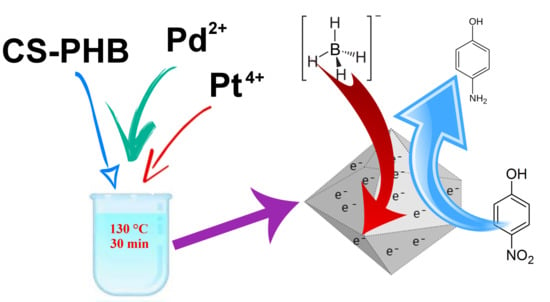
The Use of a Biopolymer Conjugate for an Eco-Friendly One-Pot Synthesis of Palladium-Platinum Alloys
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Solutions
2.2. Analytical Methods
2.3. Preparation of Cs-PHB Conjugate
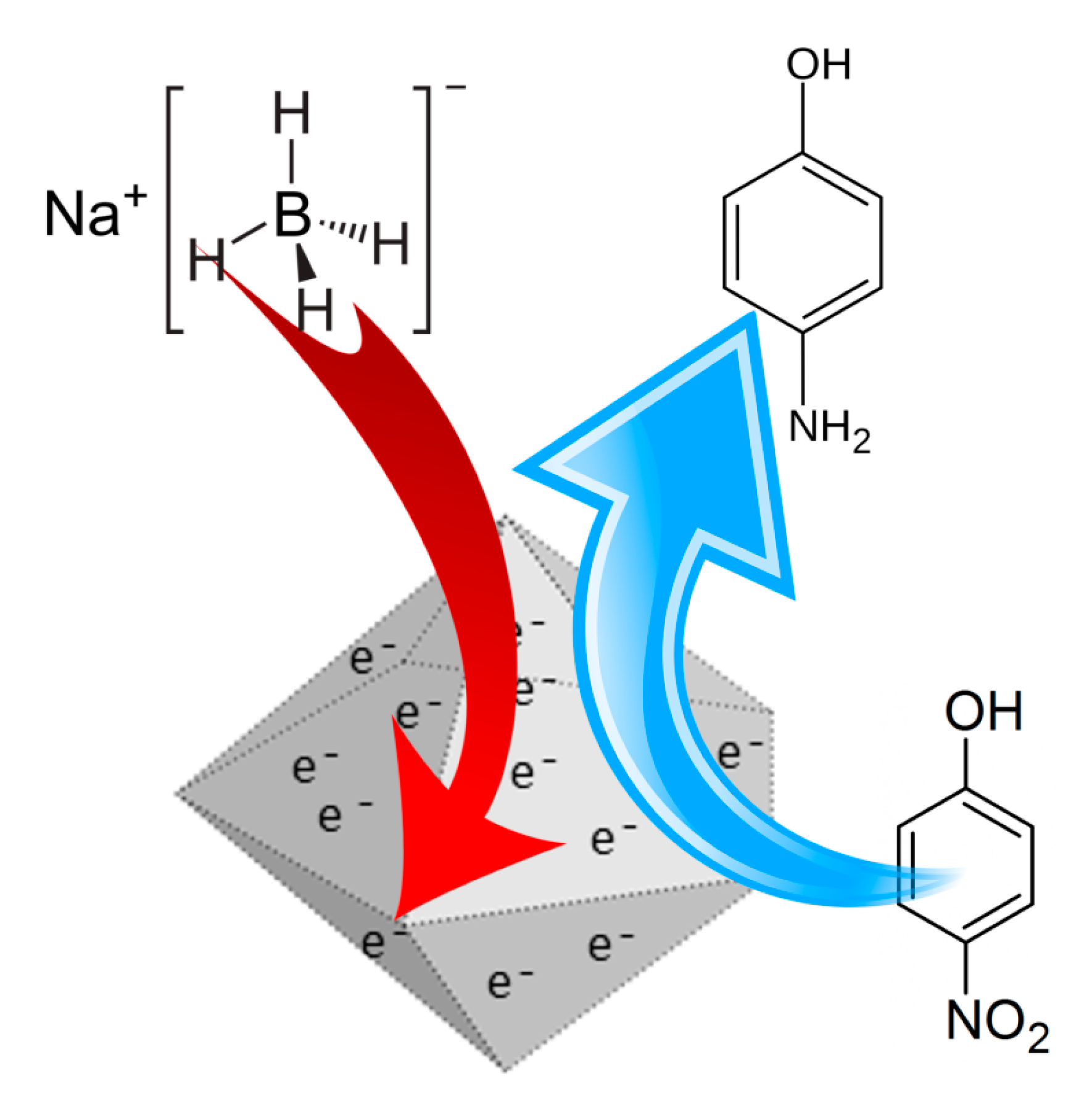
2.4. Synthesis of Bimetallic Nanoparticles
2.5. Catalytic Test
3. Results and Discussion
3.1. Characterization of the Nanoparticles
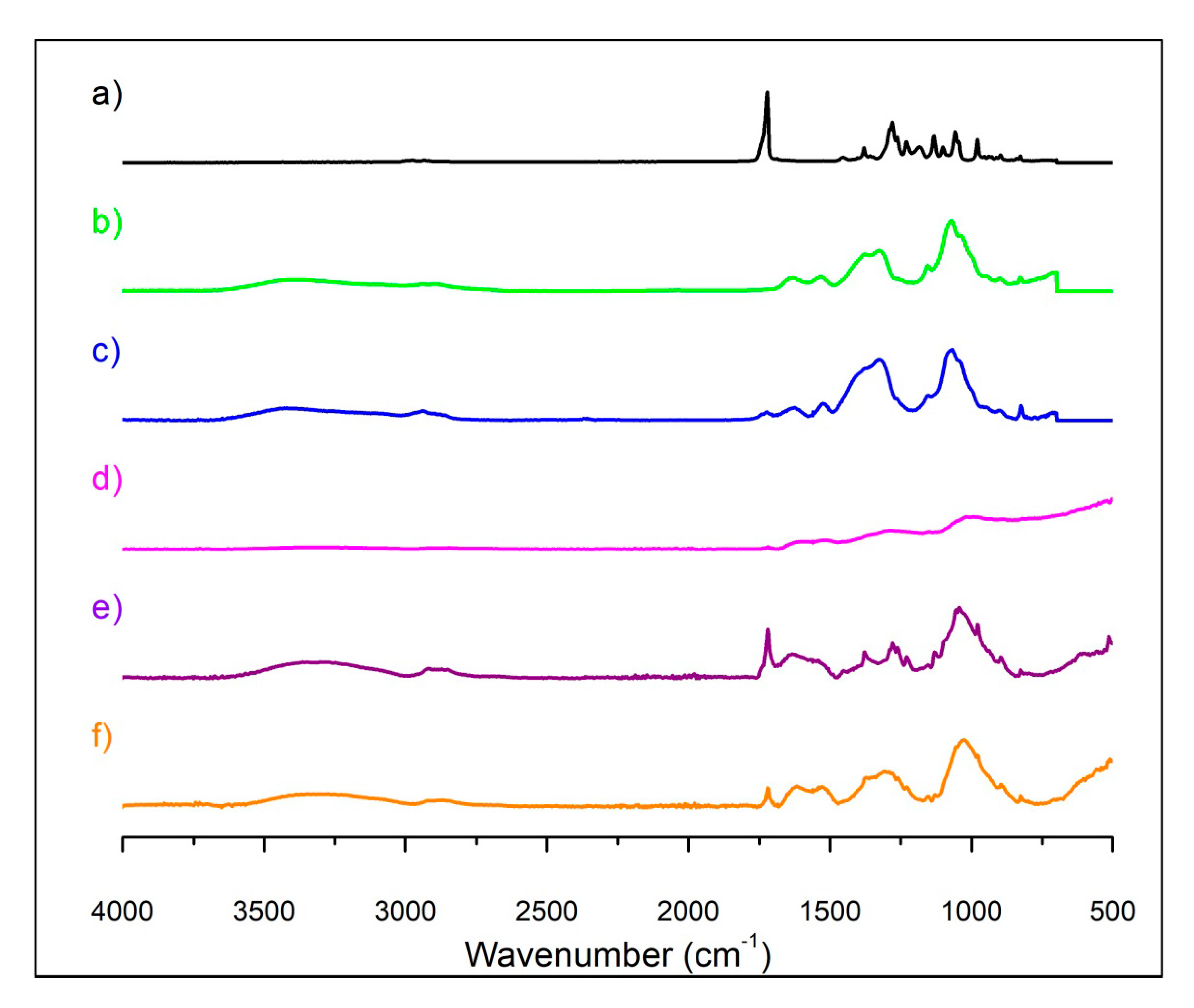
3.1.1. ATR-FTIR
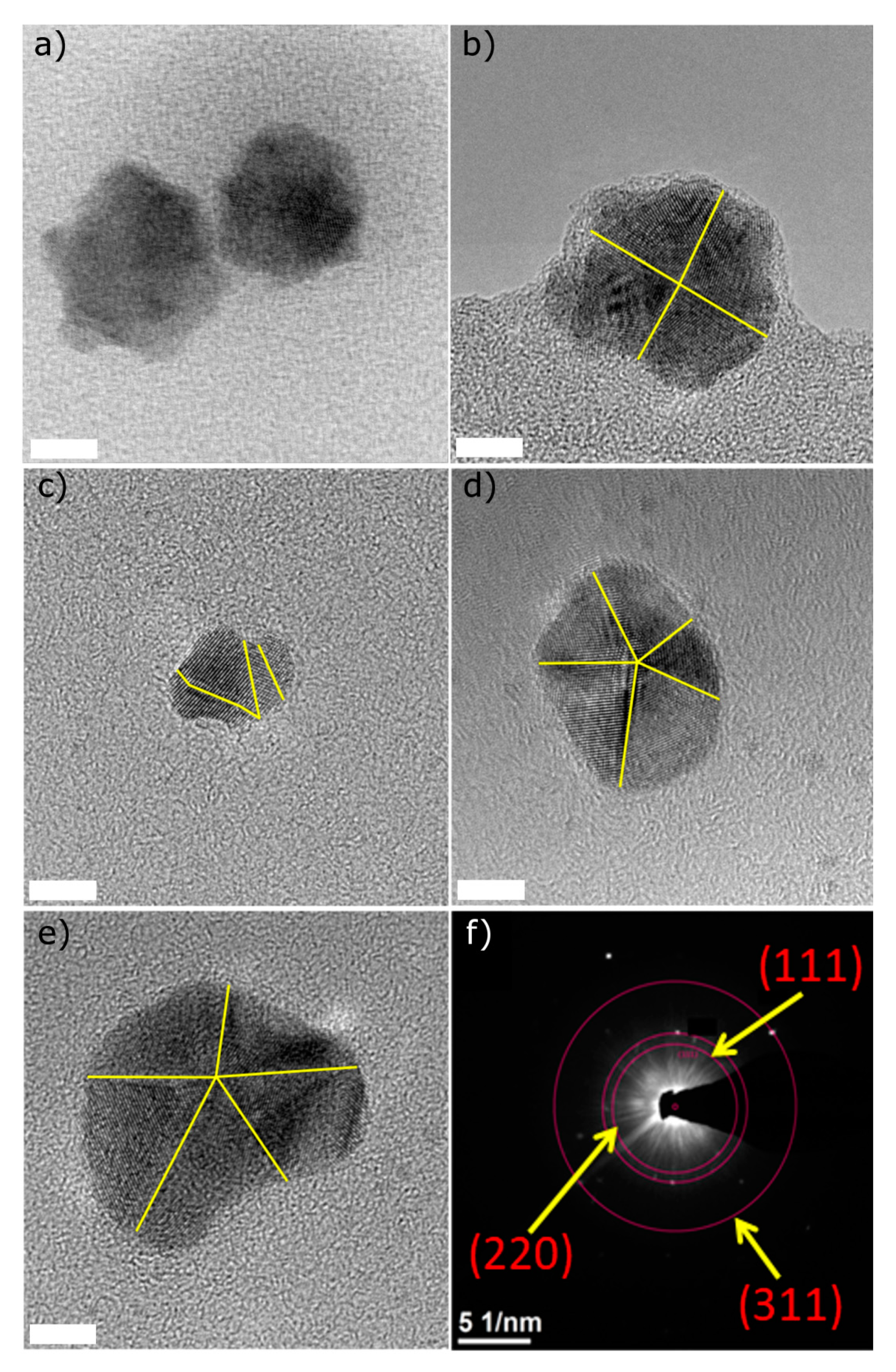
3.1.2. HR-TEM
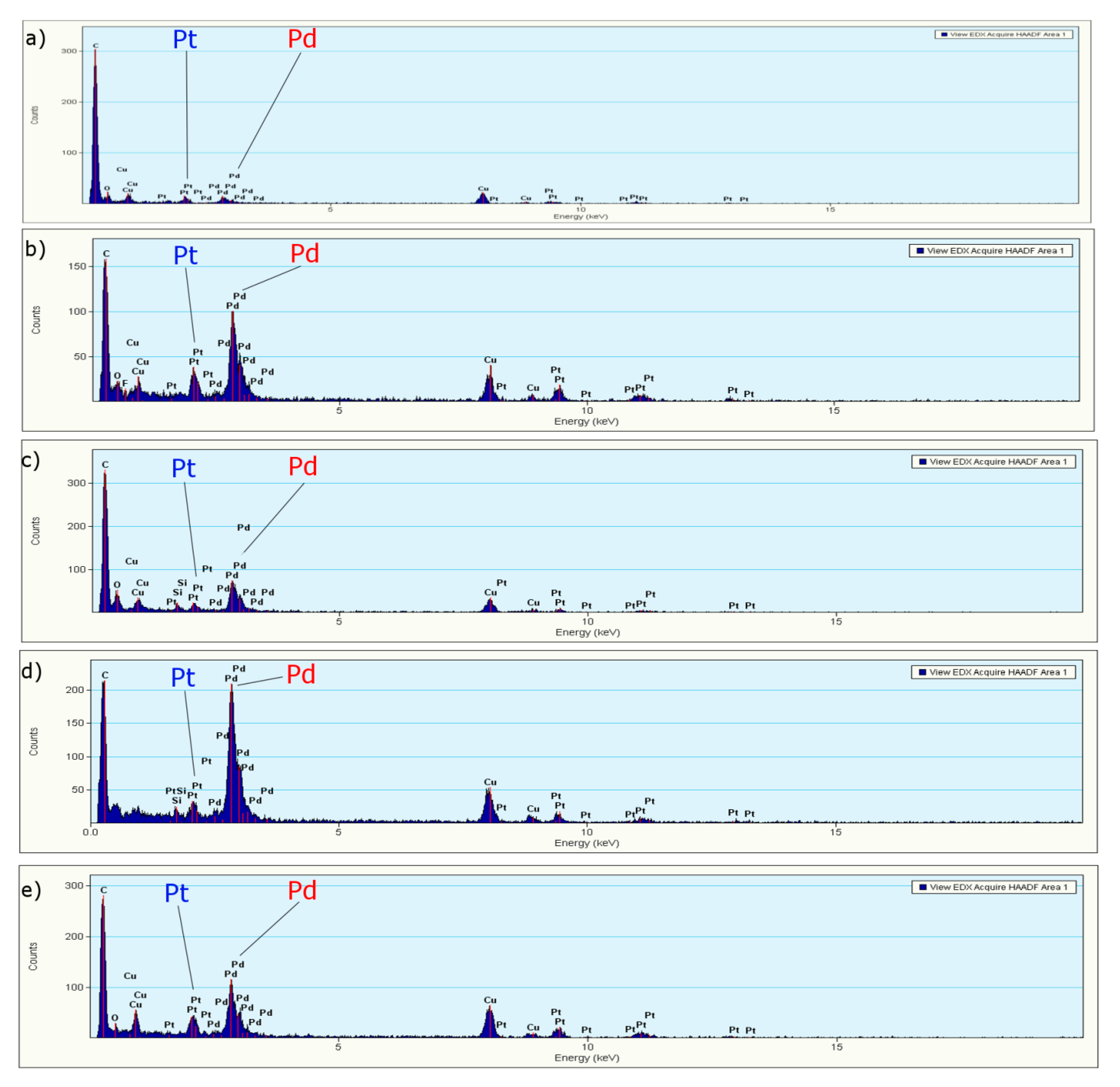
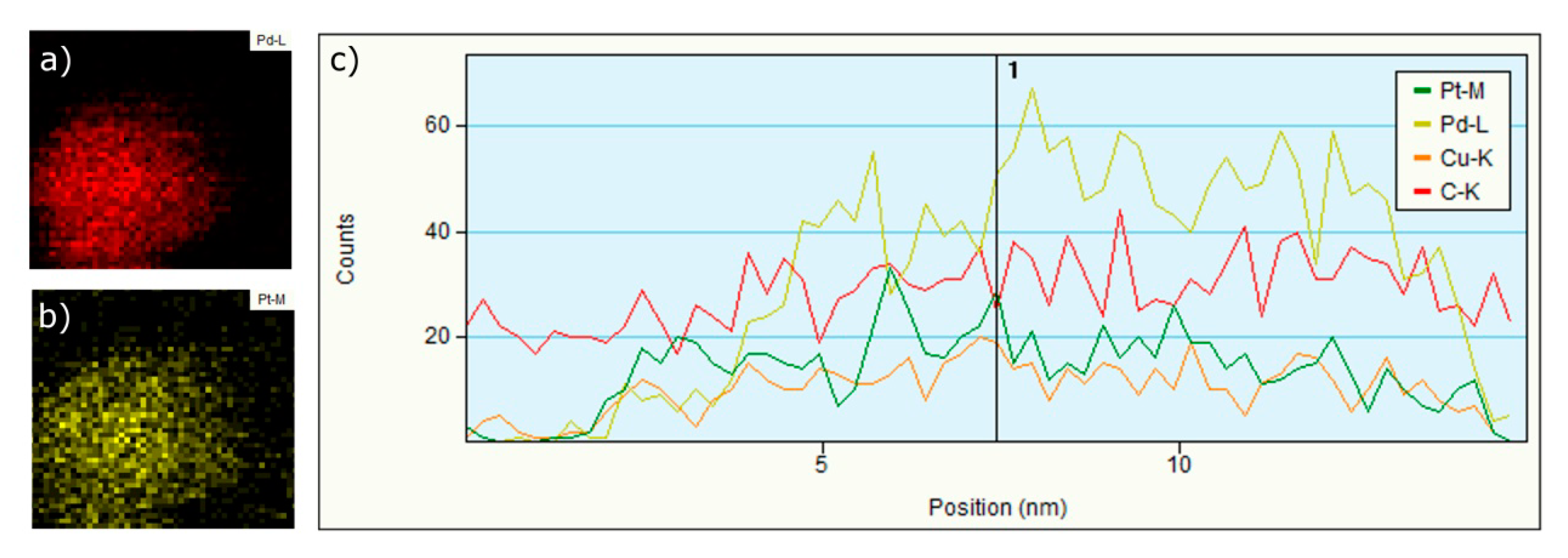
3.1.3. EDS, Mapping and Profile
3.2. Catalysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Virkutyte, J.; Varma, R.S. Green synthesis of metal nanoparticles: Biodegradable polymers and enzymes in stabilization and surface functionalization. Chem. Sci. 2011, 2, 837–846. [Google Scholar] [CrossRef]
- Moulton, M.C.; Braydich-Stolle, L.K.; Nadagouda, M.N.; Kunzelman, S.; Hussain, S.M.; Varma, R.S. Synthesis, characterization and biocompatibility of “green” synthesized silver nanoparticles using tea polyphenols. Nanoscale 2010, 2, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Vinod, V.T.P.; Saravanan, P.; Sreedhar, B.; Devi, D.K.; Sashidhar, R.B. A facile synthesis and characterization of Ag, Au and Pt nanoparticles using a natural hydrocolloid gum kondagogu (Cochlospermum gossypium). Colloids Surf. B Biointerfaces 2011, 83, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Padil, V.V.T.; Černík, M.; Thekkae Padil, V.V.; Černík, M. Green synthesis of copper oxide nanoparticles using gum karaya as a biotemplate and their antibacterial application. Int. J. Nanomed. 2013, 8, 889–898. [Google Scholar] [CrossRef]
- Nadagouda, M.N.; Speth, T.F.; Varma, R.S. Microwave-assisted green synthesis of silver nanostructures. Acc. Chem. Res. 2011, 44, 469–478. [Google Scholar] [CrossRef]
- Hebbalalu, D.; Lalley, J.; Nadagouda, M.N.; Varma, R.S. Greener techniques for the synthesis of silver nanoparticles using plant extracts, enzymes, bacteria, biodegradable polymers, and microwaves. ACS Sustain. Chem. Eng. 2013, 1, 703–712. [Google Scholar] [CrossRef]
- Seo, E.; Kim, J.; Hong, Y.; Kim, Y.S.; Lee, D.; Kim, B.S. Double hydrophilic block copolymer templated au nanoparticles with enhanced catalytic activity toward nitroarene reduction. J. Phys. Chem. C 2013, 117, 11686–11693. [Google Scholar] [CrossRef]
- Machmudah, S.; Sato, T.; Wahyudiono; Sasaki, M.; Goto, M. Silver nanoparticles generated by pulsed laser ablation in supercritical CO2 medium. High Press. Res. 2012, 32, 60–66. [Google Scholar] [CrossRef]
- Zhao, L.; Song, J.; Xue, Y.; Zhao, X.; Deng, Y.; Li, Q.; Xia, Y. Green synthesis of Ag–Au bimetallic nanoparticles with alginate for sensitive detection of H2O2. Catal. Lett. 2018, 148, 3248–3256. [Google Scholar] [CrossRef]
- Padil, V.V.T.; Wacławek, S.; Černík, M. Green Synthesis: Nanoparticles and Nanofibres Based on Tree Gums for Environmental Applications. Ecol. Chem. Eng. S 2016, 23, 533–557. [Google Scholar] [CrossRef]
- Sun, L.; Li, J.; Cai, J.; Zhong, L.; Ren, G.; Ma, Q. One pot synthesis of gold nanoparticles using chitosan with varying degree of deacetylation and molecular weight. Carbohydr. Polym. 2017, 178, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xu, X.J.; Zheng, X.C.; Guan, X.X.; Liu, P. Chitosan supported palladium nanoparticles: The novel catalysts for hydrogen generation from hydrolysis of ammonia borane. Mater. Res. Bull. 2018, 103, 89–95. [Google Scholar] [CrossRef]
- Oliveira, Â.A.S.; Medeiros, R.L.B.A.; Figueredo, G.P.; Macedo, H.P.; Braga, R.M.; Maziviero, F.V.; Melo, M.A.F.; Melo, D.M.A.; Vieira, M.M. One-step synthesis of LaNiO3 with chitosan for dry reforming of methane. Int. J. Hydrogen Energy 2018, 43, 9696–9704. [Google Scholar] [CrossRef]
- Anitha, A.; Sowmya, S.; Kumar, P.T.S.; Deepthi, S.; Chennazhi, K.P.; Ehrlich, H.; Tsurkan, M.; Jayakumar, R. Chitin and chitosan in selected biomedical applications. Prog. Polym. Sci. 2014, 39, 1644–1667. [Google Scholar] [CrossRef]
- Ali, A.; Ahmed, S. A review on chitosan and its nanocomposites in drug delivery. Int. J. Biol. Macromol. 2018, 109, 273–286. [Google Scholar] [CrossRef]
- Hahn, T.; Zibek, S. Sewage Polluted Water Treatment via Chitosan: A Review. In Chitin-Chitosan—Myriad Functionalities in Science and Technology; InTechOpen: London, UK, 2018. [Google Scholar]
- Logithkumar, R.; Keshavnarayan, A.; Dhivya, S.; Chawla, A.; Saravanan, S.; Selvamurugan, N. A review of chitosan and its derivatives in bone tissue engineering. Carbohydr. Polym. 2016, 151, 172–188. [Google Scholar] [CrossRef]
- Baig, R.B.N.; Varma, R.S. Copper on chitosan: A recyclable heterogeneous catalyst for azide-alkyne cycloaddition reactions in water. Green Chem. 2013, 15, 1839. [Google Scholar] [CrossRef]
- Sedghi, R.; Heidari, B.; Shahmohamadi, H.; Zarshenas, P.; Varma, R.S. Pd Nanocatalyst Adorned on Magnetic Chitosan@N-Heterocyclic Carbene: Eco-Compatible Suzuki Cross-Coupling Reaction. Molecules 2019, 24, 3048. [Google Scholar] [CrossRef]
- Devi, R.; Dhamodharan, R. Pretreatment in Hot Glycerol for Facile and Green Separation of Chitin from Prawn Shell Waste. ACS Sustain. Chem. Eng. 2018, 6, 846–853. [Google Scholar] [CrossRef]
- Al Rowaihi, I.S.; Paillier, A.; Rasul, S.; Karan, R.; Grötzinger, S.W.; Takanabe, K.; Eppinger, J. Poly(3-hydroxybutyrate) production in an integrated electromicrobial setup: Investigation under stress-inducing conditions. PLoS ONE 2018, 13, e0196079. [Google Scholar] [CrossRef]
- Silva, F.; Campanari, S.; Matteo, S.; Valentino, F.; Majone, M.; Villano, M. Impact of nitrogen feeding regulation on polyhydroxyalkanoates production by mixed microbial cultures. New Biotechnol. 2017, 37, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Valentino, F.; Morgan-Sagastume, F.; Campanari, S.; Villano, M.; Werker, A.; Majone, M. Carbon recovery from wastewater through bioconversion into biodegradable polymers. New Biotechnol. 2017, 37, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Baric, M.; Pierro, L.; Pietrangeli, B.; Papini, M.P. Polyhydroxyalkanoate (PHB) as a slow-release electron donor for advanced in situ bioremediation of chlorinated solvent-contaminated aquifers. New Biotechnol. 2014, 31, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Michalak, M.; Marek, A.A.; Zawadiak, J.; Kawalec, M.; Kurcok, P. Synthesis of PHB-based carrier for drug delivery systems with pH-controlled release. Eur. Polym. J. 2013, 49, 4149–4156. [Google Scholar] [CrossRef]
- Getachew, A.; Woldesenbet, F. Production of biodegradable plastic by polyhydroxybutyrate (PHB) accumulating bacteria using low cost agricultural waste material. BMC Res. Notes 2016, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wacławek, S.; Chronopoulou, L.; Petrangeli Papini, M.; Vtp, V.; Palocci, C.; Kupčík, J.; Černík, M. Enhancement of stability and reactivity of nanosized zero-valent iron with polyhydroxybutyrate. Desalin. Water Treat. 2017, 69. [Google Scholar] [CrossRef]
- Torkelson, T.R.; Oyen, F.; Rowe, V.K. The toxicity of chloroform as determined by single and repeated exposure of laboratory animals. Am. Ind. Hyg. Assoc. J. 1976, 37, 697–705. [Google Scholar] [CrossRef]
- Rannug, U. Genotoxic effects of 1,2-dibromoethane and 1,2-dichloroethane. Mutat. Res. Rev. Genet. Toxicol. 1980, 76, 269–295. [Google Scholar] [CrossRef]
- Silvestri, D.; Wacławek, S.; Sobel, B.; Torres-Mendieta, R.; Novotný, V.; Nguyen, N.H.A.; Ševců, A.; Padil, V.V.T.; Müllerová, J.; Stuchlík, M.; et al. A poly(3-hydroxybutyrate)-chitosan polymer conjugate for the synthesis of safer gold nanoparticles and their applications. Green Chem. 2018, 20, 4975–4982. [Google Scholar] [CrossRef]
- Sharma, G.; Kumar, A.; Sharma, S.; Naushad, M.; Prakash Dwivedi, R.; ALOthman, Z.A.; Mola, G.T. Novel development of nanoparticles to bimetallic nanoparticles and their composites: A review. J. King Saud Univ. Sci. 2019, 31, 257–269. [Google Scholar] [CrossRef]
- Shifrina, Z.B.; Matveeva, V.G.; Bronstein, L.M. Role of polymer structures in catalysis by transition metal and metal oxide Nanoparticle Composites. Chem. Rev. 2019. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.; Lu, Y.; Polzer, F.; Ballauff, M.; Drechsler, M. Catalytic activity of palladium nanoparticles encapsulated in spherical poly electrolyte brushes and core-shell microgels. Chem. Mater. 2007, 19, 1062–1069. [Google Scholar] [CrossRef]
- Mei, Y.; Sharma, G.; Lu, Y.; Ballauff, M.; Drechsler, M.; Irrgang, T.; Kempe, R. High catalytic activity of platinum nanoparticles immobilized on spherical polyelectrolyte brushes. Langmuir 2005, 21, 12229–12234. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yao, C.; Wang, Y.C.; Mikael, S.; Gunasekaran, S.; Ma, Z.; Cai, Z.; Wang, X. High-density platinum nanoparticle-decorated titanium dioxide nanofiber networks for efficient capillary photocatalytic hydrogen generation. J. Mater. Chem. A 2016, 4, 11672–11679. [Google Scholar] [CrossRef]
- Wu, M.C.; Hsiao, K.C.; Chang, Y.H.; Chan, S.H. Photocatalytic hydrogen evolution of palladium nanoparticles decorated black TiO2 calcined in argon atmosphere. Appl. Surf. Sci. 2018, 430, 407–414. [Google Scholar] [CrossRef]
- Elsey, J.; Bubley, J.A.; Zhu, L.; Rao, S.; Sasaki, M.; Pollack, B.P.; Yang, L.; Arbiser, J.L. Palladium based nanoparticles for the treatment of advanced melanoma. Sci. Rep. 2019, 9, 3255. [Google Scholar] [CrossRef] [PubMed]
- Samadi, A.; Klingberg, H.; Jauffred, L.; Kjær, A.; Bendix, P.M.; Oddershede, L.B. Platinum nanoparticles: A non-toxic, effective and thermally stable alternative plasmonic material for cancer therapy and bioengineering. Nanoscale 2018, 10, 9097–9107. [Google Scholar] [CrossRef]
- Lai, J.; Luque, R.; Xu, G. Recent Advances in the Synthesis and Electrocatalytic Applications of Platinum-Based Bimetallic Alloy Nanostructures. ChemCatChem 2015, 7, 3206–3228. [Google Scholar] [CrossRef]
- Wang, L.; Yamauchi, Y. Metallic nanocages: Synthesis of bimetallic Pt-Pd hollow nanoparticles with dendritic shells by selective chemical etching. J. Am. Chem. Soc. 2013, 135, 16762–16765. [Google Scholar] [CrossRef]
- Lim, B.; Wang, J.; Camargo, P.H.C.; Cobley, C.M.; Kim, M.J.; Xia, Y. Twin-induced growth of palladium-platinum alloy nanocrystals. Angew. Chem. Int. Ed. 2009, 48, 6304–6308. [Google Scholar] [CrossRef]
- Venkateshaiah, A.; Silvestri, D.; Ramakrishnan, R.K.; Wacławek, S.; Padil, V.V.T.; Černík, M.; Varma, R.S. Gum Kondagogu/Reduced Graphene Oxide Framed Platinum Nanoparticles and Their Catalytic Role. Molecules 2019, 24, 3643. [Google Scholar] [CrossRef] [PubMed]
- Baruah, B.; Gabriel, G.J.; Akbashev, M.J.; Booher, M.E. Facile synthesis of silver nanoparticles stabilized by cationic polynorbornenes and their catalytic activity in 4-nitrophenol reduction. Langmuir 2013, 29, 4225–4234. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, M.F.; Melo, K.R.T.; Sabry, D.A.; Sassaki, G.L.; Rocha, H.A.O. Does the use of chitosan contribute to oxalate kidney stone formation? Mar. Drugs 2015, 13, 141–158. [Google Scholar] [CrossRef] [PubMed]
- Dang Nguyen Vô, K.; Kowandy, C.; Dupont, L.; Coqueret, X. Evidence of chitosan-mediated reduction of Au(III) to Au(0) nanoparticles under electron beam by using OH and e-aq scavengers. Chem. Commun. 2015, 51, 4017–4020. [Google Scholar] [CrossRef]
- Dorjnamjin, D.; Ariunaa, M.; Shim, Y.K. Synthesis of silver nanoparticles using hydroxyl functionalized ionic liquids and their antimicrobial activity. Int. J. Mol. Sci. 2008, 9, 807–820. [Google Scholar] [CrossRef]
- Hu, P.; Song, Y.; Rojas-Andrade, M.D.; Chen, S. Platinum Nanoparticles Functionalized with Ethynylphenylboronic Acid Derivatives: Selective Manipulation of Nanoparticle Photoluminescence by Fluoride Ions. Langmuir 2014, 30, 5224–5229. [Google Scholar] [CrossRef]
- Mendoza-Pérez, R.; Guisbiers, G. Bimetallic Pt-Pd nano-catalyst: Size, shape and composition matter. Nanotechnology 2019, 30, 305702. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, N.; Goebl, J.; Lu, Z.; Yin, Y. A systematic study of the synthesis of silver nanoplates: Is citrate a “magic” reagent? J. Am. Chem. Soc. 2011, 133, 18931–18939. [Google Scholar] [CrossRef]
- Ghosh, A.; Dutta, S.; Mukherjee, I.; Biswas, S.; Chatterjee, S.; Saha, R. Template-free synthesis of flower-shaped zero-valent iron nanoparticle: Role of hydroxyl group in controlling morphology and nitrate reduction. Adv. Powder Technol. 2017, 28, 2256–2264. [Google Scholar] [CrossRef]
- Long, N.V.; Hien, T.D.; Asaka, T.; Ohtaki, M.; Nogami, M. Synthesis and characterization of Pt–Pd nanoparticles with core-shell morphology: Nucleation and overgrowth of the Pd shells on the as-prepared and defined Pt seeds. J. Alloys Compd. 2011, 509, 7702–7709. [Google Scholar] [CrossRef]
- Tuo, Y.; Liu, G.; Dong, B.; Yu, H.; Zhou, J.; Wang, J.; Jin, R. Microbial synthesis of bimetallic PdPt nanoparticles for catalytic reduction of 4-nitrophenol. Environ. Sci. Pollut. Res. 2017, 24, 5249–5258. [Google Scholar] [CrossRef]
- Li, H.; Han, L.; Cooper-White, J.; Kim, I. Palladium nanoparticles decorated carbon nanotubes: Facile synthesis and their applications as highly efficient catalysts for the reduction of 4-nitrophenol. Green Chem. 2012, 14, 586. [Google Scholar] [CrossRef]
- Huang, X.; Li, Y.; Li, Y.; Zhou, H.; Duan, X.; Huang, Y. Synthesis of PtPd bimetal nanocrystals with controllable shape, composition, and their tunable catalytic properties. Nano Lett. 2012, 12, 4265–4270. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Guo, S.; Dong, S. Rapid, general synthesis of pdpt bimetallic alloy nanosponges and their enhanced catalytic performance for ethanol/methanol electrooxidation in an alkaline medium. Chem. A Eur. J. 2013, 19, 1104–1111. [Google Scholar] [CrossRef] [PubMed]
- Yin, A.X.; Min, X.Q.; Zhang, Y.W.; Yan, C.H. Shape-selective synthesis and facet-dependent enhanced electrocatalytic activity and durability of monodisperse Sub-10 nm Pt-Pd tetrahedrons and cubes. J. Am. Chem. Soc. 2011, 133, 3816–3819. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.W.; Ko, A.R.; Han, S.B.; Kim, H.S.; Park, K.W. Synthesis of octahedral Pt–Pd alloy nanoparticles for improved catalytic activity and stability in methanol electrooxidation. Phys. Chem. Chem. Phys. 2011, 13, 5569. [Google Scholar] [CrossRef]
- Liu, X.Y.; Zhang, Y.; Gong, M.X.; Tang, Y.W.; Lu, T.H.; Chen, Y.; Lee, J.M. Facile synthesis of corallite-like Pt-Pd alloy nanostructures and their enhanced catalytic activity and stability for ethanol oxidation. J. Mater. Chem. A 2014, 2, 13840–13844. [Google Scholar] [CrossRef]
- Scott, R.W.J.; Datye, A.K.; Crooks, R.M. Bimetallic Palladium-Platinum Dendrimer-Encapsulated Catalysts. J. Am. Chem. Soc. 2003, 125, 3708–3709. [Google Scholar] [CrossRef]
- Datta, K.J.; Datta, K.K.R.; Gawande, M.B.; Ranc, V.; Čépe, K.; Malgras, V.; Yamauchi, Y.; Varma, R.S.; Zboril, R. Pd@Pt Core-Shell Nanoparticles with Branched Dandelion-like Morphology as Highly Efficient Catalysts for Olefin Reduction. Chem. A Eur. J. 2016, 22, 1577–1581. [Google Scholar] [CrossRef]
- Wang, X.; Vara, M.; Luo, M.; Huang, H.; Ruditskiy, A.; Park, J.; Bao, S.; Liu, J.; Howe, J.; Chi, M.; et al. Pd@Pt Core—Shell Concave Decahedra: A Class of Catalysts for the Oxygen Reduction Reaction with Enhanced Activity and Durability. J. Am. Chem. Soc. 2015, 137, 15036–15042. [Google Scholar] [CrossRef]
- Lim, B.; Jiang, M.; Camargo, P.H.C.; Cho, E.C.; Tao, J.; Lu, X.; Zhu, Y.; Xia, Y. Pd-Pt bimetallic nanodendrites with high activity for oxygen reduction. Science 2009, 324, 1302–1305. [Google Scholar] [CrossRef]
- Wacławek, S.; Gončuková, Z.; Adach, K.; Fijałkowski, M.; Černík, M. Green synthesis of gold nanoparticles using Artemisia dracunculus extract: Control of the shape and size by varying synthesis conditions. Environ. Sci. Pollut. Res. 2018, 25, 24210–24219. [Google Scholar] [CrossRef] [PubMed]
- Stumm, W.; Morgan, J.J. Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters; Wiley: Hoboken, NJ, USA, 2012; ISBN 1118591488. [Google Scholar]
- Kästner, C.; Thünemann, A.F. Catalytic Reduction of 4-Nitrophenol Using Silver Nanoparticles with Adjustable Activity. Langmuir 2016, 32, 7383–7391. [Google Scholar] [CrossRef] [PubMed]
- Gangula, A.; Podila, R.; Karanam, L.; Janardhana, C.; Rao, A.M. Catalytic reduction of 4-nitrophenol using biogenic gold and silver nanoparticles derived from breynia rhamnoides. Langmuir 2011, 27, 15268–15274. [Google Scholar] [CrossRef] [PubMed]
- Lara, L.R.S.; Zottis, A.D.; Elias, W.C.; Faggion, D.; Maduro De Campos, C.E.; Acuña, J.J.S.; Domingos, J.B. The catalytic evaluation of in situ grown Pd nanoparticles on the surface of Fe3O4@dextran particles in the p-nitrophenol reduction reaction. RSC Adv. 2015, 5, 8289–8296. [Google Scholar] [CrossRef]
- Ma, T.; Liang, F.; Chen, R.; Liu, S.; Zhang, H. Synthesis of Au-Pd bimetallic nanoflowers for catalytic reduction of 4-nitrophenol. Nanomaterials 2017, 7, 239. [Google Scholar] [CrossRef]
- Chen, X.; Cai, Z.; Chen, X.; Oyama, M. AuPd bimetallic nanoparticles decorated on graphene nanosheets: Their green synthesis, growth mechanism and high catalytic ability in 4-nitrophenol reduction. J. Mater. Chem. A 2014, 2, 5668–5674. [Google Scholar] [CrossRef]
- Wu, W.; Lei, M.; Yang, S.; Zhou, L.; Liu, L.; Xiao, X.; Jiang, C.; Roy, V.A.L. A one-pot route to the synthesis of alloyed Cu/Ag bimetallic nanoparticles with different mass ratios for catalytic reduction of 4-nitrophenol. J. Mater. Chem. A 2015, 3, 3450–3455. [Google Scholar] [CrossRef]
- El-Bahy, Z.M.; Hanafy, A.I.; El-Bahy, S.M. Preparation of Pt, Pd and Cu nano single and bimetallic systems-supported NaY zeolite and test their activity in p-nitrophenol reduction and as anticancer agents. J. Environ. Chem. Eng. 2019, 7, 103117. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Q.; Zhang, P.; O’Connor, D.; Varma, R.S.; Yu, M.; Hou, D. One-pot green synthesis of bimetallic hollow palladium-platinum nanotubes for enhanced catalytic reduction of p-nitrophenol. J. Colloid Interf. Sci. 2019, 539, 161–167. [Google Scholar] [CrossRef]
| Shape | Solvent | Precursors | Molar Pd/Pt Ratio | Reducing Agent | Temperature (°C) | Synthesis Time (min) | Reference |
|---|---|---|---|---|---|---|---|
| Cube | DMF | Na2PdCl4 K2PtCl6 | 1:1 | - | 130 | 300 | [54] |
| Nanosponges | Water | H2PdCl4 K2PtCl6 | 1:1 | NaBH4 | Room temperature | ~5 | [55] |
| Tetrahedron | Water | Na2PdCl4 K2PtCl6 | 1:1 | HCHO | 180 | 120 | [56] |
| Octahedron | Water | Na2PdCl4 H2PtCl6 | 1:1 | Glycerol | 100 | 180 | [57] |
| Corallite-like structure | Water | K2PdCl4 K2Pt(CN)4 | 2.05:1 | NaBH4 | Room temperature | 120 | [58] |
| Branched Dandelion-like | Water | Na2PdCl4 K2PtCl6 | 1:7 | Ascorbic acid | Room temperature | 30 | [55] |
| Nanocages | Water | K2PdBr4 Na2PtBr6 | 1:2 | Ascorbic acid | Room temperature | 480 | [40] |
| Irregular polyhedron | Water | K2PdCl4 PtCl4 | 1:1 | Cs-PHB | 130 | 30 | This work |
| Decahedron | Water | K2PdCl4 PtCl4 | 1:2 | Cs-PHB | 130 | 30 | This work |
| Decahedron | Water | K2PdCl4 PtCl4 | 2:1 | Cs-PHB | 130 | 30 | This work |
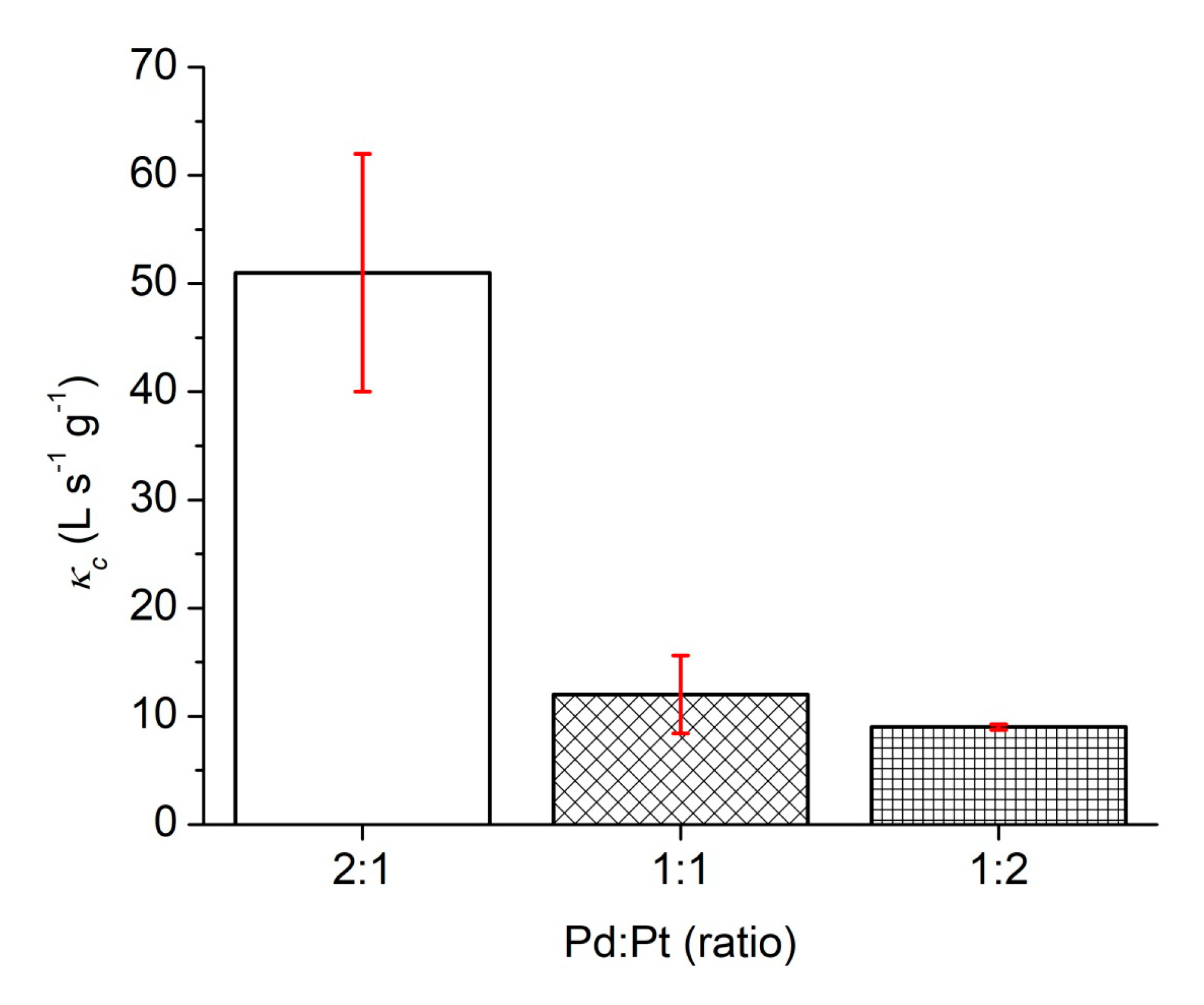
| Catalysts | Synthesis Temperature (°C) | Concentration (mg/L) | kapp (min−1) | κc (L s−1 g−1) |
|---|---|---|---|---|
| Pd/Pt (1:1) | 130 | 0.379 | 0.038 | 12 ± 4 |
| 0.757 | 0.546 | |||
| 1.515 | 0.897 | |||
| Pd/Pt (1:2) | 130 | 0.147 | 0.066 | 9 ± 1 |
| 0.293 | 0.152 | |||
| 0.586 | 0.305 | |||
| Pd/Pt (2:1) | 130 | 0.202 | 0.198 | 51 ± 11 |
| 0.404 | 0.424 | |||
| 0.809 | 1.967 |
| Catalysts | Catalyst Concentration (mg/L) | 4-NP Concentration (mM) | NaBH4 Concentration (mM) | kapp (s−1) | κc (L s−1 g−1) | Ref. |
|---|---|---|---|---|---|---|
| Pd/Au | 8 | 0.07 | 21 | 0.258 | 32 | [68] |
| Au53Pd47/graphene nanosheets | 0.06 | 0.05 | 5 | 0.014 | 240 | [69] |
| Cu/Ag | 0.48 | 0.096 | 11.2 | 0.0003 | 7.18 | [70] |
| PdCuY | 20 | 0.72 | 1.5 | 0.002 | 0.12 | [71] |
| Pd/Pt nanotubes | 3.4 | 0.09 | 100 | 0.008 | 25 | [72] |
| Pd/Pt (2:1) | 0.809 | 0.12 | 12 | 0.033 | 51 ± 11 | This work |
| Pd/Pt (1:1) | 0.757 | 0.12 | 12 | 0.009 | 12 ± 4 | This work |
| Pd/Pt (1:2) | 0.586 | 0.12 | 12 | 0.005 | 9 ± 1 | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silvestri, D.; Wacławek, S.; K. Ramakrishnan, R.; Venkateshaiah, A.; Krawczyk, K.; Padil, V.V.T.; Sobel, B.; Černík, M. The Use of a Biopolymer Conjugate for an Eco-Friendly One-Pot Synthesis of Palladium-Platinum Alloys. Polymers 2019, 11, 1948. https://doi.org/10.3390/polym11121948
Silvestri D, Wacławek S, K. Ramakrishnan R, Venkateshaiah A, Krawczyk K, Padil VVT, Sobel B, Černík M. The Use of a Biopolymer Conjugate for an Eco-Friendly One-Pot Synthesis of Palladium-Platinum Alloys. Polymers. 2019; 11(12):1948. https://doi.org/10.3390/polym11121948
Chicago/Turabian StyleSilvestri, Daniele, Stanisław Wacławek, Rohith K. Ramakrishnan, Abhilash Venkateshaiah, Kamil Krawczyk, Vinod V. T. Padil, Bartłomiej Sobel, and Miroslav Černík. 2019. "The Use of a Biopolymer Conjugate for an Eco-Friendly One-Pot Synthesis of Palladium-Platinum Alloys" Polymers 11, no. 12: 1948. https://doi.org/10.3390/polym11121948
APA StyleSilvestri, D., Wacławek, S., K. Ramakrishnan, R., Venkateshaiah, A., Krawczyk, K., Padil, V. V. T., Sobel, B., & Černík, M. (2019). The Use of a Biopolymer Conjugate for an Eco-Friendly One-Pot Synthesis of Palladium-Platinum Alloys. Polymers, 11(12), 1948. https://doi.org/10.3390/polym11121948