A Combination of Aqueous Extraction and Polymeric Membranes as a Sustainable Process for the Recovery of Polyphenols from Olive Mill Solid Wastes
Abstract
:1. Introduction
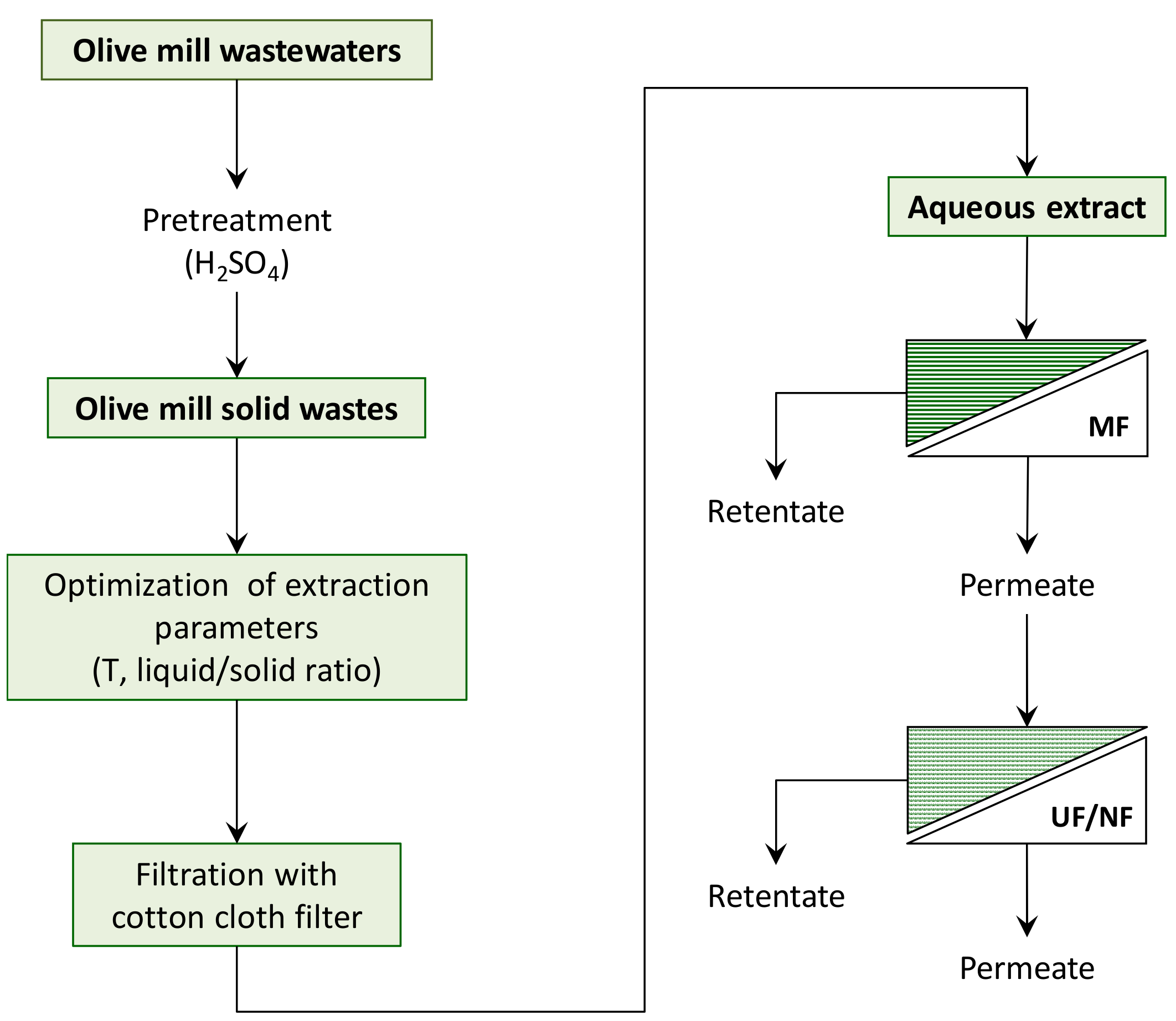
2. Materials and Methods
2.1. Materials
2.2. Extraction of Phenolic Compounds from Olive Mill Solid Wastes
2.3. MF Pre-treatment of OMSW Extracts: Equipment and Procedures
2.4. Treatment of Clarified OMSW Extracts with UF and NF Membranes: Equipment and Procedures
2.5. Measurement of Hydraulic Permeability and Membrane Cleaning
2.6. Analytical Methods
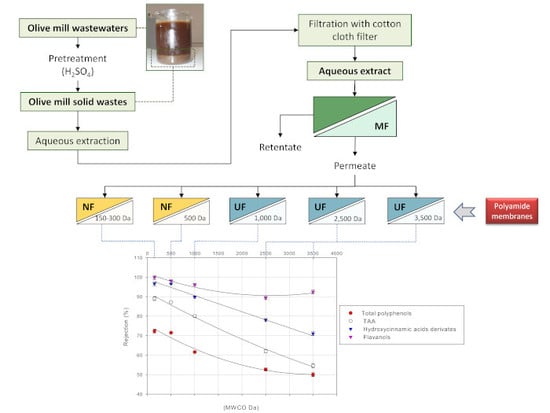
3. Results and Discussion
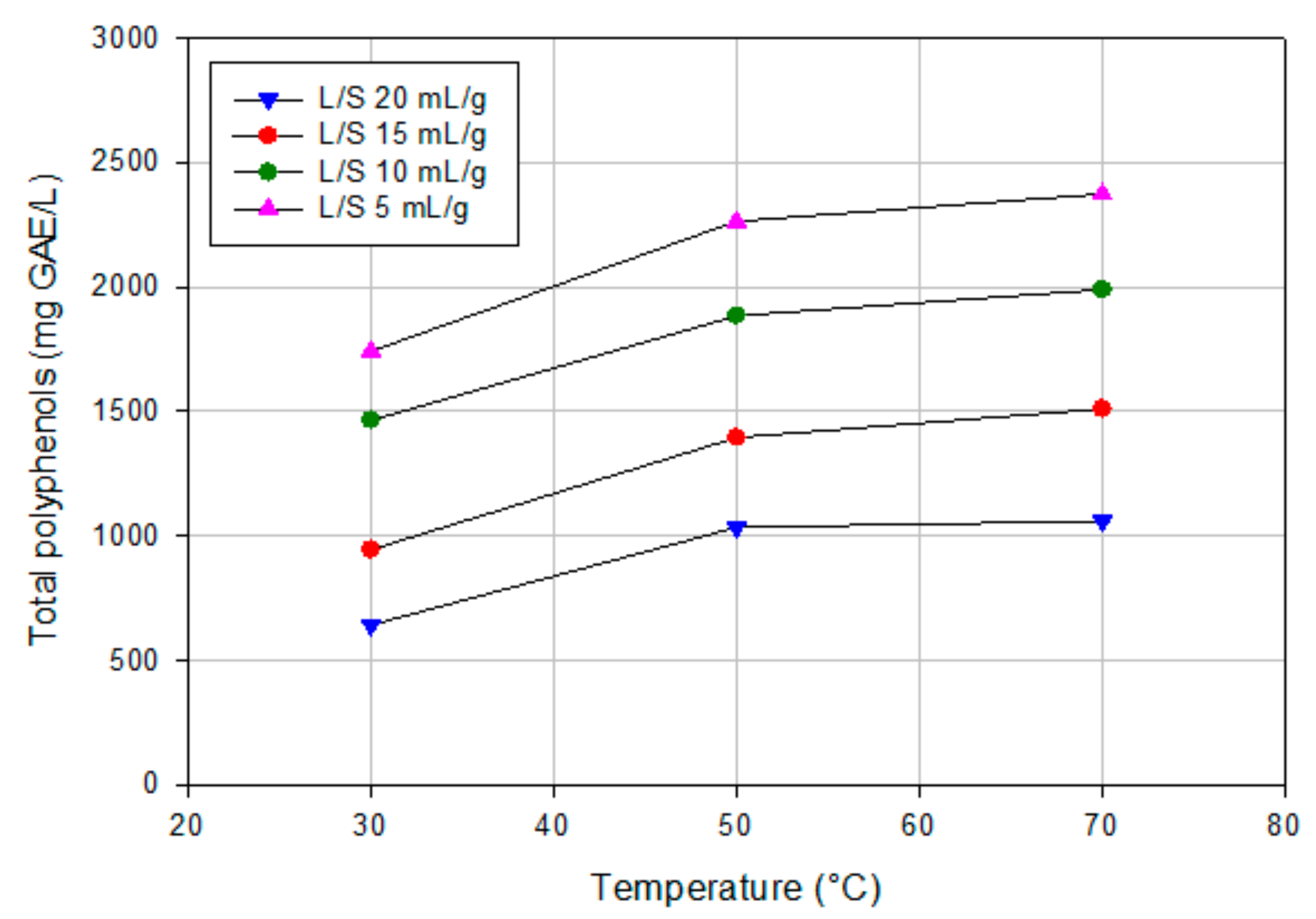
3.1. Aqueous Extraction of OMSWs
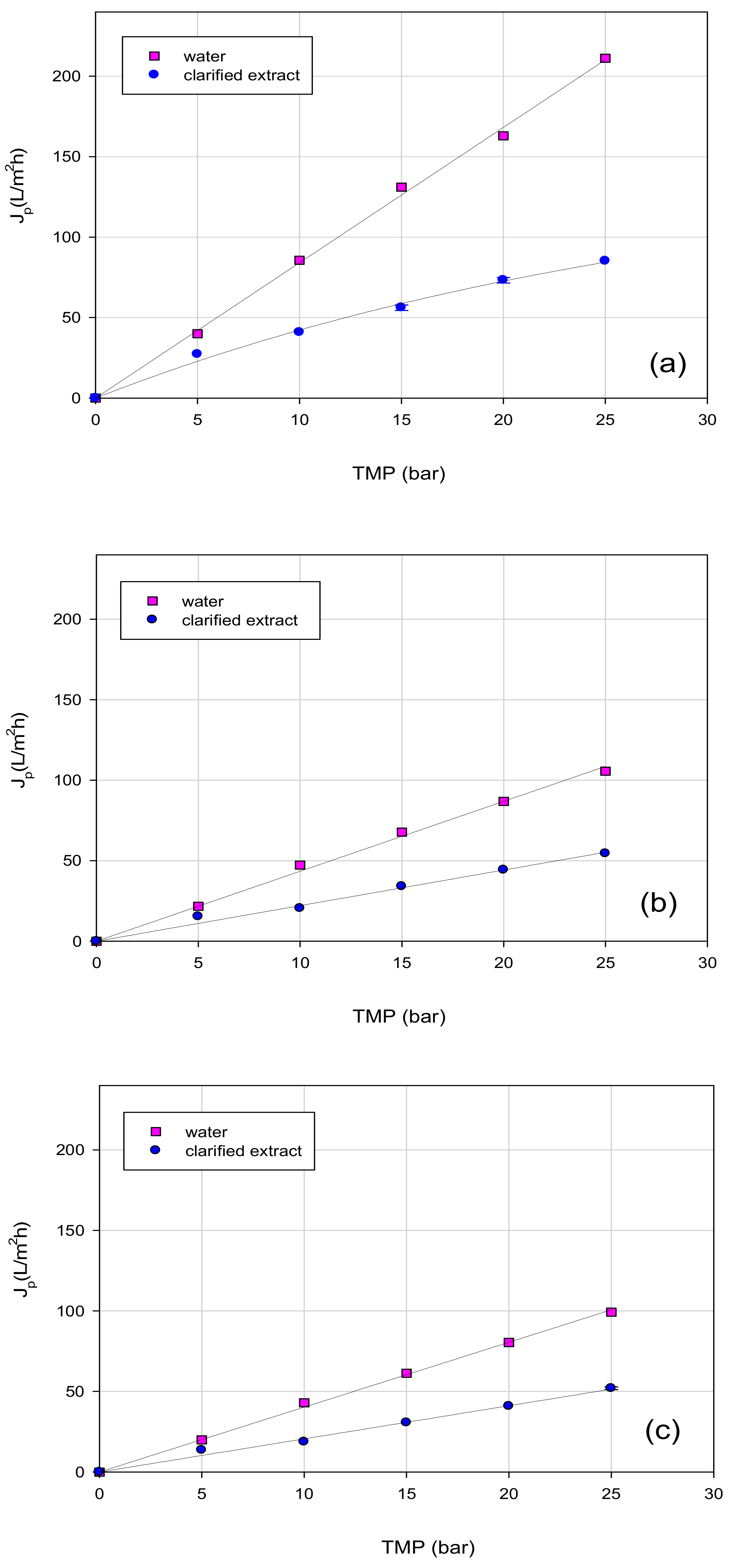
3.2. Microfiltration of OMSW Aqueous Extract
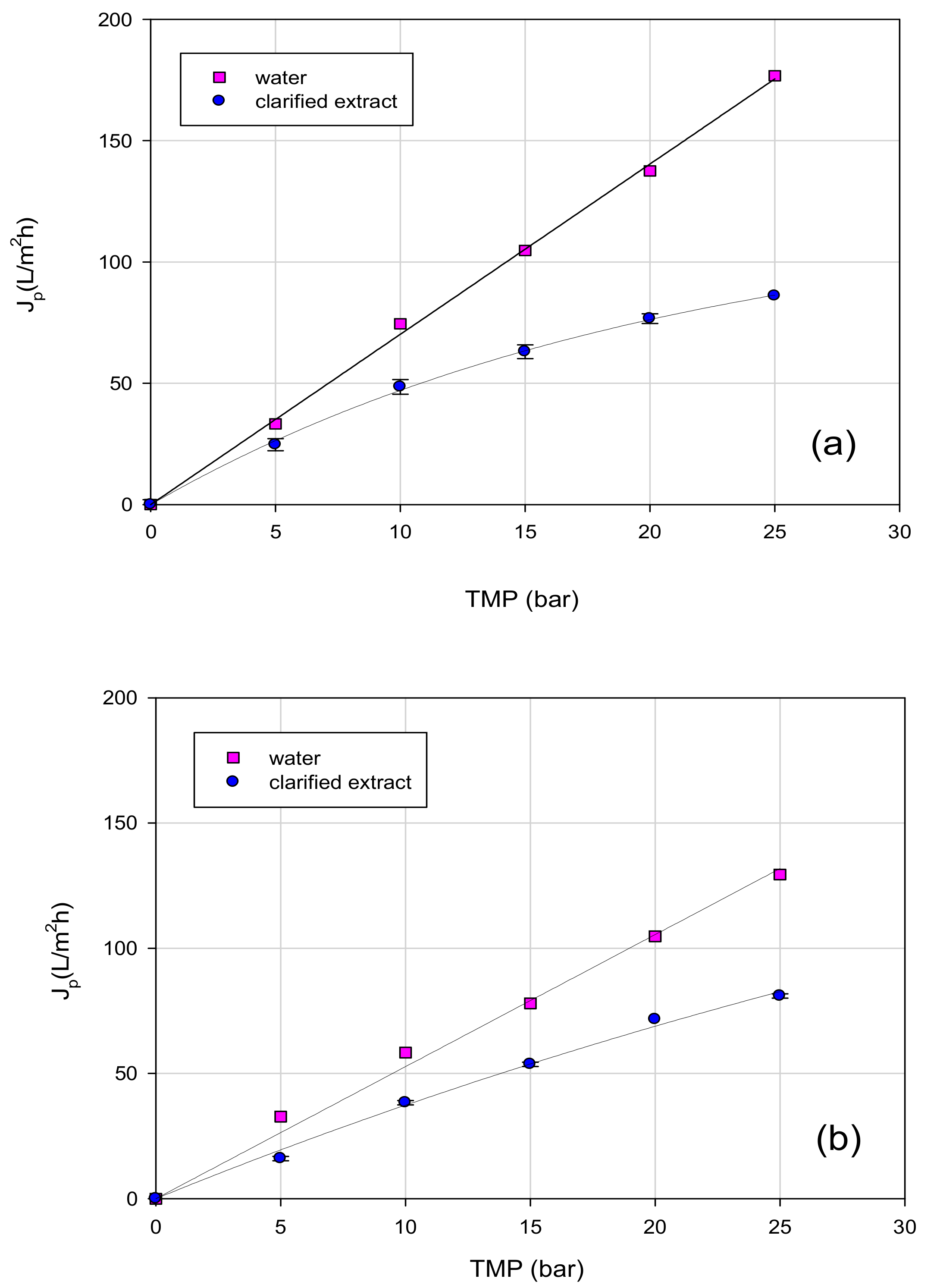
3.3. Treatment of Aqueous Extract with UF and NF Membranes: Effect of TMP on Permeate Flux
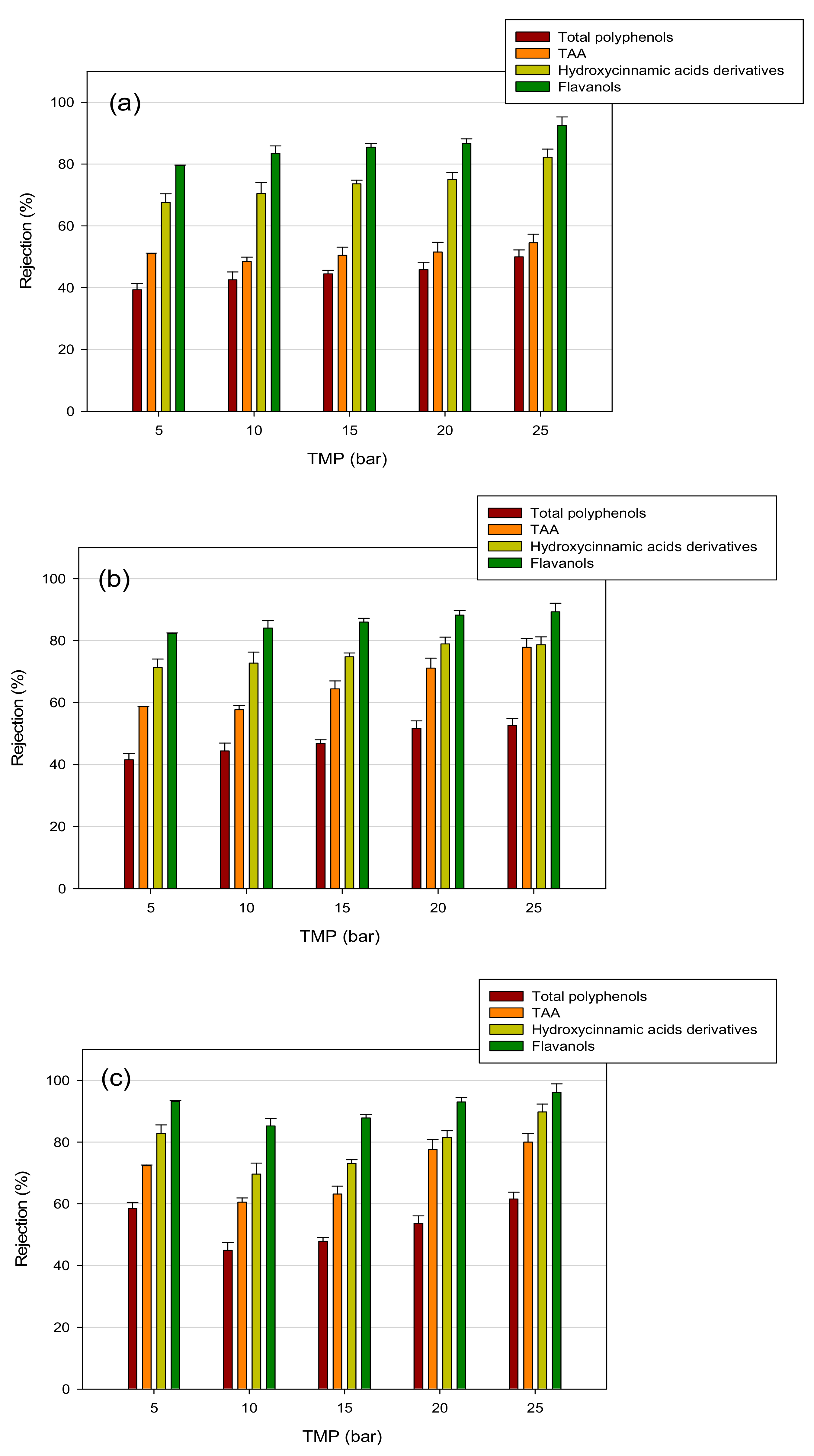
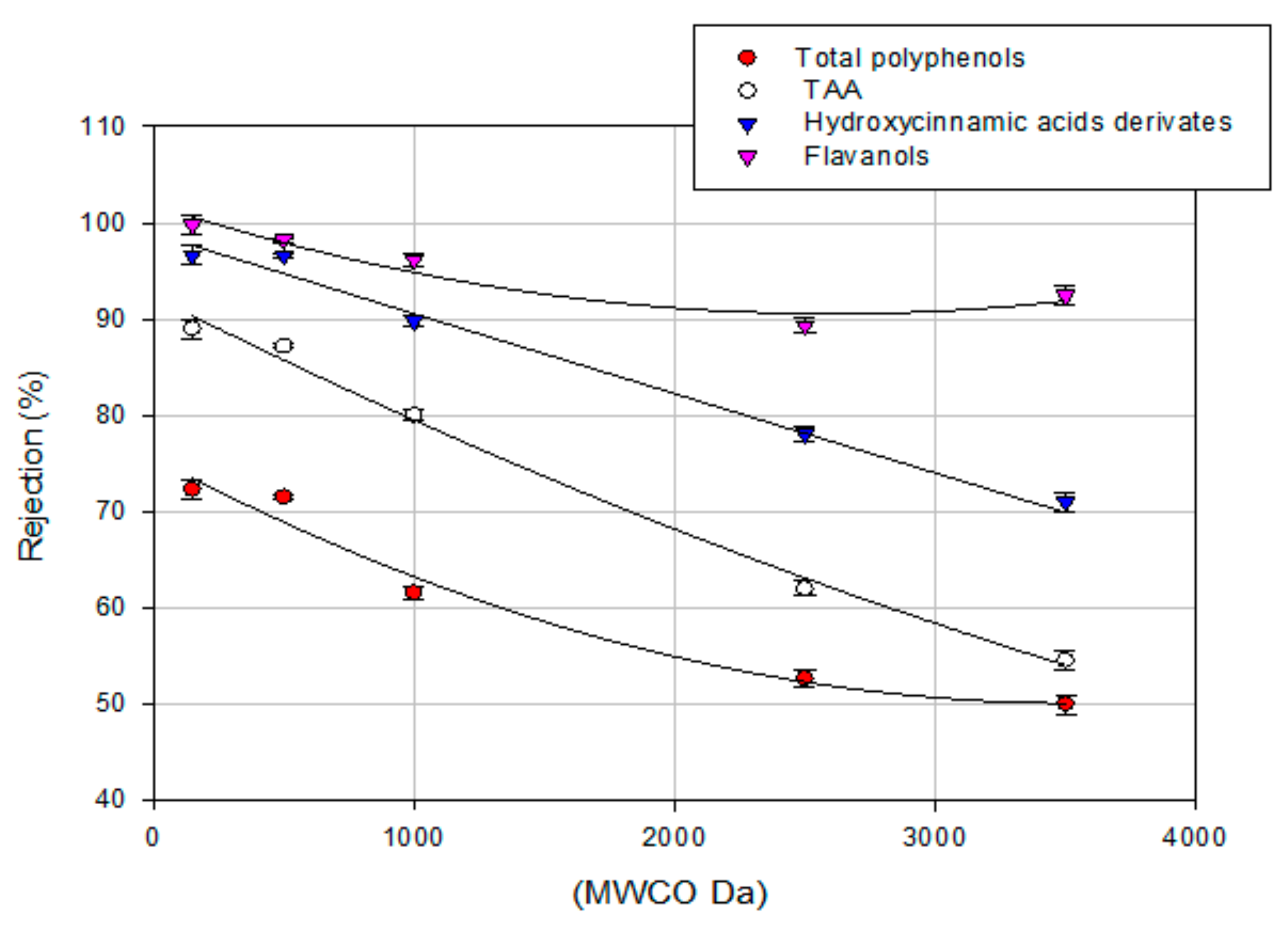
3.4. Treatment of Aqueous Extract with UF and NF Membranes: Effect of TMP on Solute Rejection
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- FAOSTAT. 2014. Available online: http://www.fao.org/faostat/en/#data/QD/visualize (accessed on 10 October 2019).
- Aliakbarian, B.; Casazza, A.A.; Perego, P. Valorization of olive oil solid waste using high pressure-high temperature reactor. Food Chem. 2011, 128, 704–710. [Google Scholar] [CrossRef]
- DellaGreca, M.; Monaco, P.; Pinto, G.; Pollio, A.; Previtera, L.; Temussi, F. Phytotoxicity of low-molecular-weight phenols from olive mill wastewaters. Bull. Environ. Contam. Toxicol. 2001, 67, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Dermeche, S.; Nadour, M.; Larroche, C.; Moulti-Mati, F.; Michaud, P. Olive mill wastes: Biochemical characterizations and valorization strategies. Process Biochem. 2013, 48, 1532–1552. [Google Scholar] [CrossRef]
- Rozzi, A.; Malpei, F. Treatment and disposal of olive mill effluents. Int. Biodeterior. Biodegrad. 1996, 38, 135–144. [Google Scholar] [CrossRef]
- Paraskeva, C.A.; Papadakis, V.G.; Tsrouchi, E.; Kanellopoulou, D.G.; Koutsoukos, P.G. Membrane processing for olive mill wastewater fractionation. Desalination 2007, 213, 218–229. [Google Scholar] [CrossRef]
- Kalogerakis, N.; Politi, M.; Foteinis, S.; Chatzisymeon, E.; Mantzavinos, D. Recovery of antioxidants from olive mill wastewaters: A viable solution that promotes their overall sustainable management. J. Environ. Manag. 2013, 128, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Obied, H.K.; Allen, M.S.; Bedgood, D.R.; Prenzler, P.D.; Robards, K.; Stockmann, R. Bioactivity and analysis of biophenols recovered from olive mill waste. J. Agric. Food Chem. 2005, 53, 823–827. [Google Scholar] [CrossRef] [PubMed]
- Araújo, M.; Pimentel, F.B.; Alves, R.C.; Oliveira, M.B.P.P. Phenolic compounds from olive mill wastes: Health effects, analytical approach and application as food antioxidants. Trends Food Sci. Technol. 2015, 45, 200–211. [Google Scholar] [CrossRef]
- Delisi, R.; Ciriminna, R.; Arvati, S.; Meneguzzo, F.; Pagliaro, M. Olive biophenol integral extraction at a two-phase olive mill. J. Clean Prod. 2018, 174, 1487–1491. [Google Scholar] [CrossRef]
- Ciriminna, R.; Meneguzzo, F.; Delisi, R.; Pagliaro, M. Olive biophenols as new antioxidant additives in food and beverage. ChemistrySelect 2017, 2, 1360–1365. [Google Scholar] [CrossRef]
- Nunes, M.A.; Pimentel, F.B.; Costa, A.S.G.; Alves, R.C.; Oliveira, M.B.P.P. Olive by-products for functional and food applications: Challenging opportunities to face environmental constraints. Innov. Food Sci. Emerg. Technol. 2016, 35, 139–148. [Google Scholar] [CrossRef]
- Galanakis, C. Recovery of high added-value components from food wastes: Conventional, emerging technologies and commercialized applications. Trends Food Sci. Technol. 2012, 26, 68–87. [Google Scholar] [CrossRef]
- Soquetta, B.M.; de Marsillac Terra, L.; Peixoto Bastos, C. Green technologies for the extraction of bioactive compounds in fruits and vegetables. CyTA J. Food 2018, 16, 400–412. [Google Scholar] [CrossRef]
- García-Castello, E.; Cassano, A.; Criscuoli, A.; Drioli, E. Recovery and concentration of polyphenols from olive mil wastewaters by integrated membrane system. Water Res. 2010, 44, 3883–3892. [Google Scholar] [CrossRef] [PubMed]
- Russo, C. A new membrane process for the selective fractionation and total recovery of polyphenols, water and organic substances from vegetation waters (VW). J. Membr. Sci. 2007, 288, 239–246. [Google Scholar] [CrossRef]
- Cassano, A.; Conidi, C.; Giorno, L.; Drioli, E. Fractionation of olive mill wastewaters by membrane separation techniques. J. Hazard. Mater. 2013, 248, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Khemakhem, I.; Gargouri, O.D.; Dhouib, A.; Ayadi, M.A.; Bouaziz, M. Oleuropein rich extract from olive leaves by combining microfiltration, ultrafiltration and nanofiltration. Sep. Purif. Technol. 2017, 172, 310–317. [Google Scholar] [CrossRef]
- Nunes, M.A.; Pawlowski, S.; Costa, A.S.G.; Alves, R.C.; Oliveira, M.B.P.P.; Velizarov, S. Valorization of olive pomace by a green integrated approach applying sustainable extraction and membrane-assisted concentration. Sci. Total Environ. 2019, 652, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Sygouni, V.; Pantziaros, A.G.; Iakovides, I.C.; Sfetsa, E.; Bogdou, P.I.; Christoforou, E.A.; Paraskeva, C.A. Treatment of two-phase olive mill wastewater and recovery of phenolic compounds using membrane technology. Membranes 2019, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Sousa, L.S.; Cabral, B.V.; Madrona, G.S.; Cardoso, V.L.; Reis, M.H.M. Purification of polyphenols from green tea leaves by ultrasound assisted ultrafiltration process. Sep. Purif. Technol. 2016, 168, 188–198. [Google Scholar] [CrossRef]
- Acero, J.L.; Benitez, F.J.; Leal, A.I.; Real, F.J.; Teva, F. Membrane filtration technologies applied to municipal secondary effluents for potential reuse. J. Hazard. Mater. 2010, 177, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Córdova, A.; Astudillo, C.; Giorno, L.; Guerrero, C.; Conidi, C.; Illanes, A.; Cassano, A. Nanofiltration potential for the purification of highly concentrated enzymatically produced oligosaccharides. Food Bioprod. Process. 2016, 98, 50–61. [Google Scholar] [CrossRef]
- Strathmann, H.; Giorno, L.; Drioli, E. An Introduction to Membrane Science and Technology; Consiglio Nazionale delle Ricerche: Rome, Italy, 2006; pp. 19–31. [Google Scholar]
- Mänttäri, M.; Nyström, M. Membrane filtration for tertiary treatment of biologically treated effluents from the pulp and paper industry. Water Sci. Technol. 2007, 55, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Obied, H.K.; Allen, H.S.; Bedgood, D.R.; Prenzler, P.; Robards, K. Investigation of Australian olive mill waste for recovery of biophenols. J. Agric. Food Chem. 2005, 53, 9911–9920. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical catión decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1337. [Google Scholar] [CrossRef]
- Wissam, Z.; Ghada, B.; Wassim, A.; Warid, K. Effective extraction of polyphenols and proanthocyanidins from pomegranate’s peel. Int. J. Pharm. Pharm. Sci. 2012, 4, 675–682. [Google Scholar]
- Piwowarska, N.; González-Alvarez, J. Extraction of antioxidants from forestry biomass: Kinetics and optimization of extraction conditions. Biomass Bioenergy 2012, 43, 42–51. [Google Scholar] [CrossRef]
- Gironi, F.; Piemonte, V. Temperature and solvent effects on polyphenol extraction process from chestnut tree wood. Chem. Eng. Res. Des. 2011, 89, 857–862. [Google Scholar] [CrossRef]
- Alu’datt, M.H.; Alli, I.; Ereifej, K.; Alhamad, M.; Al-Tawaha, A.R.; Rababah, T. Optimization, characterization and quantification of phenolic compounds in olive cake. Food Chem. 2010, 123, 117–122. [Google Scholar] [CrossRef]
- Vuong, Q.V.; Golding, J.B.; Stathopoulos, C.E.; Nguyen, M.H.; Roach, P.D. Optimizing conditions for the extraction of catechins from green tea using hot water. J. Sep. Sci. 2011, 34, 3099–3106. [Google Scholar] [CrossRef] [PubMed]
- Rajha, H.N.; El Darra, N.; Hobaika, Z.; Boussetta, N.; Vorobiev, E.; Maroun, R.G.; Louka, N. Extraction of total phenolic compounds, flavonoids, anthocyanins and tannins from grape byproducts by response surface methodology. Influence of solid-liquid ratio, particle size, time, temperature and solvent mixtures on the optimization process. Food Nutr. Sci. 2014, 5, 397–409. [Google Scholar] [CrossRef]
- Stamatopoulos, K.; Chatzilazarou, A.; Katsoyannos, E. Optimization of multistage extraction of olive leaves for recovery of phenolic compounds at moderated temperatures and short extraction times. Foods 2014, 3, 66–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maciel Bindes, M.M.; Cardoso, V.L.; Miranda Reis, M.H.; Boffito, D.C. Maximisation of the polyphenols extraction yield from green tea leaves and sequential clarification. J. Food Eng. 2019, 241, 97–104. [Google Scholar] [CrossRef]
- Cassano, A.; Donato, L.; Conidi, C.; Drioli, E. Recovery of bioactive compounds in kiwifruit juice by ultrafiltration. Innov. Food Sci. Emerg. Technol. 2008, 9, 556–562. [Google Scholar] [CrossRef]
- Mondal, S.; Majumdar, G.C.; De, S. Clarifications of stevia extract using cross flow ultrafiltration and concentration by nanofiltration. Sep. Purif. Technol. 2012, 89, 125–134. [Google Scholar]
- Suárez, M.; Romero, M.P.; Ramo, T.; Macià, A.; Motilva, M.J. Methods for preparing phenolic extracts from olive cake for potential application as food antioxidants. J. Agric. Food Chem. 2009, 57, 1463–1472. [Google Scholar] [CrossRef] [PubMed]
- Cassano, A.; Conidi, C.; Drioli, E. Comparison of the performance of UF membranes in olive mill wastewater treatment. Water Res. 2011, 45, 3197–3204. [Google Scholar] [CrossRef] [PubMed]
- Galanakis, C.M.; Tornberg, E.; Gekas, V. Clarification of high-added value products from olive mill wastewater. J. Food Eng. 2010, 99, 190–197. [Google Scholar] [CrossRef]
- Díaz-Reinoso, B.; Moure, A.; Domínguez, H.; Parajό, C.J. Ultra-and nanofiltration of aqueous extracts from distilled fermented grape pomace. J. Food Eng. 2009, 91, 587–593. [Google Scholar] [CrossRef]
- Xu, L.; Wang, S.Y. The Ginkgo biloba extract concentrated by nanofiltration. Desalination 2005, 184, 305–313. [Google Scholar] [CrossRef]
- Giacobbo, A.; Bernardes, A.M.; de Pinho, M.N. Sequential pressure-driven membrane operations to recover and fractionate polyphenols and polysaccharides from second racking wine lees. Sep. Purif. Technol. 2017, 173, 49–54. [Google Scholar] [CrossRef]
- Pino, L.; Vargas, C.; Schwarz, A.; Borquez, R. Influence of operating conditions on the removal of metals and sulfate from copper acid mine drainage by nanofiltration. Chem. Eng. J. 2018, 345, 114–125. [Google Scholar] [CrossRef]
- Vegas, R.; Moure, A.; Domínguez, H.; Parajό, J.C.; Alvarez, R.J.; Luque, S. Evaluation of ultra- and nanofiltration for refining soluble products from rice husk xylan. Bioresour. Technol. 2008, 99, 5341–5351. [Google Scholar] [CrossRef] [PubMed]
- Conidi, C.; Cassano, A.; Drioli, E. A membrane-based study for the recovery of polyphenols from bergamot juice. J. Membr. Sci. 2011, 375, 182–190. [Google Scholar] [CrossRef]
- Acosta, O.; Vaillant, F.; Pérez, A.M.; Dornier, M. Potential of ultrafiltration for separation and purification of ellagitannins in blackberry (Rubus adenotrichus Schltdl.) juice. Sep. Purif. Technol. 2014, 125, 120–125. [Google Scholar] [CrossRef]
- Díaz-Reinoso, B.; Moure, A.; González, J.; Domínguez, H. A membrane process for the recovery of a concentrated phenolic product from white vinasses. Chem. Eng. J. 2017, 327, 210–217. [Google Scholar] [CrossRef]
- Cissé, M.; Vaillant, F.; Pallet, D.; Dornier, M. Selecting ultrafiltration and nanofiltration membranes to concentrate anthocyanins from roselle extract (Hibiscus sabdariffa L.). Food Res. Int. 2011, 44, 2607–2614. [Google Scholar] [CrossRef]
- Bellona, C.; Drewes, J.E.; Xu, P.; Amy, G. Factors affecting the rejection of organic solutes during NF/RO treatment—A literature review. Water Res. 2004, 38, 2795–2809. [Google Scholar] [CrossRef] [PubMed]
- Rodis, P.S.; Karathanos, V.T.; Mantzavinou, A. Partitioning of olive oil antioxidants between oil and water phases. J. Agric. Food Chem. 2012, 50, 596–601. [Google Scholar] [CrossRef] [PubMed]


| Membrane Type | GK | GH | GE | NFA-12A | DK |
|---|---|---|---|---|---|
| Manufacturer | GE Osmonics | GE Osmonics | GE Osmonics | Parker | GE Osmonics |
| Membrane material | PA-TFC | PA-TFC | PA-TFC | PA-TFC | PA-TFC |
| Configuration | flat-sheet | flat-sheet | flat-sheet | flat-sheet | flat-sheet |
| Nominal MWCO (Da) | 3500 | 2500 | 1000 | 500 | 150-300 |
| pH operating range | 2–10 | 2–10 | 2–10 | 3–11 | 3–9 |
| Max. operating temperature (°C) | 50 | 50 | 50 | 63 | 50 |
| Max. operating pressure (bar) | 27.6 | 27.6 | 27.6 | 30.6 | 41 |
| Membrane surface area (m2) | 0.0035 | 0.0035 | 0.0035 | 0.0035 | 0.0035 |
| Contact angle (°) | <61 a | <61 a | 50 b | 10 b | 41 a |
| Parameters | Feed | Permeate | Retentate |
|---|---|---|---|
| Total suspended solids (%) | 5.4 ± 0.2 | n.d. | 8.6 ± 0.62 |
| Total polyphenols (mg GAE/L) | 1812.4 ± 12.6 | 1672.2 ± 22.6 | 2046.0 ± 18.6 |
| Flavanols (mg/L quercetin) | 190.4 ± 14.8 | 180.2 ± 11.3 | 205.1 ± 12.9 |
| Hydroxycinnamic acid derivatives (mg/L caffeic acid) | 180.2 ± 4.6 | 168.1 ± 10.4 | 210.2 ± 3.6 |
| TAA (mM Trolox) | 11.2 ± 1.4 | 11.0 ± 0.6 | 12.3 ± 1.2 |
| Membrane Type | |||||
|---|---|---|---|---|---|
| GK | GH | GE | NFA-12A | DK | |
| Lp0 (L/m2hbar) | 10.85 | 4.66 | 4.21 | 9.97 | 5.44 |
| Lp1 (L/m2hbar) | 4.76 | 2.61 | 2.73 | 7.02 | 4.76 |
| Lp2 (L/m2hbar) | 8.19 | 4.34 | 3.52 | 9.97 | 5.27 |
| Fouling index (%) | 46.2 | 44.1 | 35.2 | 29.6 | 12.5 |
| Flux recovery (%) | 92.5 | 93.1 | 83.6 | 100 | 96.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conidi, C.; Egea-Corbacho, A.; Cassano, A. A Combination of Aqueous Extraction and Polymeric Membranes as a Sustainable Process for the Recovery of Polyphenols from Olive Mill Solid Wastes. Polymers 2019, 11, 1868. https://doi.org/10.3390/polym11111868
Conidi C, Egea-Corbacho A, Cassano A. A Combination of Aqueous Extraction and Polymeric Membranes as a Sustainable Process for the Recovery of Polyphenols from Olive Mill Solid Wastes. Polymers. 2019; 11(11):1868. https://doi.org/10.3390/polym11111868
Chicago/Turabian StyleConidi, Carmela, Agata Egea-Corbacho, and Alfredo Cassano. 2019. "A Combination of Aqueous Extraction and Polymeric Membranes as a Sustainable Process for the Recovery of Polyphenols from Olive Mill Solid Wastes" Polymers 11, no. 11: 1868. https://doi.org/10.3390/polym11111868
APA StyleConidi, C., Egea-Corbacho, A., & Cassano, A. (2019). A Combination of Aqueous Extraction and Polymeric Membranes as a Sustainable Process for the Recovery of Polyphenols from Olive Mill Solid Wastes. Polymers, 11(11), 1868. https://doi.org/10.3390/polym11111868