Transferrin-Conjugated Docetaxel–PLGA Nanoparticles for Tumor Targeting: Influence on MCF-7 Cell Cycle
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of PLGA–EDA–Transferrin Conjugate and NMR Analysis
2.3. Formulation of Drug-Loaded Nanoparticles Using Polymer Conjugates
2.4. Optimization of the Formulation Process
2.5. Determination of Encapsulation Efficiency and Drug Loading Capacity
2.6. Fourier Transform Infrared Spectroscopy (FTIR)
2.7. Differential Scanning Calorimetry (DSC)
2.8. Powder X-ray Diffraction (PWRD)
2.9. Transmission Electron Microscopy (TEM)
2.10. Mean Particle Size, Size Distribution, and Zeta Potential
2.11. In Vitro Drug Release Studies
2.12. In Vitro Bioactivity Studies
2.12.1. MTT (3-(4, 5-Dimethylthiazol-2-yl)-2, 5-Diphenyltetrazolium Bromide) Assay
2.12.2. Estimation of Coumarin-6 Tagged Nanoparticles Uptake by Flow Cytometry
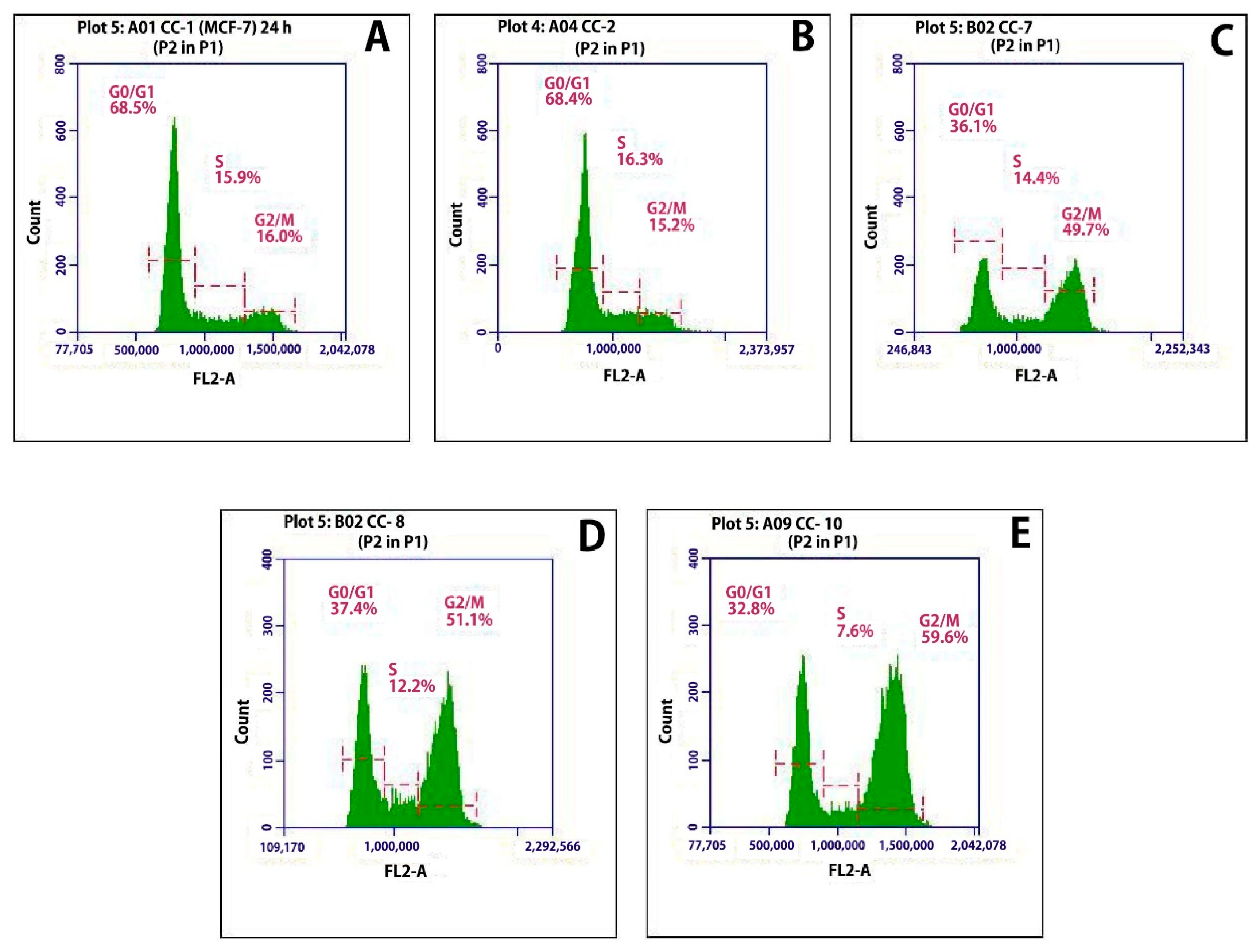
2.12.3. Cell Cycle Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, J.J.; Liu, S.P.; Zhao, J.; Wang, S.C.; Liu, T.J.; Li, X. Effects of a novel photoactivated photosensitizer on MDR1 over-expressing human breast cancer cells. J. Photochem. Photobiol. B Biol. 2017, 171, 67–74. [Google Scholar] [CrossRef]
- Fathi, M.; Barar, J. Perspective highlights on biodegradable polymeric nanosystems for targeted therapy of solid tumors. Bioimpacts 2017, 7, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Milling, L.; Zhang, Y.; Irvine, D.J. Delivering safer immunotherapies for cancer. Adv. Drug Deliv. Rev. 2017. [Google Scholar] [CrossRef] [PubMed]
- Cano, A.; Ettcheto, M.; Chang, J.H.; Barroso, E.; Espina, M.; Kuhne, B.A.; Barenys, M.; Auladell, C.; Folch, J.; Souto, E.B.; et al. Dual-drug loaded nanoparticles of Epigallocatechin-3-gallate (EGCG)/Ascorbic acid enhance therapeutic efficacy of EGCG in a APPswe/PS1dE9 Alzheimer’s disease mice model. J. Control. Release Off. J. Control. Release Soc. 2019, 301, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Lopez, E.; Egea, M.A.; Cano, A.; Espina, M.; Calpena, A.C.; Ettcheto, M.; Camins, A.; Souto, E.B.; Silva, A.M.; Garcia, M.L. PEGylated PLGA nanospheres optimized by design of experiments for ocular administration of dexibuprofen-in vitro, ex vivo and in vivo characterization. Colloids Surf. B Biointerfaces 2016, 145, 241–250. [Google Scholar] [CrossRef]
- Sanchez-Lopez, E.; Ettcheto, M.; Egea, M.A.; Espina, M.; Cano, A.; Calpena, A.C.; Camins, A.; Carmona, N.; Silva, A.M.; Souto, E.B.; et al. Memantine loaded PLGA PEGylated nanoparticles for Alzheimer’s disease: In vitro and in vivo characterization. J. Nanobiotechnol. 2018, 16, 32. [Google Scholar] [CrossRef]
- Cocco, E.; Deng, Y.; Shapiro, E.M.; Bortolomai, I.; Lopez, S.; Lin, K.; Bellone, S.; Cui, J.; Menderes, G.; Black, J.D.; et al. Dual-Targeting Nanoparticles for In Vivo Delivery of Suicide Genes to Chemotherapy-Resistant Ovarian Cancer Cells. Mol. Cancer Ther. 2017, 16, 323–333. [Google Scholar] [CrossRef]
- Harguindey, A.; Domaille, D.W.; Fairbanks, B.D.; Wagner, J.; Bowman, C.N.; Cha, J.N. Synthesis and Assembly of Click-Nucleic-Acid-Containing PEG-PLGA Nanoparticles for DNA Delivery. Adv. Mater. 2017. [Google Scholar] [CrossRef]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar]
- Li, N.; Yang, X.; Zhai, G.; Li, L. Multifunctional pluronic/poly(ethylenimine) nanoparticles for anticancer drug. J. Colloid Interface Sci. 2010, 350, 117–125. [Google Scholar] [CrossRef]
- Patil, Y.; Sadhukha, T.; Ma, L.; Panyam, J. Nanoparticle-mediated simultaneous and targeted delivery of paclitaxel and tariquidar overcomes tumor drug resistance. J. Control. Release Off. J. Control. Release Soc. 2009, 136, 21–29. [Google Scholar] [CrossRef] [PubMed]
- El-Hammadi, M.M.; Delgado, A.V.; Melguizo, C.; Prados, J.C.; Arias, J.L. Folic acid-decorated and PEGylated PLGA nanoparticles for improving the antitumour activity of 5-fluorouracil. Int. J. Pharm. 2017, 516, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; He, L.; Zhou, B.; Li, B.; Li, J. Folate-functionalized assembly of low density lipoprotein/sodium carboxymethyl cellulose nanoparticles for targeted delivery. Colloids Surf. B Biointerfaces 2017, 156, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Fan, J.; Zhao, Y.; Cheng, M.; Wang, X.; Jin, R.; Sun, T. A targeted drug delivery system based on folic acid-functionalized upconversion luminescent nanoparticles. J. Biomater. Appl. 2017, 31, 1247–1256. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Bai, Q.; Zhang, X.; Yang, H. Folate-mediated chemotherapy and diagnostics: An updated review and outlook. J. Control. Release Off. J. Control. Release Soc. 2017, 252, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.N.; Xu, Q.X.; Davoodi, P.; Wang, D.P.; Wang, C.H. Enhanced intracellular delivery and controlled drug release of magnetic PLGA nanoparticles modified with transferrin. Acta Pharmacol. Sin. 2017, 38, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Ruttala, H.B.; Ramasamy, T.; Poudal, B.K.; Choi, Y.; Choi, J.Y.; Kim, J.; Kwang Ku, S.; Choi, H.G.; Soon Yong, C.; Oh Kim, J. Molecularly targeted co-delivery of a histone deacetylase inhibitor and paclitaxel by lipid-protein hybrid nanoparticles for synergistic combinational chemotherapy. Oncotarget 2017, 8, 14925–14940. [Google Scholar] [CrossRef]
- Liu, Y.; Hui, Y.; Ran, R.; Yang, G.-Z.; Wibowo, D.; Wang, H.-F.; Middelberg, A.P.J.; Zhao, C.-X. Synergetic Combinations of Dual-Targeting Ligands for Enhanced In Vitro and In Vivo Tumor Targeting. Adv. Healthc. Mater. 2018, 7, 1800106. [Google Scholar] [CrossRef]
- Venditto, V.J.; Szoka, F.C., Jr. Cancer nanomedicines: So many papers and so few drugs! Adv. Drug Deliv. Rev. 2013, 65, 80–88. [Google Scholar] [CrossRef]
- Menditto, E.; Guerriero, F.; Orlando, V.; Crola, C.; Di Somma, C.; Illario, M.; Morisky, D.E.; Colao, A. Self-Assessment of Adherence to Medication: A Case Study in Campania Region Community-Dwelling Population. J. Aging Res. 2015, 2015, 682503. [Google Scholar] [CrossRef]
- Putignano, D.; Bruzzese, D.; Orlando, V.; Fiorentino, D.; Tettamanti, A.; Menditto, E. Differences in drug use between men and women: An Italian cross sectional study. BMC Womens Health 2017, 17, 73. [Google Scholar] [CrossRef] [PubMed]
- Menditto, E.; Cahir, C.; Aza-Pascual-Salcedo, M.; Bruzzese, D.; Poblador-Plou, B.; Malo, S.; Costa, E.; González-Rubio, F.; Gimeno-Miguel, A.; Orlando, V.; et al. Adherence to chronic medication in older populations: Application of a common protocol among three European cohorts. Patient Prefer. Adher. 2018, 12, 1975–1987. [Google Scholar] [CrossRef] [PubMed]
- Scala, D.; Menditto, E.; Armellino, M.F.; Manguso, F.; Monetti, V.M.; Orlando, V.; Antonino, A.; Makoul, G.; De Palma, M. Italian translation and cultural adaptation of the communication assessment tool in an outpatient surgical clinic. BMC Health Serv. Res. 2016, 16, 163. [Google Scholar] [CrossRef] [PubMed]
- Souto, E.B.; Dias-Ferreira, J.; Lopez-Machado, A.; Ettcheto, M.; Cano, A.; Camins Espuny, A.; Espina, M.; Garcia, M.L.; Sanchez-Lopez, E. Advanced Formulation Approaches for Ocular Drug Delivery: State-Of-The-Art and Recent Patents. Pharmaceutics 2019, 11, 460. [Google Scholar] [CrossRef] [PubMed]
- Abrego, G.; Alvarado, H.; Souto, E.B.; Guevara, B.; Bellowa, L.H.; Garduno, M.L.; Garcia, M.L.; Calpena, A.C. Biopharmaceutical profile of hydrogels containing pranoprofen-loaded PLGA nanoparticles for skin administration: In vitro, ex vivo and in vivo characterization. Int. J. Pharm. 2016, 501, 350–361. [Google Scholar] [CrossRef]
- Abrego, G.; Alvarado, H.; Souto, E.B.; Guevara, B.; Bellowa, L.H.; Parra, A.; Calpena, A.; Garcia, M.L. Biopharmaceutical profile of pranoprofen-loaded PLGA nanoparticles containing hydrogels for ocular administration. Eur. J. Pharm. Biopharm. 2015, 95, 261–270. [Google Scholar] [CrossRef]
- Araujo, J.; Vega, E.; Lopes, C.; Egea, M.A.; Garcia, M.L.; Souto, E.B. Effect of polymer viscosity on physicochemical properties and ocular tolerance of FB-loaded PLGA nanospheres. Colloids Surf. B Biointerfaces 2009, 72, 48–56. [Google Scholar] [CrossRef]
- Canadas, C.; Alvarado, H.; Calpena, A.C.; Silva, A.M.; Souto, E.B.; Garcia, M.L.; Abrego, G. In vitro, ex vivo and in vivo characterization of PLGA nanoparticles loading pranoprofen for ocular administration. Int. J. Pharm. 2016, 511, 719–727. [Google Scholar] [CrossRef]
- Jose, S.; Sowmya, S.; Cinu, T.A.; Aleykutty, N.A.; Thomas, S.; Souto, E.B. Surface modified PLGA nanoparticles for brain targeting of Bacoside-A. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2014, 63, 29–35. [Google Scholar] [CrossRef]
- Sanchez-Lopez, E.; Egea, M.A.; Davis, B.M.; Guo, L.; Espina, M.; Silva, A.M.; Calpena, A.C.; Souto, E.M.B.; Ravindran, N.; Ettcheto, M.; et al. Memantine-Loaded PEGylated Biodegradable Nanoparticles for the Treatment of Glaucoma. Small 2018, 14. [Google Scholar] [CrossRef]
- Wani, M.C.; Taylor, H.L.; Wall, M.E.; Coggon, P.; McPhail, A.T. Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J. Am. Chem. Soc. 1971, 93, 2325–2327. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Wang, L.; Han, Z.; Zhou, L.; Shan, L.; Ding, Y.; Xu, W.; Li, J.; Su, Y.; Cai, R.; et al. Different functions of DEPTOR in modulating sensitivity to chemotherapy for esophageal squamous cell carcinoma. Exp. Cell Res. 2017, 353, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, E.; Cacheux, W.; Frossard, J.L.; Koessler, T.; Abou, M.; Moniez, M.; Huber, O.; Puppa, G.; Roth, A. Exclusive neoadjuvant chemotherapy in locally advanced resectable gastric and gastro-esophageal junction adenocarcinoma. Dig. Liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital. Assoc. Study Liver 2017, 49, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, P.; Qvortrup, C.; Krogh, M.; Schoennemann, K.; Vestermark, L.W.; Jensen, H.A.; Bjerregaard, J.K. S-1 in combination with docetaxel and oxaliplatin in patients with advanced gastro-esophageal adenocarcinoma: Two parallel phase 1/2a studies. Acta Oncol. 2017, 56, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Gao, C.; Shi, F.; Feng, X.; Liu, L.; Qu, D.; Wang, C. A microemulsion co-loaded with Schizandrin A-docetaxel enhances esophageal carcinoma treatment through overcoming multidrug resistance. Drug Deliv. 2017, 24, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, K.; Hosoda, K.; Moriya, H.; Katada, C.; Sugawara, M.; Mieno, H.; Komori, S.; Katada, N.; Watanabe, M. Prognostic Advantage of Docetaxel/Cisplatin/ 5-Fluorouracil Neoadjuvant Chemotherapy in Clinical Stage II/III Esophageal Squamous Cell Carcinoma due to Excellent Control of Preoperative Disease and Postoperative Lymph Node Recurrence. Oncology 2017, 92, 221–228. [Google Scholar] [CrossRef]
- Liang, D.S.; Zhang, W.J.; Wang, A.T.; Su, H.T.; Zhong, H.J.; Qi, X.R. Treating metastatic triple negative breast cancer with CD44/neuropilin dual molecular targets of multifunctional nanoparticles. Biomaterials 2017, 137, 23–36. [Google Scholar] [CrossRef]
- Okines, A.F. T-DM1 in the Neo-Adjuvant Treatment of HER2-Positive Breast Cancer: Impact of the KRISTINE (TRIO-021) Trial. Rev. Recent Clin. Trials 2017. [Google Scholar] [CrossRef]
- Schraa, S.J.; Frerichs, K.A.; Agterof, M.J.; Hunting, J.C.B.; Los, M.; de Jong, P.C. Relative dose intensity as a proxy measure of quality and prognosis in adjuvant chemotherapy for breast cancer in daily clinical practice. Eur. J. Cancer 2017, 79, 152–157. [Google Scholar] [CrossRef]
- Zhao, G.; Li, Y.; Wang, T. Potentiation of docetaxel sensitivity by miR-638 via regulation of STARD10 pathway in human breast cancer cells. Biochem. Biophys. Res. Commun. 2017, 487, 255–261. [Google Scholar] [CrossRef]
- Silva, A.M.; Alvarado, H.L.; Abrego, G.; Martins-Gomes, C.; Garduno-Ramirez, M.L.; Garcia, M.L.; Calpena, A.C.; Souto, E.B. In Vitro Cytotoxicity of Oleanolic/Ursolic Acids-Loaded in PLGA Nanoparticles in Different Cell Lines. Pharmaceutics 2019, 11, 362. [Google Scholar] [CrossRef] [PubMed]
- Jose, S.; Fangueiro, J.F.; Smitha, J.; Cinu, T.A.; Chacko, A.J.; Premaletha, K.; Souto, E.B. Cross-linked chitosan microspheres for oral delivery of insulin: Taguchi design and in vivo testing. Colloids Surf. B Biointerfaces 2012, 92, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Mattheolabakis, G.; Taoufik, E.; Haralambous, S.; Roberts, M.L.; Avgoustakis, K. In vivo investigation of tolerance and antitumor activity of cisplatin-loaded PLGA-mPEG nanoparticles. Eur. J. Pharm. Biopharm. Off. J. Arb. Pharm. Verfahr. 2009, 71, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Andrade, L.N.; Oliveira, D.M.L.; Chaud, M.V.; Alves, T.F.R.; Nery, M.; da Silva, C.F.; Gonsalves, J.K.C.; Nunes, R.S.; Correa, C.B.; Amaral, R.G.; et al. Praziquantel-Solid Lipid Nanoparticles Produced by Supercritical Carbon Dioxide Extraction: Physicochemical Characterization, Release Profile, and Cytotoxicity. Molecules 2019, 24, 3881. [Google Scholar] [CrossRef]
- Saxena, V.; Naguib, Y.; Hussain, M.D. Folate receptor targeted 17-allylamino-17-demethoxygeldanamycin (17-AAG) loaded polymeric nanoparticles for breast cancer. Colloids Surf. B Biointerfaces 2012, 94, 274–280. [Google Scholar] [CrossRef]
- Ge, Y.; Zhang, Y.; He, S.; Nie, F.; Teng, G.; Gu, N. Fluorescence Modified Chitosan-Coated Magnetic Nanoparticles for High-Efficient Cellular Imaging. Nanoscale Res. Lett. 2009, 4, 287–295. [Google Scholar] [CrossRef]
- Pozarowski, P.; Darzynkiewicz, Z. Analysis of cell cycle by flow cytometry. Methods Mol. Biol. 2004, 281, 301–311. [Google Scholar] [CrossRef]
- Reddy, N.D.; Shoja, M.H.; Jayashree, B.S.; Nayak, P.G.; Kumar, N.; Prasad, V.G.; Pai, K.S.; Rao, C.M. In vitro and in vivo evaluation of novel cinnamyl sulfonamide hydroxamate derivative against colon adenocarcinoma. Chem. Biol. Interact. 2015, 233, 81–94. [Google Scholar] [CrossRef]
- Wu, W.; Cheng, Y.; Guo, B.H.; Wu, Q. Pharmacokinetics of liver-targeted docetaxel liposomes modified with 6-O-acyl-D-galactose esters in rabbits. Biomed. Rep. 2014, 2, 545–548. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release I. Fickian and non-fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J. Control. Release 1987, 5, 23–36. [Google Scholar] [CrossRef]








| Independent Variables | Levels | ||
|---|---|---|---|
| −1 | 0 | +1 | |
| Phase ratio | 1:3 | 1:4 | 1:5 |
| Sonication time (min) | 6 | 8 | 10 |
| Dependent variables | Y1—Mean Particle Size | ||
| Y2—Encapsulation Efficiency (EE%) | |||
| Formulation Code | Factor 1 Phase Ratio | Factor 2 Sonication Time (min) | Response 1 Mean Particle Size (nm) | Response 2 | |
|---|---|---|---|---|---|
| %EE * | %LC | ||||
| 1-Tf-PLGA NPs | 1:4 | 6 | 283.4 | 50 | 25.0 |
| 2-Tf-PLGA NPs | 1:4 | 8 | 280.6 | 45.6 | 22.8 |
| 3-Tf-PLGA NPs | 1:5 | 10 | 204.2 | 32.1 | 16.1 |
| 4-Tf-PLGA NPs | 1:4 | 10 | 235.5 | 39.6 | 19.8 |
| 5-Tf-PLGA NPs | 1:5 | 6 | 230.5 | 38.4 | 19.2 |
| 6-Tf-PLGA NPs | 1:4 | 8 | 240.6 | 44.4 | 22.2 |
| 7-Tf-PLGA NPs | 1:4 | 8 | 250.2 | 43.6 | 21.8 |
| 8-Tf-PLGA NPs | 1:4 | 8 | 247.0 | 44.8 | 22.4 |
| 9-Tf-PLGA NPs | 1:5 | 8 | 219.7 | 36.8 | 18.4 |
| 10-Tf-PLGA NPs | 1:4 | 8 | 247.8 | 42.8 | 21.4 |
| 11-Tf-PLGA NPs | 1:3 | 8 | 370.8 | 56.8 | 28.4 |
| 12-Tf-PLGA NPs | 1:3 | 6 | 426.7 | 59.6 | 29.8 |
| 13-Tf-PLGA NPs | 1:3 | 10 | 311.0 | 53.6 | 26.8 |
| Source | Sum of Squares | df | F-Value | p-Value Prob > F | ||||
|---|---|---|---|---|---|---|---|---|
| Y1 | Y2 | Y1 | Y2 | Y1 | Y2 | Y1 | Y2 | |
| Sequential Model Sum of Squares | ||||||||
| Mean versus Total | 9.682 × 105 | 26,604.74 | 1 | 1 | ||||
| Linear versus Mean | 40,378.14 | 741.1 | 2 | 2 | 24.96 | 157.99 | 0.0001 | 0.0001 |
| 2FI * versus Linear | 1998.09 | 0.022 | 1 | 1 | 2.95 | 8.642 × 10−3 | 0.1198 | 0.9280 |
| Quadratic versus 2FI | 4877.38 | 10.71 | 2 | 2 | 14.08 | 2.95 | 0.0035 | 0.1179 |
| Cubic versus Quadratic | 177.92 | 6.63 | 2 | 2 | 0.43 | 2.72 | 0.6725 | 0.1587 |
| Residual | 1034.31 | 6.09 | 5 | 5 | ||||
| Lack of Fit Tests | ||||||||
| Linear | 7101.59 | 18.78 | 6 | 6 | 4.80 | 2.68 | 0.0754 | 0.1796 |
| 2FI * | 5103.50 | 18.76 | 5 | 5 | 4.14 | 3.21 | 0.0967 | 0.1406 |
| Quadratic | 226.12 | 8.05 | 3 | 3 | 0.31 | 2.30 | 0.8211 | 0.2194 |
| Cubic | 48.20 | 1.42 | 1 | 1 | 0.20 | 1.22 | 0.6812 | 0.3320 |
| Pure Error | 986.11 | 4.67 | 4 | 4 | ||||
| Model Summary Statistics | ||||||||
| Source | R-Squared | Adjusted R-Squared | Predicted R-Squared | PRESS | ||||
| Y1 | Y2 | Y1 | Y2 | Y1 | Y2 | Y1 | Y2 | |
| Linear | 28.44 | 1.53 | 0.7998 | 0.9632 | 0.6572 | 0.9476 | 16,612.71 | 40.09 |
| 2FI * | 26.01 | 1.61 | 0.8325 | 0.9591 | 0.5742 | 0.9166 | 20,636.95 | 63.73 |
| Quadratic | 13.16 | 1.35 | 0.9571 | 0.9715 | 0.9261 | 0.8901 | 3579.85 | 83.99 |
| Cubic | 14.38 | 1.10 | 0.9488 | 0.9809 | 0.8547 | 0.7752 | 7040.67 | 171.85 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value Prob > F | - |
|---|---|---|---|---|---|---|
| Response Y1: Particle Size | ||||||
| Model | 47,253.61 | 5 | 9450.72 | 54.57 | <0.0001 | significant |
| A-phase Ratio | 34,367.80 | 1 | 34,367.80 | 198.46 | <0.0001 | |
| B-sonication Time | 6010.33 | 1 | 6010.33 | 34.71 | 0.0006 | |
| AB | 1988 | 1 | 1998.09 | 11.54 | 0.0115 | |
| A2 | 3982.76 | 1 | 3982.76 | 23.0 | 0.0020 | |
| B2 | 13.06 | 1 | 13.06 | 0.075 | 0.7916 | |
| Residual | 1212.24 | 7 | 173.18 | |||
| Lack of Fit | 226.12 | 3 | 75.37 | 0.31 | 0.8211 | not significant |
| Pure Error | 986.11 | 4 | 246.53 | |||
| Cor Total | 48,465.84 | 12 | ||||
| Response Y2: Encapsulation Efficiency | ||||||
| Model | 741.1 | 2 | 370.55 | 157.99 | <0.0001 | significant |
| A-phase Ratio | 655.22 | 1 | 655.22 | 279.36 | <0.0001 | |
| B-sonication Time | 85.88 | 1 | 85.88 | 36.62 | 0.0001 | |
| Residual | 23.45 | 10 | 2.35 | |||
| Lack of Fit | 18.78 | 6 | 3.13 | 2.68 | 0.1796 | not significant |
| Pure Error | 4.67 | 4 | 1.17 | |||
| Cor Total | 764.55 | 12 | ||||
| Factors | Level | Average Particle Size (nm) | Average Encapsulation Efficiency (%) | ||
|---|---|---|---|---|---|
| Phase ratio | 1:4.7 | Predicted | Real | Predicted | Real |
| Sonication time (min) | 10 | 206.2 | 210.6 ± 2.7 | 34.1 | 36.1 ± 2.3 |
| Compound Name | Concentration (μM/mL) | Mean Cell Death | SEM | IC50 (μM/mL) |
|---|---|---|---|---|
| Docetaxel | 0.25 | 16.5 | 2.9 | 7.097 |
| 0.5 | 23.0 | 1.1 | ||
| 1 | 41.6 | 1.0 | ||
| 2 | 51.0 | 0.7 | ||
| DCT-loaded PLGA NPs | 0.25 | 25.5 | 0.9 | 6.24 |
| 0.5 | 36.4 | 3.6 | ||
| 1 | 43.8 | 0.6 | ||
| 2 | 53.0 | 1.0 | ||
| DCT-loaded Tf-conjugated PLGA NPs | 0.25 | 23.0 | 1.1 | 4.392 |
| 0.5 | 41.6 | 1.0 | ||
| 1 | 51.0 | 0.7 | ||
| 2 | 56.6 | 0.8 | ||
| Blank NPs | 100 | 18.2 | 7.2 | 800 μg/mL |
| 200 | 16.0 | 4.0 | ||
| 400 | 19.3 | 3.9 | ||
| 800 | 15.8 | 10.4 |
| Samples | Mean Fluorescence | |
|---|---|---|
| 2 h | 24 h | |
| Blank NPs | 27,294.1 | 32,914.2 |
| DCT-loaded PLGA NPs | 2,930,523.01 | 3,085,163.04 |
| DCT-loaded Tf-conjugated PLGA NPs | 4,153,708.4 | 7,550,576.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jose, S.; Cinu, T.A.; Sebastian, R.; Shoja, M.H.; Aleykutty, N.A.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B. Transferrin-Conjugated Docetaxel–PLGA Nanoparticles for Tumor Targeting: Influence on MCF-7 Cell Cycle. Polymers 2019, 11, 1905. https://doi.org/10.3390/polym11111905
Jose S, Cinu TA, Sebastian R, Shoja MH, Aleykutty NA, Durazzo A, Lucarini M, Santini A, Souto EB. Transferrin-Conjugated Docetaxel–PLGA Nanoparticles for Tumor Targeting: Influence on MCF-7 Cell Cycle. Polymers. 2019; 11(11):1905. https://doi.org/10.3390/polym11111905
Chicago/Turabian StyleJose, Sajan, Thomas A. Cinu, Rosmy Sebastian, M. H. Shoja, N. A. Aleykutty, Alessandra Durazzo, Massimo Lucarini, Antonello Santini, and Eliana B. Souto. 2019. "Transferrin-Conjugated Docetaxel–PLGA Nanoparticles for Tumor Targeting: Influence on MCF-7 Cell Cycle" Polymers 11, no. 11: 1905. https://doi.org/10.3390/polym11111905
APA StyleJose, S., Cinu, T. A., Sebastian, R., Shoja, M. H., Aleykutty, N. A., Durazzo, A., Lucarini, M., Santini, A., & Souto, E. B. (2019). Transferrin-Conjugated Docetaxel–PLGA Nanoparticles for Tumor Targeting: Influence on MCF-7 Cell Cycle. Polymers, 11(11), 1905. https://doi.org/10.3390/polym11111905









