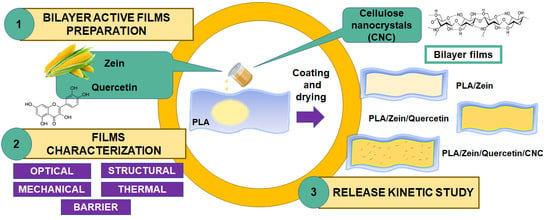
Development of Bilayer Biodegradable Composites Containing Cellulose Nanocrystals with Antioxidant Properties
Abstract
1. Introduction
2. Materials and Chemicals
2.1. Materials
2.2. Preparation of Active Bilayer PLA-Zein Composites Structures
2.2.1. Preparation of PLA Film
2.2.2. Zein Coating
2.3. Characterization of Optical and Structural Properties
2.3.1. Optical Characterization of Bilayer Structures
2.3.2. Morphological Characterization of Bilayer Structures
2.3.3. Fourier Transform Infrared (FTIR)–Attenuated Total Reflectance (ATR) Spectroscopy
2.4. Thermal Properties
2.4.1. Differential Scanning Calorimetry
2.4.2. Thermogravimetry Analysis
2.5. Mechanical Properties
2.6. Water Vapor Permeability (WVP) Analysis
2.7. Study of Release Kinetics of Quercetin from Active Bilayers Composites
2.7.1. Release Assay Procedure
2.7.2. Determination of Partition and Diffusion Coefficients of Quercetin in PLA/ZN Bilayers
2.8. Statistical Analysis
3. Results and Discussion
3.1. Optical Properties
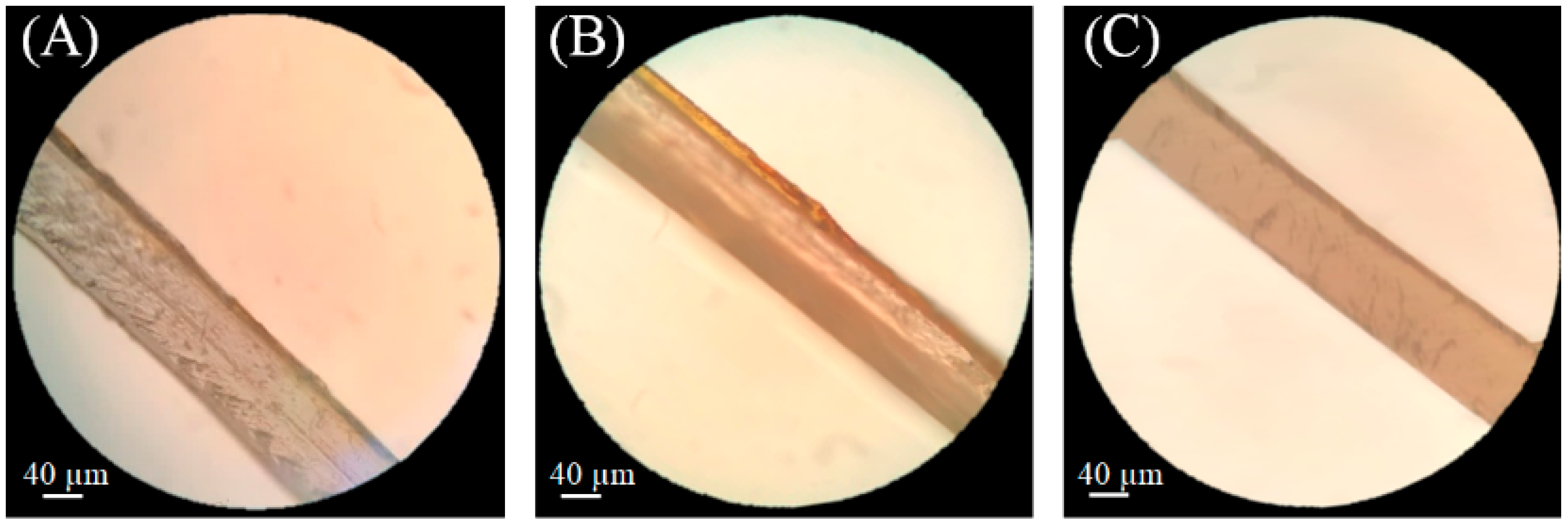
3.2. Morphological Results
3.3. FTIR Spectra Results
3.4. Thermal Characterization
3.4.1. Differential Scanning Calorimetry
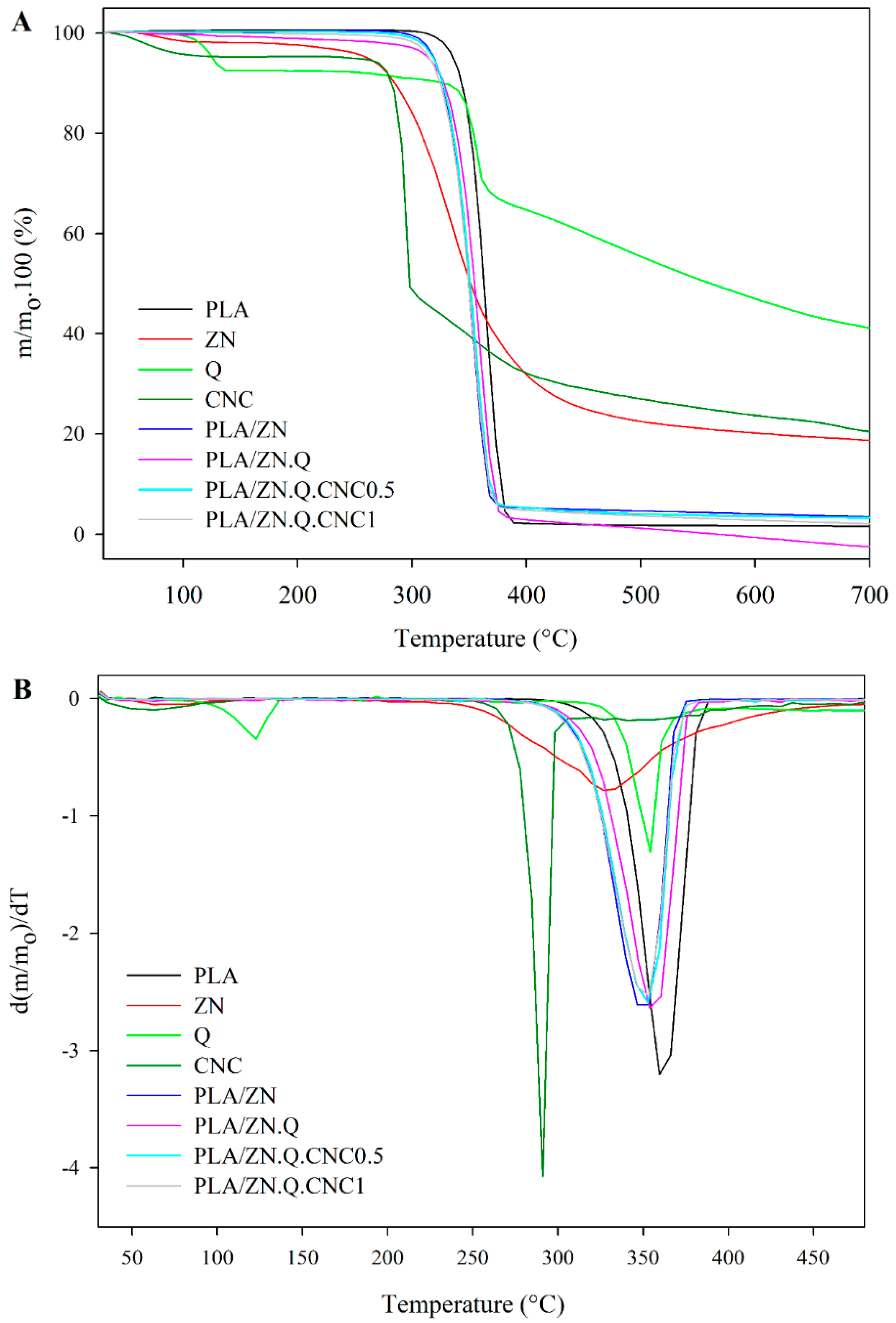
3.4.2. Thermogravimetric Analysis
3.5. Mechanical Properties
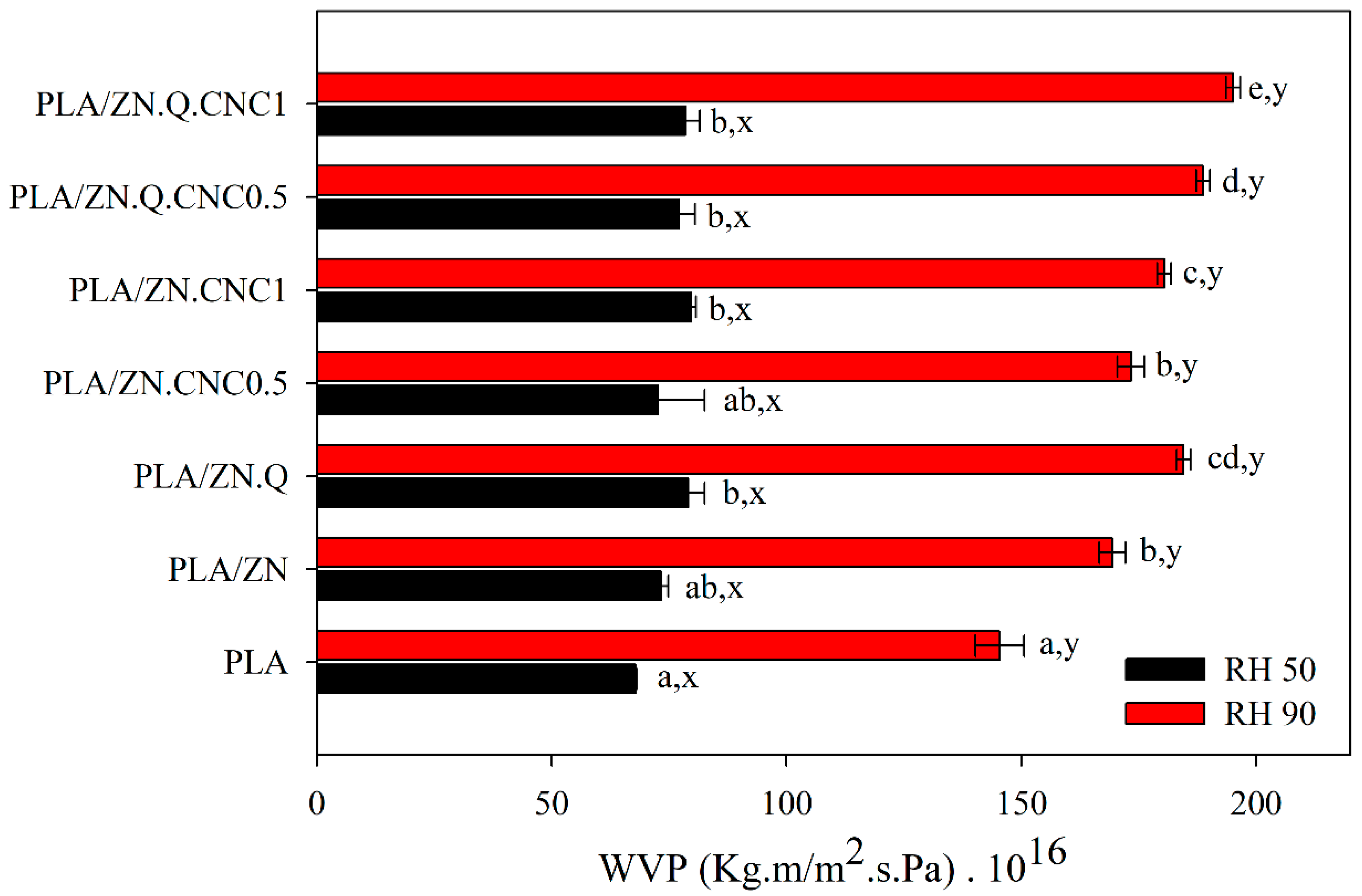
3.6. Barrier Properties
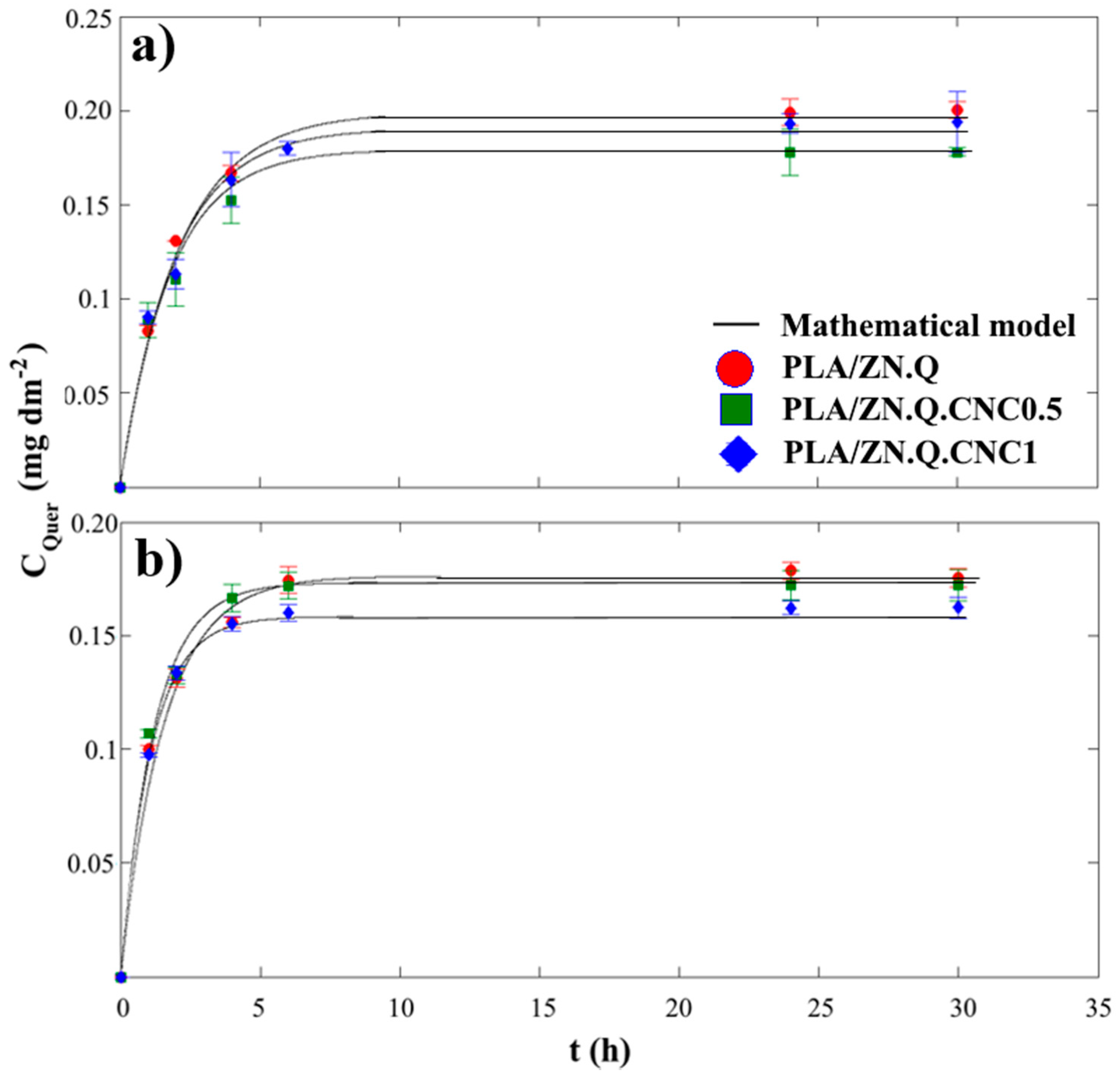
3.7. Kinetic Release of Quercetin from Bilayer Composites
Author Contributions
Funding
Conflicts of Interest
References
- González, A.; Igarzabal, C.I.A. Soy protein—Poly(lactic acid) bilayer films as biodegradable material for active food packaging. Food Hydrocoll. 2013, 33, 289–296. [Google Scholar] [CrossRef]
- Rydz, J.; Musioł, M.; Zawidlak-Węgrzyńska, B.; Sikorska, W. Present and Future of Biodegradable Polymers for Food Packaging Applications. Biopolym. Food Des. 2018, 431–467. [Google Scholar] [CrossRef]
- Castro-Aguirre, E.; Iñiguez-Franco, F.; Samsudin, H.; Fang, X.; Auras, R. Poly(lactic acid)—Mass production, processing, industrial applications, and end of life. Adv. Drug Deliv. Rev. 2016, 107, 333–366. [Google Scholar] [CrossRef]
- Bio-based Building Blocks and Polymers—Global Capacities and Trends 2017–2022—Renewable Carbon. Available online: http://bio-based.eu/downloads/bio-based-building-blocks-and-polymers-global-capacities-and-trends-2017-2022/ (accessed on 30 September 2019).
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef]
- Sin, L.T.; Rahmat, A.R.; Rahman, W.A.W. Polylactic Acid; A volume in Plastics Design Library; William Andrew. Elsevier: Amsterdam, The Netherlands, 2012; ISBN 978-1-4377-4459-0. [Google Scholar]
- Bang, G.; Kim, S.W. Biodegradable poly(lactic acid)-based hybrid coating materials for food packaging films with gas barrier properties. J. Ind. Eng. Chem. 2012, 18, 1063–1068. [Google Scholar] [CrossRef]
- Tharanathan, R. Biodegradable films and composite coatings: Past, present and future. Trends Food Sci. Technol. 2003, 14, 71–78. [Google Scholar] [CrossRef]
- Ferreira, F.V.; Pinheiro, I.F.; Gouveia, R.F.; Thim, G.P.; Lona, L.M.F. Functionalized cellulose nanocrystals as reinforcement in biodegradable polymer nanocomposites. Polym. Compos. 2018, 39, E9–E29. [Google Scholar] [CrossRef]
- López de Dicastillo, C.; Garrido, L.; Alvarado, N.; Romero, J.; Palma, J.; Galotto, M.J. Improvement of polylactide properties through cellulose Nanocrystals Embedded in Poly(Vinyl Alcohol) Electrospun Nanofibers. Nanomaterials 2017, 7, 106. [Google Scholar] [CrossRef]
- Darie, R.N.; Pâslaru, E.; Sdrobis, A.; Pricope, G.M.; Hitruc, G.E.; Poiată, A.; Baklavaridis, A.; Vasile, C. Effect of Nanoclay Hydrophilicity on the Poly(lactic acid)/Clay Nanocomposites Properties. Ind. Eng. Chem. Res. 2014, 53, 7877–7890. [Google Scholar] [CrossRef]
- Coelho, C.C.; Michelin, M.; Cerqueira, M.A.; Gonçalves, C.; Tonon, R.V.; Pastrana, L.M.; Freitas-Silva, O.; Vicente, A.A.; Cabral, L.M.; Teixeira, J.A. Cellulose nanocrystals from grape pomace: Production, properties and cytotoxicity assessment. Carbohydr. Polym. 2018, 192, 327–336. [Google Scholar] [CrossRef]
- Brinchi, L.; Cotana, F.; Fortunati, E.; Kenny, J.M. Production of nanocrystalline cellulose from lignocellulosic biomass: Technology and applications. Carbohydr. Polym. 2013, 94, 154–169. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, F.; Mariano, M.; Rabelo, S.; Gouveia, R.; Lona, L. Isolation and surface modification of cellulose nanocrystals from sugarcane bagasse waste: From a micro- to a nano-scale view. Appl. Surf. Sci. 2018, 436, 1113–1122. [Google Scholar] [CrossRef]
- Lopez-Rubio, A.; Almenar, E.; Hernández-Muñoz, P.; Lagarón, J.M.; Catalá, R.; Gavara, R. Overview of Active Polymer-Based Packaging Technologies for Food Applications. Food Rev. Int. 2004, 20, 357–387. [Google Scholar] [CrossRef]
- López-De-Dicastillo, C.; Jordá, M.; Catalá, R.; Gavara, R.; Hernández-Muñoz, P. Development of Active Polyvinyl Alcohol/β-Cyclodextrin Composites to Scavenge Undesirable Food Components. J. Agric. Food Chem. 2011, 59, 11026–11033. [Google Scholar] [CrossRef]
- De Dicastillo, C.L.; Bustos, F.; Guarda, A.; Galotto, M.J. Cross-linked methyl cellulose films with murta fruit extract for antioxidant and antimicrobial active food packaging. Food Hydrocoll. 2016, 60, 335–344. [Google Scholar] [CrossRef]
- Borzi, F.; Torrieri, E.; Wrona, M.; Nerín, C. Polyamide modified with green tea extract for fresh minced meat active packaging applications. Food Chem. 2019, 300, 125242. [Google Scholar] [CrossRef]
- Kim, S.; Baek, S.-K.; Go, E.; Song, K.; Bin Song, K. Application of Adzuki Bean Starch in Antioxidant Films Containing Cocoa Nibs Extract. Polymers 2018, 10, 1210. [Google Scholar] [CrossRef]
- Haghighi, H.; De Leo, R.; Bedin, E.; Pfeifer, F.; Siesler, H.W.; Pulvirenti, A. Comparative analysis of blend and bilayer films based on chitosan and gelatin enriched with LAE (lauroyl arginate ethyl) with antimicrobial activity for food packaging applications. Food Packag. Shelf Life 2019, 19, 31–39. [Google Scholar] [CrossRef]
- Quiles-Carrillo, L.; Montanes, N.; Lagaron, J.M.; Balart, R.; Torres-Giner, S. Bioactive Multilayer Polylactide Films with Controlled Release Capacity of Gallic Acid Accomplished by Incorporating Electrospun Nanostructured Coatings and Interlayers. Appl. Sci. 2019, 9, 533. [Google Scholar] [CrossRef]
- Muller, J.; González-Martínez, C.; Chiralt, A.; Fortunati, E. Poly(lactic) acid (PLA) and starch bilayer films, containing cinnamaldehyde, obtained by compression moulding. Eur. Polym. J. 2017, 95, 56–70. [Google Scholar] [CrossRef]
- Corradini, E.; Curti, P.S.; Meniqueti, A.B.; Martins, A.F.; Rubira, A.F.; Muniz, E.C. Recent Advances in Food-Packing, Pharmaceutical and Biomedical Applications of Zein and Zein-Based Materials. Int. J. Mol. Sci. 2014, 15, 22438–22470. [Google Scholar] [CrossRef]
- Patel, A.R.; Velikov, K.P. Zein as a source of functional colloidal nano- and microstructures. Curr. Opin. Colloid Interface Sci. 2014, 19, 450–458. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, B.; Whent, M.; Yu, L.L.; Wang, Q. Preparation and characterization of zein/chitosan complex for encapsulation of α-tocopherol, and its in vitro controlled release study. Colloids Surf. B Biointerfaces 2011, 85, 145–152. [Google Scholar] [CrossRef]
- Scramin, J.A.; De Britto, D.; Forato, L.A.; Bernardes-Filho, R.; Colnago, L.A.; Assis, O.B.G.; Bernardes-Filho, R. Characterisation of zein-oleic acid films and applications in fruit coating. Int. J. Food Sci. Technol. 2011, 46, 2145–2152. [Google Scholar] [CrossRef]
- Galotto, M.; Torres, A.; Guarda, A.; Moraga, N.; Romero, J. Experimental and theoretical study of LDPE versus different concentrations of Irganox 1076 and different thickness. Food Res. Int. 2011, 44, 566–574. [Google Scholar] [CrossRef]
- Rojas, A.; Cerro, D.; Torres, A.; Galotto, M.J.; Guarda, A.; Romero, J. Supercritical impregnation and kinetic release of 2-nonanone in LLDPE films used for active food packaging. J. Supercrit. Fluids 2015, 104, 76–84. [Google Scholar] [CrossRef]
- Torres, A.; Romero, J.; Macan, A.; Guarda, A.; Galotto, M.J. Near critical and supercritical impregnation and kinetic release of thymol in LLDPE films used for food packaging. J. Supercrit. Fluids 2014, 85, 41–48. [Google Scholar] [CrossRef]
- López-De-Dicastillo, C.; Alonso, J.M.; Catalá, R.; Gavara, R.; Hernández-Muñoz, P. Improving the Antioxidant Protection of Packaged Food by Incorporating Natural Flavonoids into Ethylene−Vinyl Alcohol Copolymer (EVOH) Films. J. Agric. Food Chem. 2010, 58, 10958–10964. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion; Clarendon Press: Oxford, England, 1975; ISBN 0198533446. [Google Scholar]
- Torres, A.; Guarda, A.; Moraga, N.; Romero, J.; Galotto, M.J. Experimental and theoretical study of thermodynamics and transport properties of multilayer polymeric food packaging. Eur. Food Res. Technol. 2012, 234, 713–722. [Google Scholar] [CrossRef]
- Lopez de Dicastillo, C.; Roa, K.; Garrido, L.; Pereira, A.; Galotto, M.J. Novel Polyvinyl Alcohol/Starch Electrospun Fibers as a Strategy to Disperse Cellulose Nanocrystals into Poly(lactic acid). Polymers 2017, 9, 117. [Google Scholar] [CrossRef]
- Alehosseini, A.; Gómez-Mascaraque, L.G.; Martínez-Sanz, M.; López-Rubio, A. Electrospun curcumin-loaded protein nanofiber mats as active/bioactive coatings for food packaging applications. Food Hydrocoll. 2019, 87, 758–771. [Google Scholar] [CrossRef]
- Souzandeh, H.; Netravali, A.N. Toughening of thermoset green zein resin: A comparison between natural rubber-based additives and plasticizers. J. Appl. Polym. Sci. 2019, 136, 48512. [Google Scholar] [CrossRef]
- Aguayo, M.G.; Pérez, A.F.; Reyes, G.; Oviedo, C.; Gacitúa, W.; Gonzalez, R.; Uyarte, O. Isolation and Characterization of Cellulose Nanocrystals from Rejected Fibers Originated in the Kraft Pulping Process. Polymers 2018, 10, 1145. [Google Scholar] [CrossRef] [PubMed]
- Anwar, B.; Bundjali, B.; Arcana, I.M. Isolation of Cellulose Nanocrystals from Bacterial Cellulose Produced from Pineapple Peel Waste Juice as Culture Medium. Procedia Chem. 2015, 16, 279–284. [Google Scholar] [CrossRef]
- Iñiguez-Franco, F.; Soto-Valdez, H.; Peralta, E.; Ayala-Zavala, J.F.; Auras, R.; Gámez-Meza, N. Antioxidant Activity and Diffusion of Catechin and Epicatechin from Antioxidant Active Films Made of Poly(l-lactic acid). J. Agric. Food Chem. 2012, 60, 6515–6523. [Google Scholar] [CrossRef]
- Da Silva, A.L.N.; Cipriano, T.F.; Silva, A.H.; Sousa, A.M.; Silva, G.M.; Rocha, M.G. Thermal, rheological and morphological properties of poly(lactic acid) (PLA) and talc composites. Polímeros Ciência Tecnol. 2014, 24, 276–282. [Google Scholar] [CrossRef]
- Bortoletto-Santos, R.; Plotegher, F.; Roncato, V.; Majaron, R.F.; Majaron, V.F.; Polito, W.L.; Ribeiro, C. Strategy for Multinutrient Application in Integrated Granules Using Zein as a Coating Layer. J. Agric. Food Chem. 2018, 66, 9582–9587. [Google Scholar] [CrossRef]
- De Almeida, C.B.; Corradini, E.; Forato, L.A.; Fujihara, R.; Filho, J.F.L. Microstructure and thermal and functional properties of biodegradable films produced using zein. Polímeros 2018, 28, 30–37. [Google Scholar] [CrossRef]
- Costa, L.A.; Fonseca, A.F.; Pereira, F.V.; Druzian, J.I. Extraction and characterization of cellulose nanocrystals from corn stover. Cell. Chem. Technol. 2015, 49, 127–133. [Google Scholar] [CrossRef]
- Milia, A.; Bruno, M.; Cavallaro, G.; Lazzara, G.; Milioto, S. Adsorption isotherms and thermal behavior of hybrids based on quercetin and inorganic fillers. J. Therm. Anal. Calorim. 2019, 138, 1971–1977. [Google Scholar] [CrossRef]
- Wu, Q.; Qin, Y.-F.; Chen, L.; Qin, Z.-Y. Conductive Polyaniline/Cellulose Nanocrystals Composite for Ammonia Gas Detection. In Computer Science and Engineering Technology (CSET2015), Medical Science and Biological Engineering (MSBE2015); World Scientific: Singapore, 2016; pp. 670–675. [Google Scholar]
- Huan, S.; Bai, L.; Liu, G.; Cheng, W.; Han, G. Electrospun nanofibrous composites of polystyrene and cellulose nanocrystals: Manufacture and characterization. RSC Adv. 2015, 5, 50756–50766. [Google Scholar] [CrossRef]
- Resano-Goizueta, I.; Ashokan, B.K.; Trezza, T.A.; Padua, G.W. Effect of Nano-Fillers on Tensile Properties of Biopolymer Films. J. Polym. Environ. 2018, 26, 3817–3823. [Google Scholar] [CrossRef]
- Chen, B.-K.; Shen, C.-H.; Chen, S.-C.; Chen, A.F. Ductile PLA modified with methacryloyloxyalkyl isocyanate improves mechanical properties. Polymer 2010, 51, 4667–4672. [Google Scholar] [CrossRef]
- Shogren, R. Water vapor permeability of biodegradable polymers. J. Polym. Environ. 1997, 5, 91–95. [Google Scholar] [CrossRef]
- Siracusa, V. Food Packaging Permeability Behaviour: A Report. Int. J. Polym. Sci. 2012, 2012, 302029. [Google Scholar] [CrossRef]
- Cerisuelo, J.P.; Gavara, R.; Hernández-Muñoz, P.; Hernández-Muñoz, P. Antimicrobial-releasing films and coatings for food packaging based on carvacrol and ethylene copolymers. Polym. Int. 2015, 64, 1747–1753. [Google Scholar] [CrossRef]
- Kim, Y.-M.; An, D.-S.; Park, H.-J.; Park, J.-M.; Lee, D.S. Properties of nisin-incorporated polymer coatings as antimicrobial packaging materials. Packag. Technol. Sci. 2002, 15, 247–254. [Google Scholar] [CrossRef]
- Santonicola, S.; Ibarra, V.G.; Sendón, R.; Mercogliano, R.; De Quirós, A.R.-B. Antimicrobial Films Based on Chitosan and Methylcellulose Containing Natamycin for Active Packaging Applications. Coatings 2017, 7, 177. [Google Scholar] [CrossRef]
- Samsudin, H. Migration of Antioxidants from Poly(lactic Acid), PLA, Films into Food Simulants: A Parameter Estimation Approach. Ph.D. Thesis, Michigan State University, East Lansing, MI, USA, 2015. [Google Scholar]
- Kashiri, M.; Cerisuelo, J.P.; Domínguez, I.; López-Carballo, G.; Muriel-Gallet, V.; Gavara, R.; Hernández-Muñoz, P. Zein films and coatings as carriers and release systems of Zataria multiflora Boiss. essential oil for antimicrobial food packaging. Food Hydrocoll. 2017, 70, 260–268. [Google Scholar] [CrossRef]

| Films | L* | a* | b* | ΔE |
|---|---|---|---|---|
| PLA | 98.3 ± 0.2 d | −0.06 ± 0.01 d | 2.27 ± 0.02 a | - |
| PLA/ZN | 97.9 ± 0.2 bc | −0.57 ± 0.03 c | 4.10 ± 0.11 b | 1.95 ± 0.15 a |
| PLA/ZN.CNC0.5 | 98.0 ± 0.1 c | −0.60 ± 0.04 c | 4.24 ± 0.16 b | 2.07 ± 0.17 a |
| PLA/ZN.CNC1 | 98.0 ± 0.2 c | −0.60 ± 0.02 c | 4.25 ± 0.04 b | 2.08 ± 0.06 a |
| PLA/ZN.Q | 97.8 ± 0.2 ab | −1.60 ± 0.23 b | 6.65 ± 0.71 c | 4.68 ± 0.75 b |
| PLA/ZN.Q.CNC0.5 | 97.7 ± 0.2 a | −1.67 ± 0.08 b | 6.85 ± 0.22 c | 4.90 ± 0.26 b |
| PLA/ZN.Q.CNC1 | 97.7 ± 0.1 a | −1.81 ± 0.07 a | 7.28 ± 0.22 d | 5.36 ± 0.24 c |
| Films | Tg (°C) | Tcc (°C) | ΔHcc (J g−1) | Tm1 (°C) | Tm2 (°C) | ΔHm (J g−1) |
|---|---|---|---|---|---|---|
| PLA | 64.2 ± 0.8 | 117.8 ± 0.1 | 18.2 ± 8.2 | 149.4 ± 0.5 | 154.4 ± 0.9 | 20.2 ± 8.8 |
| PLA/ZN | 63.7 ± 0.6 | 118.1 ± 0.2 | 24.1 ± 0.6 | 150.3 ± 0.1 | 153.6 ± 1.0 | 25.7 ± 0.3 |
| PLA/ZN.Q.CNC0.5 | 62.8 ± 3.9 | 115.4 ± 0.7 | 20.5 ± 0.6 | 147.7 ± 2.6 | 154.6 ± 0.4 | 22.7 ± 0.1 |
| PLA/ZN.Q.CNC1 | 65.4 ± 0.2 | 115.6 ± 1.5 | 23.9 ± 1.7 | 149.3 ± 0.2 | 154.2 ± 0.4 | 25.4 ± 1.6 |
| Films | Tonset | Td,max | Weight Loss between Tonset and Td,max (%) | Volatile Release Rate (wt %/°C) |
|---|---|---|---|---|
| PLA | 348.0 | 365.9 | 44.0 | 2.5 |
| PLA/ZN | 330.5 | 353.3 | 45.9 | 2.0 |
| PLA/ZN.Q.CNC0.5 | 330.6 | 354.8 | 46.9 | 1.9 |
| PLA/ZN.Q.CNC1 | 331.0 | 354.0 | 44.7 | 1.9 |
| Films | Young’s Modulus (MPa) | Maximum Resistance (MPa) | Elongation at Break (%) |
|---|---|---|---|
| PLA | 2768 ± 154 b | 58.7 ± 2.0 d | 6.1 ± 2.3 a |
| PLA/ZN | 2410 ± 393 a | 50.5 ± 3.4 c | 10.1 ± 5.3 b |
| PLA/ZN.Q | 2627 ± 138 ab | 48.1 ± 2.1 b,c | 11.8 ± 2.7 b,c |
| PLA/ZN.CNC0.5 | 2602 ± 250 a,b | 49.2 ± 3.2 b,c | 11.6 ± 4.2 b,c |
| PLA/ZN.CNC1 | 2566 ± 355 a,b | 48.7 ± 2.7 b,c | 12.1 ± 3.2 b,c |
| PLA/ZN.Q.CNC0.5 | 2631 ± 75 a,b | 48.1 ± 2.7 b | 11.3 ± 3.1 b,c |
| PLA/ZN.Q.CNC1 | 2453 ± 300 a | 44.8 ± 2.0 a | 14.5 ± 4.7 c |
| Simulant | Bilayer PLA/ZN Composite | KP.FS | Dp (m2 s−1) | RMSE |
|---|---|---|---|---|
| 10% EtOH | PLA/ZN.Q | 5422 ± 139 | 1.5 × 10−15 | 1.20 |
| PLA/ZN.Q.CNC0.5 | 5353 ± 45 | 2.0 × 10−15 | 1.05 | |
| PLA/ZN.Q.CNC1 | 5869 ± 103 | 3.0 × 10−15 | 0.43 | |
| 3% acetic acid | PLA/ZN.Q | 6330 ± 220 | 2.0 × 10−15 | 0.67 |
| PLA/ZN.Q.CNC0.5 | 5869 ± 150 | 3.5 × 10−15 | 0.34 | |
| PLA/ZN.Q.CNC1 | 6171 ± 150 | 4.5 × 10−15 | 0.71 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velásquez, E.; Rojas, A.; Piña, C.; Galotto, M.J.; López de Dicastillo, C. Development of Bilayer Biodegradable Composites Containing Cellulose Nanocrystals with Antioxidant Properties. Polymers 2019, 11, 1945. https://doi.org/10.3390/polym11121945
Velásquez E, Rojas A, Piña C, Galotto MJ, López de Dicastillo C. Development of Bilayer Biodegradable Composites Containing Cellulose Nanocrystals with Antioxidant Properties. Polymers. 2019; 11(12):1945. https://doi.org/10.3390/polym11121945
Chicago/Turabian StyleVelásquez, Eliezer, Adrián Rojas, Constanza Piña, María José Galotto, and Carol López de Dicastillo. 2019. "Development of Bilayer Biodegradable Composites Containing Cellulose Nanocrystals with Antioxidant Properties" Polymers 11, no. 12: 1945. https://doi.org/10.3390/polym11121945
APA StyleVelásquez, E., Rojas, A., Piña, C., Galotto, M. J., & López de Dicastillo, C. (2019). Development of Bilayer Biodegradable Composites Containing Cellulose Nanocrystals with Antioxidant Properties. Polymers, 11(12), 1945. https://doi.org/10.3390/polym11121945