Cellulose in Ionic Liquids and Alkaline Solutions: Advances in the Mechanisms of Biopolymer Dissolution and Regeneration
Abstract
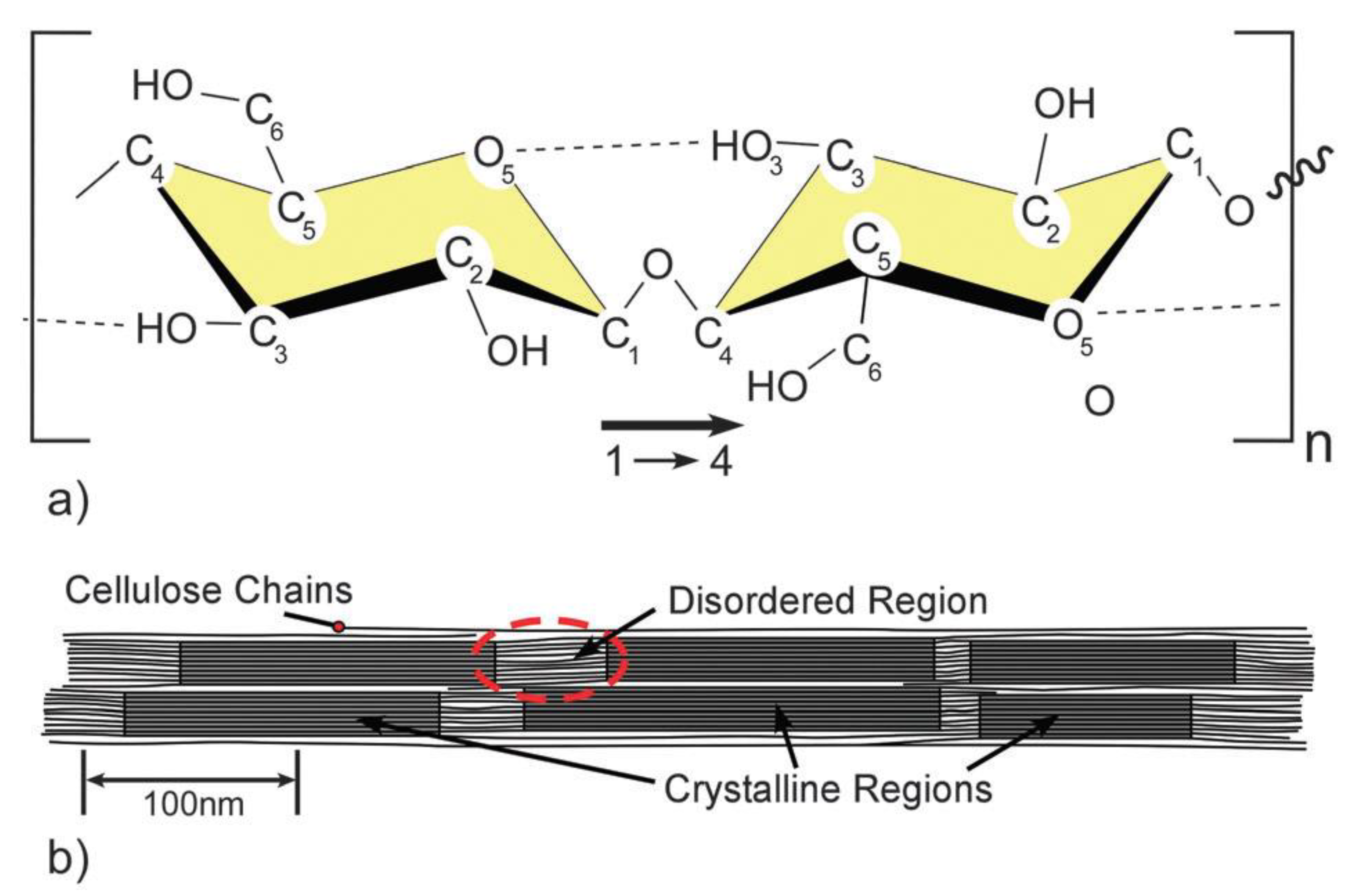
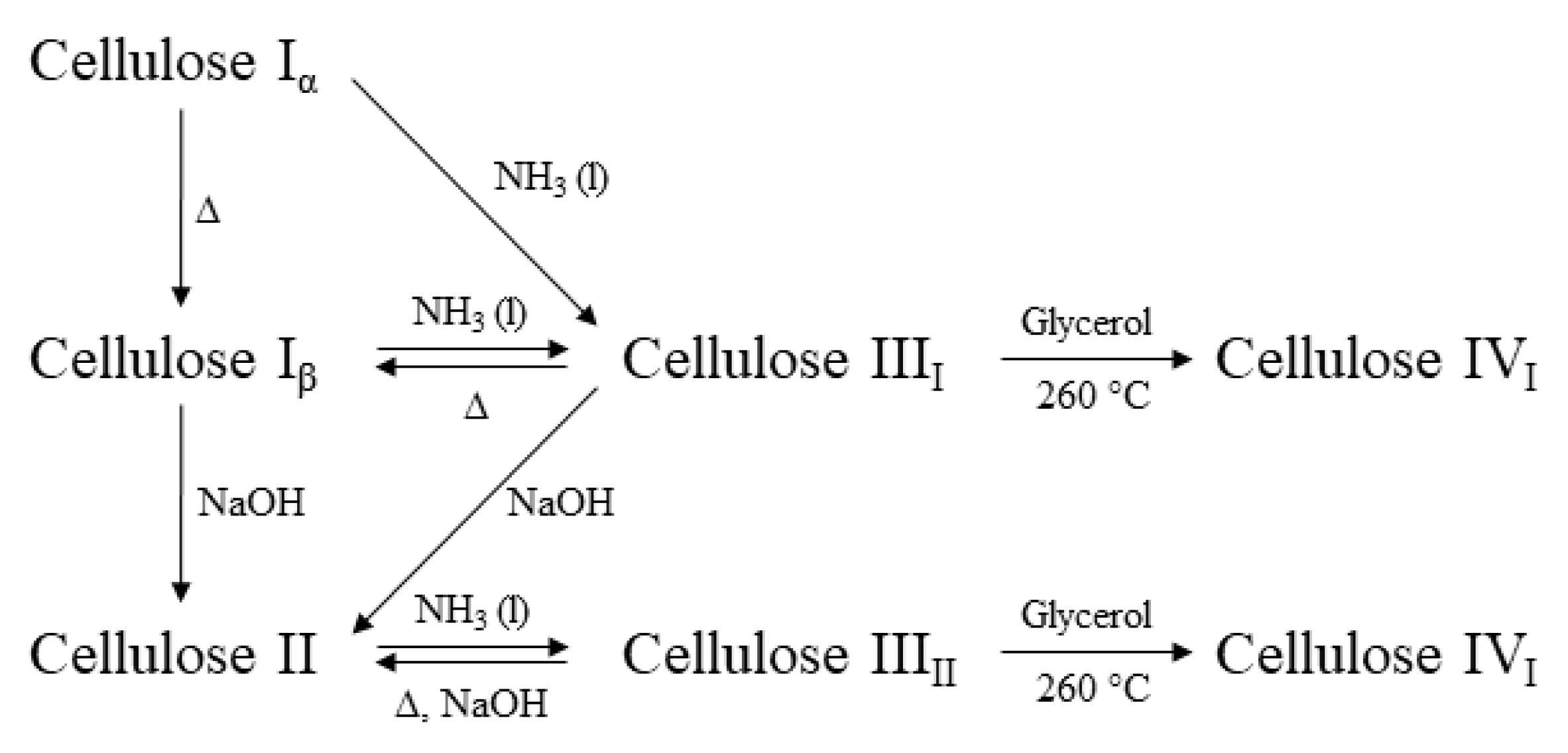
1. Cellulose Dissolution and Regeneration: A Principal Strategy for Meeting the Increased Demand on Cellulosic Fibers
2. Assessing Solvent Efficiency for Physical Dissolution of Cellulose
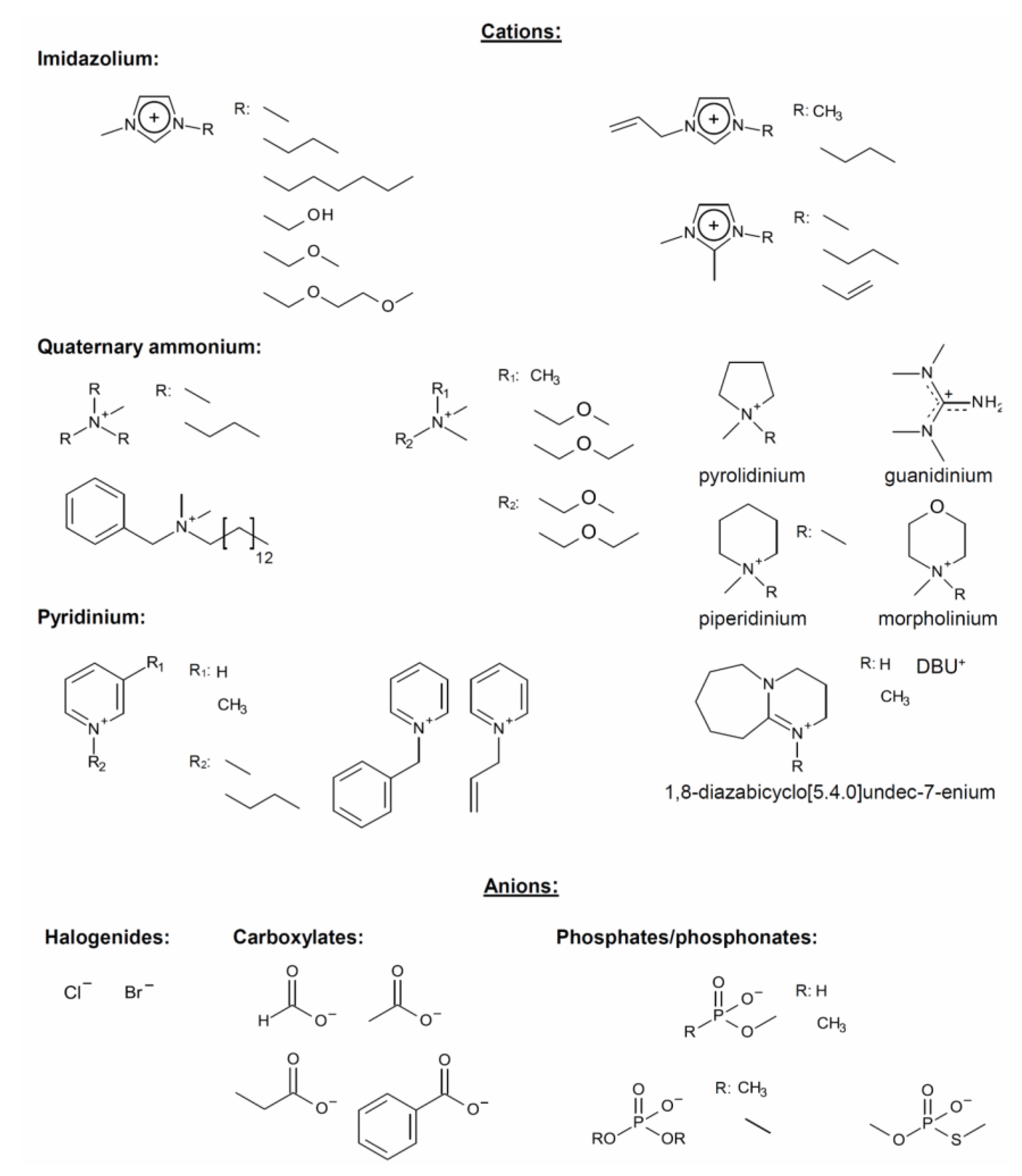
2.1. Ionic Liquids, Quaternary Ammonium Electrolytes, and Salts of Super-Bases: Versatile Cellulose Solvents
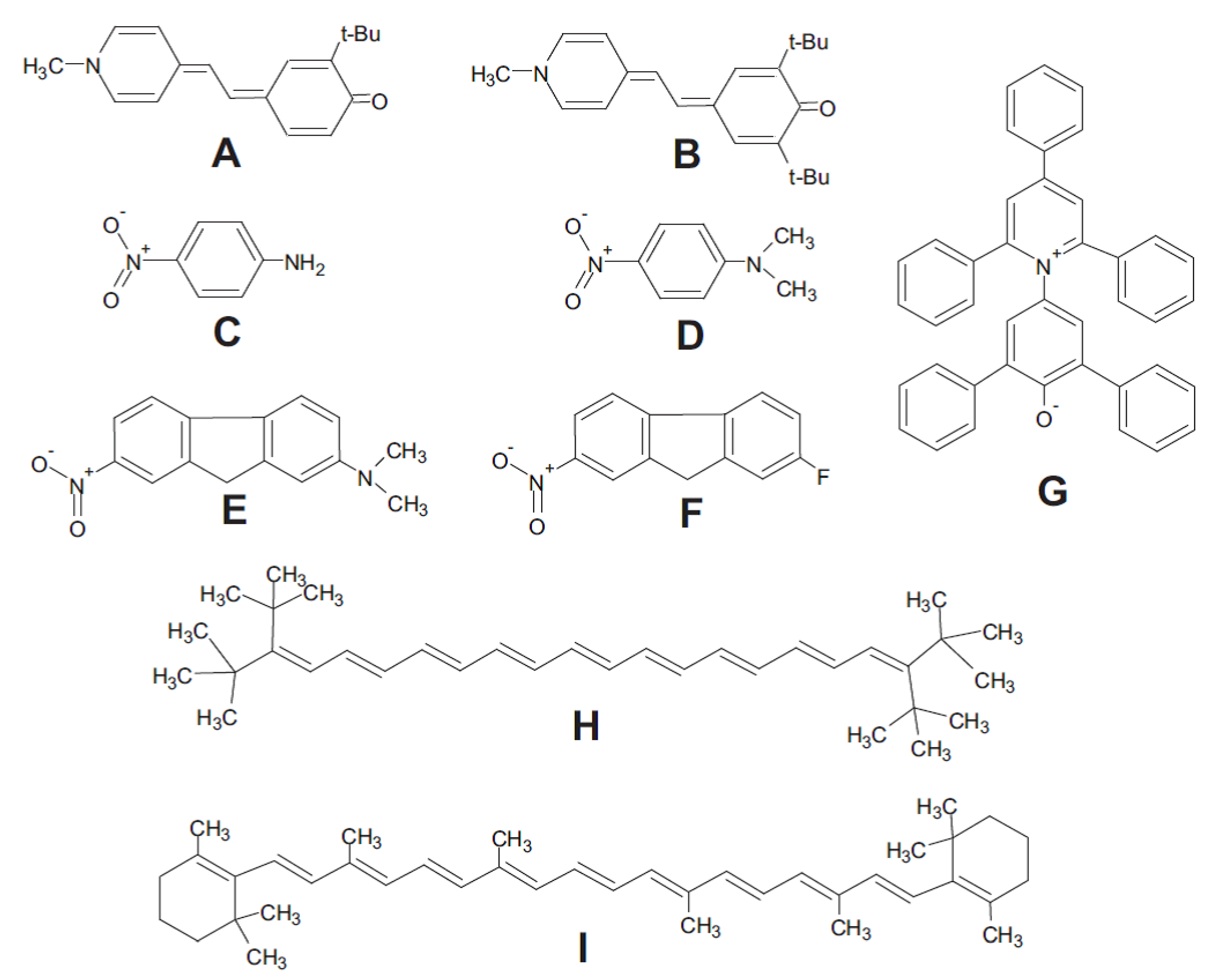
2.2. Solvatochromism and Calculation of Solvatochromic Parameters
2.2.1. Use of Solvatochromic Parameters to Assess the Efficiency of Cellulose Solvents
2.2.2. Pure Ionic Liquids as Cellulose Solvents
2.2.3. Binary Mixtures of Ionic Liquids-Molecular Solvents as Cellulose Solvents
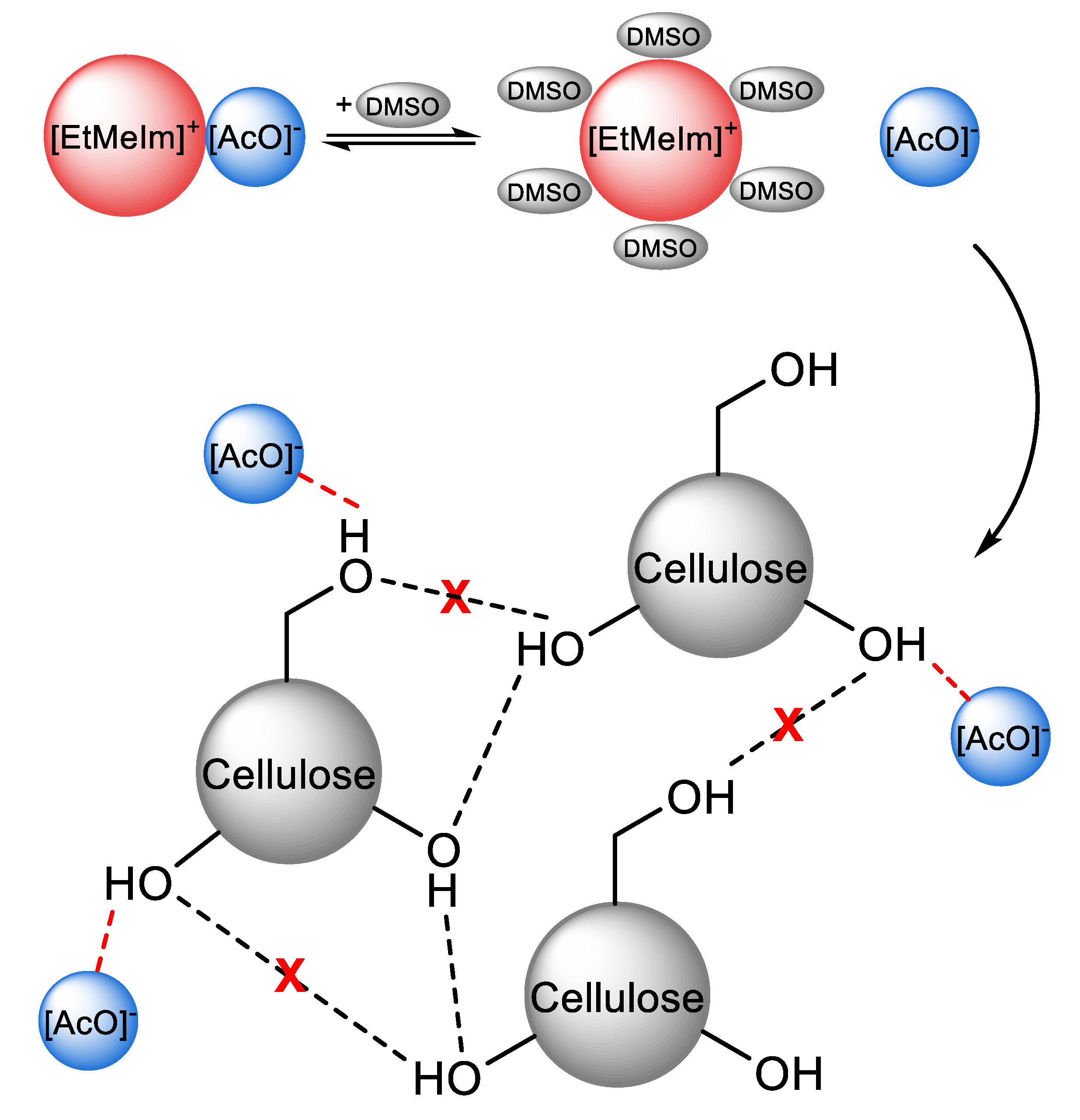
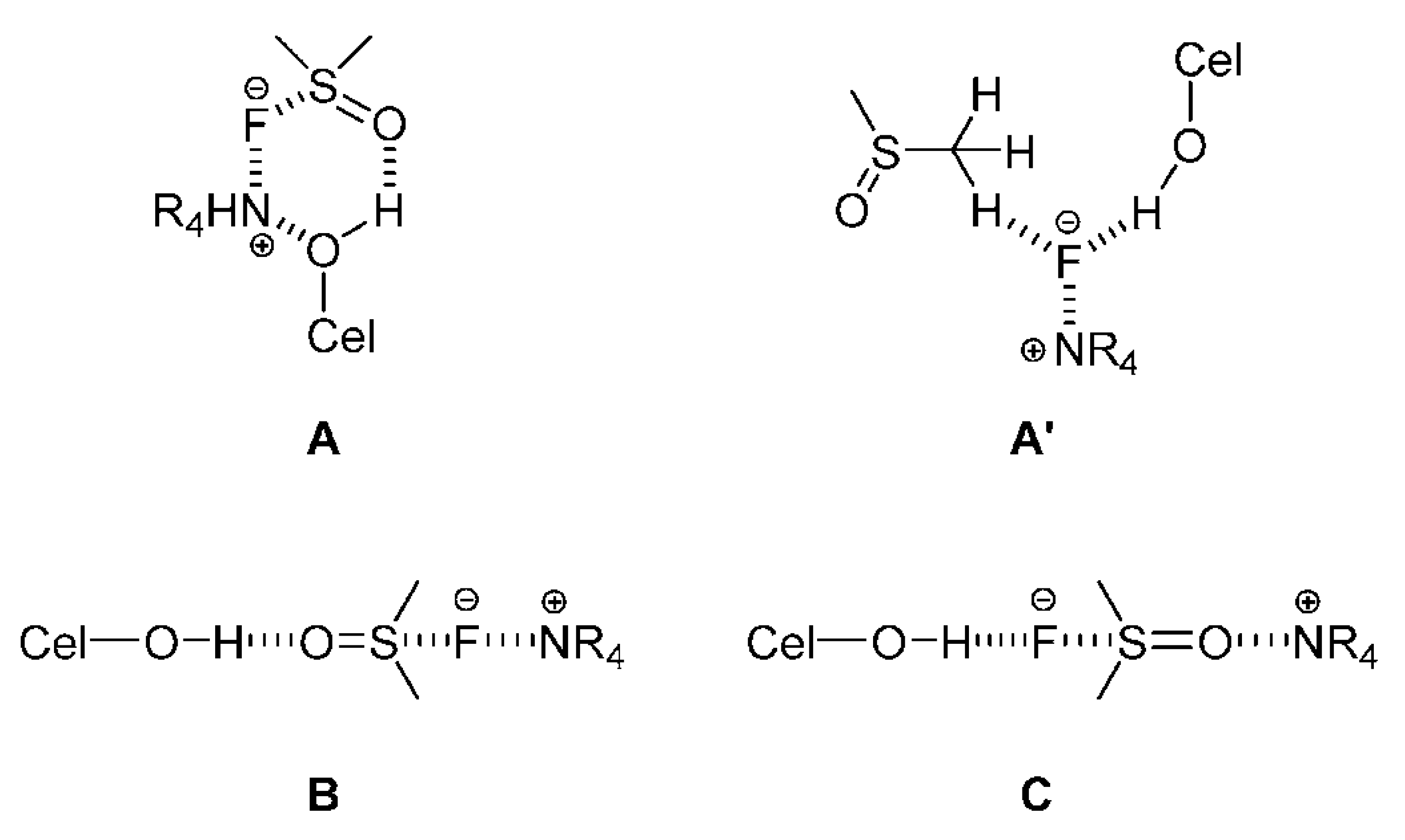
2.3. Use of NMR and Theoretical Calculations to Assess Ionic Liquids and Their Mixtures with Molecular Solvents as Cellulose Solvents
3. Mechanism of Regeneration of Dissolved Cellulose
- (i)
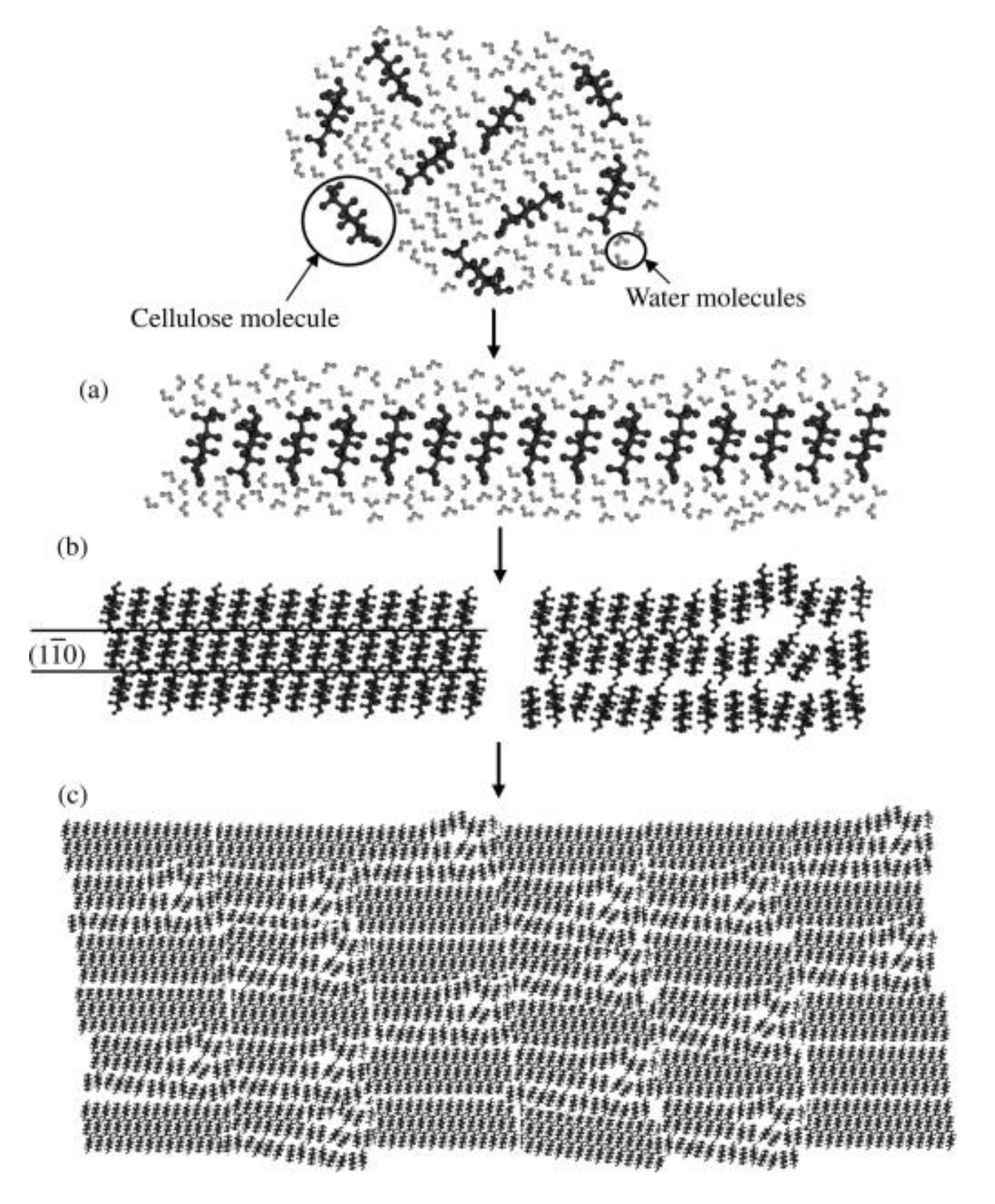
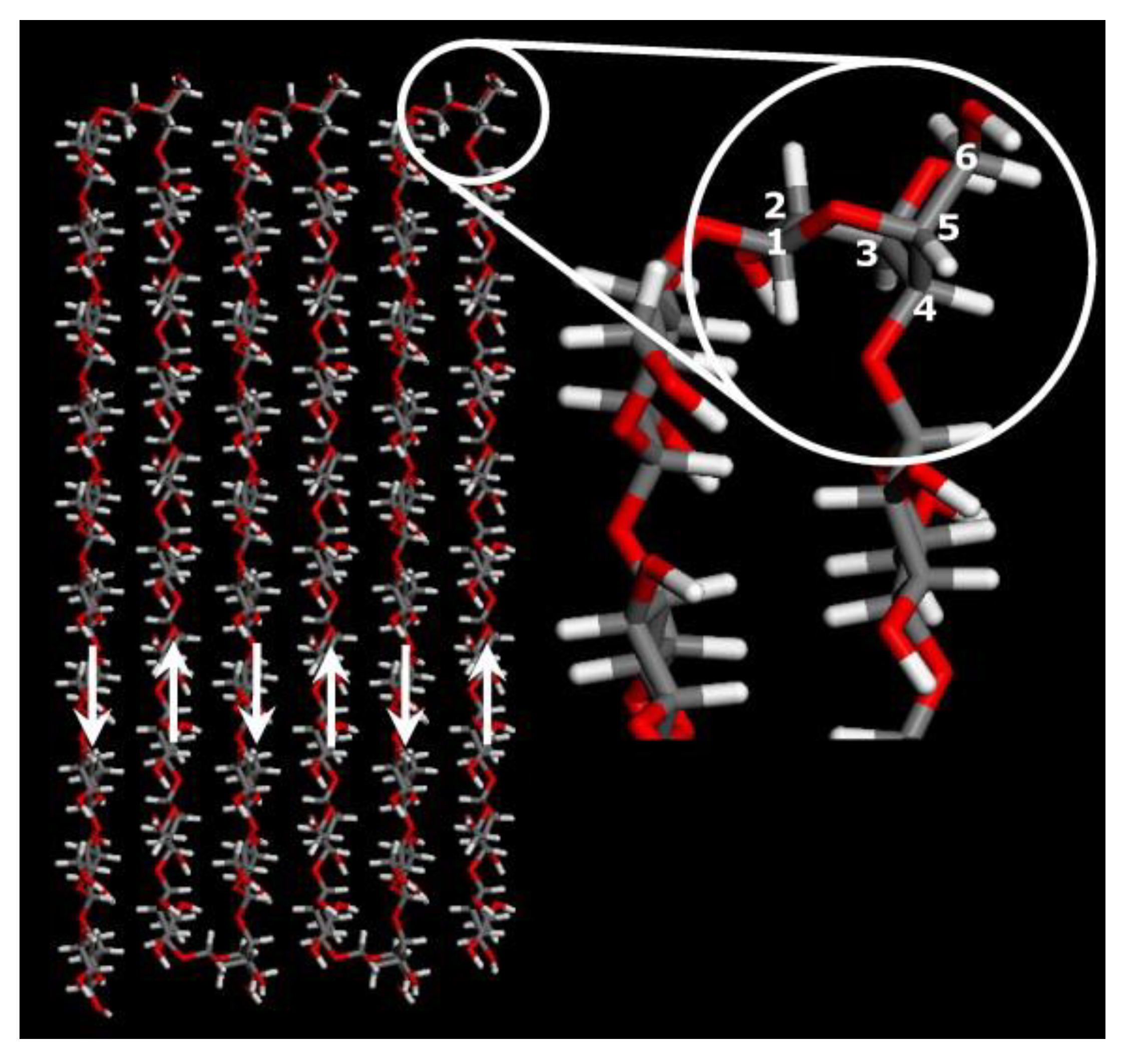
- Cellulose chains aggregate side-by-side, with glucopyranose ring stacking by hydrophobic interactions and van der Waals forces, and the formation of molecular sheets of the cellulose in solution (Figure 14a);
- (ii)
- (iii)
- Diffusion controlled aggregation of bigger three-dimensional clusters of the randomly dispersed structures in solution to form a mixture of crystal and amorphous regions (Figure 14c).
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AcO | Acetate group |
| AGU | Anhydroglucose unit |
| Bu | 1-(n-Butyl) group |
| BuMeImX | 1-(n-Butyl)-3-methylimidazolium electrolyte, X = anion |
| BuO | Butanoate |
| CB | Cellobiose |
| CT | Charge transfer |
| DBN | 1,5-Diazabicyclo [4.3.0]non-5-ene |
| DBU | 1,8-Diazabicyclo [5.4.0]undec-7-ene |
| DMAc | N,N-Dimethylacetamide |
| DMF | N,N-Dimethylformamide |
| DMI | 1,3-Dimethyl-2-imidazolidinone |
| DMSO | Dimethylsulfoxide |
| DP | Average degree of polymerization |
| ET(probe) | Solvent empirical polarity in kcal·mol−1, calculated from solvatochromic data |
| ET(RB) | Solvent empirical polarity scale based on the probe 2,6-diphenyl-4-(2,4,6-triphenylpyridinium-1-yl)phenolate |
| ET(WB) | Solvent empirical polarity scale based on the probe 2,6-dichloro-4-(2,4,6-triphenylpyridinium-1-yl)phenolate |
| EtMeImAcO | 1-Ethyl-3-methylimidazolium acetate |
| EtMeIm(EtO)2PO2 | 1-Ethyl-3-methylimidazolium diethylphosphate |
| IL | Ionic liquid |
| Im | Imidazole |
| MCC | Microcrystalline cellulose |
| MD | Molecular dynamics |
| Me | Methyl group |
| MS | Molecular solvent |
| N(CN)2 | Dicyanamide anion |
| PrO | Propionate group |
| PrOMeImAcO | 1-(2-Methoxyethyl)-3-methylimidazolium acetate |
| QAE | Quaternary ammonium electrolytes, a sub-class of ionic liquids. |
| S | Solvent |
| SA | Solvent Lewis acidity; calculated from solvatochromic data |
| SB | Solvent Lewis Basicity; calculated from solvatochromic data |
| SD | Solvent dipolarity; calculated from solvatochromic data |
| SP | Solvent polarizability; calculated from solvatochromic data |
| Sulf | Sulfolane |
| TBAF·xH2O | Tetra(n-butyl)ammonium fluoride hydrate |
| Tf2N | Bistriflimide anion |
| TMG | Tetramethylguanidine |
| V | Volume |
| χ | Mole fraction |
| λ | Wavelength |
References
- Hämmerle, F.M. The cellulose gap (The future of cellulose fibres). Lenzing. Ber. 2011, 89, 12–21. [Google Scholar]
- The Fiber Year 2018—World Survey on Textiles & Nonwovens, The Fiber Year GmbH. 2018. Available online: https://www.google.com.hk/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&ved=2ahUKEwicwrLQytnlAhU6xosBHTpfCgoQFjAAegQIABAC&url=https%3A%2F%2Fwww.thefiberyear.com%2Ffileadmin%2Fpdf%2FTFY2018TOC.pdf&usg=AOvVaw3IO3ShqlRKqGRG8vY7RD49 (accessed on 15 October 2019).
- Micklin, P. The aral sea disaster. Annu. Rev. Earth Planet. Sci. 2007, 35, 47–72. [Google Scholar] [CrossRef]
- Eichinger, D. A vision of the world of cellulosic fibers in 2020. Lenzing. Ber. 2012, 90, 1–7. [Google Scholar]
- Gericke, M.; Fardim, P.; Heinze, T. Ionic liquids—Promising but challenging solvents for homogeneous derivatization of cellulose. Molecules 2012, 17, 7458–7502. [Google Scholar] [CrossRef] [PubMed]
- King, A.W.T.; Parviainen, A.; Karhunen, P.; Matikainen, J.; Hauru, L.K.J.; Sixta, H.; Kilpeläinen, I. Relative and inherent reactivities of imidazolium-based ionic liquids: The implications for lignocellulose processing applications. RSC Adv. 2012, 2, 8020–8026. [Google Scholar] [CrossRef]
- El Seoud, O.A.; Koschella, A.; Fidale, L.C.; Dorn, S.; Heinze, T. Applications of ionic liquids in carbohydrate chemistry: A window of opportunities. Biomacromolecules 2007, 8, 2629–2647. [Google Scholar] [CrossRef]
- Cao, Y.; Wu, J.; Zhang, J.; Li, H.; Zhang, Y.; He, J. Room temperature ionic liquids (RTILs): A new and versatile platform for cellulose processing and derivatization. Chem. Eng. J. 2009, 147, 13–21. [Google Scholar] [CrossRef]
- Pinkert, A.; Marsh, K.N.; Pang, S.; Staiger, M.P. Ionic liquids and their interaction with cellulose. Chem. Rev. 2009, 109, 6712–6728. [Google Scholar] [CrossRef]
- Lindman, B.; Karlström, G.; Stigsson, L. On the mechanism of dissolution of cellulose. J. Mol. Liq. 2010, 156, 76–81. [Google Scholar] [CrossRef]
- Mäki-Arvela, P.; Anugwom, I.; Virtanen, P.; Sjöholm, R.; Mikkola, J.P. Dissolution of lignocellulosic materials and its constituents using ionic liquids—A review. Ind. Crops Prod. 2010, 32, 175–201. [Google Scholar] [CrossRef]
- Meng, Z.; Zheng, X.; Tang, K.; Liu, J.; Qin, S. Dissolution of natural polymers in ionic liquids: A review. e-Polymers 2012, 12. [Google Scholar] [CrossRef]
- Stepan, A.M.; Monshizadeh, A.; Hummel, M.; Roselli, A.; Sixta, H. Cellulose fractionation with IONCELL-P. Carbohydr. Polym. 2016, 150, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Hummel, M.; Michud, A.; Tanttu, M.; Asaadi, S.; Ma, Y.; Hauru, L.K.J.; Parviainen, A.; King, A.W.T.; Kilpeläinen, I.; Sixta, H. Ionic liquids for the production of man-made cellulosic fibers: Opportunities and challenges. Adv. Polym. Sci. 2015, 271, 133–168. [Google Scholar]
- Kostag, M.; Jedvert, K.; Achtel, C.; Heinze, T.; El Seoud, O.A. Recent advances in solvents for the dissolution, shaping and derivatization of cellulose: Quaternary ammonium electrolytes and their solutions in water and molecular solvents. Molecules 2018, 23, 511. [Google Scholar] [CrossRef] [PubMed]
- Kostag, M.; Gericke, M.; Heinze, T.; El Seoud, O.A. Twenty-five years of cellulose chemistry: Innovations in the dissolution of the biopolymer and its transformation into esters and ethers. Cellulose 2019, 26, 139–184. [Google Scholar] [CrossRef]
- Heinze, T.; El Seoud, O.A.; Koschella, A. Cellulose activation and dissolution. In Cellulose Derivatives; Springer Series on Polymer and Composite Materials; Springer: Cham, Switzerland, 2018; pp. 173–257. ISBN 978-3-319-73167-4. [Google Scholar]
- Stolte, S.; Steudte, S.; Igartua, A.; Stepnowski, P. The biodegradation of ionic liquids—The view from a chemical structure perspective. Curr. Org. Chem. 2012, 15, 1946–1973. [Google Scholar] [CrossRef]
- Jordan, A.; Gathergood, N. Biodegradation of ionic liquids—A critical review. Chem. Soc. Rev. 2015, 44, 8200–8237. [Google Scholar] [CrossRef]
- Meine, N.; Benedito, F.; Rinaldi, R. Thermal stability of ionic liquids assessed by potentiometric titration. Green Chem. 2010, 12, 1711–1714. [Google Scholar] [CrossRef]
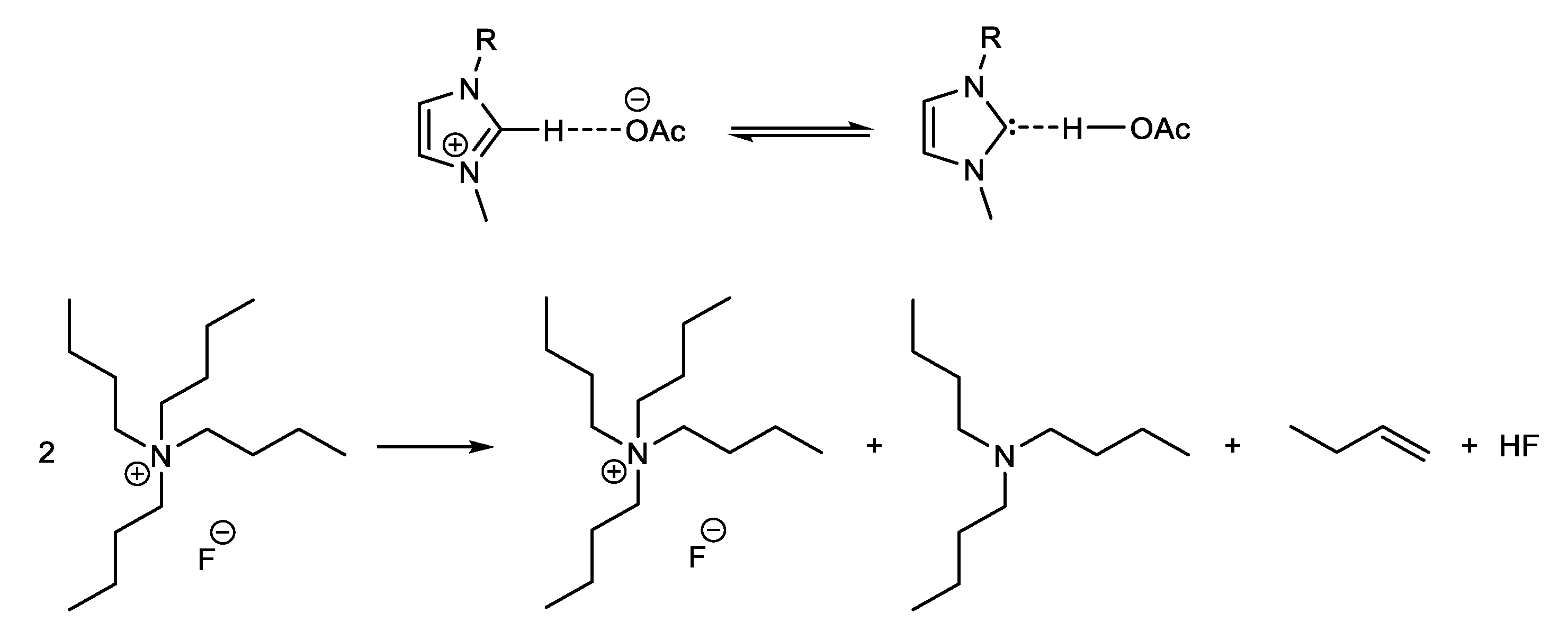
- Chiarotto, I.; Mattiello, L.; Pandolfi, F.; Rocco, D.; Feroci, M. NHC in imidazolium acetate ionic liquids: Actual or potential presence? Front. Chem. 2018, 6, 355. [Google Scholar] [CrossRef]
- Ebner, G.; Schiehser, S.; Potthast, A.; Rosenau, T. Side reaction of cellulose with common 1-alkyl-3-methylimidazolium-based ionic liquids. Tetrahedron Lett. 2008, 49, 7322–7324. [Google Scholar] [CrossRef]
- Luis, P.; Neves, L.A.; Afonso, C.A.M.; Coelhoso, I.M.; Crespo, J.G.; Garea, A.; Irabien, A. Facilitated transport of CO2 and SO2 through Supported Ionic Liquid Membranes (SILMs). Desalination 2009, 245, 485–493. [Google Scholar] [CrossRef]
- Sharma, R.K.; Fry, J.L. Instability of anhydrous tetra-n-alkylammonium fluorides. J. Org. Chem. 1983, 48, 2112–2114. [Google Scholar] [CrossRef]
- Yang, J.; Lu, X.; Yao, X.; Li, Y.; Yang, Y.; Zhou, Q.; Zhang, S. Inhibiting degradation of cellulose dissolved in ionic liquids via amino acids. Green Chem. 2019, 21, 2777–2787. [Google Scholar] [CrossRef]
- Sowmiah, S.; Srinivasadesikan, V.; Tseng, M.C.; Chu, Y.H. On the chemical stabilities of ionic liquids. Molecules 2009, 14, 3780–3813. [Google Scholar] [CrossRef]
- Weiss, I.M.; Muth, C.; Drumm, R.; Kirchner, H.O.K. Thermal decomposition of the amino acids glycine, cysteine, aspartic acid, asparagine, glutamic acid, glutamine, arginine and histidine. BMC Biophys. 2018, 11, 1–15. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, N.; He, X.; Lu, X.; Zhang, X. Physical properties of ionic liquids: Database and evaluation. J. Phys. Chem. Ref. Data 2006, 35, 1475–1517. [Google Scholar] [CrossRef]
- Sescousse, R.; Le, K.A.; Ries, M.E.; Budtova, T. Viscosity of cellulose-imidazolium-based ionic liquid solutions. J. Phys. Chem. B 2010, 114, 7222–7228. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Y.; Wang, H.; Wang, S.-W.; Liang, S.; Colby, R.H. Solution rheology of cellulose in 1-butyl-3-methyl imidazolium chloride. J. Rheol. 2011, 55, 485–494. [Google Scholar] [CrossRef]
- Shakeel, A.; Mahmood, H.; Farooq, U.; Ullah, Z.; Yasin, S.; Iqbal, T.; Chassagne, C.; Moniruzzaman, M. Rheology of pure ionic liquids and their complex fluids: A review. ACS Sustain. Chem. Eng. 2019, 7, 13586–13626. [Google Scholar] [CrossRef]
- Russo, T.; D’Amora, U.; Gloria, A.; Tunesi, M.; Sandri, M.; Rodilossi, S.; Albani, D.; Forloni, G.; Giordano, C.; Cigada, A.; et al. Systematic Analysis of Injectable Materials and 3D Rapid Prototyped Magnetic Scaffolds: From CNS Applications to Soft and Hard Tissue Repair/Regeneration. Procedia Eng. 2013, 59, 233–239. [Google Scholar] [CrossRef]
- Druel, L.; Niemeyer, P.; Milow, B.; Budtova, T. Rheology of cellulose-[DBNH][CO2Et] solutions and shaping into aerogel beads. Green Chem. 2018, 20, 3993–4002. [Google Scholar] [CrossRef]
- FitzPatrick, M.; Champagne, P.; Cunningham, M.F. The effect of subcritical carbon dioxide on the dissolution of cellulose in the ionic liquid 1-ethyl-3-methylimidazolium acetate. Cellulose 2012, 19, 37–44. [Google Scholar] [CrossRef]
- Reichardt, C.; Welton, T. Empirical parameters of solvent polarity. In Solvents and Solvent Effects in Organic Chemistry, 4th ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2010; pp. 425–508. ISBN 978-3-527-63222-0. [Google Scholar]
- King, A.W.T.; Asikkala, J.; Mutikainen, I.; Järvi, P.; Kilpeläinen, I. Distillable acid-base conjugate ionic liquids for cellulose dissolution and processing. Angew. Chem. Int. Ed. 2011, 50, 6301–6305. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Chi, Y.; Mu, T. Studies on staged precipitation of cellulose from an ionic liquid by compressed carbon dioxide. Green Chem. 2014, 16, 2736–2744. [Google Scholar] [CrossRef]
- Fidale, L.C.; Possidonio, S.; El Seoud, O.A. Application of 1-allyl-3-(1-butyl) imidazolium chloride in the synthesis of cellulose esters: Properties of the ionic liquid, and comparison with other solvents. Macromol. Biosci. 2009, 9, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Mai, N.L.; Ahn, K.; Koo, Y.M. Methods for recovery of ionic liquids—A review. Process Biochem. 2014, 49, 872–881. [Google Scholar] [CrossRef]
- Lindman, B.; Medronho, B.; Alves, L.; Costa, C.; Edlund, H.; Norgren, M. The relevance of structural features of cellulose and its interactions to dissolution, regeneration, gelation and plasticization phenomena. Phys. Chem. Chem. Phys. 2017, 19, 23704–23718. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Tang, J.; Lan, A.; Yang, Y.; Gibril, M.; Yu, M. Efficient preparation of high concentration cellulose solution with complex DMSO/ILs solvent. J. Polym. Res. 2016, 23, 32. [Google Scholar] [CrossRef]
- Nazari, B.; Utomo, N.W.; Colby, R.H. The effect of water on rheology of native cellulose/ionic liquids solutions. Biomacromolecules 2017, 18, 2849–2857. [Google Scholar] [CrossRef]
- Andanson, J.-M.; Bordes, E.; Devémy, J.; Leroux, F.; Pádua, A.A.H.; Gomes, M.F.C. Understanding the role of co-solvents in the dissolution of cellulose in ionic liquids. Green Chem. 2014, 16, 2528–2538. [Google Scholar] [CrossRef]
- Khan, I.; Kurnia, K.A.; Mutelet, F.; Pinho, S.P.; Coutinho, J.A.P. Probing the interactions between ionic liquids and water: Experimental and quantum chemical approach. J. Phys. Chem. B 2014, 118, 1848–1860. [Google Scholar] [CrossRef] [PubMed]
- Dhumal, N.R. Electronic structure, molecular electrostatic potential and hydrogen bonding in DMSO–X complexes (X=ethanol, methanol and water). Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 79, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.; Zhang, Y.; Zhao, Y.; Wang, J. Cellulose dissolution at ambient temperature: Role of preferential solvation of cations of ionic liquids by a cosolvent. Carbohydr. Polym. 2013, 92, 540–544. [Google Scholar] [CrossRef] [PubMed]
- Parviainen, A.; King, A.W.T.; Mutikainen, I.; Hummel, M.; Selg, C.; Hauru, L.K.J.; Sixta, H.; Kilpeläinen, I. Predicting cellulose solvating capabilities of acid-base conjugate ionic liquids. ChemSusChem 2013, 6, 2161–2169. [Google Scholar] [CrossRef] [PubMed]
- Kostag, M.; Dignani, M.T.; Lourenço, M.C.; de Almeida Bioni, T.; El Seoud, O.A. Assessing cellulose dissolution efficiency in solvent systems based on a robust experimental quantification protocol and enthalpy data. Holzforschung 2019. [Google Scholar] [CrossRef]
- Loffredo, C.; Pires, P.A.R.; Imran, M.; El Seoud, O.A. β-Carotene: A green, inexpensive, and convenient solvatochromic probe for the determination of solvent polarizability. Dyes Pigments 2013, 96, 16–24. [Google Scholar] [CrossRef]
- El Seoud, O.A. Understanding solvation. Pure Appl. Chem. 2009, 81, 697–707. [Google Scholar] [CrossRef]
- Reichardt, C.; Welton, T. Solvents and Solvent Effects in Organic Chemistry; Wiley-VCH: Weinheim, Germany, 2010; p. 360. ISBN 9783527324736. [Google Scholar]
- MacHado, V.G.; Stock, R.I.; Reichardt, C. Pyridinium N-phenolate betaine dyes. Chem. Rev. 2014, 114, 10429–10475. [Google Scholar] [CrossRef]
- Lungwitz, R.; Friedrich, M.; Linert, W.; Spange, S. New aspects on the hydrogen bond donor (HBD) strength of 1-butyl-3-methylimidazolium room temperature ionic liquids. New J. Chem. 2008, 32, 1493–1499. [Google Scholar] [CrossRef]
- Chiappe, C.; Pomelli, C.S.; Rajamani, S. Influence of structural variations in cationic and anionic moieties on the polarity of ionic liquids. J. Phys. Chem. B 2011, 115, 9653–9661. [Google Scholar] [CrossRef]
- Jessop, P.G.; Jessop, D.A.; Fu, D.; Phan, L. Solvatochromic parameters for solvents of interest in green chemistry. Green Chem. 2012, 14, 1245–1259. [Google Scholar] [CrossRef]
- Schmeisser, M.; Van Eldik, R. Elucidation of inorganic reaction mechanisms in ionic liquids: The important role of solvent donor and acceptor properties. Dalton Trans. 2014, 43, 15675–15692. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hauru, L.K.J.; Hummel, M.; King, A.W.T.; Kilpeläinen, I.; Sixta, H. Role of solvent parameters in the regeneration of cellulose from ionic liquid solutions. Biomacromolecules 2012, 13, 2896–2905. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Sale, K.L.; Holmes, B.M.; Simmons, B.A.; Singh, S. Understanding the interactions of cellulose with ionic liquids: A molecular dynamics study. J. Phys. Chem. B 2010, 114, 4293–4301. [Google Scholar] [CrossRef]
- Fukaya, Y.; Hayashi, K.; Wada, M.; Ohno, H. Cellulose dissolution with polar ionic liquids under mild conditions: Required factors for anions. Green Chem. 2008, 10, 44–46. [Google Scholar] [CrossRef]
- Ohno, H.; Fukaya, Y. Task specific ionic liquids for cellulose technology. Chem. Lett. 2009, 38, 2–7. [Google Scholar] [CrossRef]
- Abe, M.; Fukaya, Y.; Ohno, H. Extraction of polysaccharides from bran with phosphonate or phosphinate-derived ionic liquids under short mixing time and low temperature. Green Chem. 2010, 12, 1274–1280. [Google Scholar] [CrossRef]
- Stolarska, O.; Pawlowska-Zygarowicz, A.; Soto, A.; Rodríguez, H.; Smiglak, M. Mixtures of ionic liquids as more efficient media for cellulose dissolution. Carbohydr. Polym. 2017, 178, 277–285. [Google Scholar] [CrossRef]
- Catalán, J. Toward a generalized treatment of the solvent effect based on four empirical scales: Dipolarity (SdP, a new scale), polarizability (SP), acidity (SA), and basicity (SB) of the medium. J. Phys. Chem. B 2009, 113, 5951–5960. [Google Scholar] [CrossRef]
- Rinaldi, R. Instantaneous dissolution of cellulose in organic electrolyte solutions. Chem. Commun. 2011, 47, 511–513. [Google Scholar] [CrossRef]
- Achtel, C.; Jedvert, K.; Kosan, B.; El Seoud, O.A.; Heinze, T. Dissolution capacity of novel cellulose solvents based on triethyloctylammonium chloride. Macromol. Chem. Phys. 2017, 218, 1700208. [Google Scholar] [CrossRef]
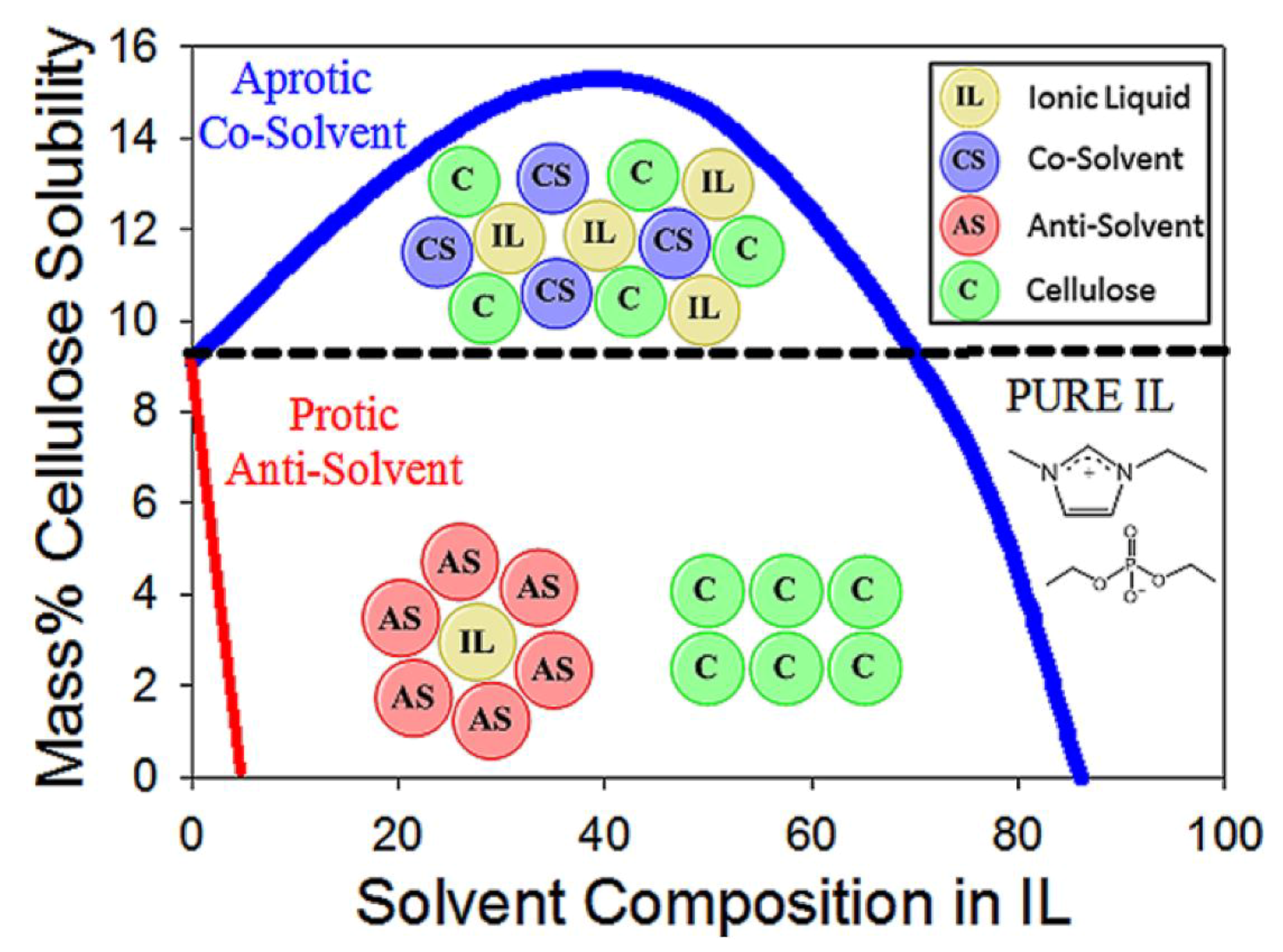
- Minnick, D.L.; Flores, R.A.; Destefano, M.R.; Scurto, A.M. Cellulose solubility in ionic liquid mixtures: Temperature, cosolvent, and antisolvent effects. J. Phys. Chem. B 2016, 120, 7906–7919. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Sale, K.L.; Simmons, B.A.; Singh, S. Molecular dynamics study of polysaccharides in binary solvent mixtures of an ionic liquid and water. J. Phys. Chem. B 2011, 115, 10251–10258. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Yuan, C.; Shi, W.; Chen, P.; Chen, H.; Xie, H.; Xu, Q.; Guo, Y.; Zheng, Q. Propylene carbonate based-organic electrolytes for cellulose dissolution processing and derivatization. ChemistrySelect 2017, 2, 3783–3787. [Google Scholar] [CrossRef]
- Bioni, T.A.; Arêas, E.P.G.; Couto, L.G.; Favarin, G.; El Seoud, O.A. Dissolution of cellulose in mixtures of ionic liquid and molecular solvents: Relevance of solvent-solvent and cellulose-solvent interactions. Nord. Pulp Pap. Res. J. 2015, 30, 105–111. [Google Scholar] [CrossRef]
- Zhong, C.; Cheng, F.; Zhu, Y.; Gao, Z.; Jia, H.; Wei, P. Dissolution mechanism of cellulose in quaternary ammonium hydroxide: Revisiting through molecular interactions. Carbohydr. Polym. 2017, 174, 400–408. [Google Scholar] [CrossRef]
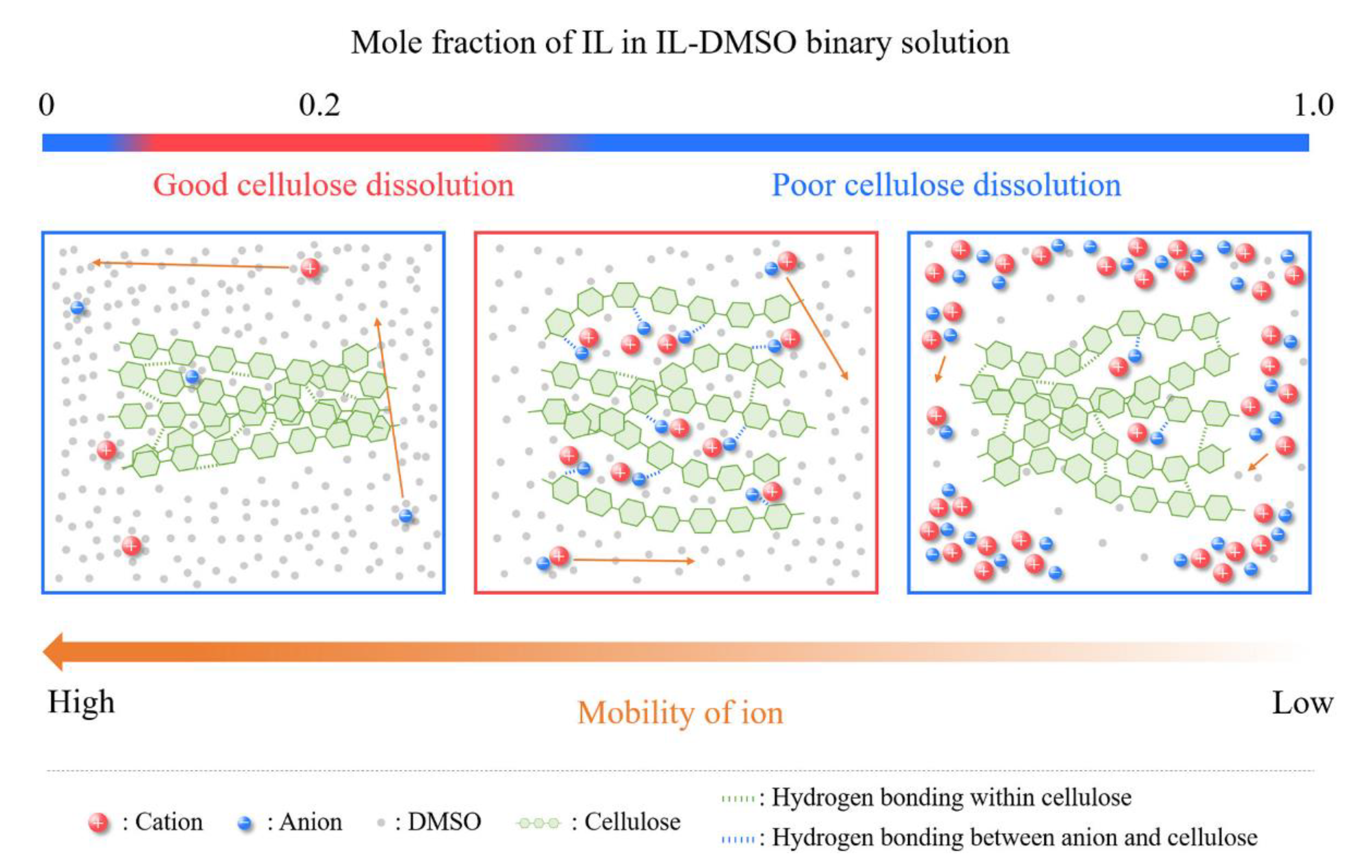
- Tomimatsu, Y.; Suetsugu, H.; Yoshimura, Y.; Shimizu, A. The solubility of cellulose in binary mixtures of ionic liquids and dimethyl sulfoxide: Influence of the anion. J. Mol. Liq. 2019, 279, 120–126. [Google Scholar] [CrossRef]
- Nawaz, H.; Pires, P.A.R.; Arêas, E.P.G.; Malek, N.I.; El Seoud, O.A. Probing cellulose acetylation in binary mixtures of an ionic liquid with dimethylsulfoxide and sulfolane by chemical kinetics, viscometry, spectroscopy, and molecular dynamics simulations. Macromol. Chem. Phys. 2015, 216, 2368–2376. [Google Scholar] [CrossRef]
- Pires, P.A.R.; Malek, N.I.; Teixeira, T.C.; Bioni, T.A.; Nawaz, H.; El Seoud, O.A. Imidazole-catalyzed esterification of cellulose in ionic liquid/molecular solvents: A multi-technique approach to probe effects of the medium. Ind. Crop. Prod. 2015, 77, 180–189. [Google Scholar] [CrossRef]
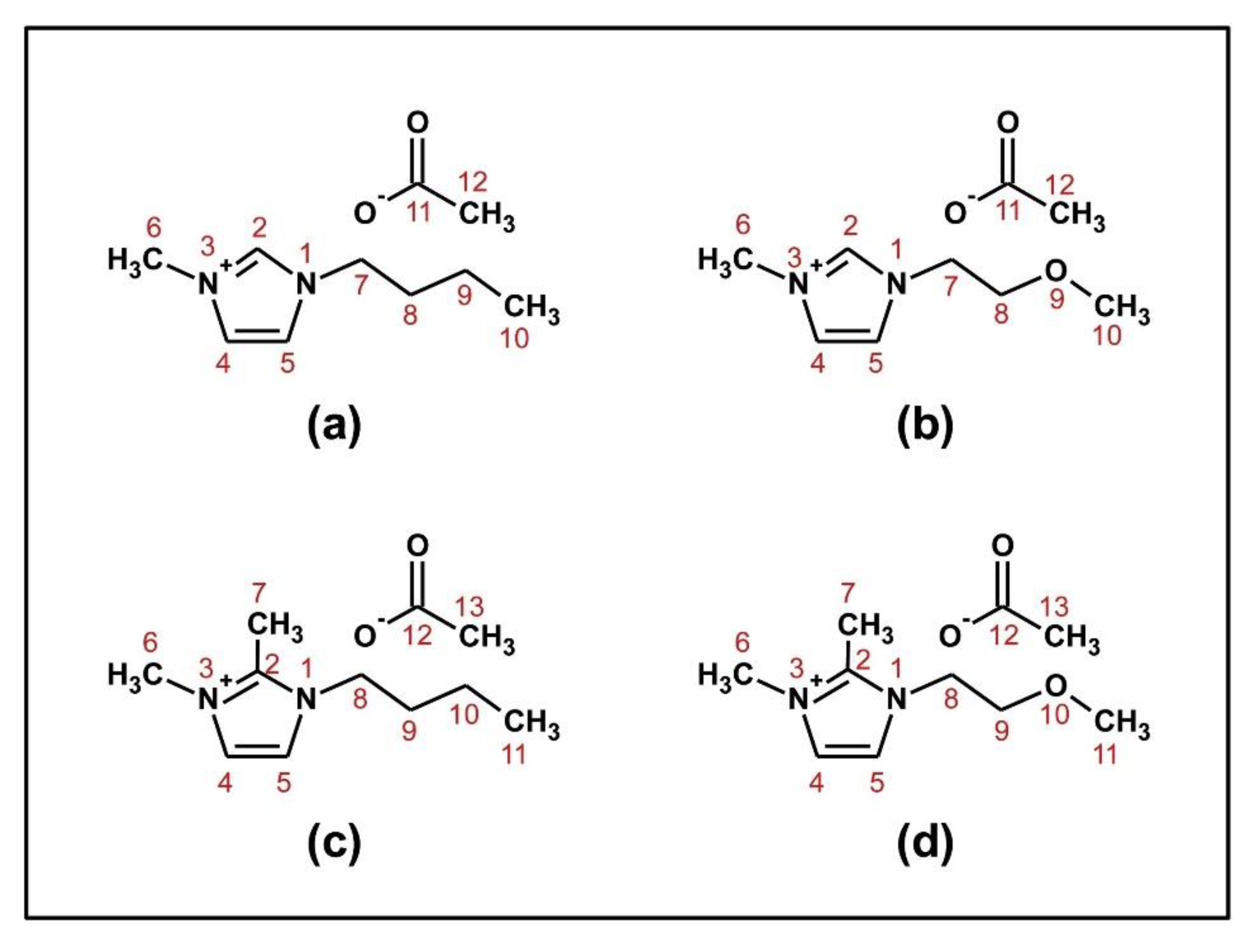
- Ferreira, D.C.; Oliveira, M.L.; Bioni, T.A.; Nawaz, H.; King, A.W.T.; Kilpeläinen, I.; Hummel, M.; Sixta, H.; El Seoud, O.A. Binary mixtures of ionic liquids-DMSO as solvents for the dissolution and derivatization of cellulose: Effects of alkyl and alkoxy side chains. Carbohydr. Polym. 2019, 212, 206–214. [Google Scholar] [CrossRef]
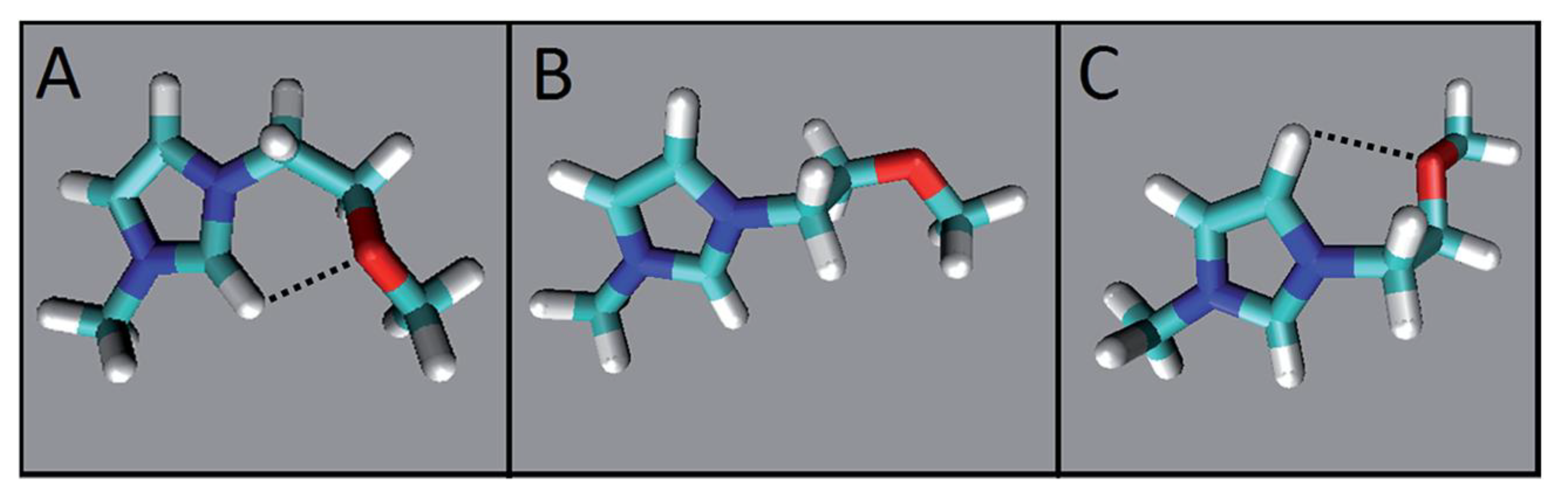
- de Jesus, J.C.; Pires, P.A.R.; Mustafa, R.; Riaz, N.; El Seoud, O.A. Experimental and theoretical studies on solvation in aqueous solutions of ionic liquids carrying different side chains: The n-butyl-group versus the methoxyethyl group. RSC Adv. 2017, 7, 15952–15963. [Google Scholar] [CrossRef]
- Wang, B.; Qin, L.; Mu, T.; Xue, Z.; Gao, G. Are ionic liquids chemically stable? Chem. Rev. 2017, 117, 7113–7131. [Google Scholar] [CrossRef] [PubMed]
- Remsing, R.C.; Swatloski, R.P.; Rogers, R.D.; Moyna, G. Mechanism of cellulose dissolution in the ionic liquid 1-n-butyl-3- methylimidazolium chloride: A 13C and 35/37Cl NMR relaxation study on model systems. Chem. Commun. 2006, 1271–1273. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, H.; Wu, J.; Zhang, J.; He, J.; Xiang, J. NMR spectroscopic studies of cellobiose solvation in EmimAc aimed to understand the dissolution mechanism of cellulose in ionic liquids. Phys. Chem. Chem. Phys. 2010, 12, 1941–1947. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, L.; Yu, J.; Wu, J.; Zhang, X.; He, J.; Zhang, J. Understanding cellulose dissolution: Effect of the cation and anion structure of ionic liquids on the solubility of cellulose. Sci. China Chem. 2016, 59, 1421–1429. [Google Scholar] [CrossRef]
- Gunnarsson, M.; Theliander, H.; Hasani, M. Chemisorption of air CO2 on cellulose: An overlooked feature of the cellulose/NaOH(aq) dissolution system. Cellulose 2017, 24, 2427–2436. [Google Scholar] [CrossRef]
- Östlund, Å.; Lundberg, D.; Nordstierna, L.; Holmberg, K.; Nydén, M. Dissolution and gelation of cellulose in TBAF/DMSO solutions: The roles of fluoride ions and water. Biomacromolecules 2009, 10, 2401–2407. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Liu, X.; Zhang, S. Towards a molecular understanding of cellulose dissolution in ionic liquids: Anion/cation effect, synergistic mechanism and physicochemical aspects. Chem. Sci. 2018, 9, 4027–4043. [Google Scholar] [CrossRef]
- Kan, Z.; Zhu, Q.; Yang, L.; Huang, Z.; Jin, B.; Ma, J. Polarization effects on the cellulose dissolution in ionic liquids: Molecular dynamics simulations with polarization model and integrated tempering enhanced sampling method. J. Phys. Chem. B 2017, 121, 4319–4332. [Google Scholar] [CrossRef]
- Casarano, R.; Pires, P.A.R.; El Seoud, O.A. Acylation of cellulose in a novel solvent system: Solution of dibenzyldimethylammonium fluoride in DMSO. Carbohydr. Polym. 2014, 101, 444–450. [Google Scholar] [CrossRef]
- Gupta, K.M.; Hu, Z.; Jiang, J. Mechanistic understanding of interactions between cellulose and ionic liquids: A molecular simulation study. Polymer 2011, 52, 5904–5911. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, D.; Duan, C.; Liu, C. Probing anion-cellulose interactions in imidazolium-based room temperature ionic liquids: A density functional study. Carbohydr. Res. 2010, 345, 2201–2205. [Google Scholar] [CrossRef] [PubMed]
- Casarano, R.; Pires, P.A.R.; Borin, A.C.; Seoud, O.A.E. Novel solvents for cellulose: Use of dibenzyldimethylammonium fluoride/dimethyl sulfoxide (DMSO) as solvent for the etherification of the biopolymer and comparison with tetra(1-butyl)ammonium fluoride/DMSO. Ind. Crops Prod. 2014, 54, 185–191. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Zhang, Y.; Jiang, K.; Wang, J.; Zhang, S. Why only ionic liquids with unsaturated heterocyclic cations can dissolve cellulose: A simulation study. ACS Sustain. Chem. Eng. 2017, 5, 3417–3428. [Google Scholar] [CrossRef]
- Potthast, A.; Rosenau, T.; Buchner, R.; Röder, T.; Ebner, G.; Bruglachner, H.; Sixta, H.; Kosma, P. The cellulose solvent system N,N-dimethylacetamide/lithium chloride revisited: The effect of water on physicochemical properties and chemical stability. Cellulose 2002, 9, 41–53. [Google Scholar] [CrossRef]
- Burchard, W. Solubility and solution structure of cellulose derivatives. Cellulose 2003, 10, 213–225. [Google Scholar] [CrossRef]
- Medronho, B.; Lindman, B. Brief overview on cellulose dissolution/regeneration interactions and mechanisms. Adv. Colloid Interface Sci. 2015, 222, 502–508. [Google Scholar] [CrossRef]
- Parthasarathi, R.; Bellesia, G.; Chundawat, S.P.S.; Dale, B.E.; Langan, P.; Gnanakaran, S. Insights into hydrogen bonding and stacking interactions in cellulose. J. Phys. Chem. A 2011, 115, 14191–14202. [Google Scholar] [CrossRef]
- Glasser, W.G.; Atalla, R.H.; Blackwell, J.; Brown, R.M.; Burchard, W.; French, A.D.; Klemm, D.O.; Nishiyama, Y. About the structure of cellulose: Debating the Lindman hypothesis. Cellulose 2012, 19, 589–598. [Google Scholar] [CrossRef]
- Medronho, B.; Romano, A.; Miguel, M.G.; Stigsson, L.; Lindman, B. Rationalizing cellulose (in) solubility: Reviewing basic physicochemical aspects and role of hydrophobic interactions. Cellulose 2012, 19, 581–587. [Google Scholar] [CrossRef]
- Medronho, B.; Duarte, H.; Alves, L.; Antunes, F.; Romano, A.; Lindman, B. Probing cellulose amphiphilicity. Nord. Pulp Pap. Res. J. 2015, 30, 058–066. [Google Scholar] [CrossRef]
- Medronho, B.; Duarte, H.; Alves, L.; Antunes, F.E.; Romano, A.; Valente, A.J.M. The role of cyclodextrin-tetrabutylammonium complexation on the cellulose dissolution. Carbohydr. Polym. 2016, 140, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Alves, L.; Medronho, B.; Antunes, F.E.; Topgaard, D.; Lindman, B. Dissolution state of cellulose in aqueous systems. 1. Alkaline solvents. Cellulose 2016, 23, 247–258. [Google Scholar] [CrossRef]
- Alves, L.; Medronho, B.; Antunes, F.E.; Topgaard, D.; Lindman, B. Dissolution state of cellulose in aqueous systems. 2. Acidic solvents. Carbohydr. Polym. 2016, 151, 707–715. [Google Scholar] [CrossRef]
- Bialik, E.; Stenqvist, B.; Fang, Y.; Östlund, Å.; Furó, I.; Lindman, B.; Lund, M.; Bernin, D. Ionization of cellobiose in aqueous alkali and the mechanism of cellulose dissolution. J. Phys. Chem. Lett. 2016, 7, 5044–5048. [Google Scholar] [CrossRef]
- Idström, A.; Gentile, L.; Gubitosi, M.; Olsson, C.; Stenqvist, B.; Lund, M.; Bergquist, K.-E.; Olsson, U.; Köhnke, T.; Bialik, E. On the dissolution of cellulose in tetrabutylammonium acetate/dimethyl sulfoxide: A frustrated solvent. Cellulose 2017, 24, 3645–3657. [Google Scholar] [CrossRef]
- Kostag, M.; El Seoud, O.A. Dependence of cellulose dissolution in quaternary ammonium-based ionic liquids/DMSO on the molecular structure of the electrolyte. Carbohydr. Polym. 2019, 205, 524–532. [Google Scholar] [CrossRef]
- Cousins, S.K.; Brown, R.M. Cellulose I microfibril assembly: Computational molecular mechanics energy analysis favours bonding by van der Waals forces as the initial step in crystallization. Polymer 1995, 36, 3885–3888. [Google Scholar] [CrossRef]
- Heiner, A.P.; Teleman, O. Interface between monoclinic crystalline cellulose and water: Breakdown of the odd/even duplicity. Langmuir 1997, 13, 511–518. [Google Scholar] [CrossRef]
- Heiner, A.P.; Kuutti, L.; Teleman, O. Comparison of the interface between water and four surfaces of native crystalline cellulose by molecular dynamics simulations. Carbohydr. Res. 1998, 306, 205–220. [Google Scholar] [CrossRef]
- Miyamoto, H.; Umemura, M.; Aoyagi, T.; Yamane, C.; Ueda, K.; Takahashi, K. Structural reorganization of molecular sheets derived from cellulose II by molecular dynamics simulations. Carbohydr. Res. 2009, 344, 1085–1094. [Google Scholar] [CrossRef] [PubMed]
- Maurer, R.J.; Sax, A.F.; Ribitsch, V. Molecular simulation of surface reorganization and wetting in crystalline cellulose I and II. Cellulose 2013, 20, 25–42. [Google Scholar] [CrossRef]
- Gupta, K.M.; Hu, Z.; Jiang, J. Molecular insight into cellulose regeneration from a cellulose/ionic liquid mixture: Effects of water concentration and temperature. RSC Adv. 2013, 3, 4425–4433. [Google Scholar] [CrossRef]
- Yamane, C.; Miyamoto, H.; Hayakawa, D.; Ueda, K. Folded-chain structure of cellulose II suggested by molecular dynamics simulation. Carbohydr. Res. 2013, 379, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.M.; Jiang, J. Cellulose dissolution and regeneration in ionic liquids: A computational perspective. Chem. Eng. Sci. 2015, 121, 180–189. [Google Scholar] [CrossRef]
- Newman, R.H.; Hemmingson, J.A. Carbon-13 NMR distinction between categories of molecular order and disorder in cellulose. Cellulose 1995, 2, 95–110. [Google Scholar] [CrossRef]
- Yamane, C.; Aoyagi, T.; Ago, M.; Sato, K.; Okajima, K.; Takahashi, T. Two different surface properties of regenerated cellulose due to structural anisotropy. Polym. J. 2006, 38, 819. [Google Scholar] [CrossRef]
- Isobe, N.; Kimura, S.; Wada, M.; Kuga, S. Mechanism of cellulose gelation from aqueous alkali-urea solution. Carbohydr. Polym. 2012, 89, 1298–1300. [Google Scholar] [CrossRef]
- Gupta, P.K.; Uniyal, V.; Naithani, S. Polymorphic transformation of cellulose I to cellulose II by alkali pretreatment and urea as an additive. Carbohydr. Polym. 2013, 94, 843–849. [Google Scholar] [CrossRef]
- Pang, J.; Wu, M.; Zhang, Q.; Tan, X.; Xu, F.; Zhang, X.; Sun, R. Comparison of physical properties of regenerated cellulose films fabricated with different cellulose feedstocks in ionic liquid. Carbohydr. Polym. 2015, 121, 71–78. [Google Scholar] [CrossRef]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef] [PubMed]
- Klemm, D.; Heublein, B.; Fink, H.-P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef] [PubMed]
- Klemm, D.; Schmauder, H.-P.; Heinze, T. Cellulose. In Biopolymers Online; Wiley-VCH: Weinheim, Germany, 2005; ISBN 978-3-527-60003-8. [Google Scholar]
- Cellulose. In Crystalline Cellulose and Derivatives: Characterization and Structures; Springer Series in Wood Science; Zugenmaier, P., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 101–174. ISBN 978-3-540-73934-0. [Google Scholar]
- Singh, P.; Duarte, H.; Alves, L.; Antunes, F.; LeMoigne, N.; Dormanns, J.; Duchemin, B.; Medronho, B. From cellulose dissolution and regeneration to added value applications—Synergism between molecular understanding and material development. In Cellulose-Fundamental Aspects and Current Trends; IntechOpen: London, UK, 2015. [Google Scholar]
- Yamane, C. Structure formation of regenerated cellulose from its solution and resultant features of high wettability: A review. Nord. Pulp Pap. Res. J. 2015, 30, 78–91. [Google Scholar] [CrossRef]
- Hermans, P.H. Degree of lateral order in various rayons as deduced from x-ray measurements. J. Polym. Sci. 1949, 4, 145–151. [Google Scholar] [CrossRef]
- Hayashi, J.; Masuda, S.; Watanabe, S. Plane Lattice Structure in Amorphous Region of Cellulose Fibers. Nippon Kagaku Kaishi 1974, 1974, 948–954. [Google Scholar] [CrossRef]
- Hishikawa, Y.; Togawa, E.; Kondo, T. Characterization of individual hydrogen bonds in crystalline regenerated cellulose using resolved polarized FTIR spectra. ACS Omega 2017, 2, 1469–1476. [Google Scholar] [CrossRef]
- Mansikkamäki, P.; Lahtinen, M.; Rissanen, K. The conversion from cellulose I to cellulose II in NaOH mercerization performed in alcohol–water systems: An X-ray powder diffraction study. Carbohydr. Polym. 2007, 68, 35–43. [Google Scholar] [CrossRef]
- Kim, N.-H.; Imai, T.; Wada, M.; Sugiyama, J. Molecular directionality in cellulose polymorphs. Biomacromolecules 2006, 7, 274–280. [Google Scholar] [CrossRef]
- Mazlan, N.S.N.; Zakaria, S.; Gan, S.; Hua, C.C.; Baharin, K.W.; Mazlan, N.S.N.; Zakaria, S.; Gan, S.; Hua, C.C.; Baharin, K.W. Comparison of regenerated cellulose membrane coagulated in sulphate based coagulant. CERNE 2019, 25, 18–24. [Google Scholar] [CrossRef]
- Ruan, D.; Zhang, L.; Lue, A.; Zhou, J.; Chen, H.; Chen, X.; Chu, B.; Kondo, T. A rapid process for producing cellulose multi-filament fibers from a NaOH/thiourea solvent system. Macromol. Rapid Commun. 2006, 27, 1495–1500. [Google Scholar] [CrossRef]
- Cai, J.; Zhang, L.; Zhou, J.; Qi, H.; Chen, H.; Kondo, T.; Chen, X.; Chu, B. Multifilament Fibers Based on Dissolution of Cellulose in NaOH/Urea Aqueous Solution: Structure and Properties. Adv. Mater. 2007, 19, 821–825. [Google Scholar] [CrossRef]
- Chen, X.; Burger, C.; Wan, F.; Zhang, J.; Rong, L.; Hsiao, B.S.; Chu, B.; Cai, J.; Zhang, L. Structure study of cellulose fibers wet-spun from environmentally friendly NaOH/urea aqueous solutions. Biomacromolecules 2007, 8, 1918–1926. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Zhu, K.; Zhou, X.; Luo, L.; Zeng, J.; Huang, R.; Lu, A.; Liu, X.; Chen, F.; Zhang, L.; et al. Influences of coagulation conditions on the structure and properties of regenerated cellulose filaments via wet-spinning in LiOH/urea solvent. ACS Sustain. Chem. Eng. 2018, 6, 4056–4067. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Seoud, O.A.; Kostag, M.; Jedvert, K.; Malek, N.I. Cellulose in Ionic Liquids and Alkaline Solutions: Advances in the Mechanisms of Biopolymer Dissolution and Regeneration. Polymers 2019, 11, 1917. https://doi.org/10.3390/polym11121917
El Seoud OA, Kostag M, Jedvert K, Malek NI. Cellulose in Ionic Liquids and Alkaline Solutions: Advances in the Mechanisms of Biopolymer Dissolution and Regeneration. Polymers. 2019; 11(12):1917. https://doi.org/10.3390/polym11121917
Chicago/Turabian StyleEl Seoud, Omar A., Marc Kostag, Kerstin Jedvert, and Naved I. Malek. 2019. "Cellulose in Ionic Liquids and Alkaline Solutions: Advances in the Mechanisms of Biopolymer Dissolution and Regeneration" Polymers 11, no. 12: 1917. https://doi.org/10.3390/polym11121917
APA StyleEl Seoud, O. A., Kostag, M., Jedvert, K., & Malek, N. I. (2019). Cellulose in Ionic Liquids and Alkaline Solutions: Advances in the Mechanisms of Biopolymer Dissolution and Regeneration. Polymers, 11(12), 1917. https://doi.org/10.3390/polym11121917





