Dielectric, Thermal and Mechanical Properties of l,d-Poly(Lactic Acid) Modified by 4′-Pentyl-4-Biphenylcarbonitrile and Single Walled Carbon Nanotube
Abstract
:1. Introduction
- (i)
- (ii)
- (iii)
- (iv)
- (v)
- liquid crystalline poly [4,4′-bis(6-hydroxyhexyloxy) biphenyl phenylsuccinate] as functional chain to copolymerize with PLA, towards improve the flexibility of PLA and caused interactions with multiwalled carbon nanotubes via π–π interaction [49];
- (vi)
- magnesium as filament based 3D printing [50];
- (vii)
- TiO2 for antibacterial packaging [51];
- (viii)
- ZnO as potential antimicrobial food packaging materials [52];
- (ix)
- high-density polyethylene/carbon black composites as electrically conductive composites with a low percolation threshold [53].
2. Experimental
2.1. Materials
2.1.1. Preparation of l,d-PLA:5CB Hybrid Materials
2.1.2. Preparation of l,d-PLA:5CB:SWCN Hybrid Materials
2.2. Characterization of Methods
3. Results and Discussion
3.1. DSC Measurements and Texture Observations Before and After Mechanical Deformation
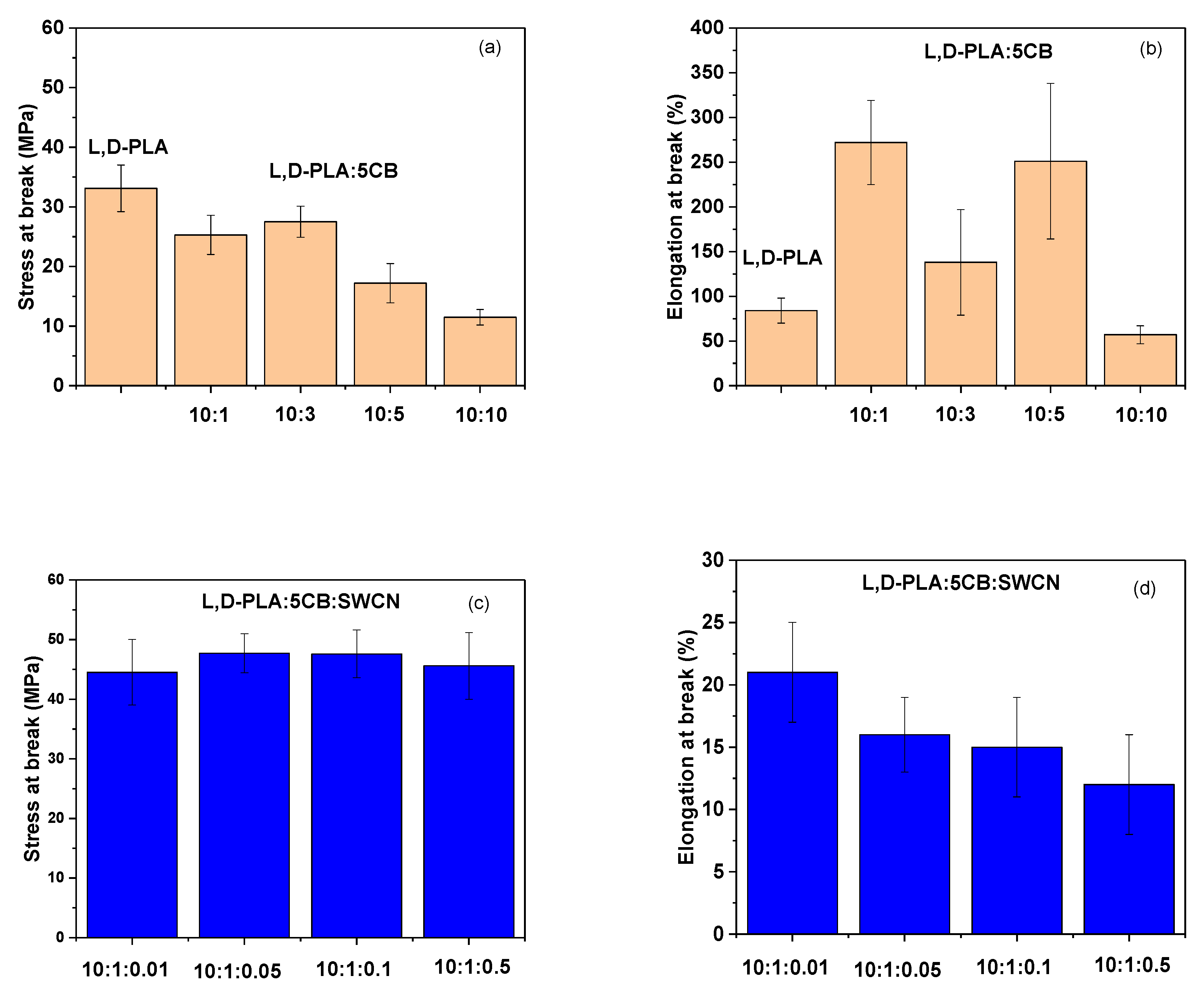
3.2. Mechanical Properties of l,d-PLA with 5CB and l,d-PLA:5CB:SWCN
- (i)
- the influence of 5CB component and its amount on the properties of thin film;
- (ii)
- select the ratio of l,d-PLA:5CB forming the thin film with the best mechanical properties;
- (iii)
- the influence of SWCN on selected l,d-PLA:5CB composite with required mechanical properties on the properties of thin film.
3.3. Thermal Images of l,d-PLA with 5CB and SWCN
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Büyüktanir, E.A.; Gheorghiu, N.; West, J.L.; Mitrokhin, M.; Holter, B.; Glushchenko, A. Field-induced polymer wall formation in a bistable smectic-a liquid crystal display. Appl. Phys. Lett. 2006, 89, 031101. [Google Scholar] [CrossRef]
- Stephenson, S.W.; Johnson, D.M.; Kilburn, J.I.; Mi, X.-D.; Rankin, C.M.; Capurso, R.G. 16.3: Development of a flexible electronic display using photographic technology. SID Symp. Dig. Tech. Papers 2004, 35, 774–777. [Google Scholar] [CrossRef]
- West, J.L.; Zhang, K.; Zhang, M.; Buyuktanir, E.; Glushchenko, A. Stressed liquid crystals and their application. In Proceedings of the SPIE 5936, Liquid Crystals IX, 59360L, San Diego, CA, USA, 20 August 2005; SPIE: Washington, WA, USA, 2005. [Google Scholar]
- Koncar, V. Optical fiber fabric displays. Opt. Photon. News 2005, 16, 40–44. [Google Scholar] [CrossRef]
- Yase, K.; Suzuki, K.; Hiroshima, M.; Mimura, A.; Shuu, Y.M.; Toda, S.; Koaizawa, H. 64.5l: Late-news paper: Large area flexible display of fiber oled. SID Symp. Dig. Tech. Papers 2006, 37, 1870–1873. [Google Scholar] [CrossRef]
- Humphries, R.L.; Luckhurst, G.R.; Carrington, A. A statistical theory of liquid crystalline mixtures: Phase separation. Math. Phys. Sci. 1976, 352, 41–56. [Google Scholar] [CrossRef]
- Flory, P.J. Molecular Theory of Liquid Crystals. In Advances in Polymer Science; Springer: Berlin, Germany, 2005; pp. 1–36. [Google Scholar]
- Mucha, M. Polymer as an important component of blends and composites with liquid crystals. Prog. Polym. Sci. 2003, 28, 837–873. [Google Scholar] [CrossRef]
- Soule, E.R.; Rey, A.D. Modelling complex liquid crystal mixtures: From polymer dispersed mesophase to nematic nanocolloids. Mol. Simul. 2012, 38, 735–750. [Google Scholar] [CrossRef]
- Gorkunov, M.V.; Shandryuk, G.A.; Shatalova, A.M.; Kutergina, I.Y.; Merekalov, A.S.; Kudryavtsev, Y.V.; Talroze, R.V.; Osipov, M.A. Phase separation effects and the nematic–isotropic transition in polymer and low molecular weight liquid crystals doped with nanoparticles. Soft Matter 2013, 9, 3578–3588. [Google Scholar] [CrossRef]
- Milette, J.; Toader, V.; Soulé, E.R.; Lennox, R.B.; Rey, A.D.; Reven, L. A molecular and thermodynamic view of the assembly of gold nanoparticles in nematic liquid crystal. Langmuir 2013, 29, 1258–1263. [Google Scholar] [CrossRef] [PubMed]
- Kasch, N.; Dierking, I. Phase transitions and separations in a distorted liquid crystalline mixture. J. Chem. Phys. 2015, 143, 064907. [Google Scholar] [CrossRef] [PubMed]
- Buyuktanir, E.A.; West, J.L.; Frey, M.W. Liquid crystal microfibers lead to responsive optoelectronic textiles. In Proceedings of the SPIE 7955, Emerging Liquid Crystal Technologies VI, 79550P, San Diego, CA, USA, 2 February 2011; SPIE: Washington, WA, USA, 2011. [Google Scholar]
- Wu, Y.; An, Q.; Yin, J.; Hua, T.; Xie, H.; Li, G.; Tang, H. Liquid crystal fibers produced by using electrospinning technique. Colloid. Polym. Sci. 2008, 286, 897–905. [Google Scholar] [CrossRef]
- Lagerwall, J.P.F.; McCann, J.T.; Formo, E.; Scalia, G.; Xia, Y. Coaxial electrospinning of microfibres with liquid crystal in the core. Chem. Commun. 2008, 42, 5420–5422. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Huang, H.; Jing, Y.; Fu, H.; Chang, P.; Li, D.; Yao, Y.; Fan, Z. Flexible photovoltaic technologies. J. Mater. Chem. C 2014, 2, 1233–1247. [Google Scholar] [CrossRef]
- Zheng, Q.; Fang, G.; Bai, W.; Sun, N.; Qin, P.; Fan, X.; Cheng, F.; Yuan, L.; Zhao, X. Efficiency improvement in organic solar cells by inserting a discotic liquid crystal. Sol. Energy Mater. Sol. Cells 2011, 95, 2200–2205. [Google Scholar] [CrossRef]
- Iwan, A.; Boharewicz, B.; Tazbir, I.; Hamplová, V.; Bubnov, A. Effect of chiral photosensitive liquid crystalline dopants on the performance of organic solar cells. Solid-State Electron. 2015, 104, 53–60. [Google Scholar] [CrossRef]
- Krebs, F.C.; Biancardo, M.; Winther-Jensen, B.; Spanggard, H.; Alstrup, J. Strategies for incorporation of polymer photovoltaics into garments and textiles. Sol. Energy Mater. Sol. Cells 2006, 90, 1058–1067. [Google Scholar] [CrossRef]
- Lee, Y.-H.; Kim, J.-S.; Noh, J.; Lee, I.; Kim, H.J.; Choi, S.; Seo, J.; Jeon, S.; Kim, T.S.; Lee, J.Y.; et al. Wearable textile battery rechargeable by solar energy. Nano Lett. 2013, 13, 5753–5761. [Google Scholar] [CrossRef] [PubMed]
- Buyuktanir, E.A.; Frey, M.W.; West, J.L. Self-assembled, optically responsive nematic liquid crystal/polymer core-shell fibers: Formation and characterization. Polymer 2010, 51, 4823–4830. [Google Scholar] [CrossRef]
- Gray, G.W.; Harrison, K.J.; Nash, J.A. New family of nematic liquid crystals for displays. Electron. Lett. 1973, 9, 130–131. [Google Scholar] [CrossRef]
- Serrano, L.A.; Fornerod, M.J.; Yang, Y.; Gaisford, S.; Stellacci, F.; Guldin, S. Phase behaviour and applications of a binary liquid mixture of methanol and a thermotropic liquid crystal. Soft Matter 2018, 14, 4615–4620. [Google Scholar] [CrossRef] [PubMed]
- Hori, H.; Urakawa, O.; Adachi, K. Dielectric relaxation in phase-segregated mixtures of polystyrene and liquid crystal 5cb. Macromolecules 2004, 37, 1583–1590. [Google Scholar] [CrossRef]
- Javadian, S.; Dalir, N.; Kakemam, J. Non-covalent intermolecular interactions of colloidal nematic liquid crystals doped with graphene oxide. Liq. Cryst. 2017, 44, 1341–1355. [Google Scholar] [CrossRef]
- Fryń, P.; Bogdanowicz, K.A.; Górska, N.; Rysz, J.; Krysiak, P.; Marzec, M.; Marzec, M.; Iwan, A.; Januszko, A. Hybrid materials based on l,d-poly(lactic acid) and single-walled carbon nanotubes as flexible substrate for organic devices. Polymers 2018, 10, 1271. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Mallegni, N.; Phuong, T.V.; Coltelli, M.-B.; Cinelli, P.; Lazzeri, A. Poly(lactic acid) (PLA) Based Tear Resistant and Biodegradable Flexible Films by Blown Film Extrusion. Materials 2018, 11, 148. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, V.; Mohanty, A.K.; Misra, M. Perspective on Polylactic Acid (PLA) based Sustainable Materials for Durable Applications: Focus on Toughness and Heat Resistance. ACS Sustain. Chem. Eng. 2016, 4, 2899–2916. [Google Scholar] [CrossRef]
- Coltelli, M.B.; Della Maggiore, I.; Bertoldo, M.; Bronco, S.; Signori, F.; Ciardelli, F. Poly(lactic acid) properties as a consequence of poly(butylene adipate-co-terephthalate) blending and acetyl tributyl citrate plasticization. J. Appl. Polym. Sci. 2008, 110, 1250–1262. [Google Scholar] [CrossRef]
- Scatto, M.; Salmini, E.; Castiello, S.; Coltelli, M.B.; Conzatti, L.; Stagnaro, P.; Andreotti, L.; Bronco, S. Plasticized and nanofilled poly(lactic acid)-based cast films: Effect of plasticizer and organoclay on processability and final properties. J. Appl. Polym. Sci. 2013, 127, 4947–4956. [Google Scholar] [CrossRef]
- Farsetti, S.; Cioni, B.; Lazzeri, A. Physico-Mechanical Properties of Biodegradable Rubber Toughened Polymers. Macromol. Symp. 2011, 301, 82–89. [Google Scholar] [CrossRef]
- Signori, F.; Coltelli, M.B.; Bronco, S.; Ciardelli, F. Thermal degradation of poly(lactic acid) (PLA) and poly(butylene adipate-co-terephthalate) (PBAT) and their blends upon melt processing. Polym. Degrad. Stab. 2009, 94, 74–82. [Google Scholar] [CrossRef]
- Supthanyakula, R.; Kaabbuathongb, N.; Chirachanchai, S. Random poly(butylene succinate-co-lactic acid) as a multi-functional additive for miscibility, toughness, and clarity of PLA/PBS blends. Polymer 2016, 105, 1–9. [Google Scholar] [CrossRef]
- Su, S.; Kopitzky, R.; Tolga, S.; Kabasci, S. Polylactide (PLA) and Its Blends with Poly(butylene succinate) (PBS): A Brief Review. Polymers 2019, 11, 1193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Anna, A.; Arrigo, R.; Frache, A. PLA/PHB Blends: Biocompatibilizer Effects. Polymers 2019, 11, 1416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Daelemans, L.; Fiorio, R.; Gou, M.; D’hooge, D.R.; De Clerck, K.; Cardon, L. Improving Mechanical Properties for Extrusion-Based Additive Manufacturing of Poly(Lactic Acid) by Annealing and Blending with Poly(3-Hydroxybutyrate). Polymers 2019, 11, 1529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Wang, H.; Du, P.; Liu, J.; Liu, D.; Liu, P. Porous polylactic acid/carbon nanotubes/polyaniline composite film as flexible free-standing electrode for supercapacitors. Electrochim. Acta 2019, 294, 312–324. [Google Scholar] [CrossRef]
- Ma, P.; Lv, P.; Xu, P.; Du, M.; Zhu, H.; Dong, W.; Chen, M. Design of bio-based conductive and fast crystallizing nanocomposites with controllable distribution of multiwalled carbon nanotubes via interfacial stereocomplexation. Chem. Eng. J. 2018, 336, 223–232. [Google Scholar] [CrossRef]
- Shi, S.; Chen, Y.; Jing, J.; Yang, L. Preparation and 3D-printing of highly conductive polylactic acid/carbon nanotube nanocomposites via local enrichment strategy. RSC Adv. 2019, 9, 29980–29986. [Google Scholar] [CrossRef] [Green Version]
- Zare, Y.; Rhee, R.Y. Following the morphological and thermal properties of PLA/PEO blends containing carbon nanotubes (CNTs) during hydrolytic degradation. Compos. Part B 2019, 175, 107132. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, W.; Zhang, F.; Leng, J.; Pei, S.; Wang, L.; Jia, X.; Chase, C.; Suna, B.Z.; Chou, T.W. Microstructural design for enhanced shape memory behavior of 4D printed composites based on carbon nanotube/polylactic acid filament. Compos. Sci. Technol. 2019, 181, 107692. [Google Scholar] [CrossRef]
- Zhou, Y.; Lei, L.; Yang, B.; Li, J.; Ren, J. Preparation and characterization of polylactic acid (PLA) carbon nanotube nanocomposites. Polym. Test. 2018, 68, 34–38. [Google Scholar] [CrossRef]
- Yang, L.; Li, S.; Zhou, X.; Liu, J.; Li, Y.; Yang, M.; Yuan, Q.; Zhang, W. Effects of carbon nanotube on the thermal, mechanical, and electrical properties of PLA/CNT printed parts in the FDM process. Synth. Met. 2019, 253, 122–130. [Google Scholar] [CrossRef]
- Liu, S.; Wu, G.; Chen, X.; Zhang, X.; Yu, J.; Liu, M.; Zhang, Y.; Wang, P. Degradation Behavior In Vitro of Carbon Nanotubes (CNTs)/Poly(lactic acid) (PLA) Composite Suture. Polymers 2019, 11, 1015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasheminejad, K.; Montazeri, A. Enhanced interfacial characteristics in PLA/graphene composites through numerically-designed interface treatment. Appl. Surf. Sci. 2020, 502, 144150. [Google Scholar] [CrossRef]
- Ahmad, A.F.; Ab Aziz, S.; Abbas, Z.; Obaiys, S.J.; Matori, K.A.; Mohd Zaid, M.H.; Raad, H.K.; Sa’ad Aliyu, U. Chemically Reduced Graphene Oxide-Reinforced Poly(Lactic Acid)/Poly(Ethylene Glycol) Nanocomposites: Preparation, Characterization, and Applications in Electromagnetic Interference Shielding. Polymers 2019, 11, 661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, R.; Ren, Z.; Jia, X.; Bi, H.; Yang, H.; Ji, T.; Xu, M.; Cai, M. Preparation and Characterization of 3D Printed PLA-Based Conductive Composites Using Carbonaceous Fillers by Masterbatch Melting Method. Polymers 2019, 11, 1589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lia, M.-Q.; Wua, J.-M.; Songa, F.; Lic, D.-D.; Wanga, X.-L.; Chena, L.; Wang, Y.-Z. Flexible and electro-induced shape memory Poly(Lactic Acid)-based material constructed by inserting a main-chain liquid crystalline and selective localization of carbon nanotubes. Compos. Sci. Technol. 2019, 173, 1–6. [Google Scholar] [CrossRef]
- Antoniac, I.; Popescu, D.; Zapciu, A.; Antoniac, A.; Miculescu, F.; Moldovan, H. Magnesium Filled Polylactic Acid (PLA) Material for Filament Based 3D Printing. Materials 2019, 12, 719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segura González, E.A.; Olmos, D.; Lorente, M.A.; Vélaz, I.; González-Benito, J. Preparation and Characterization of Polymer Composite Materials Based on PLA/TiO2 for Antibacterial Packaging. Polymers 2018, 10, 1365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, I.; Viswanathan, K.; Kasi, G.; Sadeghi, K.; Thanakkasaranee, S.; Seo, J. Poly(Lactic Acid)/ZnO Bionanocomposite Films with Positively Charged ZnO as Potential Antimicrobial Food Packaging Materials. Polymers 2019, 11, 1427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, X.; Kang, B.; Shi, S. Selective Localization of Carbon Black in Bio-Based Poly (Lactic Acid)/Recycled High-Density Polyethylene Co–Continuous Blends to Design Electrical Conductive Composites with a Low Percolation Threshold. Polymers 2019, 11, 1583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shanbhag, A.G. Utilization of information measure as a means of image thresholding. CVGIP Graph. Models Image Process. 1994, 56, 414–419. [Google Scholar] [CrossRef]
- Różycka, A.; Bogdanowicz, K.A.; Górska, N.; Rysz, J.; Marzec, M.; Iwan, A.; Pich, R.; Januszko, A. Influence of TiO2 nanoparticles on liquid crystalline, structural and electrochemical properties of (8z)-n-(4-((z)-(4-pentylphenylimino)methyl)benzylidene)-4-pentylbenzenamine. Materials 2019, 12, 1097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luckachan, G.E.; Pillai, C.K.S. Biodegradable polymers - a review on recent trends and emerging perspectives. J. Polym. Environ. 2011, 19, 637–676. [Google Scholar] [CrossRef]
- Dichtl, C.; Sippel, P.; Krohns, S. Dielectric properties of 3d printed polylactic acid. Adv. Mater. Sci. Eng. 2017, 2017, 10. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.; Zhou, X.; Wu, J.; Liao, Y.; Zhu, J.; Xie, X.; Zhou, H. Peanut-like crystals in polycarbonate/plasticizer blends. Macromol. Chem. Phys. 2017, 218, 1600471. [Google Scholar] [CrossRef]
- Beigmoradi, R.; Samimi, A.; Mohebbi-Kalhori, D. Engineering of oriented carbon nanotubes in composite materials. Beilstein J. Nanotechnol. 2018, 9, 415–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]




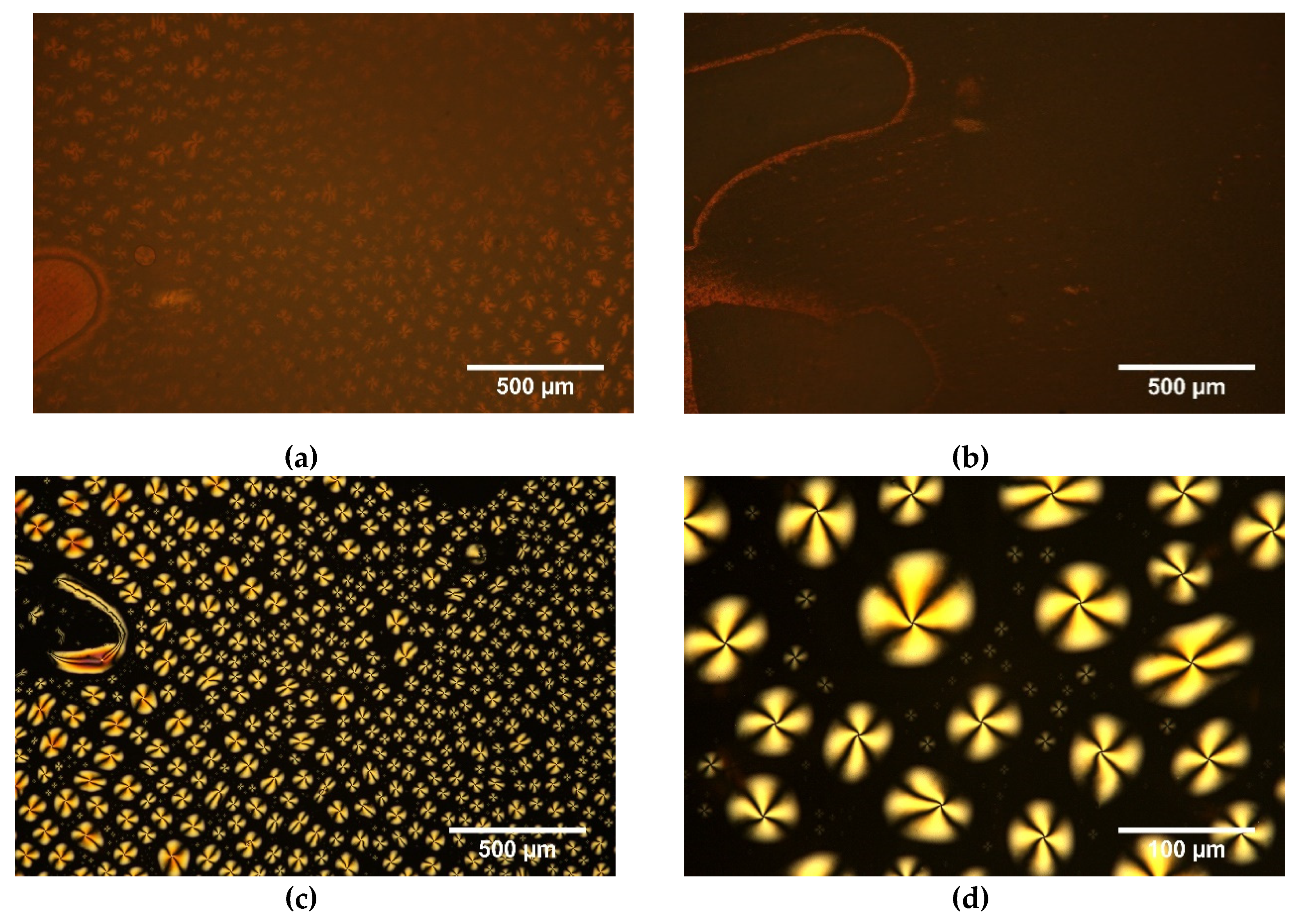
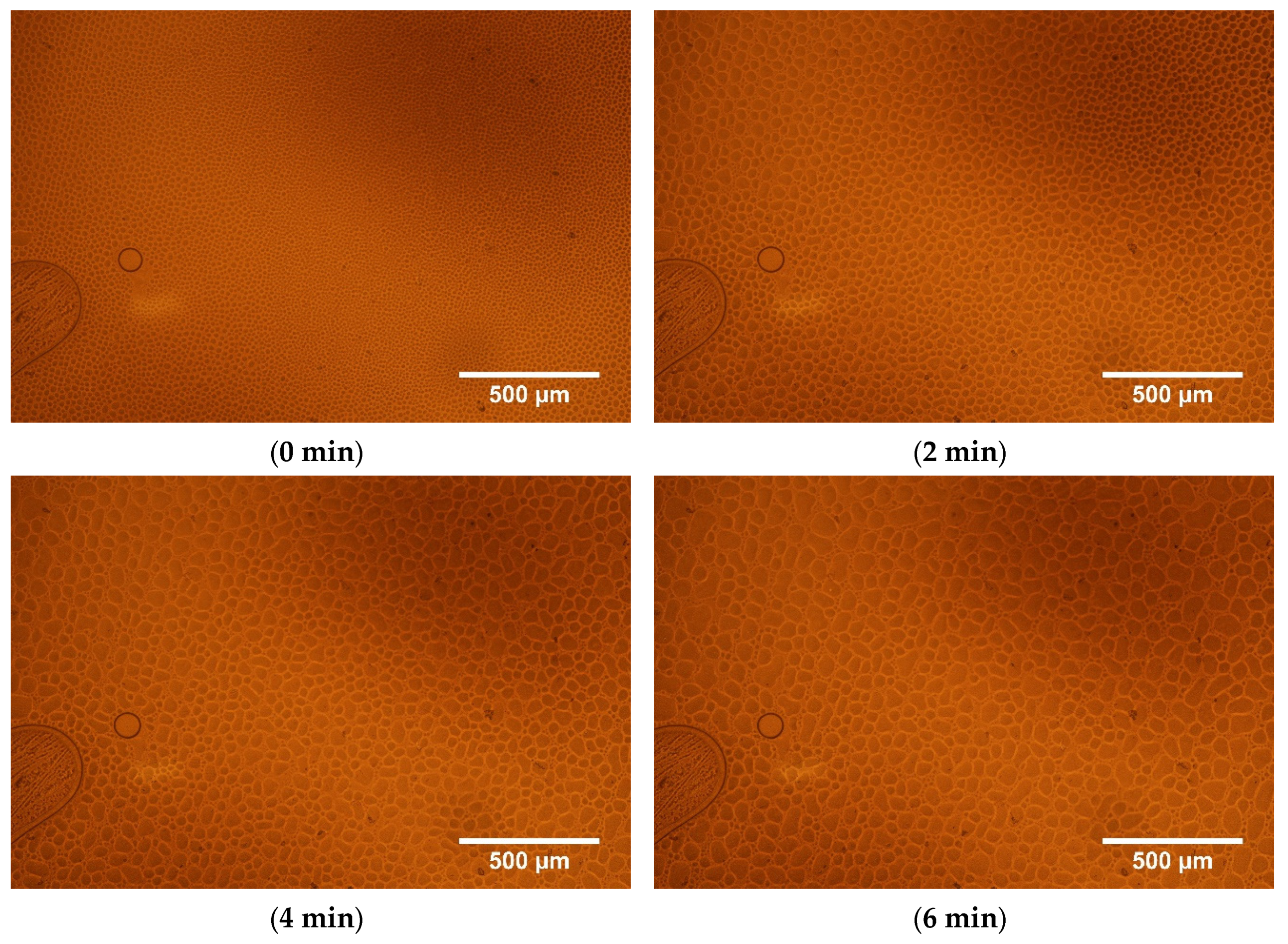
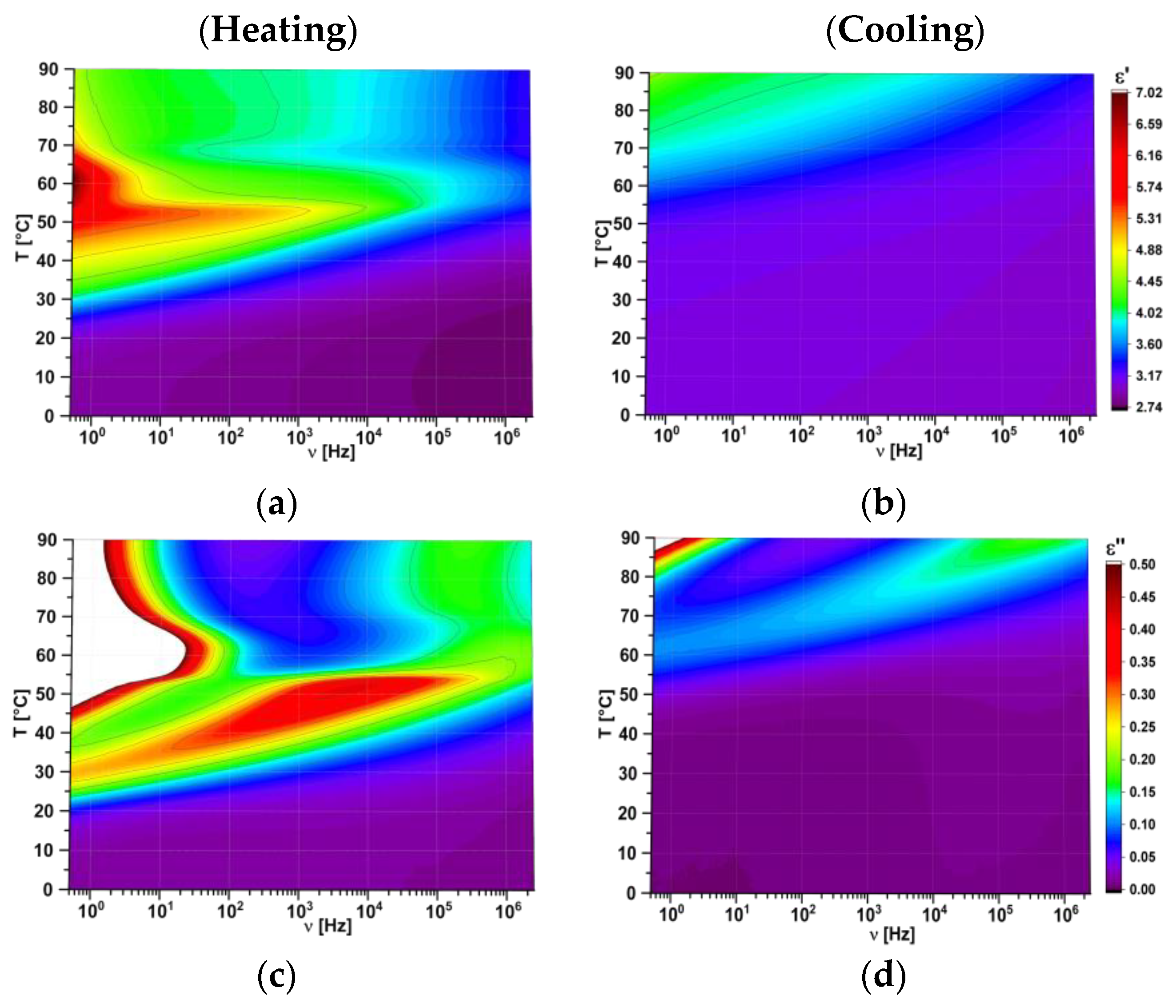
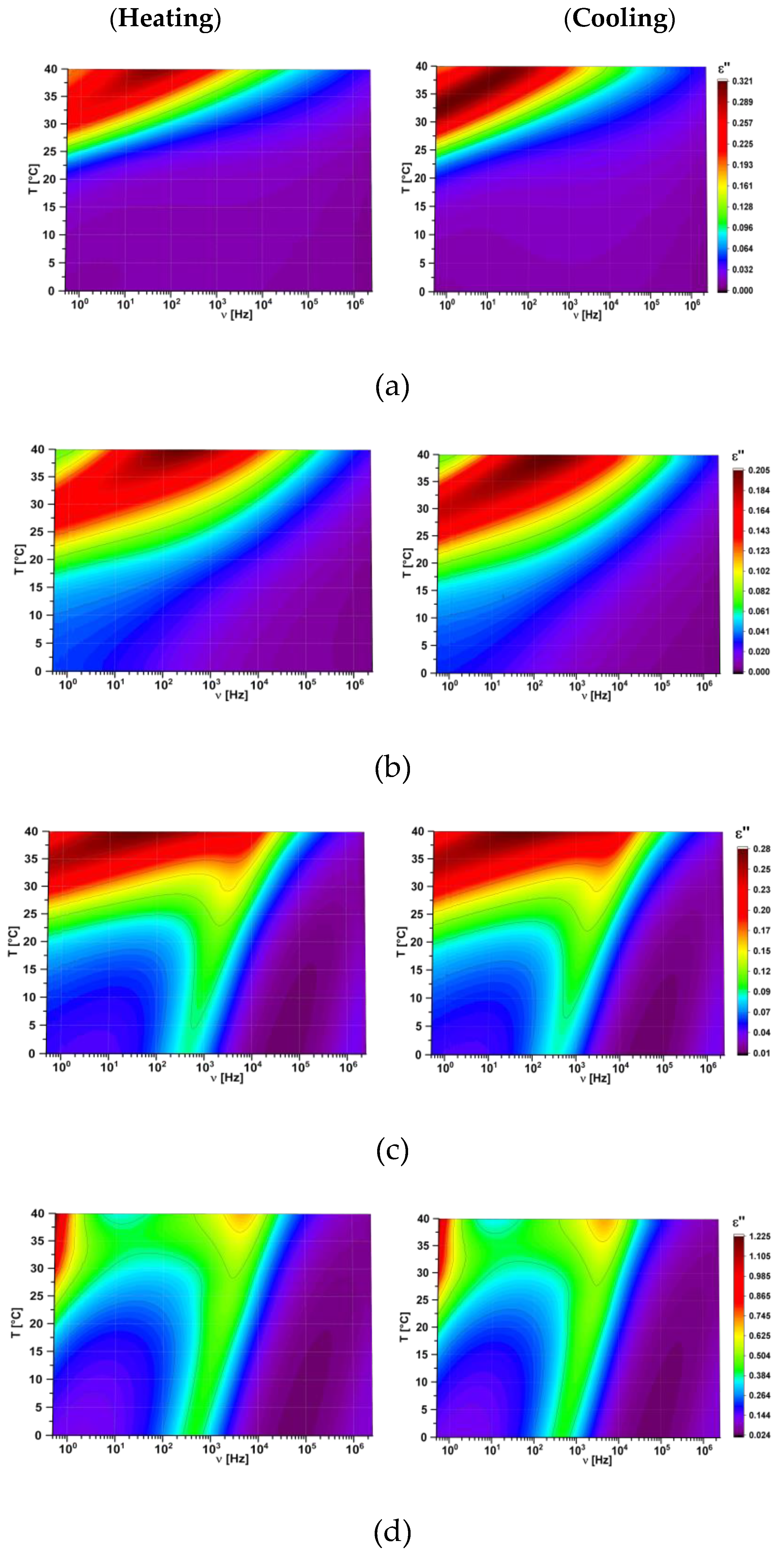

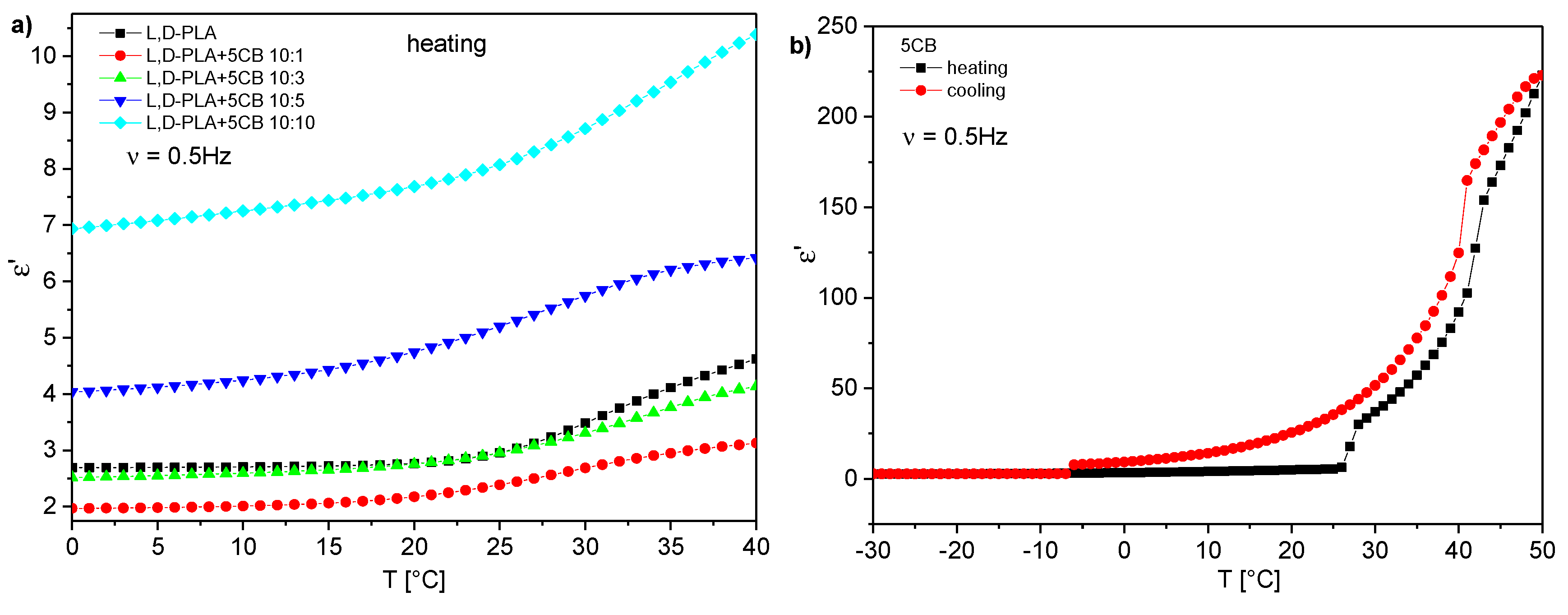



| Compositions [w/w/w] | Glass Transition [°C] | Transition to Liquid [°C] |
|---|---|---|
| l,d-PLA | 61 | 161 |
| l,d-PLA:5CB, 10:1 | 45 | 152 |
| l,d-PLA:5CB, 10:3 | 37 | 148 |
| l,d-PLA:5CB, 10:5 | 31 | 147 |
| l,d-PLA:5CB, 10:10 | 30 | 141 |
| l,d-PLA:5CB:SWCN (10:1:0.01) | 52 | 155 |
| l,d-PLA:5CB:SWCN (10:1:0.05) | 48 | 154 |
| l,d-PLA:5CB:SWCN (10:1:0.1) | 50 | 155 |
| l,d-PLA:5CB:SWCN (10:1:0.5) | 53 | 155 |
| Time [min] | Amount of Droplets | Total Area of Droplets [μm2] | Average Size [μm2] | Area Occupied by Droplets [%] | Circularity |
|---|---|---|---|---|---|
| 0 | 9372 | 1,784,464 | 190 | 55 | 0.74 |
| 1 | 7588 | 1,837,159 | 242 | 57 | 0.76 |
| 2 | 6653 | 1,997,143 | 300 | 62 | 0.76 |
| 4 | 5600 | 2,175,919 | 389 | 67 | 0.76 |
| 6 | 4980 | 2,243,426 | 450 | 69 | 0.76 |
| Composition (w/w) | Thickness (mm) | Cross-Section Area (mm2) | Length (mm) | Stress at Break (MPa) | Elongation at Break (%) |
|---|---|---|---|---|---|
| l,d-PLA | 0.080 ± 0.005 | 0.89 | 8.2 | 33.1 ± 3.9 | 84 ± 14 |
| l,d-PLA:5CB (10:1) | 0.089 ± 0.004 | 1.10 | 9.4 | 25.3 ± 3.3 | 272 ± 47 |
| l,d-PLA:5CB (10:3) | 0.048 ± 0.004 | 0.60 | 10.0 | 27.5 ± 2.6 | 138 ± 59 |
| l,d-PLA:5CB (10:5) | 0.057 ± 0.010 | 0.69 | 9.2 | 17.2 ± 3.3 | 251 ± 87 |
| l,d-PLA:5CB (10:10) | 0.072 ± 0.004 | 0.86 | 10.6 | 11.5 ± 1.3 | 57 ± 10 |
| l,d-PLA:5CB:SWCN (10:1:0.01) | 0.043 ± 0.002 | 0.50 | 9.7 | 44.5 ± 5.5 | 21 ± 4 |
| l,d-PLA:5CB:SWCN (10:1:0.05) | 0.047 ± 0.001 | 0.60 | 8.5 | 47.7 ± 3.3 | 16 ± 3 |
| l,d-PLA:5CB:SWCN (10:1:0.1) | 0.055 ± 0.002 | 0.70 | 9.7 | 47.6 ± 4.0 | 15 ± 4 |
| l,d-PLA:5CB:SWCN (10:1:0.5) | 0.050 ± 0.002 | 0.60 | 9.7 | 45.6 ± 5.6 | 12 ± 4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fryń, P.; Bogdanowicz, K.A.; Krysiak, P.; Marzec, M.; Iwan, A.; Januszko, A. Dielectric, Thermal and Mechanical Properties of l,d-Poly(Lactic Acid) Modified by 4′-Pentyl-4-Biphenylcarbonitrile and Single Walled Carbon Nanotube. Polymers 2019, 11, 1867. https://doi.org/10.3390/polym11111867
Fryń P, Bogdanowicz KA, Krysiak P, Marzec M, Iwan A, Januszko A. Dielectric, Thermal and Mechanical Properties of l,d-Poly(Lactic Acid) Modified by 4′-Pentyl-4-Biphenylcarbonitrile and Single Walled Carbon Nanotube. Polymers. 2019; 11(11):1867. https://doi.org/10.3390/polym11111867
Chicago/Turabian StyleFryń, Patryk, Krzysztof Artur Bogdanowicz, Piotr Krysiak, Monika Marzec, Agnieszka Iwan, and Adam Januszko. 2019. "Dielectric, Thermal and Mechanical Properties of l,d-Poly(Lactic Acid) Modified by 4′-Pentyl-4-Biphenylcarbonitrile and Single Walled Carbon Nanotube" Polymers 11, no. 11: 1867. https://doi.org/10.3390/polym11111867
APA StyleFryń, P., Bogdanowicz, K. A., Krysiak, P., Marzec, M., Iwan, A., & Januszko, A. (2019). Dielectric, Thermal and Mechanical Properties of l,d-Poly(Lactic Acid) Modified by 4′-Pentyl-4-Biphenylcarbonitrile and Single Walled Carbon Nanotube. Polymers, 11(11), 1867. https://doi.org/10.3390/polym11111867







