The Use of Chitosan, Alginate, and Pectin in the Biomedical and Food Sector—Biocompatibility, Bioadhesiveness, and Biodegradability
Abstract
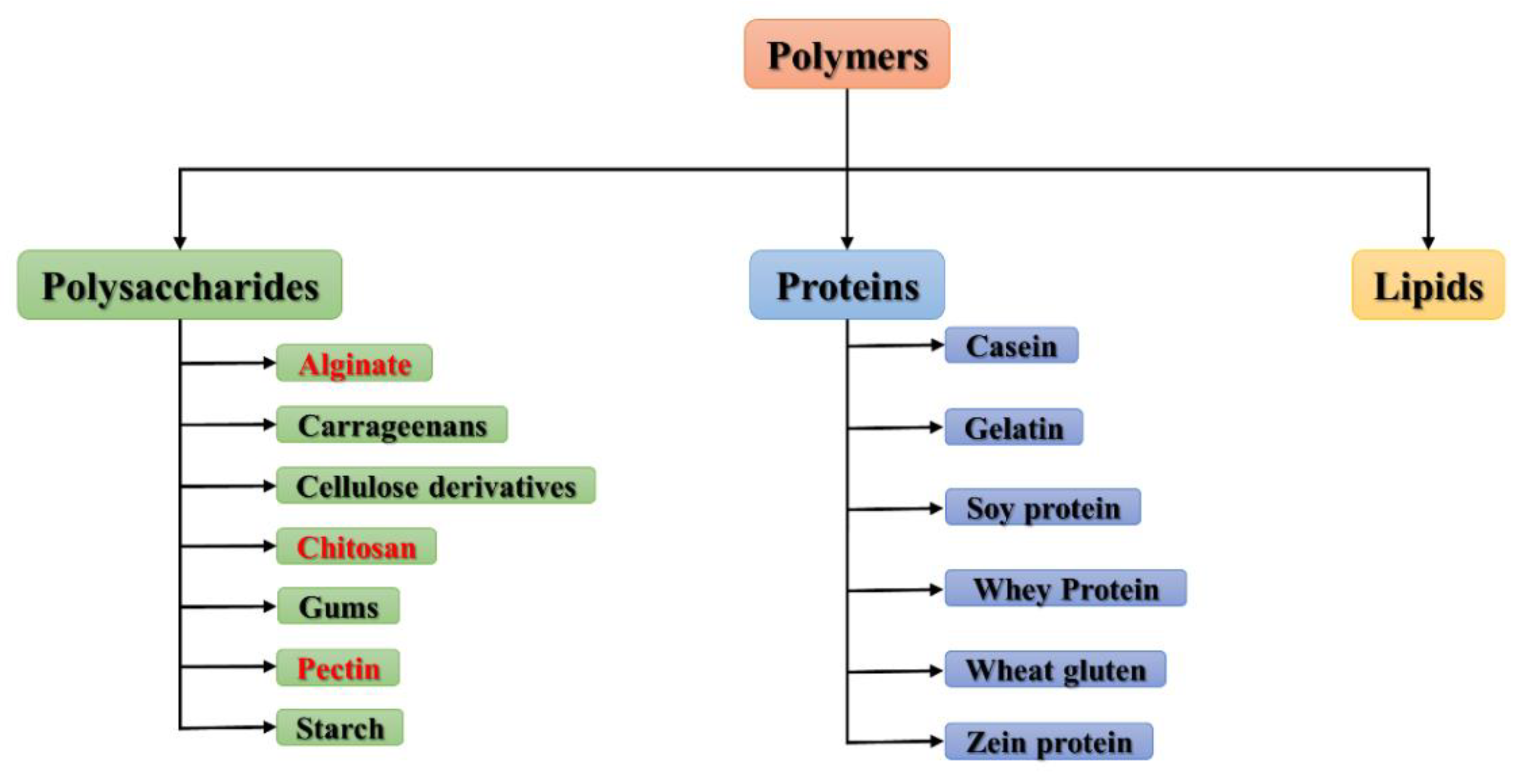
:1. Introduction
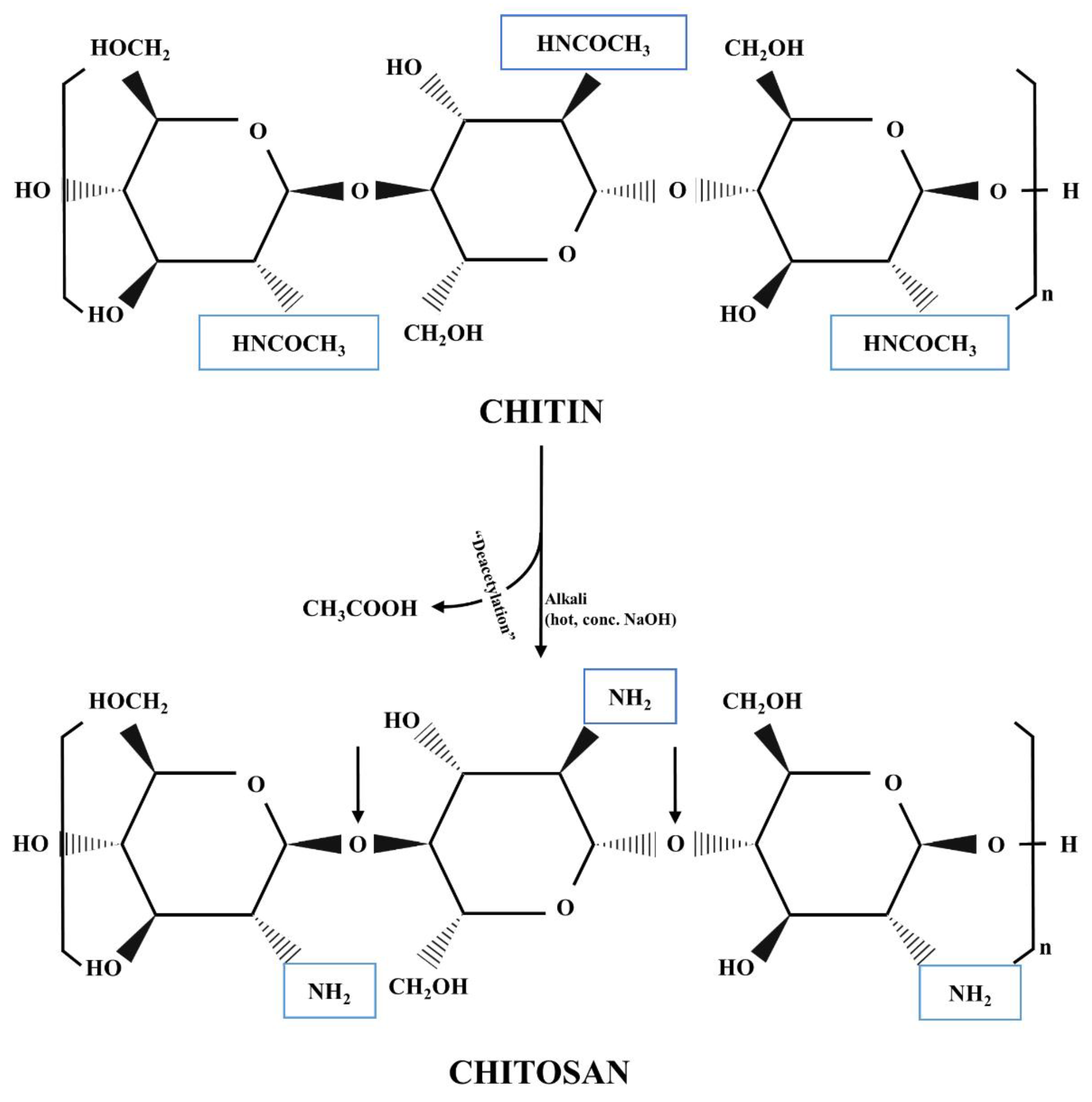
2. Chitosan
2.1. History, Structure, and Sources
2.2. Properties and Applications of Chitosan
2.2.1. Biocompatibility and Biodegradability
2.2.2. Bioadhesiveness
2.2.3. Chitosan Absorption
2.2.4. pH Sensitiveness
2.3. Limitations
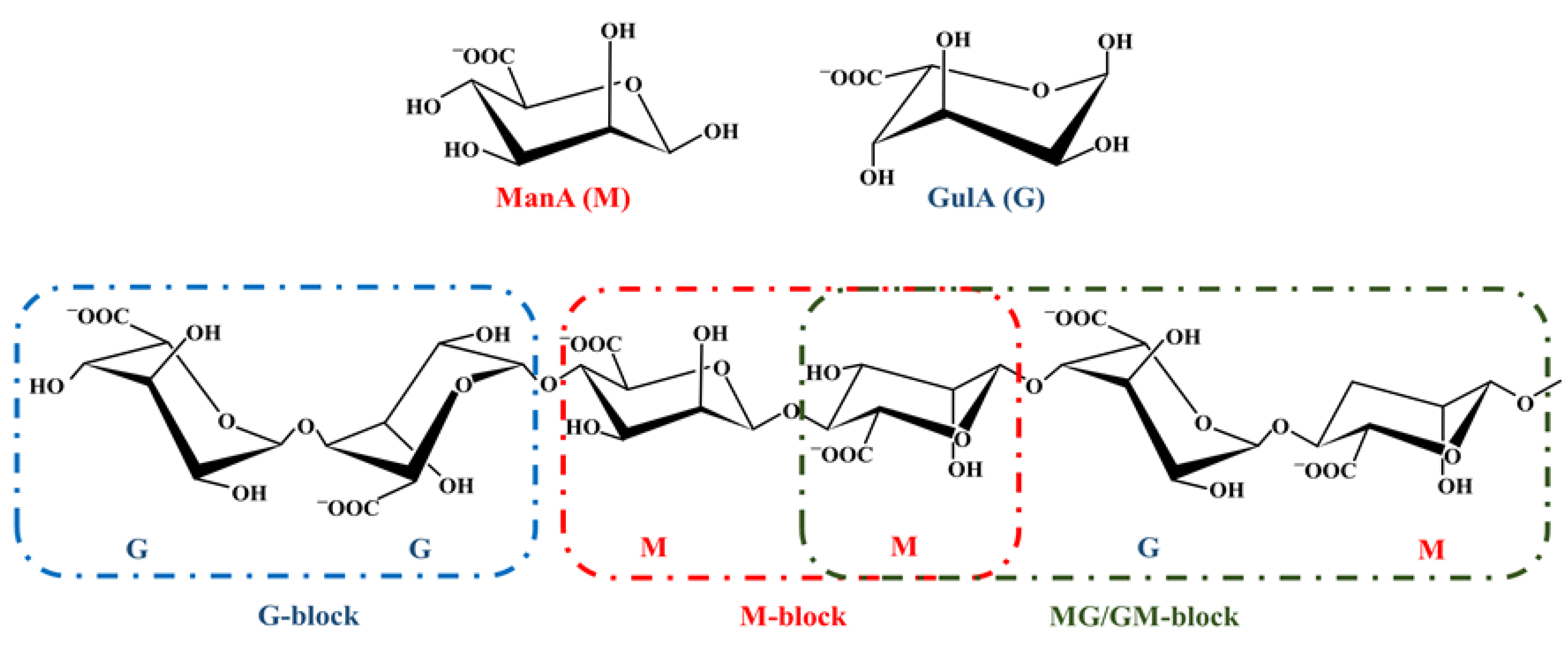
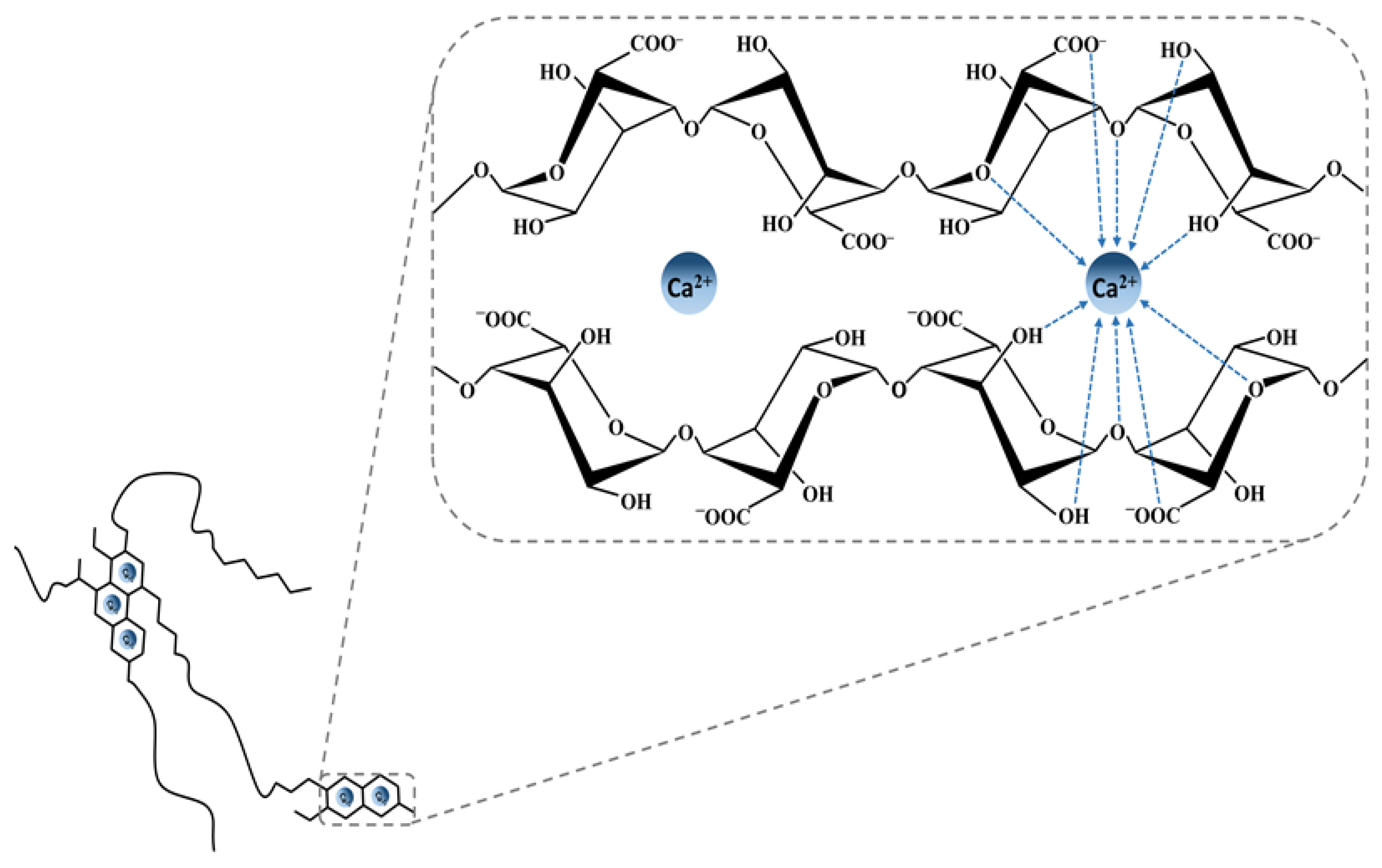
3. Alginate
3.1. History, Structure, and Sources
3.2. Properties and Applications of Alginate
3.2.1. Biocompatibility and Biodegradability
3.2.2. Bioadhesiveness
3.2.3. Alginate Absorption
3.2.4. pH Sensitivity
3.3. Limitations
4. Pectins
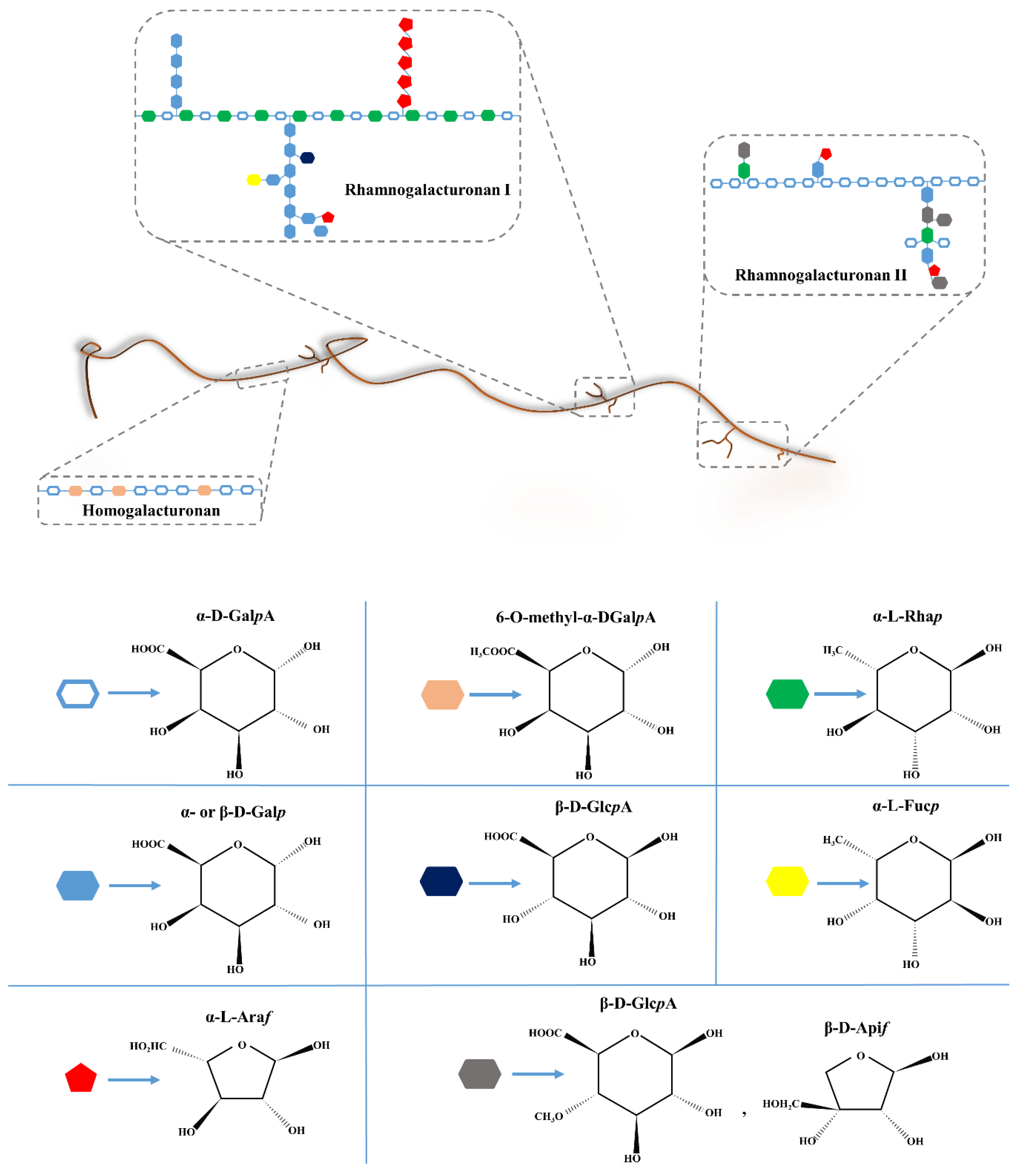
4.1. History, Structure, and Sources
4.2. Properties and Applications of Pectin
4.2.1. Biocompatibility and Biodegradability
4.2.2. Bioadhesiveness
4.2.3. Pectin Absorption
4.2.4. pH Sensitivity
4.3. Limitations
5. Perspectives and Future Trends
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, X.; Lin, W.; Astruc, D.; Gu, H.B. Syntheses and applications of dendronized polymers. Prog. Polym. Sci. 2019, 96, 43–105. [Google Scholar] [CrossRef]
- Rogovina, S.Z.; Prut, E.V.; Berlin, A.A. Composite Materials Based on Synthetic Polymers Reinforced with Natural Fibers. Polym. Sci. Ser. A 2019, 61, 417–438. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Jouyandeh, M.; Ganjali, M.R.; Hadavand, B.S.; Mozafari, M.; Sheiko, S.S.; Vatankhah-Varnoosfaderani, M.; Gutierrez, T.J.; Saeb, M.R. Thermo-sensitive polymers in medicine: A review. Eur. Polym. J. 2019, 117, 402–423. [Google Scholar] [CrossRef]
- Gouveia, T.I.A.; Biernacki, K.; Castro, M.C.R.; Goncalves, M.P.; Souza, H.K.S. A new approach to develop biodegradable films based on thermoplastic pectin. Food Hydrocoll. 2019, 97, 105175. [Google Scholar] [CrossRef]
- Calinoiu, L.F.; Vodnar, D.C. Whole Grains and Phenolic Acids: A Review on Bioactivity, Functionality, Health Benefits and Bioavailability. Nutrients 2018, 10, 1615. [Google Scholar] [CrossRef] [PubMed]
- Szabo, K.; Catoi, A.F.; Vodnar, D.C. Bioactive Compounds Extracted from Tomato Processing by-Products as a Source of Valuable Nutrients. Plant Foods Hum. Nutr. 2018, 73, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Setiowati, A.D.; Rwigamba, A.; Van der Meeren, P. The influence of degree of methoxylation on the emulsifying and heat stabilizing activity of whey protein-pectin conjugates. Food Hydrocoll. 2019, 96, 54–64. [Google Scholar] [CrossRef]
- Smith, A.M.; Moxon, S.; Morris, G.A. Biopolymers as wound healing materials. In Wound Healing Biomaterials; Ågren, M.S., Ed.; Woodhead Publishing: Sawston, UK, 2016; pp. 261–287. [Google Scholar] [CrossRef]
- Coman, V.; Teleky, B.-E.; Mitrea, L.; Martău, G.A.; Szabo, K.; Călinoiu, L.-F.; Vodnar, D.C. Bioactive potential of fruit and vegetable wastes. Adv. Food Nutr. Res. 2019, in press. [Google Scholar] [CrossRef]
- Teleky, B.E.; Vodnar, D.C. Biomass-Derived Production of Itaconic Acid as a Building Block in Specialty Polymers. Polymers 2019, 11, 1035. [Google Scholar] [CrossRef]
- Gonzalez-Henriquez, C.M.; Sarabia-Vallejos, M.A.; Rodriguez-Hernandez, J. Polymers for additive manufacturing and 4D-printing: Materials, methodologies, and biomedical applications. Prog. Polym. Sci. 2019, 94, 57–116. [Google Scholar] [CrossRef]
- Gong, J.; Chen, X.C.; Tang, T. Recent progress in controlled carbonization of (waste) polymers. Prog. Polym. Sci. 2019, 94, 1–32. [Google Scholar] [CrossRef]
- Top 5 Vendors in the Global Biopolymers Market from 2017–2021: Technavio. Available online: https://www.businesswire.com/news/home/20170112005066/en/Top-5-Vendors-Global-Biopolymers-Market-2017-2021 (accessed on 2 September 2019).
- Cavallaro, G.; Lazzara, G.; Milioto, S. Sustainable nanocomposites based on halloysite nanotubes and pectin/polyethylene glycol blend. Polym. Degrad. Stab. 2013, 98, 2529–2536. [Google Scholar] [CrossRef] [Green Version]
- Abdollahi, M.; Alboofetileh, M.; Rezaei, M.; Behrooz, R. Comparing physico-mechanical and thermal properties of alginate nanocomposite films reinforced with organic and/or inorganic nanofillers. Food Hydrocoll. 2013, 32, 416–424. [Google Scholar] [CrossRef]
- Sanuja, S.; Agalya, A.; Umapathy, M.J. Studies on Magnesium Oxide Reinforced Chitosan Bionanocomposite Incorporated with Clove Oil for Active Food Packaging Application. Int. J. Polym. Mater. Polym. Biomater. 2014, 63, 733–740. [Google Scholar] [CrossRef]
- Guerreiro, A.C.; Gago, C.M.L.; Miguel, M.G.C.; Faleiro, M.L.; Antunes, M.D.C. The influence of edible coatings enriched with citral and eugenol on the raspberry storage ability, nutritional and sensory quality. Food Packag. Shelf Life 2016, 9, 20–28. [Google Scholar] [CrossRef]
- Zhang, L.H.; Li, S.F.; Dong, Y.; Zhi, H.H.; Zong, W. Tea polyphenols incorporated into alginate-based edible coating for quality maintenance of Chinese winter jujube under ambient temperature. LWT-Food Sci. Technol. 2016, 70, 155–161. [Google Scholar] [CrossRef]
- Kanetis, L.; Exarchou, V.; Charalambous, Z.; Goulas, V. Edible coating composed of chitosan and Salvia fruticosa Mill. extract for the control of grey mould of table grapes. J. Sci. Food Agric. 2017, 97, 452–460. [Google Scholar] [CrossRef]
- Calinoiu, L.F.; Stefanescu, B.E.; Pop, I.D.; Muntean, L.; Vodnar, D.C. Chitosan Coating Applications in Probiotic Microencapsulation. Coatings 2019, 9, 194. [Google Scholar] [CrossRef]
- Krisanti, E.A.; Naziha, G.M.; Amany, N.S.; Mulia, K.; Handayani, N.A.; IOP. Effect of biopolymers composition on release profile of iron(II) fumarate from chitosan-alginate microparticles. IOP Conf. Ser. Mater. Sci. Eng. 2019, 509, 012100. [Google Scholar] [CrossRef]
- Gupta, B.; Tummalapalli, M.; Deopura, B.L.; Alam, M.S. Preparation and characterization of in-situ crosslinked pectin-gelatin hydrogels. Carbohydr. Polym. 2014, 106, 312–318. [Google Scholar] [CrossRef]
- Liu, L.; Liu, C.K.; Fishman, M.L.; Hicks, K.B. Composite films from pectin and fish skin gelatin or soybean flour protein. J. Agric. Food Chem. 2007, 55, 2349–2355. [Google Scholar] [CrossRef] [PubMed]
- Pranoto, Y.; Salokhe, V.M.; Rakshit, S.K. Physical and antibacte rial properties of alginate-based edible film incorporated with garlic oil. Food Res. Int. 2005, 38, 267–272. [Google Scholar] [CrossRef]
- Lee, J.B.; Ahn, J.; Lee, J.; Kwak, H.S. The microencapsulated ascorbic acid release in vitro and its effect on iron bioavailability. Arch. Pharm. Res. 2003, 26, 874–879. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, R.; Chen, L.; McClements, D.J. Encapsulation of lactase (beta-galactosidase) into kappa-carrageenan-based hydrogel beads: Impact of environmental conditions on enzyme activity. Food Chem. 2016, 200, 69–75. [Google Scholar] [CrossRef]
- Estevinho, B.N.; Rocha, F.; Santos, L.; Alves, A. Microencapsulation with chitosan by spray drying for industry applications—A review. Trends Food Sci. Technol. 2013, 31, 138–155. [Google Scholar] [CrossRef]
- Goncalves, A.; Estevinho, B.N.; Rocha, F. Microencapsulation of vitamin A: A review. Trends Food Sci. Technol. 2016, 51, 76–87. [Google Scholar] [CrossRef] [Green Version]
- Gupta, C.; Chawla, P.; Arora, S.; Tomar, S.K.; Singh, A.K. Iron microencapsulation with blend of gum arabic, maltodextrin and modified starch using modified solvent evaporation method—Milk fortification. Food Hydrocoll. 2015, 43, 622–628. [Google Scholar] [CrossRef]
- Valenzuela, C.; Hernandez, V.; Morales, M.S.; Neira-Carrillo, A.; Pizarro, F. Preparation and characterization of heme iron-alginate beads. LWT-Food Sci. Technol. 2014, 59, 1283–1289. [Google Scholar] [CrossRef]
- Zlotkin, S.H.; Schauer, C.; Owusu Agyei, S.; Wolfson, J.; Tondeur, M.C.; Asante, K.P.; Newton, S.; Serfass, R.E.; Sharieff, W. Demonstrating zinc and iron bioavailability from intrinsically labeled microencapsulated ferrous fumarate and zinc gluconate Sprinkles in young children. J. Nutr. 2006, 136, 920–925. [Google Scholar] [CrossRef]
- Călinoiu, L.F.; Mitrea, L.; Precup, G.; Bindea, M.; Rusu, B.; Szabo, K.; Dulf, F.V.; Ştefănescu, B.E.; Vodnar, D.C. Sustainable use of agro-industrial wastes for feeding 10 billion people by 2050. Prof. Food Chain. Ethics Roles Responsib. 2018, 482–486. [Google Scholar] [CrossRef]
- Bilal, M.; Iqbal, H.M.N. Naturally-derived biopolymers: Potential platforms for enzyme immobilization. Int. J. Biol. Macromol. 2019, 130, 462–482. [Google Scholar] [CrossRef] [PubMed]
- Calinoiu, L.F.; Catoi, A.F.; Vodnar, D.C. Solid-State Yeast Fermented Wheat and Oat Bran as A Route for Delivery of Antioxidants. Antioxidants 2019, 8, 372. [Google Scholar] [CrossRef] [PubMed]
- Mitrea, L.; Calinoiu, L.F.; Precup, G.; Bindea, M.; Rusu, B.; Trif, M.; Stefanescu, B.E.; Pop, I.D.; Vodnar, D.C. Isolated Microorganisms for Bioconversion of Biodiesel-Derived Glycerol Into 1,3-Propanediol. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca-Food Sci. Technol. 2017, 74, 43–49. [Google Scholar] [CrossRef] [Green Version]
- Mitrea, L.; Calinoiu, L.F.; Precup, G.; Bindea, M.; Rusu, B.; Trif, M.; Ferenczi, L.J.; Stefanescu, B.E.; Vodnar, D.C. Inhibitory Potential of Lactobacillus Plantarum on Escherichia Coli. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca-Food Sci. Technol. 2017, 74, 99–101. [Google Scholar] [CrossRef]
- Adeel, M.; Bilal, M.; Rasheed, T.; Sharma, A.; Iqbal, H.M.N. Graphene and graphene oxide: Functionalization and nano-bio-catalytic system for enzyme immobilization and biotechnological perspective. Int. J. Biol. Macromol. 2018, 120, 1430–1440. [Google Scholar] [CrossRef] [PubMed]
- Amin, F.; Bhatti, H.N.; Bilal, M.; Asgher, M. Improvement of activity, thermo-stability and fruit juice clarification characteristics of fungal exo-polygalacturonase. Int. J. Biol. Macromol. 2017, 95, 974–984. [Google Scholar] [CrossRef] [PubMed]
- Wohlgemuth, R. Biocatalysis-key to sustainable industrial chemistry. Curr. Opin. Biotechnol. 2010, 21, 713–724. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, Q.; Wang, W.; Zhou, L.; Gao, J. Preparation of Immobilized Lipase through Combination of Cross-Linked En-zyme Aggregates and Biomimetic Silicification. Chin. J. Catal. (Chin. Vers.) 2013, 33, 857–862. [Google Scholar] [CrossRef]
- Szabo, K.; Diaconeasa, Z.; Catoi, A.F.; Vodnar, D.C. Screening of Ten Tomato Varieties Processing Waste for Bioactive Components and Their Related Antioxidant and Antimicrobial Activities. Antioxidants 2019, 8, 292. [Google Scholar] [CrossRef]
- Stefanescu, B.E.; Szabo, K.; Mocan, A.; Crisan, G. Phenolic Compounds from Five Ericaceae Species Leaves and Their Related Bioavailability and Health Benefits. Molecules 2019, 24, 2046. [Google Scholar] [CrossRef]
- Vert, M.; Doi, Y.; Hellwich, K.H.; Hess, M.; Hodge, P.; Kubisa, P.; Rinaudo, M.; Schue, F. Terminology for biorelated polymers and applications (IUPAC Recommendations 2012). Pure Appl. Chem. 2012, 84, 377–408. [Google Scholar] [CrossRef]
- La Rosa, A.D. Life cycle assessment of biopolymers. In Biopolymers and Biotech Admixtures for Eco-Efficient Construction Materials; Pacheco-Torgal, F., Ivanov, V., Karak, N., Jonkers, H., Eds.; Woodhead Publishing: Sawston, UK, 2016; pp. 57–78. [Google Scholar] [CrossRef]
- Natural Polymers and Biopolymers—Polymers Produced in Nature. Available online: https://www.sigmaaldrich.com/materials-science/material-science-products.html?TablePage=16371327 (accessed on 29 July 2019).
- Rendon, R.; Ortíz-Sánchez, A.; Tovar-Sánchez, E.; Huicochea, E. The Role of Biopolymers in Obtaining Environmentally Friendly Materials. Compos. Renew. Sustain. Mater. 2016. [Google Scholar] [CrossRef] [Green Version]
- Vroman, I.; Tighzert, L. Biodegradable Polymers. Materials 2009, 2, 307–344. [Google Scholar] [CrossRef]
- Mensitieri, G.; Di Maio, E.; Buonocore, G.G.; Nedi, I.; Oliviero, M.; Sansone, L.; Iannace, S. Processing and shelf life issues of selected food packaging materials and structures from renewable resources. Trends Food Sci. Technol. 2011, 22, 72–80. [Google Scholar] [CrossRef]
- Gutiérrez, T.J. Polymers for Food Applications; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar] [CrossRef]
- Ruban, S.W. Biobased Packaging—Application in Meat Industry. Vet. World 2009, 2, 79–82. [Google Scholar] [CrossRef]
- Madhavan Nampoothiri, K.; Nair, N.R.; John, R.P. An overview of the recent developments in polylactide (PLA) research. Bioresour. Technol. 2010, 101, 8493–8501. [Google Scholar] [CrossRef]
- Sinha, V.R.; Singla, A.K.; Wadhawan, S.; Kaushik, R.; Kumria, R.; Bansal, K.; Dhawan, S. Chitosan microspheres as a potential carrier for drugs. Int. J. Pharm. 2004, 274, 1–33. [Google Scholar] [CrossRef]
- Nasui, L.; Vodnar, D.; Socaciu, C. Bioactive Labels for Fresh Fruits and Vegetables. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Food Sci. Technol. 2013, 70, 74–82. [Google Scholar] [CrossRef]
- Dong, H.Q.; Cheng, L.Y.; Tan, J.H.; Zheng, K.W.; Jiang, Y.M. Effects of chitosan coating on quality and shelf life of peeled litchi fruit. J. Food Eng. 2004, 64, 355–358. [Google Scholar] [CrossRef]
- Guo, Z.Y.; Xing, R.E.; Liu, S.; Zhong, Z.M.; Ji, X.; Wang, L.; Li, P.C. The influence of molecular weight of quaternized chitosan on antifungal activity. Carbohydr. Polym. 2008, 71, 694–697. [Google Scholar] [CrossRef]
- Athayde, A.J.A.A.; de Oliveira, P.D.L.; Guerra, I.C.D.; da Conceicao, M.L.; de Lima, M.A.B.; Arcanjo, N.M.O.; Madruga, M.S.; Berger, L.R.R.; de Souza, E.L. A coating composed of chitosan and Cymbopogon citratus (Dc. Ex Nees) essential oil to control Rhizopus soft rot and quality in tomato fruit stored at room temperature. J. Hortic. Sci. Biotechnol. 2016, 91, 582–591. [Google Scholar] [CrossRef]
- He, P.; Davis, S.S.; Illum, L. In vitro evaluation of the mucoadhesive properties of chitosan microspheres. Int. J. Pharm. 1998, 166, 75–88. [Google Scholar] [CrossRef]
- Hejazi, R.; Amiji, M. Chitosan-based gastrointestinal delivery systems. J. Control. Release Off. J. Control. Release Soc. 2003, 89, 151–165. [Google Scholar] [CrossRef]
- Suksamran, T.; Opanasopit, P.; Rojanarata, T.; Ngawhirunpat, T. Development of Alginate/Chitosan Microparticles for Dust Mite Allerge. Trop. J. Pharm. Res. 2011, 10. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.J.; Ma, L.L.; Shi, H.T.; Zhu, J.B.; Wu, J.; Ding, Z.W.; An, Y.; Zou, Y.Z.; Ge, J.B. Alginate Oligosaccharide Prevents Acute Doxorubicin Cardiotoxicity by Suppressing Oxidative Stress and Endoplasmic Reticulum-Mediated Apoptosis. Mar. Drugs 2016, 14, 231. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.S.; Cardoso, M.D.; Guimaraes, A.C.; Guerreiro, A.C.; Gago, C.M.; Vilas Boas, E.V.; Dias, C.M.; Manhita, A.C.; Faleiro, M.L.; Miguel, M.G.; et al. Effect of edible coatings with essential oils on the quality of red raspberries over shelf-life. J. Sci. Food Agric. 2017, 97, 929–938. [Google Scholar] [CrossRef]
- Ramana Rao, T.V.; Baraiya, N.S.; Vyas, P.B.; Patel, D.M. Composite coating of alginate-olive oil enriched with antioxidants enhances postharvest quality and shelf life of Ber fruit (Ziziphus mauritiana Lamk. Var. Gola). J. Food Sci. Technol. 2016, 53, 748–756. [Google Scholar] [CrossRef]
- Kobaslija, M.; McQuade, D.T. Removable colored coatings based on calcium alginate hydrogels. Biomacromolecules 2006, 7, 2357–2361. [Google Scholar] [CrossRef]
- Victoria Emerton, E.C. Essential Guide to Food Additives, 3rd ed.; Royal Society of Chemistry: London, UK, 2008. [Google Scholar]
- Hampson, F.C.; Farndale, A.; Strugala, V.; Sykes, J.; Jolliffe, I.G.; Dettmar, P.W. Alginate rafts and their characterisation. Int. J. Pharm. 2005, 294, 137–147. [Google Scholar] [CrossRef]
- Tusi, S.K.; Khalaj, L.; Ashabi, G.; Kiaei, M.; Khodagholi, F. Alginate oligosaccharide protects against endoplasmic reticulum- and mitochondrial-mediated apoptotic cell death and oxidative stress. Biomaterials 2011, 32, 5438–5458. [Google Scholar] [CrossRef]
- Zhou, R.; Shi, X.; Gao, Y.; Cai, N.; Jiang, Z.; Xu, X. Anti-inflammatory activity of guluronate oligosaccharides obtained by oxidative degradation from alginate in lipopolysaccharide-activated murine macrophage RAW 264.7 cells. J. Agric. Food Chem. 2015, 63, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Jiang, X.; Jiang, Y.; Hu, X.; Mou, H.; Li, M.; Guan, H. In vitro antioxidative activities of three marine oligosaccharides. Nat. Prod. Res. 2007, 21, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wu, Y.; Shen, Y.; Zhou, C.; Li, Y.F.; He, R.R.; Liu, M. Enhanced Therapeutic Efficacy of Doxorubicin for Breast Cancer Using Chitosan Oligosaccharide-Modified Halloysite Nanotubes. ACS Appl. Mater. Interfaces 2016, 8, 26578–26590. [Google Scholar] [CrossRef] [PubMed]
- Mesbahi, G.; Jamalian, J.; Farahnaky, A. A comparative study on functional properties of beet and citrus pectins in food systems. Food Hydrocoll. 2005, 19, 731–738. [Google Scholar] [CrossRef]
- Sriamornsak, P.; Kennedy, R.A. Swelling and diffusion studies of calcium polysaccharide gels intended for film coating. Int. J. Pharm. 2008, 358, 205–213. [Google Scholar] [CrossRef]
- Rodriguez-Garcia, I.; Cruz-Valenzuela, M.R.; Silva-Espinoza, B.A.; Gonzalez-Aguilar, G.A.; Moctezuma, E.; Gutierrez-Pacheco, M.M.; Tapia-Rodriguez, M.R.; Ortega-Ramirez, L.A.; Ayala-Zavala, J.F. Oregano (Lippia graveolens) essential oil added within pectin edible coatings prevents fungal decay and increases the antioxidant capacity of treated tomatoes. J. Sci. Food Agric. 2016, 96, 3772–3778. [Google Scholar] [CrossRef]
- Guerreiro, A.C.; Gago, C.M.L.; Faleiro, M.L.; Miguel, M.G.C.; Antunes, M.D. The effect of edible coatings on the nutritional quality of ‘Bravo de Esmolfe’ fresh-cut apple through shelf-life. LWT 2017, 75, 210–219. [Google Scholar] [CrossRef]
- Narasimman, P.; Sethuraman, P. An Overview on the Fundamentals of Pectin. Int. J. Adv. Res. 2016, 4, 1855–1860. [Google Scholar] [CrossRef]
- Sriamornsak, P. Application of pectin in oral drug delivery. Expert Opin. Drug Deliv. 2011, 8, 1009–1023. [Google Scholar] [CrossRef]
- Wong, T.W.; Colombo, G.; Sonvico, F. Pectin matrix as oral drug delivery vehicle for colon cancer treatment. AAPS PharmSciTech 2011, 12, 201–214. [Google Scholar] [CrossRef]
- Liu, L.; Fishman, M.L.; Kost, J.; Hicks, K.B. Pectin-based systems for colon-specific drug delivery via oral route. Biomaterials 2003, 24, 3333–3343. [Google Scholar] [CrossRef]
- Munarin, F.; Tanzi, M.C.; Petrini, P. Advances in biomedical applications of pectin gels. Int. J. Biol. Macromol. 2012, 51, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Rodsamran, P.; Sothornvit, R. Lime peel pectin integrated with coconut water and lime peel extract as a new bioactive film sachet to retard soybean oil oxidation. Food Hydrocoll. 2019, 97, 105173. [Google Scholar] [CrossRef]
- Shepherd, R.; Reader, S.; Falshaw, A. Chitosan functional properties. Glycoconj. J. 1997, 14, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Raafat, D.; Sahl, H.G. Chitosan and its antimicrobial potential--a critical literature survey. Microb. Biotechnol. 2009, 2, 186–201. [Google Scholar] [CrossRef]
- Harish Prashanth, K.V.; Tharanathan, R.N. Chitin/chitosan: Modifications and their unlimited application potential—An overview. Trends Food Sci. Technol. 2007, 18, 117–131. [Google Scholar] [CrossRef]
- Roberts, G.A.F. Chitin Chemistry; Palgrave: London, UK, 1992. [Google Scholar] [CrossRef]
- Rhazi, M.; Desbrieres, J.; Tolaimate, A.; Alagui, A.; Vottero, P. Investigation of different natural sources of chitin: Influence of the source and deacetylation process on the physicochemical characteristics of chitosan. Polym. Int. 2000, 49, 337–344. [Google Scholar] [CrossRef]
- Wuolijoki, E.; Hirvela, T.; Ylitalo, P. Decrease in serum LDL cholesterol with microcrystalline chitosan. Methods Find. Exp. Clin. Pharmacol. 1999, 21, 357–361. [Google Scholar] [CrossRef]
- Nwe, N.; Chandrkrachang, S.; Stevens, W.F.; Maw, T.; Tan, T.K.; Khor, E.; Wong, S.M. Production of fungal chitosan by solid state and submerged fermentation. Carbohydr. Polym. 2002, 49, 235–237. [Google Scholar] [CrossRef]
- Zhang, X.; Li, K.; Xing, R.; Liu, S.; Li, P. Metabolite Profiling of Wheat Seedlings Induced by Chitosan: Revelation of the Enhanced Carbon and Nitrogen Metabolism. Front. Plant Sci. 2017, 8, 2017. [Google Scholar] [CrossRef]
- Anthonsen, M.W.; Varum, K.M.; Smidsrod, O. Solution Properties of Chitosans–Conformation and Chain Stiffness of Chitosans with Different Degrees of N-Acetylation. Carbohydr. Polym. 1993, 22, 193–201. [Google Scholar] [CrossRef]
- Wang, W.; Bo, S.Q.; Li, S.Q.; Qin, W. Determination of the Mark-Houwink equation for chitosans with different degrees of deacetylation. Int. J. Biol. Macromol. 1991, 13, 281–285. [Google Scholar] [CrossRef]
- Muzzarelli, R.; Baldassarre, V.; Conti, F.; Ferrara, P.; Biagini, G.; Gazzanelli, G.; Vasi, V. Biological activity of chitosan: Ultrastructural study. Biomaterials 1988, 9, 247–252. [Google Scholar] [CrossRef]
- Muzzarelli, R.A. Human enzymatic activities related to the therapeutic administration of chitin derivatives. Cell. Mol. Life Sci. CMLS 1997, 53, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Arachchi, J.K.V.; Jeon, Y.J. Food applications of chitin and chitosans. Trends Food Sci. Technol. 1999, 10, 37–51. [Google Scholar] [CrossRef]
- Kanauchi, O.; Deuchi, K.; Imasato, Y.; Shizukuishi, M.; Kobayashi, E. Mechanism for the inhibition of fat digestion by chitosan and for the synergistic effect of ascorbate. Biosci. Biotechnol. Biochem. 1995, 59, 786–790. [Google Scholar] [CrossRef] [PubMed]
- George, M.; Abraham, T.E. Polyionic hydrocolloids for the intestinal delivery of protein drugs: Alginate and chitosan-a review. J. Control. Release Off. J. Control. Release Soc. 2006, 114, 1–14. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Berger, J.; Reist, M.; Mayer, J.M.; Felt, O.; Peppas, N.A.; Gurny, R. Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. Eur. J. Pharm. Biopharm. Off. J. Arb. Fur Pharm. Verfahr. E.V 2004, 57, 19–34. [Google Scholar] [CrossRef]
- Polk, A.; Amsden, B.; De Yao, K.; Peng, T.; Goosen, M.F. Controlled release of albumin from chitosan-alginate microcapsules. J. Pharm. Sci. 1994, 83, 178–185. [Google Scholar] [CrossRef]
- Agnihotri, S.A.; Mallikarjuna, N.N.; Aminabhavi, T.M. Recent advances on chitosan-based micro- and nanoparticles in drug delivery. J. Control. Release Off. J. Control. Release Soc. 2004, 100, 5–28. [Google Scholar] [CrossRef] [PubMed]
- Lehr, C.-M.; Bouwstra, J.A.; Schacht, E.H.; Junginger, H.E. In vitro evaluation of mucoadhesive properties of chitosan and some other natural polymers. Int. J. Pharm. 1992, 78, 43–48. [Google Scholar] [CrossRef]
- Gåserød, O.; Jolliffe, I.G.; Hampson, F.C.; Dettmar, P.W.; Skjåk-Bræk, G. The enhancement of the bioadhesive properties of calcium alginate gel beads by coating with chitosan. Int. J. Pharm. 1998, 175, 237–246. [Google Scholar] [CrossRef]
- Remunan-Lopez, C.; Portero, A.; Lemos, M.; Vila-Jato, J.L.; Nunez, M.J.; Riveiro, P.; Lopez, J.M.; Piso, M.; Alonso, M.J. Chitosan microspheres for the specific delivery of amoxycillin to the gastric cavity. STP Pharma Sci. 2000, 10, 69–76. [Google Scholar]
- Alexander, G.R.; Kotelchuck, M. Quantifying the adequacy of prenatal care: A comparison of indices. Public Health Rep. 1996, 111, 408–418, discussion 419. [Google Scholar] [CrossRef]
- Shimoda, J.; Onishi, H.; Machida, Y. Bioadhesive characteristics of chitosan microspheres to the mucosa of rat small intestine. Drug Dev. Ind. Pharm. 2001, 27, 567–576. [Google Scholar] [CrossRef]
- Miyazaki, S.; Nakayama, A.; Oda, M.; Takada, M.; Attwood, D. Drug-Release from Oral Mucosal Adhesive Tablets of Chitosan and Sodium Alginate. Int. J. Pharm. 1995, 118, 257–263. [Google Scholar] [CrossRef]
- Remunan-Lopez, C.; Portero, A.; Vila-Jato, J.L.; Alonso, M.J. Design and evaluation of chitosan/ethylcellulose mucoadhesive bilayered devices for buccal drug delivery. J. Control. Release Off. J. Control. Release Soc. 1998, 55, 143–152. [Google Scholar] [CrossRef]
- Fiebrig, I.; Harding, S.E.; Rowe, A.J.; Hyman, S.C.; Davis, S.S. Transmission electron microscopy studies on pig gastric mucin and its interactions with chitosan. Carbohydr. Polym. 1995, 28, 239–244. [Google Scholar] [CrossRef]
- Deacon, M.P.; McGurk, S.; Roberts, C.J.; Williams, P.M.; Tendler, S.J.; Davies, M.C.; Davis, S.S.; Harding, S.E. Atomic force microscopy of gastric mucin and chitosan mucoadhesive systems. Biochem. J. 2000, 348 Pt 3, 557–563. [Google Scholar] [CrossRef]
- Bernkop-Schnurch, A.; Humenberger, C.; Valenta, C. Basic studies on bioadhesive delivery systems for peptide and protein drugs. Int. J. Pharm. 1998, 165, 217–225. [Google Scholar] [CrossRef]
- Genta, I.; Perugini, P.; Pavanetto, F.; Modena, T.; Conti, B.; Muzzarelli, R.A. Microparticulate drug delivery systems. Exs 1999, 87, 305–313. [Google Scholar]
- Herrera, N.; Salaberria, A.M.; Mathew, A.P.; Oksman, K. Plasticized polylactic acid nanocomposite films with cellulose and chitin nanocrystals prepared using extrusion and compression molding with two cooling rates: Effects on mechanical, thermal and optical properties. Compos. Part A-Appl. Sci. Manuf. 2016, 83, 89–97. [Google Scholar] [CrossRef] [Green Version]
- Rivero, S.; Giannuzzi, L.; Garcia, M.A.; Pinotti, A. Controlled delivery of propionic acid from chitosan films for pastry dough conservation. J. Food Eng. 2013, 116, 524–531. [Google Scholar] [CrossRef]
- Bonjean, M.; Prime, A.; Avon, P. [Pelada in 2 homozygous twins]. Bull Soc Fr Derm. Syphiligr 1968, 75, 521–522. [Google Scholar] [CrossRef]
- Liu, J.; Liu, S.; Chen, Y.; Zhang, L.; Kan, J.; Jin, C.H. Physical, mechanical and antioxidant properties of chitosan films grafted with different hydroxybenzoic acids. Food Hydrocoll. 2017, 71, 176–186. [Google Scholar] [CrossRef]
- Corbel, M.J.; Rondle, C.J.; Bird, R.G. Degradation of influenza virus by non-ionic detergent. Epidemiol. Infect. 1970, 68, 77–80. [Google Scholar] [CrossRef] [Green Version]
- Correa-Pacheco, Z.N.; Bautista-Baños, S.; Valle-Marquina, M.Á.; Hernández-López, M. The Effect of Nanostructured Chitosan and Chitosan-thyme Essential Oil Coatings on Colletotrichum gloeosporioidesGrowthin vitroand on cv Hass Avocado and Fruit Quality. J. Phytopathol. 2017, 165, 297–305. [Google Scholar] [CrossRef]
- Hosseini, M.H.; Razavi, S.H.; Mousavi, M.A. Antimicrobial, Physical and Mechanical Properties of Chitosan-Based Films Incorporated with Thyme, Clove and Cinnamon Essential Oils. J. Food Process. Preserv. 2009, 33, 727–743. [Google Scholar] [CrossRef]
- Ham-Pichavant, F.; Sebe, G.; Pardon, P.; Coma, V. Fat resistance properties of chitosan-based paper packaging for food applications. Carbohydr. Polym. 2005, 61, 259–265. [Google Scholar] [CrossRef]
- Oh, Y.A.; Oh, Y.J.; Song, A.Y.; Won, J.S.; Song, K.B.; Min, S.C. Comparison of effectiveness of edible coatings using emulsions containing lemongrass oil of different size droplets on grape berry safety and preservation. LWT-Food Sci. Technol. 2017, 75, 742–750. [Google Scholar] [CrossRef]
- Siripatrawan, U.; Harte, B.R. Physical properties and antioxidant activity of an active film from chitosan incorporated with green tea extract. Food Hydrocoll. 2010, 24, 770–775. [Google Scholar] [CrossRef]
- Illum, L.; Farraj, N.F.; Davis, S.S. Chitosan as a novel nasal delivery system for peptide drugs. Pharm. Res. 1994, 11, 1186–1189. [Google Scholar] [CrossRef] [PubMed]
- Kotze, A.F.; de Leeuw, B.J.; Luessen, H.L.; de Boer, A.G.; Verhoef, J.C.; Junginger, H.E. Chitosans for enhanced delivery of therapeutic peptides across intestinal epithelia: In vitro evaluation in Caco-2 cell monolayers. Int. J. Pharm. 1997, 159, 243–253. [Google Scholar] [CrossRef]
- Luessen, H.L.; de Leeuw, B.J.; Langemeyer, M.W.; de Boer, A.B.; Verhoef, J.C.; Junginger, H.E. Mucoadhesive polymers in peroral peptide drug delivery. VI. Carbomer and chitosan improve the intestinal absorption of the peptide drug buserelin in vivo. Pharm. Res. 1996, 13, 1668–1672. [Google Scholar] [CrossRef] [PubMed]
- Junginger, H.E.; Verhoef, J.C. Macromolecules as safe penetration enhancers for hydrophilic drugs—A fiction? Pharm. Sci. Technol. Today 1998, 1, 370–376. [Google Scholar] [CrossRef]
- Schipper, N.G.; Olsson, S.; Hoogstraate, J.A.; deBoer, A.G.; Varum, K.M.; Artursson, P. Chitosans as absorption enhancers for poorly absorbable drugs 2: Mechanism of absorption enhancement. Pharm. Res. 1997, 14, 923–929. [Google Scholar] [CrossRef]
- Lehr, C.M. From sticky stuff to sweet receptors--achievements, limits and novel approaches to bioadhesion. Eur. J. Drug Metab. Pharm. 1996, 21, 139–148. [Google Scholar] [CrossRef]
- Artursson, P.; Lindmark, T.; Davis, S.S.; Illum, L. Effect of chitosan on the permeability of monolayers of intestinal epithelial cells (Caco-2). Pharm Res. 1994, 11, 1358–1361. [Google Scholar] [CrossRef]
- Borchard, G.; Lueβen, H.L.; de Boer, A.G.; Verhoef, J.C.; Lehr, C.-M.; Junginger, H.E. The potential of mucoadhesive polymers in enhancing intestinal peptide drug absorption. III: Effects of chitosan-glutamate and carbomer on epithelial tight junctions in vitro. J. Control. Release 1996, 39, 131–138. [Google Scholar] [CrossRef]
- Dodane, V.; Amin Khan, M.; Merwin, J.R. Effect of chitosan on epithelial permeability and structure. Int. J. Pharm. 1999, 182, 21–32. [Google Scholar] [CrossRef]
- Smith, J.; Wood, E.; Dornish, M. Effect of chitosan on epithelial cell tight junctions. Pharm. Res. 2004, 21, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Natsume, H.; Iwata, S.; Ohtake, K.; Miyamoto, M.; Yamaguchi, M.; Hosoya, K.; Kobayashi, D.; Sugibayashi, K.; Morimoto, Y. Screening of cationic compounds as an absorption enhancer for nasal drug delivery. Int. J. Pharm. 1999, 185, 1–12. [Google Scholar] [CrossRef]
- Senel, S.; Hincal, A.A. Drug permeation enhancement via buccal route: Possibilities and limitations. J. Control. Release Off. J. Control. Release Soc. 2001, 72, 133–144. [Google Scholar] [CrossRef]
- Grabnar, I.; Bogataj, M.; Mrhar, A. Influence of chitosan and polycarbophil on permeation of a model hydrophilic drug into the urinary bladder wall. Int. J. Pharm. 2003, 256, 167–173. [Google Scholar] [CrossRef]
- Sinswat, P.; Tengamnuay, P. Enhancing effect of chitosan on nasal absorption of salmon calcitonin in rats: Comparison with hydroxypropyl- and dimethyl-beta-cyclodextrins. Int. J. Pharm. 2003, 257, 15–22. [Google Scholar] [CrossRef]
- Sandri, G.; Rossi, S.; Ferrari, F.; Bonferoni, M.C.; Muzzarelli, C.; Caramella, C. Assessment of chitosan derivatives as buccal and vaginal penetration enhancers. Eur. J. Pharm. Sci. 2004, 21, 351–359. [Google Scholar] [CrossRef]
- Yao, K.D.; Peng, T.; Feng, H.B.; He, Y.Y. Swelling kinetics and release characteristic of crosslinked chitosan: Polyether polymer network (semi-IPN) hydrogels. J. Polym. Sci. Part A Polym. Chem. 1994, 32, 1213–1223. [Google Scholar] [CrossRef]
- Van der Merwe, S.M.; Verhoef, J.C.; Verheijden, J.H.; Kotze, A.F.; Junginger, H.E. Trimethylated chitosan as polymeric absorption enhancer for improved peroral delivery of peptide drugs. Eur. J. Pharm. Biopharm. Off. J. Arb. Fur Pharm. Verfahr. E.V 2004, 58, 225–235. [Google Scholar] [CrossRef]
- Stanford, Elliptic Curve Cryptography (E.C.C). British Patent 142, 1881.
- Gomez, C.G.; Perez Lambrecht, M.V.; Lozano, J.E.; Rinaudo, M.; Villar, M.A. Influence of the extraction-purification conditions on final properties of alginates obtained from brown algae (Macrocystis pyrifera). Int. J. Biol. Macromol. 2009, 44, 365–371. [Google Scholar] [CrossRef]
- Smidsrod, O.; Skjakbraek, G. Alginate as Immobilization Matrix for Cells. Trends Biotechnol. 1990, 8, 71–78. [Google Scholar] [CrossRef]
- Sutherland, I.W. Alginates. In Biomaterials; Byrom, D., Ed.; Palgrave Macmillan: London, UK, 1991; pp. 307–331. [Google Scholar] [CrossRef]
- Skjak-Braek, G.; Grasdalen, H.; Larsen, B. Monomer sequence and acetylation pattern in some bacterial alginates. Carbohydr. Res. 1986, 154, 239–250. [Google Scholar] [CrossRef]
- Mærk, M. Looking for the Big Picture: Genome-Based Approaches to Improve Alginate Production in Azotobacter vinelandii; Norwegian University of Science and Technology: Trondheim, Norway, 2014. [Google Scholar]
- Nussinovitch, A. Hydrocolloid Applications; Springer: Boston, MA, USA, 1997. [Google Scholar] [CrossRef]
- Smith, A.M.; Miri, T. Alginates in Foods. In Practical Food Rheology; John Wiley & Sons: Hoboken, NJ, USA, 2010; pp. 113–132. [Google Scholar] [CrossRef]
- Espevik, T.; Otterlei, M.; Skjak-Braek, G.; Ryan, L.; Wright, S.D.; Sundan, A. The involvement of CD14 in stimulation of cytokine production by uronic acid polymers. Eur. J. Immunol. 1993, 23, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Cole, D.R.; Waterfall, M.; McIntyre, M.; Baird, J.D. Microencapsulated islet grafts in the BB/E rat: A possible role for cytokines in graft failure. Diabetologia 1992, 35, 231–237. [Google Scholar] [CrossRef] [PubMed]
- De Vos, P.; De Haan, B.; Pater, J.; Van Schilfgaarde, R. Association between capsule diameter, adequacy of encapsulation, and survival of microencapsulated rat islet allografts. Transplantation 1996, 62, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, U.; Klock, G.; Federlin, K.; Hannig, K.; Kowalski, M.; Bretzel, R.G.; Horcher, A.; Entenmann, H.; Sieber, U.; Zekorn, T. Production of mitogen-contamination free alginates with variable ratios of mannuronic acid to guluronic acid by free flow electrophoresis. Electrophoresis 1992, 13, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Mumper, R.J.; Huffman, A.S.; Puolakkainen, P.A.; Bouchard, L.S.; Gombotz, W.R. Calcium-alginate beads for the oral delivery of transforming growth factor-β1 (TGF-β1): Stabilization of TGF-β1 by the addition of polyacrylic acid within acid-treated beads. J. Control. Release 1994, 30, 241–251. [Google Scholar] [CrossRef]
- Gombotz, W.R.; Wee, S.F. Protein release from alginate matrices. Adv. Drug Deliv. Rev. 1998, 31, 267–285. [Google Scholar] [CrossRef]
- Chickering, D.E.; Mathiowitz, E. Bioadhesive microspheres: I. A novel electrobalance-based method to study adhesive interactions between individual microspheres and intestinal mucosa. Control. Release 1995, 34, 251–261. [Google Scholar] [CrossRef]
- Ch’ng, H.S.; Park, H.; Kelly, P.; Robinson, J.R. Bioadhesive polymers as platforms for oral controlled drug delivery II: Synthesis and evaluation of some swelling, water-insoluble bioadhesive polymers. J. Pharm. Sci. 1985, 74, 399–405. [Google Scholar] [CrossRef]
- Kwok, K.K.; Groves, M.J.; Burgess, D.J. Production of 5-15 microns diameter alginate-polylysine microcapsules by an air-atomization technique. Pharm. Res. 1991, 8, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Wawer, A.A.; Harvey, L.J.; Dainty, J.R.; Perez-Moral, N.; Sharp, P.; Fairweather-Tait, S.J. Alginate inhibits iron absorption from ferrous gluconate in a randomized controlled trial and reduces iron uptake into Caco-2 cells. PLoS ONE 2014, 9, e112144. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.M.; Liu, X.F.; Sun, X.; Li, Y.; Nie, X.Y.; Tang, W.K.; Yu, R.H.; Shui, J.L. Alginate-templated synthesis of CoFe/carbon fiber composite and the effect of hierarchically porous structure on electromagnetic wave absorption performance. Carbon 2019, 151, 36–45. [Google Scholar] [CrossRef]
- Harrison, J.; McNeill, K.G.; Janiga, A. The effect of sodium alginate on the absorption of strontium and calcium in human subjects. Can. Med. Assoc. J. 1966, 95, 532–534. [Google Scholar] [PubMed]
- Falkeborg, M.; Cheong, L.Z.; Gianfico, C.; Sztukiel, K.M.; Kristensen, K.; Glasius, M.; Xu, X.; Guo, Z. Alginate oligosaccharides: Enzymatic preparation and antioxidant property evaluation. Food Chem. 2014, 164, 185–194. [Google Scholar] [CrossRef]
- Chen, J.; Hu, Y.; Zhang, L.; Wang, Y.; Wang, S.; Zhang, Y.; Guo, H.; Ji, D.; Wang, Y. Alginate Oligosaccharide DP5 Exhibits Antitumor Effects in Osteosarcoma Patients following Surgery. Front. Pharm. 2017, 8, 623. [Google Scholar] [CrossRef]
- Qu, Y.; Wang, Z.; Zhou, H.; Kang, M.; Dong, R.; Zhao, J. Oligosaccharide nanomedicine of alginate sodium improves therapeutic results of posterior lumbar interbody fusion with cages for degenerative lumbar disease in osteoporosis patients by downregulating serum miR-155. Int. J. Nanomed. 2017, 12, 8459–8469. [Google Scholar] [CrossRef]
- Wan, J.; Zhang, J.; Chen, D.W.; Yu, B.; He, J. Effects of alginate oligosaccharide on the growth performance, antioxidant capacity and intestinal digestion-absorption function in weaned pigs. Anim. Feed Sci. Technol. 2017, 234, 118–127. [Google Scholar] [CrossRef]
- Jiao, L.F.; Song, Z.H.; Ke, Y.L.; Xiao, K.; Hu, C.H.; Shi, B. Cello-oligosaccharide influences intestinal microflora, mucosal architecture and nutrient transport in weaned pigs. Anim. Feed Sci. Technol. 2014, 195, 85–91. [Google Scholar] [CrossRef]
- Wan, J.; Li, Y.; Chen, D.; Yu, B.; Zheng, P.; Mao, X.; Yu, J.; He, J. Expression of a Tandemly Arrayed Plectasin Gene from Pseudoplectania nigrella in Pichia pastoris and its Antimicrobial Activity. J. Microbiol. Biotechnol. 2016, 26, 461–468. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.K.; Lee, E.J. The Controlled Release of Blue Dextran from Alginate Beads. Int. J. Pharm. 1992, 79, 11–19. [Google Scholar] [CrossRef]
- Sugawara, S.; Imai, T.; Otagiri, M. The controlled release of prednisolone using alginate gel. Pharm. Res. 1994, 11, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.C.; Wu, Y.C.; Mi, F.L.; Lin, Y.H.; Yu, L.C.; Sung, H.W. A novel pH-sensitive hydrogel composed of N,O-carboxymethyl chitosan and alginate cross-linked by genipin for protein drug delivery. J. Control. Release Off. J. Control. Release Soc. 2004, 96, 285–300. [Google Scholar] [CrossRef] [PubMed]
- Carbone, M.; Donia, D.T.; Sabbatella, G.; Antiochia, R. Silver nanoparticles in polymeric matrices for fresh food packaging. J. King Saud Univ. Sci. 2016, 28, 273–279. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez, T.J. Biodegradability and Compostability of Food Nanopackaging Materials. In Composites Materials for Food Packaging; Wiley: Hoboken, NJ, USA, 2018; pp. 269–296. [Google Scholar] [CrossRef]
- Gutiérrez, T.J. Chitosan Applications for the Food Industry. In Chitosan; Wiley: Hoboken, NJ, USA, 2017; pp. 183–232. [Google Scholar] [CrossRef]
- Gutierrez, T.J.; Alvarez, V.A. Cellulosic materials as natural fillers in starch-containing matrix-based films: A review. Polym. Bull. 2017, 74, 2401–2430. [Google Scholar] [CrossRef]
- Gutierrez, T.J.; Alvarez, V.A. Bionanocomposite films developed from corn starch and natural and modified nano-clays with or without added blueberry extract. Food Hydrocoll. 2018, 77, 407–420. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez, T.J.; Seligra, P.G.; Jaramillo, C.M.; Jaramillo, C.M.; Famá, L.; Goyanes, S. Effect of Filler Properties on the Antioxidant Response of Thermoplastic Starch Composites. In Handbook of Composites from Renewable Materials; Wiley: Hoboken, NJ, USA, 2017; pp. 337–369. [Google Scholar] [CrossRef]
- Chen, C.Y.; Peng, X.; Zeng, R.; Chen, M.; Wan, C.P.; Chen, J.Y. Ficus hirta fruits extract incorporated into an alginate-based edible coating for Nanfeng mandarin preservation. Sci. Hortic. 2016, 202, 41–48. [Google Scholar] [CrossRef] [Green Version]
- Aloui, H.; Khwaldia, K.; Sanchez-Gonzalez, L.; Muneret, L.; Jeandel, C.; Hamdi, M.; Desobry, S. Alginate coatings containing grapefruit essential oil or grapefruit seed extract for grapes preservation. Int. J. Food Sci. Technol. 2014, 49, 952–959. [Google Scholar] [CrossRef]
- Liu, P.; Krishnan, T.R. Alginate-pectin-poly-L-lysine particulate as a potential controlled release formulation. J. Pharm. Pharmacol. 1999, 51, 141–149. [Google Scholar] [CrossRef]
- Torre, M.L.; Giunchedi, P.; Maggi, L.; Stefli, R.; Machiste, E.O.; Conte, U. Formulation and characterization of calcium alginate beads containing ampicillin. Pharm. Dev. Technol. 1998, 3, 193–198. [Google Scholar] [CrossRef]
- Chan, L.W.; Heng, P.W. Effects of aldehydes and methods of cross-linking on properties of calcium alginate microspheres prepared by emulsification. Biomaterials 2002, 23, 1319–1326. [Google Scholar] [CrossRef]
- Kulkarni, A.R.; Soppimath, K.S.; Aralaguppi, M.I.; Aminabhavi, T.M.; Rudzinski, W.E. Preparation of cross-linked sodium alginate microparticles using glutaraldehyde in methanol. Drug Dev. Ind. Pharm. 2000, 26, 1121–1124. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.R.; Soppimath, K.S.; Aminabhavi, T.M.; Dave, A.M.; Mehta, M.H. Glutaraldehyde crosslinked sodium alginate beads containing liquid pesticide for soil application. J. Control. Release Off. J. Control. Release Soc. 2000, 63, 97–105. [Google Scholar] [CrossRef]
- Kulkarni, A.R.; Soppimath, K.S.; Aminabhavi, T.M.; Rudzinski, W.E. In-vitro release kinetics of cefadroxil-loaded sodium alginate interpenetrating network beads. Eur. J. Pharm. Biopharm. Off. J. Arb. Fur Pharm. Verfahr. E.V 2001, 51, 127–133. [Google Scholar] [CrossRef]
- Keppler, F.; Hamilton, J.T.; Brass, M.; Rockmann, T. Methane emissions from terrestrial plants under aerobic conditions. Nature 2006, 439, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Henri, B. Recherches sur un nouvel acide universellement répandu dans tous les vegetaux. Ann. De Chim. Et De Phys. 1825, 2, 173–178. [Google Scholar]
- WHO. Principles and Methods for the Risk Assessment of Chemicals in Food; WHO: Geneva, Switzerland, 2009. [Google Scholar]
- Yapo, B.M. Pectic substances: From simple pectic polysaccharides to complex pectins—A new hypothetical model. Carbohydr. Polym. 2011, 86, 373–385. [Google Scholar] [CrossRef]
- Mohnen, D. Pectin structure and biosynthesis. Curr. Opin. Plant Biol. 2008, 11, 266–277. [Google Scholar] [CrossRef]
- Willats, W.G.; McCartney, L.; Mackie, W.; Knox, J.P. Pectin: Cell biology and prospects for functional analysis. Plant Mol. Biol. 2001, 47, 9–27. [Google Scholar] [CrossRef]
- Monsoor, M.A.; Kalapathy, U.; Proctor, A. Improved method for determination of pectin degree of esterification by diffuse reflectance Fourier transform infrared spectroscopy. J. Agric. Food Chem. 2001, 49, 2756–2760. [Google Scholar] [CrossRef]
- Sungthongjeen, S.; Sriamornsak, P.; Pitaksuteepong, T.; Somsiri, A.; Puttipipatkhachorn, S. Effect of degree of esterification of pectin and calcium amount on drug release from pectin-based matrix tablets. AAPS PharmSciTech 2004, 5, E9. [Google Scholar] [CrossRef] [PubMed]
- Devries, J.; Denuijl, C.; Voragen, A.; Rombouts, F.; Pilnik, W. Structural features of the neutral sugar side chains of apple pectic substances. Carbohydr. Polym. 1983, 3, 193–205. [Google Scholar] [CrossRef]
- Coenen, G.J.; Bakx, E.J.; Verhoef, R.P.; Schols, H.A.; Voragen, A.G.J. Identification of the connecting linkage between homo- or xylogalacturonan and rhamnogalacturonan type I. Carbohydr. Polym. 2007, 70, 224–235. [Google Scholar] [CrossRef]
- Ridley, B.L.; O’Neill, M.A.; Mohnen, D. Pectins: Structure, biosynthesis, and oligogalacturonide-related signaling. Phytochemistry 2001, 57, 929–967. [Google Scholar] [CrossRef]
- Mishra, R.; Banthia, A.; Majeed, A. Pectin based formulations for biomedical applications: A review. Asian J. Pharm. Clin. Res. 2012, 5, 1–7. [Google Scholar] [CrossRef]
- Mishra, R.K.; Majeed, A.B.A.; Banthia, A.K. Development and characterization of pectin/gelatin hydrogel membranes for wound dressing. Int. J. Plast. Technol. 2011, 15, 82–95. [Google Scholar] [CrossRef]
- Coimbra, P.; Ferreira, P.; de Sousa, H.C.; Batista, P.; Rodrigues, M.A.; Correia, I.J.; Gil, M.H. Preparation and chemical and biological characterization of a pectin/chitosan polyelectrolyte complex scaffold for possible bone tissue engineering applications. Int. J. Biol. Macromol. 2011, 48, 112–118. [Google Scholar] [CrossRef]
- McCann, M.C.; Roberts, K. Plant cell wall architecture: The role of pectins and pectinases. In Pectins and Pectinases; Visser, J., Voragen, A.G.J., Eds.; Elsevier Science: Amsterdam, The Netherlands, 1996; pp. 91–107. [Google Scholar]
- Ashford, M.; Fell, J.; Attwood, D.; Sharma, H.; Woodhead, P. An Evaluation of Pectin as a Carrier for Drug Targeting to the Colon. J. Control. Release 1993, 26, 213–220. [Google Scholar] [CrossRef]
- Liu, L.; Fishman, M.L.; Hicks, K.B. Pectin in controlled drug delivery – a review. Cellulose 2006, 14, 15–24. [Google Scholar] [CrossRef]
- Graham, N.B.; McNeill, M.E. Hydrogels for controlled drug delivery. Biomaterials 1984, 5, 27–36. [Google Scholar] [CrossRef]
- Tiwari, S.B.; Murthy, T.K.; Pai, M.R.; Mehta, P.R.; Chowdary, P.B. Controlled release formulation of tramadol hydrochloride using hydrophilic and hydrophobic matrix system. AAPS PharmSciTech 2003, 4, E31. [Google Scholar] [CrossRef]
- Paharia, A.; Yadav, A.K.; Rai, G.; Jain, S.K.; Pancholi, S.S.; Agrawal, G.P. Eudragit-coated pectin microspheres of 5-fluorouracil for colon targeting. AAPS PharmSciTech 2007, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Sungthongjeen, S.; Pitaksuteepong, T.; Somsiri, A.; Sriamornsak, P. Studies on pectins as potential hydrogel matrices for controlled-release drug delivery. Drug Dev. Ind. Pharm. 1999, 25, 1271–1276. [Google Scholar] [CrossRef] [PubMed]
- Watts, P.; Smith, A. PecSys: In situ gelling system for optimised nasal drug delivery. Expert Opin. Drug Del. 2009, 6, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Beneke, C.E.; Viljoen, A.M.; Hamman, J.H. Polymeric plant-derived excipients in drug delivery. Molecules 2009, 14, 2602–2620. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, Z.; Sharma, S.; Khuller, G.K. Inhalable alginate nanoparticles as antitubercular drug carriers against experimental tuberculosis. Int. J. Antimicrob. Agents 2005, 26, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Ravi, V.; TM Pramod Kumar, S. Novel colon targeted drug delivery system using natural polymers. Indian J. Pharm. Sci. 2008, 70, 111–113. [Google Scholar] [CrossRef] [PubMed]
- Sriamornsak, P.; Wattanakorn, N.; Takeuchi, H. Study on the mucoadhesion mechanism of pectin by atomic force microscopy and mucin-particle method. Carbohydr. Polym. 2010, 79, 54–59. [Google Scholar] [CrossRef]
- Ludwig, A. The use of mucoadhesive polymers in ocular drug delivery. Adv. Drug Deliv. Rev. 2005, 57, 1595–1639. [Google Scholar] [CrossRef]
- Glinsky, V.V.; Raz, A. Modified citrus pectin anti-metastatic properties: One bullet, multiple targets. Carbohydr. Res. 2009, 344, 1788–1791. [Google Scholar] [CrossRef]
- Dhalleine, C.; Assifaoui, A.; Moulari, B.; Pellequer, Y.; Cayot, P.; Lamprecht, A.; Chambin, O. Zinc-pectinate beads as an in vivo self-assembling system for pulsatile drug delivery. Int. J. Pharm. 2011, 414, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Sriamornsak, P.; Puttipipatkhachorn, S. Chitosan-pectin composite gel spheres: Effect of some formulation variables on drug release. Macromol. Symp. 2004, 216, 17–21. [Google Scholar] [CrossRef]
- Perera, G.; Barthelmes, J.; Bernkop-Schnurch, A. Novel pectin-4-aminothiophenole conjugate microparticles for colon-specific drug delivery. J. Control. Release Off. J. Control. Release Soc. 2010, 145, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, A.; Jain, A.; Khare, P.; Agrawal, R.K.; Jain, S.K. Metronidazole loaded pectin microspheres for colon targeting. J. Pharm. Sci. 2009, 98, 4229–4236. [Google Scholar] [CrossRef] [PubMed]
- Jitpukdeebodintra, S.; Jangwang, A. Instant noodles with pectin for weight reduction. J. Food Agric. Environ. 2009, 7, 126–129. [Google Scholar]
- Radi, M.; Akhavan-Darabi, S.; Akhavan, H.R.; Amiri, S. The use of orange peel essential oil microemulsion and nanoemulsion in pectin-based coating to extend the shelf life of fresh-cut orange. J. Food Process. Preserv. 2017, 42, e13441. [Google Scholar] [CrossRef]
- Loth, F. Industrial Gums: Polysaccharides and Their Derivatives. 3rd edition. Edited by Roy L. Whistler and James N. BeMiller. ISBN 0-12-746253-8. Academic Press, Inc., San Diego/New York/Boston/London/Sidney/Tokyo/Toronto 1993. 642P. Acta Polym. 1993, 44, 172. [Google Scholar] [CrossRef]
- Oakenfull, D.G. The Chemistry of High-Methoxyl Pectins. In The Chemistry and Technology of Pectin; Walter, R.H., Ed.; Academic Press: San Diego, CA, USA, 1991; pp. 87–108. [Google Scholar] [CrossRef]
- Platt, D.; Raz, A. Modulation of the lung colonization of B16-F1 melanoma cells by citrus pectin. J. Natl. Cancer Inst. 1992, 84, 438–442. [Google Scholar] [CrossRef]
- Baldus, S.E.; Zirbes, T.K.; Weingarten, M.; Fromm, S.; Glossmann, J.; Hanisch, F.G.; Monig, S.P.; Schroder, W.; Flucke, U.; Thiele, J.; et al. Increased galectin-3 expression in gastric cancer: Correlations with histopathological subtypes, galactosylated antigens and tumor cell proliferation. Tumour Biol. 2000, 21, 258–266. [Google Scholar] [CrossRef]
- Kuwabara, I.; Liu, F.T. Galectin-3 promotes adhesion of human neutrophils to laminin. J. Immunol. 1996, 156, 3939–3944. [Google Scholar]
- Nangia-Makker, P.; Honjo, Y.; Sarvis, R.; Akahani, S.; Hogan, V.; Pienta, K.J.; Raz, A. Galectin-3 induces endothelial cell morphogenesis and angiogenesis. Am. J. Pathol. 2000, 156, 899–909. [Google Scholar] [CrossRef]
- Stegmayr, J.; Lepur, A.; Kahl-Knutson, B.; Aguilar-Moncayo, M.; Klyosov, A.A.; Field, R.A.; Oredsson, S.; Nilsson, U.J.; Leffler, H. Low or No Inhibitory Potency of the Canonical Galectin Carbohydrate-binding Site by Pectins and Galactomannans. J. Biol. Chem. 2016, 291, 13318–13334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johannes, L.; Jacob, R.; Leffler, H. Galectins at a glance. J. Cell Sci. 2018, 131. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Sun, L.; Yang, S.; He, C.; Tai, G.; Zhou, Y. The roles and mechanisms of homogalacturonan and rhamnogalacturonan I pectins on the inhibition of cell migration. Int. J. Biol. Macromol. 2018, 106, 207–217. [Google Scholar] [CrossRef]
- Minzanova, S.T.; Mironov, V.F.; Arkhipova, D.M.; Khabibullina, A.V.; Mironova, L.G.; Zakirova, Y.M.; Milyukov, V.A. Biological Activity and Pharmacological Application of Pectic Polysaccharides: A Review. Polymers 2018, 10, 1407. [Google Scholar] [CrossRef]
- Liu, S.Y.; Shi, X.J.; Xu, L.L.; Yi, Y.T. Optimization of pectin extraction and antioxidant activities from Jerusalem artichoke. Chin. J. Oceanol. Limnol. 2016, 34, 372–381. [Google Scholar] [CrossRef]
- Tharanathan, R.N. Biodegradable films and composite coatings: Past, present and future. Trends Food Sci. Technol. 2003, 14, 71–78. [Google Scholar] [CrossRef]
| Composite Material | Effect | Possible Application | Reference |
|---|---|---|---|
| Chitin nanocrystals | - Improve mechanical properties and transparency. | Packaging and food packaging. | [110] |
| Chitosan/MgO | - Improves mechanical properties; - Increases opacity; - Decreases swelling, permeability, and solubility; - Antimicrobial properties. | Food active packaging. | [16] |
| Chitosan with additional compounds of Propionic acid | - Propionic acid incorporation into chitosan films inhibits Candida spp and Penicillium spp growth; - Extended food shelf life by maintaining microbial growth in the latency period. | Antimicrobial films and coatings. | [111] |
| Chitosan with additional compounds of Microemulsions formed from C–BF–G as emulsifier additive with AIT and LAE as antimicrobials | - Micro emulsions create micro pores and micro channels that hold antimicrobials effectively; - Facilitates antimicrobial release from the center to the surface of films or coatings, thus enhancing their antimicrobial efficacy; - Films with 1% AIT reduced Listeria innocua populations in ready-to-eat meat and strawberries; - Films with 1% LAE reduced Escherichia coli and Salmonella spp. populations in strawberries. | Antimicrobial films and coatings. | [55] |
| Chitosan with additional compounds of PA | - Chitosan/PA composite films present more TPC and AA than chitosan films. | Antimicrobial films and coatings. | [112] |
| Chitosan with additional compounds of Hydroxybenzoic acids: GLA, GTA, PA, SA, and VA | - AA assays show that chitosan films with hydroxybenzoic acid have higher DPPH scavenging activity than films consisting of chitosan only; - GLA provides higher antioxidant activity. | Antimicrobial films and coatings. | [113] |
| Chitosan with additional compounds of Cymbopogon citratus (lemongrass) essential oil | - Coating decreases the severity of Rhizopus soft rot; - More significantly delays the infection when the fruit were artificially contaminated after coating application; - The application of the coating preserves the general quality of tomato fruit. | Applying coatings on fresh and cut fruits and vegetables. | [56] |
| Chitosan with additional compounds of Natamycin, nisin, pomegranate, and grape seed extract | - Coating reduces the O2 consumption of the fruit; - Shows better effects on delaying changes of pH, water activity, and TMC; - The incorporation of different antimicrobial agents into chitosan matrix does not reveal any significant effect. | Applying coatings on fresh and cut fruits and vegetables. | [114] |
| Chitosan with additional compounds of Salvia fruticosa Mill. extract | - The efficacy of the coating against grey mold is statistically equal to the synthetic fungicide thiabendazole; - Coating decreases the rate of fruit WL during cold storage, while preserved; - Coatings do not affect quality attributes and the bioactive compounds in table grapes. | Applying coatings on fresh and cut fruits and vegetables. | [19] |
| Chitosan with additional compounds of thyme essential oil nanoparticles | - The coating reduces the incidence of C. gloeosporioides on avocado; - Coating does not affect the quality of avocado; - Fruit is better maintained than untreated fruit. | Applying coatings on fresh and cut fruits and vegetables. | [115] |
| Chitosan with GP | - Casting method and film physical form. | Antimicrobial films and coatings. | [116] |
| Chitosan with FAA | - Coating physical form. | Oil barrier packaging. | [117] |
| Chitosan with additional compounds of Lemongrass oil | - Coating with nanodroplet of oil shows higher initial inhibition of Salmonella typhimurium; - Greater growth inhibition of microorganisms and higher retention of color; - AA and better SE during storage. | Applying coatings on fresh and cut fruits and vegetables. | [118] |
| Chitosan with GP and GTE | - Casting method and film physical form. | Active food packaging. | [119] |
| Edible polymers pectin–fish gelatin with glycerol plasticizer and Glutaraldehyde additives | - Casting method and film physical form. | Packaging or coating of food or drugs. | [23] |
| Composite Material | Effect | Possible Application | Reference |
|---|---|---|---|
| Alginate with additional compounds of Ag nanoparticles | - Provide antimicrobial and antiviral properties. | Fresh food packaging, packaging for agricultural products. | [166,167,168,169,170,171] |
| Alginate/nano-clays Mnt and CNC from MCC | - Decrease water solubility; - Increase surface hydrophobicity with CNC and decrease of this parameter with nanoclay addition; - Reduction in WVP; - Tensile properties improved. | Food packaging. | [15] |
| Alginate with additional compounds of LEO or OEO | - The lower capacity for scavenging ABTS free radicals or quenching singlet oxygen; - The coatings with the essential orange oil are very efficient for controlling yeast and mold growth. | Applying coatings on fresh and cut fruits and vegetables. | [61] |
| Alginate with additional compounds of OO | - Coatings decrease DR, WL, and total sugars and increase the level of antioxidants; - The delayed activity of PG, PL, and PME was noticed in coated fruit representing the reduced softening and ripening process. | Applying coatings on fresh and cut fruits and vegetables. | [62] |
| Alginate with additional compounds of tea polyphenols | - Coatings decrease red indices, TCC, RR, electrolyte leakage, and malonaldehyde content and maintain the AAC, TPC, and the activities of antioxidant enzymes while have no significant effect on firmness. | Applying coatings on fresh and cut fruits and vegetables. | [18] |
| Alginate with additional compounds of Ficus hirta fruit extract | - The DR, WL, RR, and MDA content is much lower in the coated samples; - The coating treatment enhances the activities of antioxidant and defense-related enzymes such as SOD, CAT, CHI, GLU, and PAL and the accumulation of phenolic compounds. | Applying coatings on fresh and cut fruits and vegetables. | [172] |
| Alginate with additional compounds of GSE or GEO | - Coatings reduce WL, maintain firmness during storage, preserve the antioxidant activity of treated grapes, and decrease DR in inoculated fruit. | Applying coatings on fresh and cut fruits and vegetables. | [173] |
| Sodium Alginate with GP and garlic oil additives | - Casting method and film physical form. | Antibacterial food applications. | [24] |
| Sodium alginate with calcium chloride additives | - Sprayer methods and coating physical form. | Food protection. | [63] |
| Composite Material | Effect | Possible Application | Reference |
|---|---|---|---|
| Pectin PEG Halloysite nanotubes | - Decrease wettability; - Improve mechanical properties. | Coatings for food conservation. | [14] |
| Pectin with additional compounds of AAC, CAC and SC | - Coatings reduce microbial spoilage; - They do not significantly influence sensory and nutritional qualities. | Applying coatings on fresh and cut fruits and vegetables. | [73] |
| Pectin with additional compounds of citral and eugenol | - Coatings are not cytotoxic and do not considerably change the general physicochemical and nutritional characteristics of raspberries; - The impact is mainly on decreasing food spoilage microorganisms and accordingly extending shelf-life. | Applying coatings on fresh and cut fruits and vegetables. | [17] |
| Pectin with additional compounds of OEO | - Coatings with OEO exhibit antifungal influence on inoculated tomatoes; - Increase TPC and AA; - The sensorial acceptability of the coated tomatoes is well accepted by panelists. | Applying coatings on fresh and cut fruits and vegetables. | [72] |
| Pectin with additional compounds of OPEO | - Coatings reduce the quality loss and improve the sensory scores during storage; - Nano emulsion-based nano coatings containing essential oil have been effective in bacterial and fungal inactivation. | Applying coatings on fresh and cut fruits and vegetables. | [213] |
| Pectin–gelatin with GP | Crosslinking than air drying method and film physical form. | Biomedical product. | [22] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martău, G.A.; Mihai, M.; Vodnar, D.C. The Use of Chitosan, Alginate, and Pectin in the Biomedical and Food Sector—Biocompatibility, Bioadhesiveness, and Biodegradability. Polymers 2019, 11, 1837. https://doi.org/10.3390/polym11111837
Martău GA, Mihai M, Vodnar DC. The Use of Chitosan, Alginate, and Pectin in the Biomedical and Food Sector—Biocompatibility, Bioadhesiveness, and Biodegradability. Polymers. 2019; 11(11):1837. https://doi.org/10.3390/polym11111837
Chicago/Turabian StyleMartău, Gheorghe Adrian, Mihaela Mihai, and Dan Cristian Vodnar. 2019. "The Use of Chitosan, Alginate, and Pectin in the Biomedical and Food Sector—Biocompatibility, Bioadhesiveness, and Biodegradability" Polymers 11, no. 11: 1837. https://doi.org/10.3390/polym11111837
APA StyleMartău, G. A., Mihai, M., & Vodnar, D. C. (2019). The Use of Chitosan, Alginate, and Pectin in the Biomedical and Food Sector—Biocompatibility, Bioadhesiveness, and Biodegradability. Polymers, 11(11), 1837. https://doi.org/10.3390/polym11111837







