S33. [Google Scholar] [CrossRef]
- Magill, S.S.; Edwards, J.R.; Bamberg, W.; Beldavs, Z.G.; Dumyati, G.; Kainer, M.A.; Lynfield, R.; Maloney, M.; McAllister-Hollod, L.; Nadle, J.; et al. Multistate Point-Prevalence Survey of Health Care–Associated Infections. N. Engl. J. Med. 2014, 370, 1198–1208. [Google Scholar] [CrossRef]
- Salat, M.; Petkova, P.; Hoyo, J.; Perelshtein, I.; Gedanken, A.; Tzanov, T. Durable antimicrobial cotton textiles coated sonochemically with ZnO nanoparticles embedded in an in-situ enzymatically generated bioadhesive. Carbohydr. Polym. 2018, 189, 198–203. [Google Scholar] [CrossRef]
- Bauer-Savage, J.; Pittet, D.; Kim, E.; Allegranzi, B. Local production of WHO-recommended alcohol-based handrubs: Feasibility, advantages, barriers and costs. Bull. World Health Organ. 2013, 91, 963–969. [Google Scholar] [CrossRef]
- Tacconelli, E.; Pezzani, M.D. Public health burden of antimicrobial resistance in Europe. Lancet Infect. Dis. 2019, 19, 4–6. [Google Scholar] [CrossRef]
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modelling analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef]
- Qiu, Y.; Chan, S.T.; Lin, L.; Shek, T.L.; Tsang, T.F.; Barua, N.; Zhang, Y.; Ip, M.; Chan, P.K.; Blanchard, N.; et al. Design, synthesis and biological evaluation of antimicrobial diarylimine and –amine compounds targeting the interaction between the bacterial NusB and NusE proteins. Eur. J. Med. Chem. 2019, 178, 214–231. [Google Scholar] [CrossRef] [PubMed]
- Udayraj, U.; Wang, F.; Song, W.; Ke, Y.; Xu, P.; Chow, C.S.W.; Noor, N. Performance enhancement of hybrid personal cooling clothing in a hot environment: PCM cooling energy management with additional insulation. Ergonomics 2019, 62, 928–939. [Google Scholar] [CrossRef] [PubMed]
- Fijan, S.; Turk, S.Š. Hospital textiles, are they a possible vehicle for healthcare-associated infections? Int. J. Environ. Res. Public Health 2012, 9, 3330–3343. [Google Scholar] [CrossRef] [PubMed]
- Noimark, S.; Weiner, J.; Noor, N.; Allan, E.; Williams, C.K.; Shaffer, M.S.P.P.; Parkin, I.P. Dual-mechanism antimicrobial polymer-ZnO nanoparticle & crystal violet-encapsulated silicone. Adv. Funct. Mater. 2015, 25, 1367–1373. [Google Scholar]
- Hwang, G.B.; Allan, E.; Parkin, I.P. White Light-Activated Antimicrobial Paint using Crystal Violet. ACS Appl. Mater. Interfaces 2016, 8, 15033–15039. [Google Scholar] [CrossRef]
- Ristić, T.; Zemljič, L.F.; Novak, M.; Kunčič, M.K.; Sonjak, S.; Cimerman, N.G.; Strnad, S. Antimicrobial efficiency of functionalized cellulose fibres as potential medical textiles. Sci. Against Microb. Pathog. Commun. Curr. Res. Technol. Adv. 2011, 6, 36–51. [Google Scholar]
- Borkow, G.; Gabbay, J. Biocidal textiles can help fight nosocomial infections. Med. Hypotheses 2008, 70, 990–994. [Google Scholar] [CrossRef]
- Pulit-Prociak, J.; Chwastowski, J.; Kucharski, A.; Banach, M. Functionalization of textiles with silver and zinc oxide nanoparticles. Appl. Surf. Sci. 2016, 385, 543–553. [Google Scholar] [CrossRef]
- Naskar, A.; Khan, H.; Sarkar, R.; Kumar, S.; Halder, D.; Jana, S. Anti-biofilm activity and food packaging application of room temperature solution process based polyethylene glycol capped Ag-ZnO-graphene nanocomposite. Mater. Sci. Eng. C 2018, 91, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Shin, B.; Lee, K.R.; Moon, M.W.; Kim, H.Y. Extreme water repellency of nanostructured low-surface-energy non-woven fabrics. Soft Matter 2012, 8, 1817–1823. [Google Scholar] [CrossRef]
- Del Valle, L.J.; Franco, M.; Katsarava, R.; Puiggalí, J. Electrospun biodegradable polymers loaded with bactericide agents. AIMS Mol. Sci. 2016, 3, 52–87. [Google Scholar] [CrossRef][Green Version]
- Kafizas, A.; Noor, N.; Carmichael, P.; Scanlon, D.O.; Carmalt, C.J.; Parkin, I.P. Combinatorial atmospheric pressure chemical vapor deposition of F:TiO2; The relationship between photocatalysis & transparent conducting oxide properties. Adv. Funct. Mater. 2014, 24, 1758–1771. [Google Scholar]
- Dixon, S.; Noor, N.; Sathasivam, S.; Lu, Y.; Parkin, I. Synthesis of superhydrophobic polymer/tungsten (VI) oxide nanocomposite thin films. Eur. J. Chem. 2016, 7, 139–145. [Google Scholar] [CrossRef]
- Sotelo-Vazquez, C.; Noor, N.; Kafizas, A.; Quesada-Cabrera, R.; Scanlon, D.O.; Taylor, A.; Durrant, J.R.; Parkin, I.P. Multifunctional P-doped TiO2 Films: A new approach to self-cleaning, transparent conducting oxide materials. Chem. Mater. 2015, 27, 3234–3242. [Google Scholar] [CrossRef]
- Liu, S.; Sun, G. New Refreshable N -Halamine Polymeric Biocides: N -Chlorination of Acyclic Amide Grafted Cellulose. Ind. Eng. Chem. Res. 2009, 48, 613–618. [Google Scholar] [CrossRef]
- Openshaw, J.J.; Morris, W.M.; Lowry, G.V.; Nazmi, A. Reduction in bacterial contamination of hospital textiles by a novel silver-based laundry treatment. Am. J. Infect. Control 2016, 44, 1705–1708. [Google Scholar] [CrossRef][Green Version]
- Lazary, A.; Weinberg, I.; Vatine, J.; Jefidoff, A.; Bardenstein, R.; Borkow, G.; Ohana, N. Reduction of healthcare-associated infections in a long-term care brain injury ward by replacing regular linens with biocidal copper oxide impregnated linens. Int. J. Infect. Dis. 2014, 24, 23–29. [Google Scholar] [CrossRef]
- Liu, H.; Zhong, L.; Yun, K.; Samal, M. Synthesis, characterization, and antibacterial properties of silver nanoparticles-graphene and graphene oxide composites. Biotechnol. Bioprocess Eng. 2016, 21, 1–18. [Google Scholar] [CrossRef]
- Wang, R.; Wang, X.; Xin, J.H. Advanced visible-light-driven self-cleaning cotton by Au/TiO2/SiO2 photocatalysts. ACS Appl. Mater. Interfaces 2010, 2, 82–85. [Google Scholar] [CrossRef]
- Navik, R.; Thirugnanasampanthan, L.; Venkatesan, H.; Kamruzzaman, M.; Shafiq, F.; Cai, Y. Synthesis and application of magnesium peroxide on cotton fabric for antibacterial properties. Cellulose 2017, 24, 3573–3587. [Google Scholar] [CrossRef]
- Liu, R.; Wang, X.; Ye, J.; Xue, X.; Zhang, F.; Zhang, H.; Hou, X.; Liu, X.; Zhang, Y. Enhanced antibacterial activity of silver-decorated sandwich-like mesoporous silica/reduced graphene oxide nanosheets through photothermal effect. Nanotechnology 2018, 29, 105704. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.M.; Yau, S.K.W.; Lok, C.N.; So, M.H.; Che, C.M. Oxidative dissolution of silver nanoparticles by biologically relevant oxidants: A kinetic & mechanistic study. Chem. Asian J. 2010, 5, 285–293. [Google Scholar] [PubMed]
- Hicks, A.L.; Reed, R.B.; Theis, T.L.; Hanigan, D.; Huling, H.; Zaikova, T.; Hutchison, J.E.; Miller, J. Environmental impacts of reusable nanoscale silver-coated hospital gowns compared to single-use, disposable gowns. Environ. Sci. Nano 2016, 3, 1124–1132. [Google Scholar] [CrossRef]
- Ge, L.; Li, Q.; Wang, M.; Ouyang, J.; Li, X.; Xing, M.M.Q. Nanosilver particles in medical applications: Synthesis, performance, and toxicity. Int. J. Nanomed. 2014, 9, 2399–2407. [Google Scholar]
- Karahan, H.E.; Wiraja, C.; Xu, C.; Wei, J.; Wang, Y.; Wang, L.; Liu, F.; Chen, Y. Graphene Materials in Antimicrobial Nanomedicine: Current Status and Future Perspectives. Adv. Healthc. Mater. 2018, 7, 1701406. [Google Scholar] [CrossRef]
- Miao, H.; Teng, Z.; Wang, C.; Chong, H.; Wang, G. Recent Progress in Two-Dimensional Antimicrobial Nanomaterials. Chem. Eur. J. 2019, 25, 929–944. [Google Scholar] [CrossRef]
- Tan, C.; Cao, X.; Wu, X.J.; He, Q.; Yang, J.; Zhang, X.; Chen, J.; Zhao, W.; Han, S.; Nam, G.H.; et al. Recent Advances in Ultrathin Two-Dimensional Nanomaterials. Chem. Rev. 2017, 117, 6225–6331. [Google Scholar] [CrossRef]
- Georgakilas, V.; Tiwari, J.N.; Kemp, K.C.; Perman, J.A.; Bourlinos, A.B.; Kim, K.S.; Zboril, R. Noncovalent Functionalization of Graphene and Graphene Oxide for Energy Materials, Biosensing, Catalytic, and Biomedical Applications. Chem. Rev. 2016, 116, 5464–5519. [Google Scholar] [CrossRef]
- Sun, S.; Tang, S.; Chang, X.; Wang, N.; Wang, D.; Liu, T.; Lei, Y.; Zhu, Y. A bifunctional melamine sponge decorated with silver-reduced graphene oxide nanocomposite for oil-water separation and antibacterial applications. Appl. Surf. Sci. 2019, 473, 1049–1061. [Google Scholar] [CrossRef]
- Deng, C.H.; Gong, J.L.; Zhang, P.; Zeng, G.M.; Song, B.; Liu, H.Y. Preparation of melamine sponge decorated with silver nanoparticles-modified graphene for water disinfection. J. Colloid Interface Sci. 2017, 488, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, W.; Jiu, J.; Suganuma, K. Efficient assembly of high-performance reduced graphene oxide/silver nanowire transparent conductive film based on in situ light-induced reduction technology. Appl. Surf. Sci. 2018, 459, 732–740. [Google Scholar] [CrossRef]
- Ng, S.W.; Noor, N.; Zheng, Z. Graphene-based two-dimensional Janus materials. NPG Asia Mater. 2018, 10, 217–237. [Google Scholar] [CrossRef]
- Park, C.M.; Wang, D.; Han, J.; Heo, J.; Su, C. Evaluation of the colloidal stability and adsorption performance of reduced graphene oxide–elemental silver/magnetite nanohybrids for selected toxic heavy metals in aqueous solutions. Appl. Surf. Sci. 2019, 471, 8–17. [Google Scholar] [CrossRef]
- Alwahib, A.A.; Sadrolhosseini, A.R.; An’amt, M.N.; Lim, H.N.; Yaacob, M.H.; Abu Bakar, M.H.; Ming, H.N.; Mahdi, M.A. Reduced Graphene Oxide/Maghemite Nanocomposite for Detection of Hydrocarbon Vapor Using Surface Plasmon Resonance. IEEE Photonics J. 2016, 8, 1–9. [Google Scholar] [CrossRef]
- Pusty, M.; Sinha, L.; Shirage, P.M. A flexible self-poled piezoelectric nanogenerator based on a rGO–Ag/PVDF nanocomposite. New J. Chem. 2019, 43, 284–294. [Google Scholar] [CrossRef]
- Barua, S.; Thakur, S.; Aidew, L.; Buragohain, A.K.; Chattopadhyay, P.; Karak, N. One step preparation of a biocompatible, antimicrobial reduced graphene oxide–silver nanohybrid as a topical antimicrobial agent. RSC Adv. 2014, 4, 9777. [Google Scholar] [CrossRef]
- Sehmi, S.K.; Noimark, S.; Weiner, J.; Allan, E.; MacRobert, A.J.; Parkin, I.P. Potent Antibacterial Activity of Copper Embedded into Silicone & Polyurethane. ACS Appl. Mater. Interfaces 2015, 7, 22807–22813. [Google Scholar]
- Genslein, C.; Hausler, P.; Kirchner, E.M.; Bierl, R.; Baeumner, A.J.; Hirsch, T. Graphene-enhanced plasmonic nanohole arrays for environmental sensing in aqueous samples. Beilstein J. Nanotechnol. 2016, 7, 1564–1573. [Google Scholar] [CrossRef]
- Zhao, Y.; Xie, Y.; Hui, Y.Y.; Tang, L.; Jie, W.; Jiang, Y.; Xu, L.; Lau, S.P.; Chai, Y. Highly impermeable & transparent graphene as an ultra-thin protection barrier for Ag thin films. J. Mater. Chem. C 2013, 1, 4956–4961. [Google Scholar]
- Seltenrich, N. Nanosilver: Weighing the Risks and Benefits. Environ. Health Perspect. 2013, 121, a220–a225. [Google Scholar] [CrossRef] [PubMed]
- Zvyagina, A.I.; Melnikova, E.K.; Averin, A.A.; Baranchikov, A.E.; Tameev, A.R.; Malov, V.V.; Ezhov, A.A.; Grishanov, D.A.; Gun, J.; Ermakova, E.V.; et al. A facile approach to fabricating ultrathin layers of reduced graphene oxide on planar solids. Carbon 2018, 134, 62–70. [Google Scholar] [CrossRef]
- Kant, R.; Tabassum, R.; Gupta, B.D. Integrating nanohybrid membranes of reduced graphene oxide: Chitosan: Silica sol gel with fiber optic SPR for caffeine detection. Nanotechnology 2017, 28, 195502. [Google Scholar] [CrossRef]
- Yoon, S.S.; Lee, K.E.; Cha, H.J.; Seong, D.G.; Um, M.K.; Byun, J.H.; Oh, Y.; Oh, J.H.; Lee, W.; Lee, J.U. Highly Conductive Graphene/Ag Hybrid Fibers for Flexible Fiber-Type Transistors. Sci. Rep. 2015, 5, 16366. [Google Scholar] [CrossRef]
- Johnson, D.W.; Dobson, B.P.; Coleman, K.S. A manufacturing perspective on graphene dispersions. Curr. Opin. Colloid Interface Sci. 2015, 20, 367–382. [Google Scholar] [CrossRef]
- Shao, W.; Liu, X.; Min, H.; Dong, G.; Feng, Q.; Zuo, S. Preparation, Characterization, and Antibacterial Activity of Silver Nanoparticle-Decorated Graphene Oxide Nanocomposite. ACS Appl. Mater. Interfaces 2015, 7, 6966–6973. [Google Scholar] [CrossRef]
- Baruah, B. In situ & facile synthesis of silver nanoparticles on baby wipes & their applications in catalysis & SERS. RSC Adv. 2016, 6, 5016–5023. [Google Scholar]
- Beech, S.; Noimark, S.; Page, K.; Noor, N.; Allan, E.; Parkin, I. Incorporation of crystal violet, methylene blue & safranin O into a copolymer emulsion; the development of a novel antimicrobial paint. RSC Adv. 2015, 5, 26364. [Google Scholar]
- Thakur, S.; Karak, N. Green reduction of graphene oxide by aqueous phytoextracts. Carbon 2012, 50, 5331–5339. [Google Scholar] [CrossRef]
- Burgess, H.D. The stabilization of cellulosic fibres by borohydride derivatives. In Proceedings of the 9th Trienn. Meet. ICOM-CC, Dresden, German, 26–31 August 1990; pp. 447–452. [Google Scholar]
- Heritage, N.R.I.C. Conservation of Papers and Textiles; National Research Institute of Cultural Heritage: Daejeon, Korea, 2012; ISBN 9788963258041.
- Ringgaard, M.G. An investigation of the effects of borohydride treatments of oxidized cellulose textiles. In Proceedings of the Strengthening the Bond: Science & Textiles: North American Textile Conservation Conference, Philadelphia, PA, USA, 5–6 April 2002; pp. 91–100. [Google Scholar]
- Ballard, M.; Tímár-Balázsy, Á.; Eastop, D.; Timar-Balazsy, A. Chemical Principles of Textile Conservation. Stud. Conserv. 2000, 45, 215. [Google Scholar] [CrossRef]
- Baber, R.; Mazzei, L.; Thanh, N.T.K.; Gavriilidis, A. Synthesis of silver nanoparticles in a microfluidic coaxial flow reactor. RSC Adv. 2015, 5, 95585–95591. [Google Scholar] [CrossRef]
- Polte, J.; Tuaev, X.; Wuithschick, M.; Fischer, A.; Thuenemann, A.F.; Rademann, K.; Kraehnert, R.; Emmerling, F. Formation mechanism of colloidal silver nanoparticles: Analogies and differences to the growth of gold nanoparticles. ACS Nano 2012, 6, 5791–5802. [Google Scholar] [CrossRef] [PubMed]
- Takesue, M.; Tomura, T.; Yamada, M.; Hata, K.; Kuwamoto, S.; Yonezawa, T. Size of elementary clusters and process period in silver nanoparticle formation. J. Am. Chem. Soc. 2011, 133, 14164–14167. [Google Scholar] [CrossRef] [PubMed]
- Guex, L.G.; Sacchi, B.; Peuvot, K.F.; Andersson, R.L.; Pourrahimi, A.M.; Ström, V.; Farris, S.; Olsson, R.T. Experimental review: Chemical reduction of graphene oxide (GO) to reduced graphene oxide (rGO) by aqueous chemistry. Nanoscale 2017, 9, 9562–9571. [Google Scholar] [CrossRef] [PubMed]
- MacInnes, M.M.; Hlynchuk, S.; Acharya, S.; Lehnert, N.; Maldonado, S. Reduction of Graphene Oxide Thin Films by Cobaltocene and Decamethylcobaltocene. ACS Appl. Mater. Interfaces 2018, 10, 2004–2015. [Google Scholar] [CrossRef]
- Hu, C.; Zhai, X.; Liu, L.; Zhao, Y.; Jiang, L.; Qu, L. Spontaneous Reduction and Assembly of Graphene oxide into Three-Dimensional Graphene Network on Arbitrary Conductive Substrates. Sci. Rep. 2013, 3, 2065. [Google Scholar] [CrossRef]
- Luo, X.; Liu, S.; Zhou, J.; Zhang, L. In situ synthesis of Fe3O4/cellulose microspheres with magnetic-induced protein delivery. J. Mater. Chem. 2009, 19, 3538–3545. [Google Scholar] [CrossRef]
- Chung, K.; Rani, A.; Lee, J.E.; Kim, J.E.; Kim, Y.; Yang, H.; Kim, S.O.; Kim, D.; Kim, D.H. Systematic study on the sensitivity enhancement in graphene plasmonic sensors based on layer-by-layer self-assembled graphene oxide multilayers and their reduced analogues. ACS Appl. Mater. Interfaces 2015, 7, 144–151. [Google Scholar] [CrossRef]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. J. Biol. Chem. 2015, 290, 1712–1720. [Google Scholar] [CrossRef]
- Sanders, E.R. Aseptic Laboratory Techniques: Plating Methods. J. Vis. Exp. 2012, 11, e3064. [Google Scholar] [CrossRef] [PubMed]
- Hasnain, S.; Zulfequar, M.; Nishat, N. Adsorption properties of thermally stable & biologically active polyurea: Its synthesis & spectral aspects. Polym. Adv. Technol. 2012, 23, 1002–1010. [Google Scholar]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Hunter, L. Engineering Apparel Fabrics and Garments; Woodhead Publishing Ltd.: Cambridge, UK, 2009; ISBN 1845696441. [Google Scholar]
- Wu, M.; Ma, B.; Pan, T.; Chen, S.; Sun, J. Silver-Nanoparticle-Colored Cotton Fabrics with Tunable Colors & Durable Antibacterial & Self-Healing Superhydrophobic Properties. Adv. Funct. Mater. 2016, 26, 569–576. [Google Scholar]
- Yang, J.; Bai, J.; Liu, M.; Chen, Y.; Wang, S.; Yang, Q. Determination of Phosphorus in Soil by ICP-OES Using an Improved Standard Addition Method. J. Anal. Methods Chem. 2018, 2018, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fitzsimmons, J. Analytical Method Validation: ICP-OES. Electron. Suppl. Mater. J. Anal. At. Spectrom. 2015. [Google Scholar]
- Xi, J.; Xiao, J.; Xiao, F.; Jin, Y.; Dong, Y.; Jing, F.; Wang, S. Mussel-inspired Functionalization of Cotton for Nano-catalyst Support and Its Application in a Fixed-bed System with High Performance. Sci. Rep. 2016, 6, 21904. [Google Scholar] [CrossRef]
- Papa, L.; De Freitas, I.C.; Geonmonond, R.S.; De Aquino, C.B.; Pieretti, J.C.; Domingues, S.H.; Ando, R.A.; Camargo, P.H.C. Supports matter: Unraveling the role of charge transfer in the plasmonic catalytic activity of silver nanoparticles. J. Mater. Chem. A 2017, 5, 11720–11729. [Google Scholar] [CrossRef]
- Zeng, X.; McCarthy, D.T.; Deletic, A.; Zhang, X. Silver/Reduced Graphene Oxide Hydrogel as Novel Bactericidal Filter for Point-of-Use Water Disinfection. Adv. Funct. Mater. 2015, 25, 4344–4351. [Google Scholar] [CrossRef]
- Zhang, H.; Fan, X.; Quan, X.; Chen, S.; Yu, H. Graphene sheets grafted Ag@AgCl hybrid with enhanced plasmonic photocatalytic activity under visible light. Environ. Sci. Technol. 2011, 45, 5731–5736. [Google Scholar] [CrossRef]
- Losurdo, M.; Bergmair, I.; Dastmalchi, B.; Kim, T.H.; Giangregroio, M.M.; Jiao, W.; Bianco, G.V.; Brown, A.S.; Hingerl, K.; Bruno, G. Graphene as an electron shuttle for silver deoxidation: Removing a key barrier to plasmonics and metamaterials for sers in the visible. Adv. Funct. Mater. 2014, 24, 1864–1878. [Google Scholar] [CrossRef]
- Ren, J.; Wang, C.; Zhang, X.; Carey, T.; Chen, K.; Yin, Y.; Torrisi, F. Environmentally-friendly conductive cotton fabric as flexible strain sensor based on hot press reduced graphene oxide. Carbon 2017, 111, 622–630. [Google Scholar] [CrossRef]
- Kumar, A.; Vemula, P.K.; Ajayan, P.M.; John, G. Silver-nanoparticle-embedded antimicrobial paints based on vegetable oil. Nat. Mater. 2008, 7, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Fang, R.; Ge, X.; Du, M.; Li, Z.; Yang, C.; Fang, B.; Liang, Y. Preparation of silver/graphene/polymer hybrid microspheres and the study of photocatalytic degradation. Colloid Polym. Sci. 2014, 292, 985–990. [Google Scholar] [CrossRef]
- Bhunia, S.K.; Jana, N.R. Reduced graphene oxide-silver nanoparticle composite as visible light photocatalyst for degradation of colorless endocrine disruptors. ACS Appl. Mater. Interfaces 2014, 6, 20085–20092. [Google Scholar] [CrossRef]
- Ponja, S.D.; Sehmi, S.K.; Allan, E.; MacRobert, A.J.; Parkin, I.P.; Carmalt, C.J. Enhanced Bactericidal Activity of Silver Thin Films Deposited via Aerosol-Assisted Chemical Vapor Deposition. ACS Appl. Mater. Interfaces 2015, 7, 28616–28623. [Google Scholar] [CrossRef]
- Ding, D.; Chen, L.; Dong, S.; Cai, H.; Chen, J.; Jiang, C.; Cai, T. Natural ageing process accelerates the release of Ag from functional textile in various exposure scenarios. Sci. Rep. 2016, 6, 37314. [Google Scholar] [CrossRef]
- Liu, H.; Lv, M.; Deng, B.; Li, J.; Yu, M.; Huang, Q.; Fan, C. Laundering durable antibacterial cotton fabrics grafted with pomegranate-shaped polymer wrapped in silver nanoparticle aggregations. Sci. Rep. 2015, 4, 5920. [Google Scholar] [CrossRef]
- Zeng, Q.; Ding, C.; Li, Q.; Yuan, W.; Peng, Y.; Hu, J.; Zhang, K.Q. Rapid fabrication of robust, washable, self-healing superhydrophobic fabrics with non-iridescent structural color by facile spray coating. RSC Adv. 2017, 7, 8443–8452. [Google Scholar] [CrossRef]
- Üreyen, M.E.; Aslan, C. Determination of silver release from antibacterial finished cotton and polyester fabrics into water. J. Text. Inst. 2016, 108, 1128–1135. [Google Scholar] [CrossRef]
- Nischala, K.; Rao, T.N.; Hebalkar, N. Silica–silver core–shell particles for antibacterial textile application. Colloids Surf. B Biointerfaces 2011, 82, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Montazer, M.; Alimohammadi, F.; Shamei, A.; Rahimi, M.K. In situ synthesis of nano silver on cotton using Tollens’ reagent. Carbohydr. Polym. 2012, 87, 1706–1712. [Google Scholar] [CrossRef]
- Kebede, M.A.; Imae, T.; Wu, C.M.; Cheng, K.B. Cellulose fibers functionalized by metal nanoparticles stabilized in dendrimer for formaldehyde decomposition and antimicrobial activity. Chem. Eng. J. 2017, 311, 340–347. [Google Scholar] [CrossRef]
- Abou-Okeil, A.; Sheta, A.M.; Amr, A.; Ali, M.A. Wound dressing based on nonwoven viscose fabrics. Carbohydr. Polym. 2012, 90, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Wei, S.S.; Hu, S.Q.; Tang, J.X. Antimicrobial finishing of cotton textile with nanosized silver colloids synthesized using polyethylene glycol. J. Text. Inst. 2011, 102, 150–156. [Google Scholar] [CrossRef]
- Vinoth, R.; Babu, S.G.; Bharti, V.; Gupta, V.; Navaneethan, M.; Bhat, S.V.; Muthamizhchelvan, C.; Ramamurthy, P.C.; Sharma, C.; Aswal, D.K.; et al. Ruthenium based metallopolymer grafted reduced graphene oxide as a new hybrid solar light harvester in polymer solar cells. Sci. Rep. 2017, 7, 43133. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Han, J.W.; Dayem, A.A.; Eppakayala, V.; Kim, J.H. Oxidative stress-mediated antibacterial activity of graphene oxide and reduced graphene oxide in Pseudomonas aeruginosa. Int. J. Nanomed. 2012, 7, 5901–5914. [Google Scholar] [CrossRef]
- Bharti, S.; Mukherji, S.; Mukherji, S. Water disinfection using fixed bed reactors packed with silver nanoparticle immobilized glass capillary tubes. Sci. Total Environ. 2019, 689, 991–1000. [Google Scholar] [CrossRef]
- Benn, T.M.; Westerhoff, P. Nanoparticle Silver Released into Water from Commercially Available Sock Fabrics. Environ. Sci. Technol. 2008, 42, 4133–4139. [Google Scholar] [CrossRef]
- Panyala, N.R.; Peña-méndez, E.M.; Havel, J. Silver or silver nanoparticles: A hazardous threat to the environment and human health? J. Appl. Biomed. 2008, 6, 117–129. [Google Scholar] [CrossRef]
- Xie, X.; Mao, C.; Liu, X.; Zhang, Y.; Cui, Z.; Yang, X.; Yeung, K.W.K.; Pan, H.; Chu, P.K.; Wu, S. Synergistic Bacteria Killing through Photodynamic and Physical Actions of Graphene Oxide/Ag/Collagen Coating. ACS Appl. Mater. Interfaces 2017, 9, 26417–26428. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xiang, C.; Tan, L.; Lan, J.; Peng, L.; Jiang, S.; Guo, R. Preparation of silver/reduced graphene oxide coated polyester fabric for electromagnetic interference shielding. RSC Adv. 2017, 7, 40452–40461. [Google Scholar] [CrossRef]
- Rivero, P.J.; Urrutia, A.; Goicoechea, J.; Arregui, F.J. Nanomaterials for Functional Textiles and Fibers. Nanoscale Res. Lett. 2015, 10, 501. [Google Scholar] [CrossRef] [PubMed]
- Semwal, V.; Gupta, B.D. Highly sensitive surface plasmon resonance based fiber optic pH sensor utilizing rGO-Pani nanocomposite prepared by in situ method. Sens. Actuators B Chem. 2019, 283, 632–642. [Google Scholar] [CrossRef]
- Kelly, F.M.; Johnston, J.H. Colored & functional silver nanoparticle-wool fiber composites. ACS Appl. Mater. Interfaces 2011, 3, 1083–1092. [Google Scholar]
- Tang, B.; Yao, Y.; Li, J.; Qin, S.; Zhu, H.; Kaur, J.; Chen, W.; Sun, L.; Wang, X. Functional Application of Noble Metal Nanoparticles In Situ Synthesized on Ramie Fibers. Nanoscale Res. Lett. 2015, 10, 366. [Google Scholar] [CrossRef]
- Xiao, F.X.; Hung, S.F.; Tao, H.B.; Miao, J.; Yang, H.B.; Liu, B. Spatially branched hierarchical ZnO nanorod-TiO2 nanotube array heterostructures for versatile photocatalytic and photoelectrocatalytic applications: Towards intimate integration of 1D–1D hybrid nanostructures. Nanoscale 2014, 6, 14950–14961. [Google Scholar] [CrossRef]
- Friend, C.S.; Lal, M.; Biswas, A.; Winiarz, J.; Zhang, L.; Prasad, P.N. Multifunctional organic-inorganic nanocomposites for photonics. In Organic-Inorganic Hybrid Materials for Photonics; SPIE: Bellingham, WA, USA, 1998; Volume 3469, pp. 100–107. [Google Scholar]
- Shabnam, N.; Sharmila, P.; Kim, H.; Pardha-Saradhi, P.; Zhi, X.; Wang, K. Light Mediated Generation of Silver Nanoparticles by Spinach Thylakoids/Chloroplasts. PLoS ONE 2016, 11, e0167937. [Google Scholar] [CrossRef]
- Kim, B.H.; Oh, J.H.; Han, S.H.; Yun, Y.J.; Lee, J.S. Combinatorial polymer library approach for the synthesis of silver nanoplates. Chem. Mater. 2012, 24, 4424–4433. [Google Scholar] [CrossRef]
- Shahid-ul-Islam, S.I.; Butola, B.S.; Mohammad, F. Silver nanomaterials as future colorants and potential antimicrobial agents for natural and synthetic textile materials. RSC Adv. 2016, 6, 44232–44247. [Google Scholar] [CrossRef]
- Abd Aziz, N.S.; Nakajima, Y.; Sato, H.; Maekawa, T.; Hashim, A.M. One-pot green synthesis of Ag nanoparticle-decorated reduced graphene oxide composites: Effect of Ag/graphene oxide volume ratio and its demonstration as low-voltage on-chip photodetector. J. Mater. Sci. 2018, 53, 11620–11632. [Google Scholar] [CrossRef]
- Maiti, R.; Sinha, T.K.; Mukherjee, S.; Adhikari, B.; Ray, S.K. Enhanced and Selective Photodetection Using Graphene-Stabilized Hybrid Plasmonic Silver Nanoparticles. Plasmonics 2016, 11, 1297–1304. [Google Scholar] [CrossRef]
- Pereira, M.L.D.O.; Grasseschi, D.; Toma, H.E. Photocatalytic Activity of Reduced Graphene Oxide-Gold Nanoparticle Nanomaterials: Interaction with Asphaltene and Conversion of a Model Compound. Energy Fuels 2018, 32, 2673–2680. [Google Scholar] [CrossRef]
- Ben-Jaber, S.; Peveler, W.J.; Quesada-Cabrera, R.; Sol, C.W.O.; Papakonstantinou, I.; Parkin, I.P. Sensitive and specific detection of explosives in solution and vapour by surface-enhanced Raman spectroscopy on silver nanocubes. Nanoscale 2017, 9, 16459–16466. [Google Scholar] [CrossRef] [PubMed]
- Avila-Alfaro, J.A.; Sánchez-Valdes, S.; Ramos-deValle, L.F.; Ortega-Ortiz, H.; Méndez-Nonell, J.; Patiño-Soto, A.P.; Narro-Cespedes, R.I.; Perera-Mercado, Y.A.; Avalos-Belmontes, F. Ultrasound Irradiation Coating of Silver Nanoparticle on ABS Sheet Surface. J. Inorg. Organomet. Polym. Mater. 2013, 23, 673–683. [Google Scholar] [CrossRef]
- Sadezky, A.; Muckenhuber, H.; Grothe, H.; Niessner, R.; Pöschl, U. Raman microspectroscopy of soot and related carbonaceous materials: Spectral analysis and structural information. Carbon 2005, 43, 1731–1742. [Google Scholar] [CrossRef]
- Wan, F.; Shi, H.; Chen, W.; Gu, Z.; Du, L.; Wang, P.; Wang, J.; Huang, Y. Charge Transfer Effect on Raman and Surface Enhanced Raman Spectroscopy of Furfural Molecules. Nanomaterials 2017, 7, 210. [Google Scholar] [CrossRef]
- Nardo, V.M.; Sinopoli, A.; Kabalan, L.; Ponterio, R.C.; Saija, F.; Trusso, S. SERS and DFT study of indigo adsorbed on silver nanostructured surface. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 205, 465–469. [Google Scholar] [CrossRef]
- Kazarian, S.G.; Chan, K.L.A. ATR-FTIR spectroscopic imaging: Recent advances and applications to biological systems. Analyst 2013, 138, 1940. [Google Scholar] [CrossRef]
- Gurunathan, S.; Han, J.; Park, J.; Kim, E.; Choi, Y.; Kwon, D.; Kim, J. Reduced graphene oxide–silver nanoparticle nanocomposite: A potential anticancer nanotherapy. Int. J. Nanomed. 2015, 5, 6257–6276. [Google Scholar] [CrossRef]
- Gurunathan, S.; Park, J.H.; Han, J.W.; Kim, J.H. Comparative assessment of the apoptotic potential of silver nanoparticles synthesized by Bacillus tequilensis and Calocybe indica in MDA-MB-231 human breast cancer cells: Targeting p53 for anticancer therapy. Int. J. Nanomed. 2015, 10, 4203. [Google Scholar] [CrossRef] [PubMed]
- Díez, N.; Śliwak, A.; Gryglewicz, S.; Grzyb, B.; Gryglewicz, G. Enhanced reduction of graphene oxide by high-pressure hydrothermal treatment. RSC Adv. 2015, 5, 81831–81837. [Google Scholar] [CrossRef]
- Yu, H.; Miller, C.J.; Ikeda-Ohno, A.; Waite, T.D. Photodegradation of contaminants using Ag@AgCl/rGO assemblages: Possibilities and limitations. Catal. Today 2014, 224, 122–131. [Google Scholar] [CrossRef]
- Das, A.K.; Srivastav, M.; Layek, R.K.; Uddin, M.E.; Jung, D.; Kim, N.H.; Lee, J.H. Iodide-mediated room temperature reduction of graphene oxide: A rapid chemical route for the synthesis of a bifunctional electrocatalyst. J. Mater. Chem. A 2014, 2, 1332–1340. [Google Scholar] [CrossRef]
- Sengupta, I.; Bhattacharya, P.; Talukdar, M.; Neogi, S.; Pal, S.K.; Chakraborty, S. Bactericidal effect of graphene oxide and reduced graphene oxide: Influence of shape of bacteria. Colloid Interface Sci. Commun. 2019, 28, 60–68. [Google Scholar] [CrossRef]
- Liang, M.; Su, R.; Huang, R.; Qi, W.; Yu, Y.; Wang, L.; He, Z. Facile in Situ Synthesis of Silver Nanoparticles on Procyanidin-Grafted Eggshell Membrane and Their Catalytic Properties. ACS Appl. Mater. Interfaces 2014, 6, 4638–4649. [Google Scholar] [CrossRef]
- Aragaw, B.A.; Su, W.N.; Rick, J.; Hwang, B.J. Highly efficient synthesis of reduced graphene oxide–Nafion nanocomposites with strong coupling for enhanced proton and electron conduction. RSC Adv. 2013, 3, 23212. [Google Scholar] [CrossRef]
- Cui, J.; Yang, Y.; Zheng, M.; Liu, Y.; Xiao, Y.; Lei, B.; Chen, W. Facile fabrication of graphene oxide loaded with silver nanoparticles as antifungal materials. Mater. Res. Express 2014, 1, 045007. [Google Scholar] [CrossRef]
- Xing, Y.; Yang, X.; Dai, J. Antimicrobial finishing of cotton textile based on water glass by sol–gel method. J. Sol Gel Sci. Technol. 2007, 43, 187–192. [Google Scholar] [CrossRef]
- Moghayedi, M.; Goharshadi, E.K.; Ghazvini, K.; Ahmadzadeh, H.; Ranjbaran, L.; Masoudi, R.; Ludwig, R. Kinetics and mechanism of antibacterial activity and cytotoxicity of Ag-RGO nanocomposite. Colloids Surf. B Biointerfaces 2017, 1, 366–374. [Google Scholar] [CrossRef]
- Szunerits, S.; Boukherroub, R. Antibacterial activity of graphene-based materials. J. Mater. Chem. B 2016, 4, 6892–6912. [Google Scholar] [CrossRef]
- Liu, C.; Shen, J.; Yeung, K.W.K.; Tjong, S.C. Development and Antibacterial Performance of Novel Polylactic Acid-Graphene Oxide-Silver Nanoparticle Hybrid Nanocomposite Mats Prepared By Electrospinning. ACS Biomater. Sci. Eng. 2017, 3, 471–486. [Google Scholar] [CrossRef]
- Yun, S.H.; Kwok, S.J.J. Light in diagnosis, therapy and surgery. Nat. Biomed. Eng. 2017, 1, 0008. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Aslan, K.; Previte, M.J.R.; Geddes, C.D. Plasmonic engineering of singlet oxygen generation. Proc. Natl. Acad. Sci. USA 2008, 105, 1798–1802. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.; Agrawal, T.; Khan, U.; Gupta, G.K.; Rai, V.; Huang, Y.; Hamblin, M.R. Antimicrobial photodynamic inactivation in nanomedicine: Small light strides against bad bugs. Nanomedicine 2015, 10, 2379–2404. [Google Scholar] [CrossRef]
- Chandrasekhar, P.S.; Chander, N.; Anjaneyulu, O.; Komarala, V.K. Plasmonic effect of Ag@TiO2 core–shell nanocubes on dye-sensitized solar cell performance based on reduced graphene oxide–TiO2 nanotube composite. Thin Solid Films 2015, 594, 45–55. [Google Scholar] [CrossRef]
- Zhong, S.; Jiang, W.; Han, M.; Liu, G.; Zhang, N.; Lu, Y. Graphene supported silver@silver chloride & ferroferric oxide hybrid, a magnetically separable photocatalyst with high performance under visible light irradiation. Appl. Surf. Sci. 2015, 347, 242–249. [Google Scholar]
- Tang, J.; Chen, Q.; Xu, L.; Zhang, S.; Feng, L.; Cheng, L.; Xu, H.; Liu, Z.; Peng, R. Graphene Oxide–Silver Nanocomposite As a Highly Effective Antibacterial Agent with Species-Specific Mechanisms. ACS Appl. Mater. Interfaces 2013, 5, 3867–3874. [Google Scholar] [CrossRef]
- Nazari, F.; Movafeghi, A.; Jafarirad, S.; Kosari-Nasab, M.; Divband, B. Synthesis of Reduced Graphene Oxide-Silver Nanocomposites and Assessing Their Toxicity on the Green Microalga Chlorella vulgaris. Bionanoscience 2018, 8, 997–1007. [Google Scholar] [CrossRef]
- Cao, M.; Wang, M.; Li, L.; Qiu, H.; Padhiar, M.A.; Yang, Z. Wearable rGO-Ag NW@cotton fiber piezoresistive sensor based on the fast charge transport channel provided by Ag nanowire. Nano Energy 2018, 50, 528–535. [Google Scholar] [CrossRef]
- Xiu, Z.; Zhang, Q.; Puppala, H.L.; Colvin, V.L.; Alvarez, P.J.J. Negligible Particle-Specific Antibacterial Activity of Silver Nanoparticles. Nano Lett. 2012, 12, 4271–4275. [Google Scholar] [CrossRef]
- Mirzajani, F.; Ghassempour, A.; Aliahmadi, A.; Esmaeili, M.A. Antibacterial effect of silver nanoparticles on Staphylococcus aureus. Res. Microbiol. 2011, 162, 542–549. [Google Scholar] [CrossRef]


| P < 0.05 | Control | Blank Substrate | rGO | rGO: PW | Ag | Ag: PW | Ag-rGO | Ag-rGO: PW |
|---|---|---|---|---|---|---|---|---|
| Control | / | + | + | − | + | + | + | + |
| Blank Substrate | + | / | + | − | + | − | + | + |
| rGO | + | + | / | + | − | + | + | + |
| rGO: PW | − | − | + | / | + | + | + | + |
| Ag | + | + | − | + | / | + | − | + |
| Ag: PW | + | − | + | + | + | / | + | − |
| Ag-rGO | + | + | + | + | − | + | / | + |
| Ag-rGO: PW | + | + | + | + | + | − | + | / |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noor, N.; Mutalik, S.; Younas, M.W.; Chan, C.Y.; Thakur, S.; Wang, F.; Yao, M.Z.; Mou, Q.; Leung, P.H.-m. Durable Antimicrobial Behaviour from Silver-Graphene Coated Medical Textile Composites. Polymers 2019, 11, 2000. https://doi.org/10.3390/polym11122000
Noor N, Mutalik S, Younas MW, Chan CY, Thakur S, Wang F, Yao MZ, Mou Q, Leung PH-m. Durable Antimicrobial Behaviour from Silver-Graphene Coated Medical Textile Composites. Polymers. 2019; 11(12):2000. https://doi.org/10.3390/polym11122000
Chicago/Turabian StyleNoor, Nuruzzaman, Suhas Mutalik, Muhammad Waseem Younas, Cheuk Ying Chan, Suman Thakur, Faming Wang, Mian Zhi Yao, Qianqian Mou, and Polly Hang-mei Leung. 2019. "Durable Antimicrobial Behaviour from Silver-Graphene Coated Medical Textile Composites" Polymers 11, no. 12: 2000. https://doi.org/10.3390/polym11122000
APA StyleNoor, N., Mutalik, S., Younas, M. W., Chan, C. Y., Thakur, S., Wang, F., Yao, M. Z., Mou, Q., & Leung, P. H.-m. (2019). Durable Antimicrobial Behaviour from Silver-Graphene Coated Medical Textile Composites. Polymers, 11(12), 2000. https://doi.org/10.3390/polym11122000