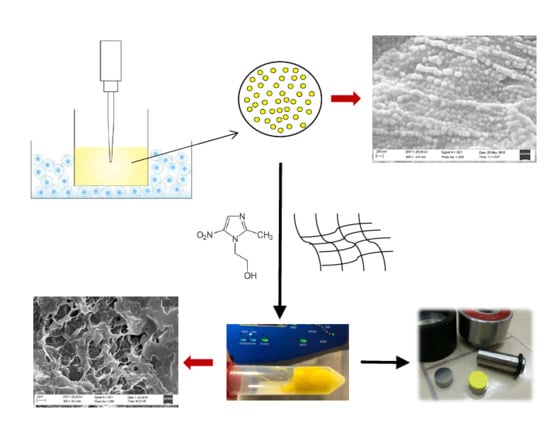
Bioadhesive Matrix Tablets Loaded with Lipophilic Nanoparticles as Vehicles for Drugs for Periodontitis Treatment: Development and Characterization
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Lipid Ratio Mixtures Screening Studies
2.3. Preparation of CUR-Loaded NLC
2.4. NLCs Characterization
2.4.1. Dynamic Light Scattering (DLS) and Z-Potential Measurements
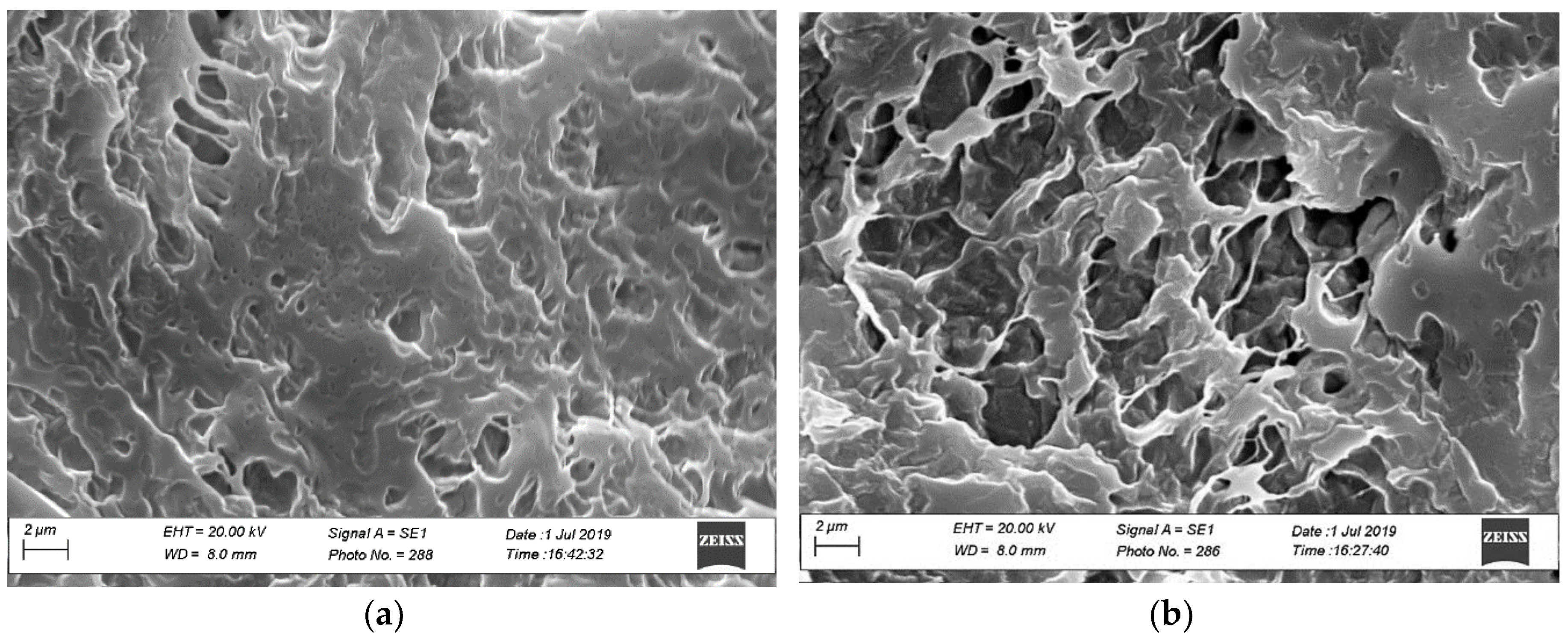
2.4.2. Morphology by Scanning Electron Microscopy (SEM)
2.4.3. Entrapment Efficacy, Drug Loading and Drug Recovery of NLCs
CUR Quantification in Fresh NLC Dispersion
- Dialysis assay: Dialysis tube (molecular weight cut off, MWCO, 12–14,000 Da, Visking Dialysis Membrane, Medicell Membranes Ltd., London, UK) was pre-activated and filled by 2 mL of CUR-NLC dispersion and submerged in 350 mL of distilled water, keeping at room temperature and under magnetic stir. After 24 h both the dispersion inside the tube and the external water were analyzed by HPLC.
- Ultrafiltration assay: Aliquots of 0.45 mL of fresh samples were centrifuged (Microfuge 22R, Beckman coulter™ Brea, CA, USA) in two Ultrafree-MC (Millipore, Burlington, MA, USA) devices, with membrane cut-off of 10,000 NMWL and 30,000 NMWL, at 8000 rpm and 4 °C for 30 min [26]. In the end, the liquid ultrafiltrate was analyzed by HPLC.
2.5. Preparation of Sponges Loaded with MTR and CUR-NLC.
Tablets Preparation
2.6. Tablets Characterization
2.6.1. Porosity
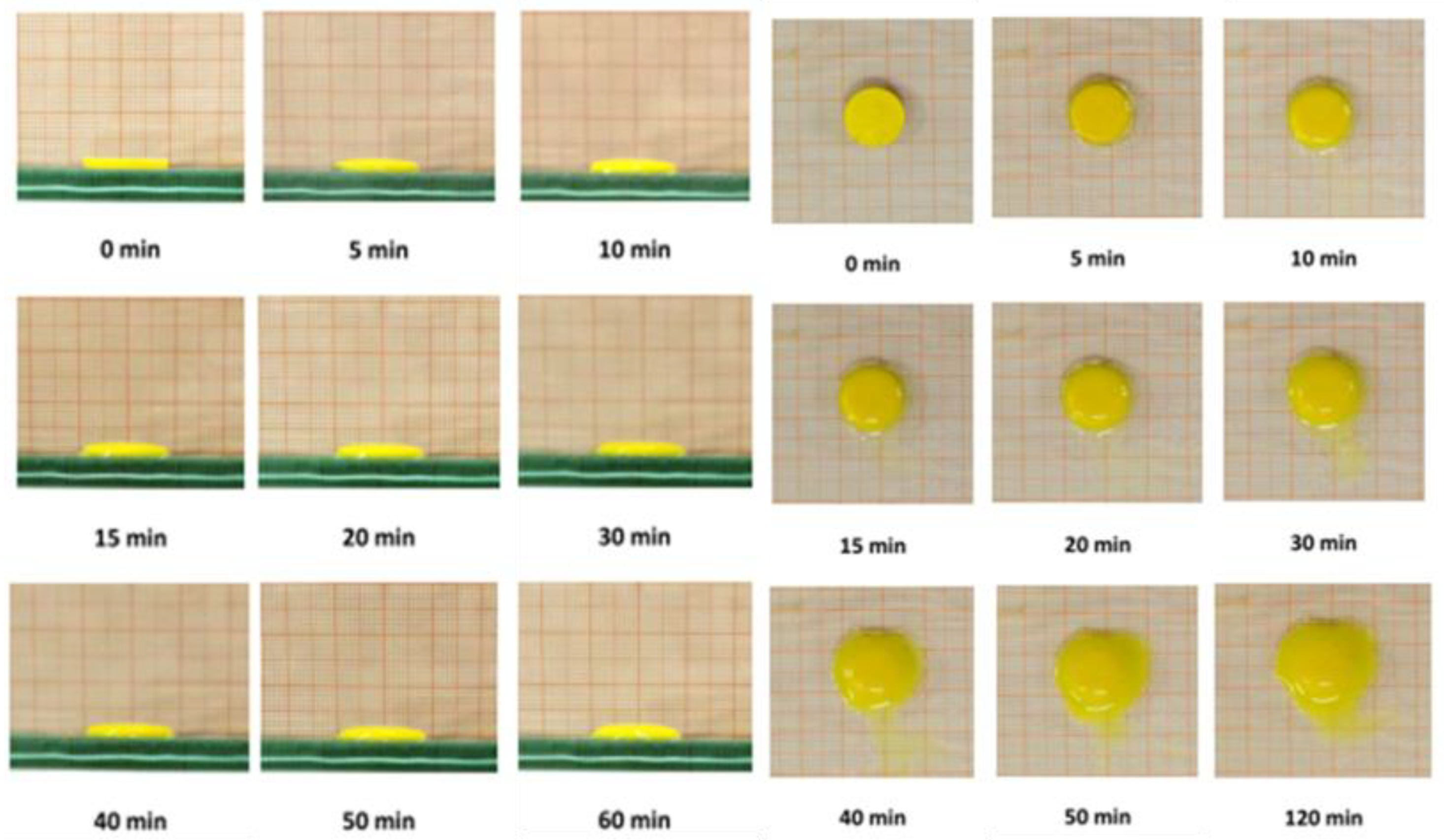
2.6.2. Swelling Test
2.6.3. Ex Vivo Mucoadhesion Strength Measurement
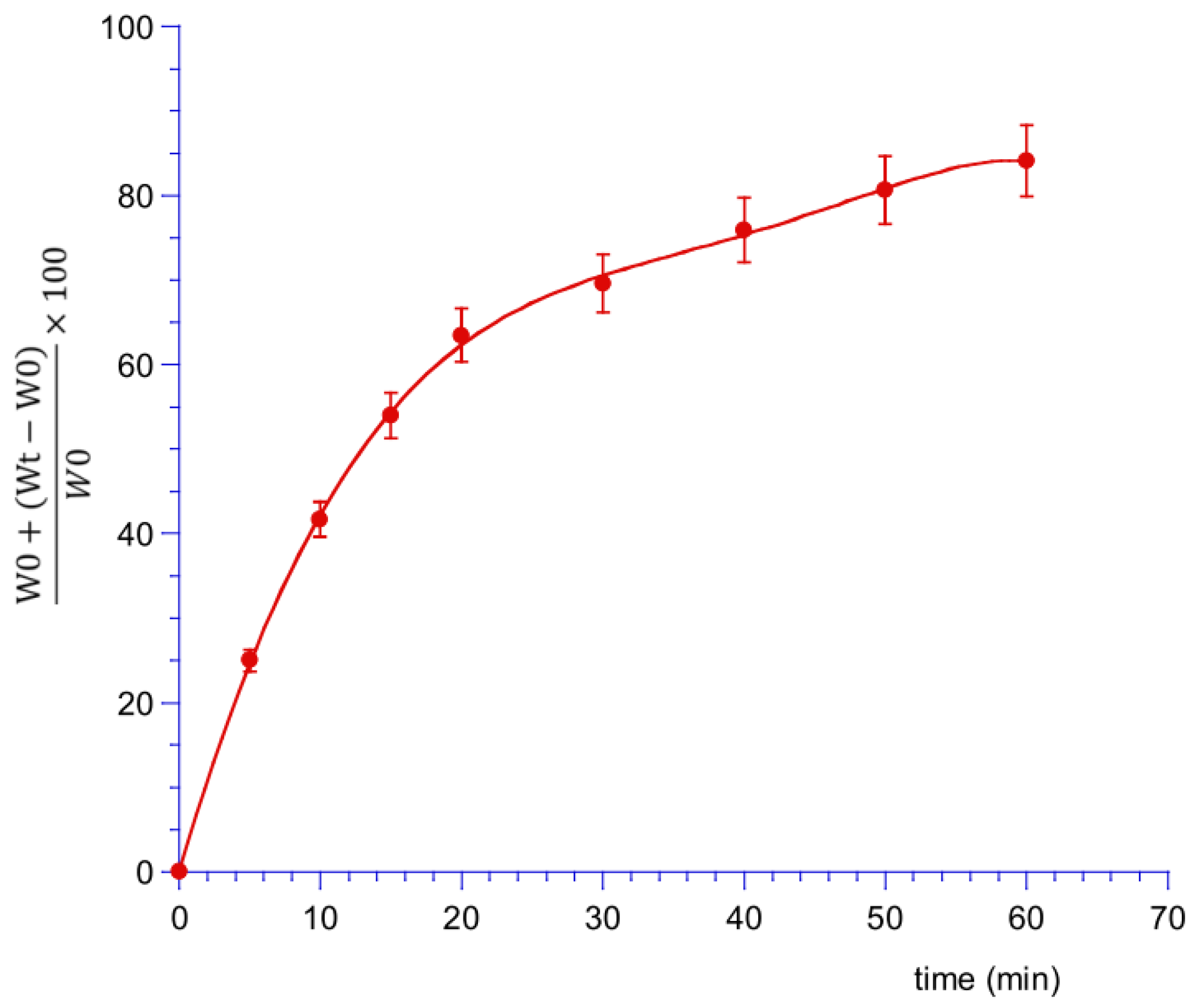
2.6.4. In Vitro CUR and MTR Release Studies
2.7. Ex Vivo Permeation and Penetration of CUR and MTR throughout Porcine Buccal Mucosa
Quantification of CUR and MTR Entrapped into Porcine Mucosa
2.8. Drugs Assay
2.8.1. UV-Vis Method
2.8.2. HPLC Method
2.9. Data Analysis
3. Results and Discussion
3.1. CUR-NLC Formulation and Characterization
3.2. Sponges Loaded with MTR and CUR-NLCs and Tablet Formulation and Characterization
3.3. Ex Vivo Mucoadhesion Strength Measurement
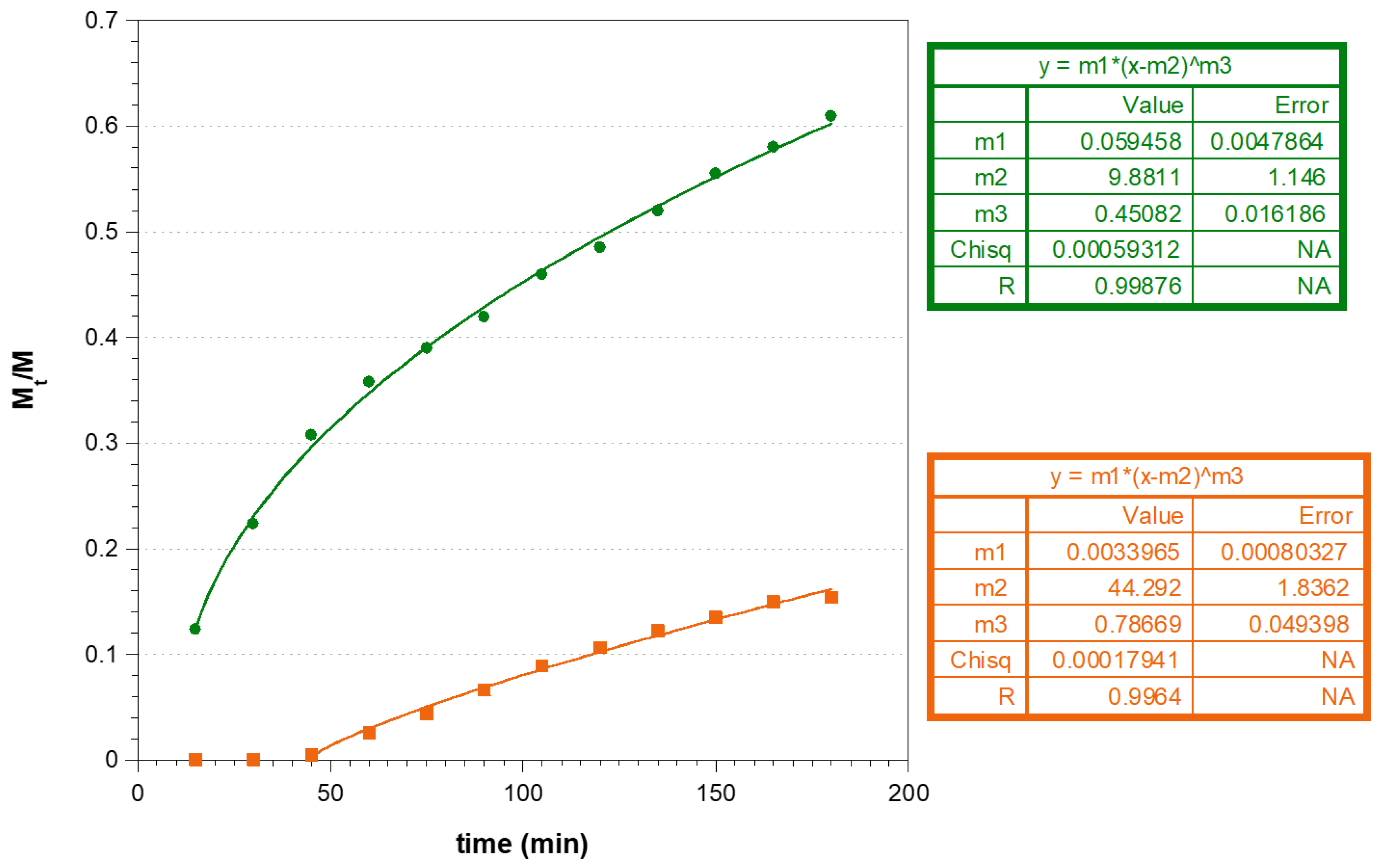
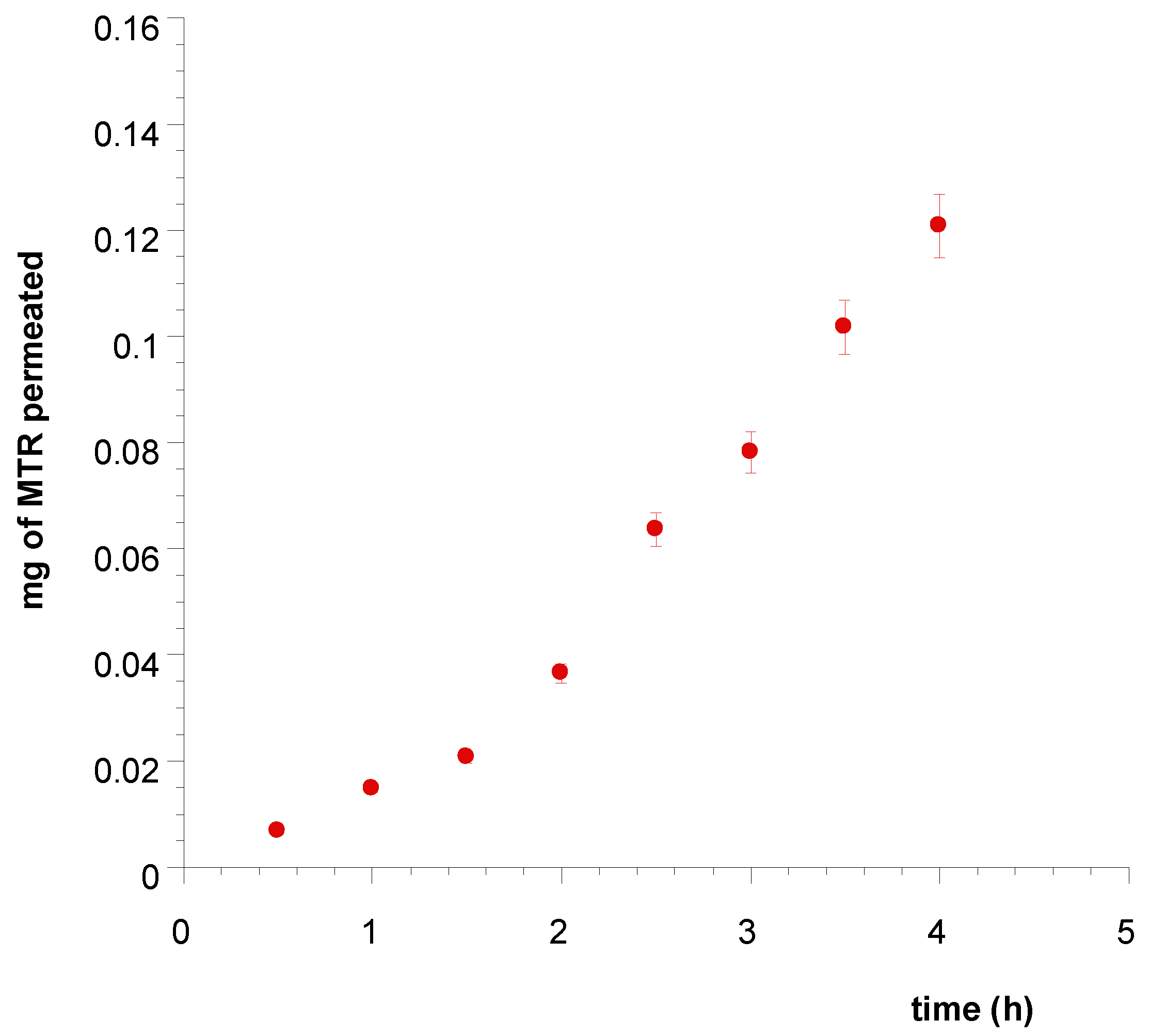
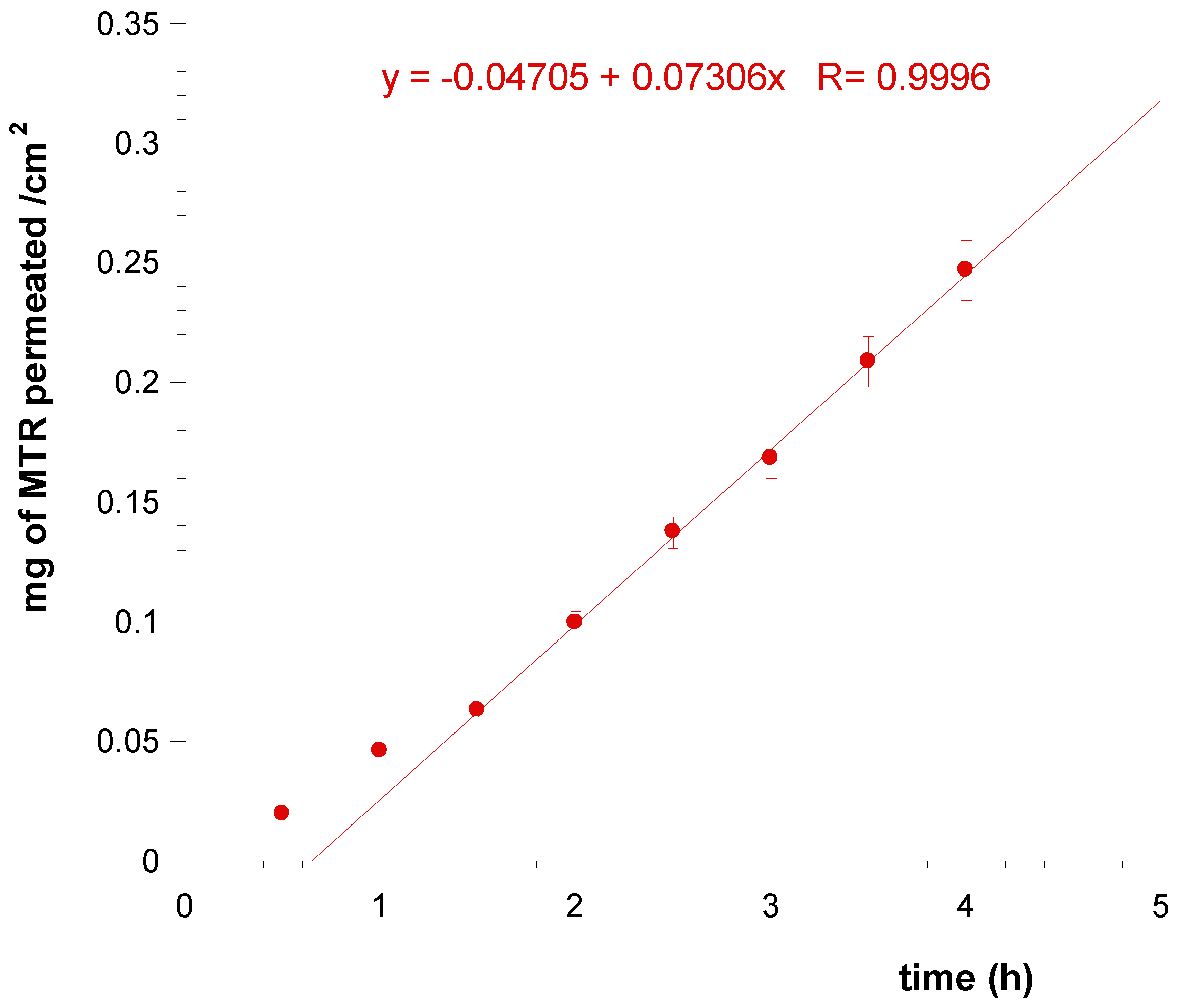
3.4. Ex Vivo Permeation and Penetration of CUR and MTR throughout Porcine Mucosa
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dahlén, G. Bacterial infections of the oral mucosa. Periodontol. 2000 2009, 49, 13–38. [Google Scholar] [CrossRef]
- Hoare, A.; Soto, C.; Rojas-Celis, V.; Bravo, D. Chronic Inflammation as a Link between Periodontitis and Carcinogenesis. Mediat. Inflamm. 2019, 2019, 1029857. [Google Scholar] [CrossRef] [PubMed]
- Cekici, A.; Kantarci, A.; Hasturk, H.; Van Dyke, T.E. Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontol. 2000 2014, 64, 57–80. [Google Scholar] [CrossRef] [PubMed]
- Kesarwala, A.H.; Krishna, M.C.; Mitchell, J.B. Oxidative stress in oral diseases. Oral Dis. 2016, 22, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Murgia, D.; Mauceri, R.; Campisi, G.; De Caro, V. Advance on resveratrol application in bone regeneration: Progress and perspectives for use in oral and maxillofacial surgery. Biomolecules 2019, 9, 94. [Google Scholar] [CrossRef]
- Sato, S.; Fonseca, M.J.V.; Del Ciampo, J.O.; Jabor, J.R.; Pedrazzi, V. Metronidazole-containing gel for the treatment of periodontitis: An in vivo evaluation. Braz. Oral Res. 2008, 22, 145–150. [Google Scholar] [CrossRef]
- Labib, G.S.; Aldawsari, H.M.; Badr-eldin, S.M. Metronidazole and Pentoxifylline films for the local treatment of chronic periodontal pockets: Preparation, in vitro evaluation and clinical assessment. Expert Opin. Drug Deliv. 2014, 11, 855–865. [Google Scholar] [CrossRef]
- Lo, S.; Edlund, C.; Nord, C.E. Metronidazole Is Still the Drug of Choice for Treatment of Anaerobic Infections. Clin. Infect. Dis. 2010, 50. [Google Scholar] [CrossRef]
- Brook, I.; Wexler, H.M.; Goldstein, E.J.C. Antianaerobic antimicrobials: Spectrum and susceptibility testing. Clin. Microbiol. Rev. 2013, 26, 526–546. [Google Scholar] [CrossRef]
- Zheng, Q.T.; Yang, Z.H.; Yu, L.Y.; Ren, Y.Y.; Huang, Q.X.; Liu, Q.; Ma, X.Y.; Chen, Z.K.; Wang, Z.B.; Zheng, X. Synthesis and antioxidant activity of curcumin analogs. J. Asian Nat. Prod. Res. 2017, 19, 489–503. [Google Scholar] [CrossRef]
- Gupta, S.C.; Patchva, S.; Aggarwal, B.B. Therapeutic roles of curcumin: Lessons learned from clinical trials. AAPS J. 2012, 15, 195–218. [Google Scholar] [CrossRef] [PubMed]
- Menon, V.P.; Sudheer, A.R. Antioxidant and Anti-Inflammatory Properties of Curcumin; Aggarwal, B.B., Surh, Y.-J., Shishodia, S., Eds.; Springer: Boston, MA, USA, 2007; Volume 595. [Google Scholar]
- Akbik, D.; Ghadiri, M.; Chrzanowski, W.; Rohanizadeh, R. Curcumin as a wound healing agent. Life Sci. 2014, 116, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Jacob, P.; Nath, S.; Sultan, O. Use of Curcumin in Periodontal Inflammation. Interdiscip. J. Microinflamm. 2014, 1. [Google Scholar] [CrossRef]
- Dave, D.H.; Patel, P.; Shah, M.; Dadawala, S.M.; Saraiya, K.; Sant, A.V. Comparative Evaluation of Efficacy of Oral Curcumin Gel as an Adjunct to Scaling and Root Planing in the Treatment of Chronic Periodontitis. Adv. Hum. Biol. 2018, 64–69. [Google Scholar] [CrossRef]
- Jain, S.; Rama, S.; Meka, K.; Chatterjee, K. Curcumin eluting nanofibers augment osteogenesis toward phytochemical based bone tissue engineering. Biomed. Mater. 2016, 11, 055007. [Google Scholar] [CrossRef]
- Joshi, D.; Garg, T.; Goyal, A.K.; Rath, G. Advanced drug delivery approaches against periodontitis. Drug Deliv. 2016, 23, 363–377. [Google Scholar] [CrossRef]
- De Caro, V.; Scaturro, A.L.; Di Prima, G.; Avellone, G.; Sutera, F.M.; Di Fede, O.; Campisi, G.; Giannola, L.I. Aloin delivery on buccal mucosa: Ex vivo studies and design of a new locoregional dosing system. Drug Dev. Ind. Pharm. 2015, 41, 1541–1547. [Google Scholar] [CrossRef]
- De Caro, V.; Ajovalasit, A.; Sutera, F.M.; Murgia, D.; Sabatino, M.A.; Dispenza, C. Development and Characterization of an Amorphous Solid Dispersion of Furosemide in the Form of a Sublingual Bioadhesive Film to Enhance Bioavailability. Pharmaceutics 2017, 9, 22. [Google Scholar] [CrossRef]
- Laffleur, F. Mucoadhesive polymers for buccal drug delivery. Drug Dev. Ind. Pharm. 2014, 40, 591–598. [Google Scholar] [CrossRef]
- Morantes, S.J.; Buitrago, D.M.; Ibla, J.F.; García, Y.M.; Lafaurie, G.I.; Parraga, J.E. 5- Composites of hydrogels and nanoparticles: A potential solution to current challenges in buccal drug delivery. In Biopolymer-Based Composites; Jana, S., Maiti, S., Jana, S., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; pp. 107–138. [Google Scholar]
- Müller, R.H.; Radtke, M.; Wissing, S. A Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv. Drug Deliv. Rev. 2002, 54 (Suppl. 1), S131–S155. [Google Scholar]
- Marques, A.C.; Rocha, A.I.; Leal, P.; Estanqueiro, M.; Lobo, J.M.S. Development and characterization of mucoadhesive buccal gels containing lipid nanoparticles of ibuprofen. Int. J. Pharm. 2017, 533, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Hazzah, H.A.; Farid, R.M.; Nasra, M.M.A.; EL-Massik, M.A.; Abdallah, O.Y. Lyophilized sponges loaded with curcumin solid lipid nanoparticles for buccal delivery: Development and characterization. Int. J. Pharm. 2015, 492, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Naksuriya, O.; Van Steenbergen, M.J.; Torano, J.S.; Okonogi, S.; Hennink, W.E. A Kinetic Degradation Study of Curcumin in Its Free Form and Loaded in Polymeric Micelles. AAPS J. 2016, 18, 777–787. [Google Scholar] [CrossRef] [PubMed]
- Pardeike, J.; Weber, S.; Zarfl, H.P.; Pagitz, M.; Zimmer, A. Itraconazole-loaded nanostructured lipid carriers (NLC) for pulmonary treatment of aspergillosis in falcons. Eur. J. Pharm. Biopharm. 2016, 108, 269–276. [Google Scholar] [CrossRef]
- Ho, M.-H.; Kuo, P.-Y.; Hsieh, H.-J.; Hsien, T.-Y.; Hou, L.-T.; Lai, J.-Y.; Wang, D.-M. Preparation of porous scaffolds by using freeze-extraction and freeze-gelation methods. Biomaterials 2004, 25, 129–138. [Google Scholar] [CrossRef]
- Kassem, M.A.; Elmeshad, A.N.; Fares, A.R. Enhanced bioavailability of buspirone hydrochloride via cup and core buccal tablets: Formulation and in vitro/in vivo evaluation. Int. J. Pharm. 2014, 463, 68–80. [Google Scholar] [CrossRef]
- Ng, S.-F.; Rouse, J.; Sanderson, D.; Eccleston, G. A Comparative Study of Transmembrane Diffusion and Permeation of Ibuprofen across Synthetic Membranes Using Franz Diffusion Cells. Pharmaceutics 2010, 2, 209–223. [Google Scholar] [CrossRef]
- Wang, D.-P.; Yeh, M.-K. Degradation kinetics of metronidazole in solution. J. Pharm. Sci. 1993, 82, 95–98. [Google Scholar] [CrossRef]
- Bruschi, M.L. 5- Mathematical models of drug release. In Strategies to Modify the Drug Release from Pharmaceutical Systems; Bruschi, M.L., Ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2015; pp. 63–86. [Google Scholar] [CrossRef]
- De Caro, V.; Giandalia, G.; Siragusa, M.G.; Sutera, F.M.; Giannola, L.I. New prospective in treatment of Parkinson’s disease: Studies on permeation of ropinirole through buccal mucosa. Int. J. Pharm. 2012, 429, 78–83. [Google Scholar] [CrossRef]
- Del Consuelo, I.D.; Pizzolato, G.P.; Falson, F.; Guy, R.H.; Jacques, Y. Evaluation of pig esophageal mucosa as a permeability barrier model for buccal tissue. J. Pharm. Sci. 2005, 94, 2777–2788. [Google Scholar] [CrossRef]
- De Caro, V.; Giandalia, G.; Siragusa, M.G.; Paderni, C.; Campisi, G.; Giannola, L.I. Evaluation of galantamine transbuccal absorption by reconstituted human oral epithelium and porcine tissue as buccal mucosa models: Part I. Eur. J. Pharm. Biopharm. 2008, 70, 869–873. [Google Scholar] [CrossRef] [PubMed]
- Di Prima, G.; Conigliaro, A.; De Caro, V. Mucoadhesive Polymeric Films to Enhance Barbaloin Penetration Into Buccal Mucosa: A Novel Approach to Chemoprevention. AAPS PharmSciTech 2019, 20, 18. [Google Scholar] [CrossRef] [PubMed]
- Shetty, A.V.; Thirugnanam, S.; Dakshinamoorthy, G.; Kajdacsy-balla, A.; Gnanasekar, M. 18α-glycyrrhetinic acid targets prostate cancer cells by down-regulating inflammation-related genes. Int. J. Oncol. 2011, 39, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Damle, M. Glycyrrhiza glabra (Liquorice)—A potent medicinal herb. Int. J. Herb. Med. 2014, 2, 132–136. [Google Scholar]
- Trotta, M.; Peira, E.; Debernardi, F.; Gallarate, M. Elastic liposomes for skin delivery of dipotassium glycyrrhizinate. Int. J. Pharm. 2002, 241, 319–327. [Google Scholar] [CrossRef]
- Long, D.R.; Mead, J.; Hendricks, J.M.; Hardy, M.E.; Voyich, J.M. 18β-glycyrrhetinic acid inhibits methicillin-resistant Staphylococcus aureus survival and attenuates virulence gene expression. Antimicrob. Agents Chemother. 2013, 57, 241–247. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Pan, M.-H.; Cheng, A.-L.; Lin, L.-I.; Ho, Y.-S.; Hsieh, C.-Y.; Lin, J.-K. Stability of curcumin in buffer solutions and characterization of its degradation products. J. Pharm. Biomed. Anal. 1997, 15, 1867–1876. [Google Scholar] [CrossRef]
- Brar, S.K.; Verma, M. Measurement of nanoparticles by light-scattering techniques. TrAC Trends Anal. Chem. 2011, 30, 4–17. [Google Scholar] [CrossRef]
- Thatipamula, R.P.; Palem, C.R.; Gannu, R.; Mudragada, S.; Yamsani, M.R. Formulation and in vitro characterization of domperidone loaded solid lipid nanoparticles and nanostructured lipid carriers. DARU J. Pharm. Sci. 2011, 19, 23–32. [Google Scholar]
- Caccavo, D.; Cascone, S.; Lamberti, G.; Barba, A.A.; Larsson, A. Swellable Hydrogel-based Systems for Controlled Drug Delivery. In Smart Drug Delivery System; IntechOpen: London, UK, 2016. [Google Scholar] [CrossRef]


| Sample | CUR % | GA % | HEXA % | IP % | Melting Point °C |
|---|---|---|---|---|---|
| Mixture 1 | 1 | 1 | 39.2 | 58.8 | 31 ± 2 °C |
| Mixture 2 | 1 | 1 | 58.8 | 39.2 | 40 ± 2 °C |
| Mixture 3 | 1 | 3 | 58 | 38 | 45 ± 2 °C |
| Mixture 4 | 2.5 | 3 | 56.7 | 37.8 | 48 ± 2 °C |
| Empty | 0 | 3 | 57.3 | 38.2 | 42 ± 2 °C |
| . | Tween 20% | Z-Average (nm) | PDI | Z-Potential (mV) |
|---|---|---|---|---|
| Mixture 3 | 0.5 | 100 | 0.242 | −24.6 |
| 1 | 100 | 0.274 | −24.4 | |
| Mixture 4 | 0.5 | 121.6 | 0.235 | −37.4 |
| 1 | 121.8 | 0.272 | −33.2 | |
| Empty | 0.5 | 129.4 | 0.216 | −8.4 |
| Contact Time (min) | Force of Adhesion (N) | Detachment Force (N/m2) |
|---|---|---|
| 5 | 0.064 | 486.81 |
| 10 | 0.073 | 549.50 |
| 15 | 0.100 | 752.35 |
| 20 | 0.146 | 1095.33 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murgia, D.; Angellotti, G.; D’Agostino, F.; De Caro, V. Bioadhesive Matrix Tablets Loaded with Lipophilic Nanoparticles as Vehicles for Drugs for Periodontitis Treatment: Development and Characterization. Polymers 2019, 11, 1801. https://doi.org/10.3390/polym11111801
Murgia D, Angellotti G, D’Agostino F, De Caro V. Bioadhesive Matrix Tablets Loaded with Lipophilic Nanoparticles as Vehicles for Drugs for Periodontitis Treatment: Development and Characterization. Polymers. 2019; 11(11):1801. https://doi.org/10.3390/polym11111801
Chicago/Turabian StyleMurgia, Denise, Giuseppe Angellotti, Fabio D’Agostino, and Viviana De Caro. 2019. "Bioadhesive Matrix Tablets Loaded with Lipophilic Nanoparticles as Vehicles for Drugs for Periodontitis Treatment: Development and Characterization" Polymers 11, no. 11: 1801. https://doi.org/10.3390/polym11111801
APA StyleMurgia, D., Angellotti, G., D’Agostino, F., & De Caro, V. (2019). Bioadhesive Matrix Tablets Loaded with Lipophilic Nanoparticles as Vehicles for Drugs for Periodontitis Treatment: Development and Characterization. Polymers, 11(11), 1801. https://doi.org/10.3390/polym11111801