Preparation and Evaluation of Glucose Based Non-Isocyanate Polyurethane Self-Blowing Rigid Foams
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of the Glucose Based Non-Isocyanate Polyurethane(g-NIPU)
2.3. Preparation of NIPU Foams
2.4. FTIR
2.5. MALDI-TOF Analysis
2.6. Water Absorption (24 h)
2.7. Compression
2.8. Ignition Test
3. Results and Discussion
3.1. Effect of Amount of Maleic Acid Addition on Performance of the Foams
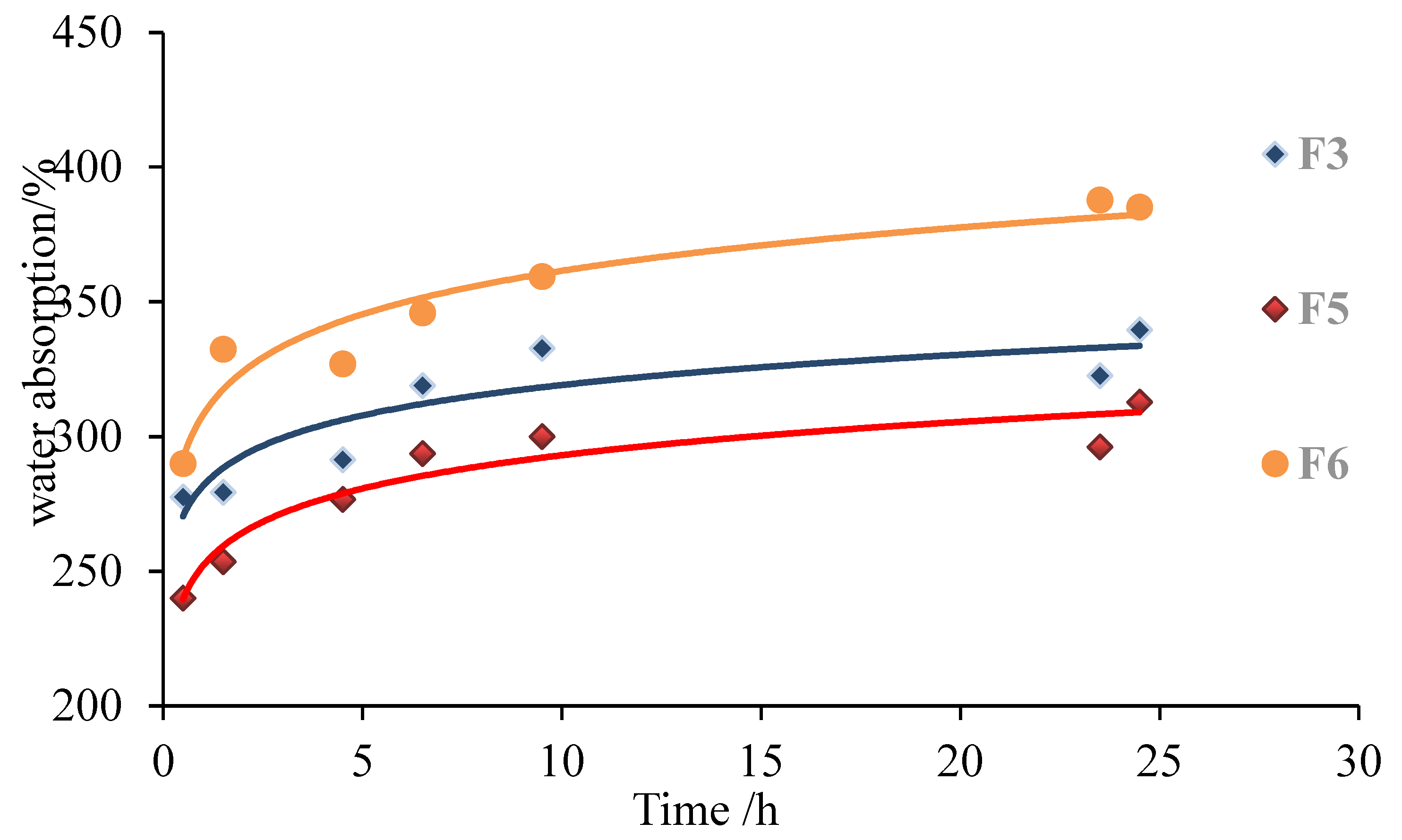
3.2. Effect of Glutaraldehyde Addition Amounts on Basic Performance of Foams
3.3. Foam Compression Resistance
3.4. SEM Analysis
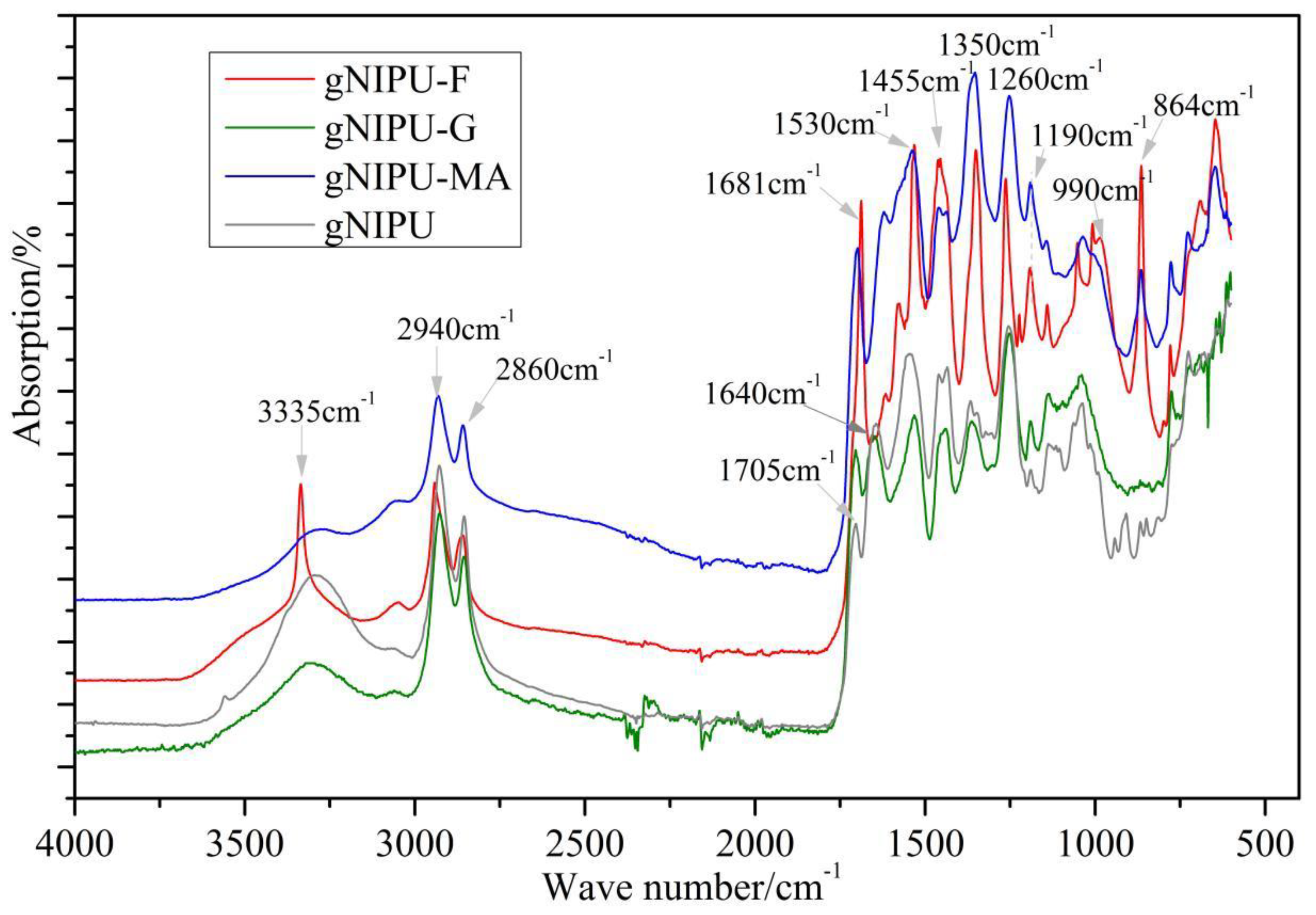
3.5. FTIR Analysis
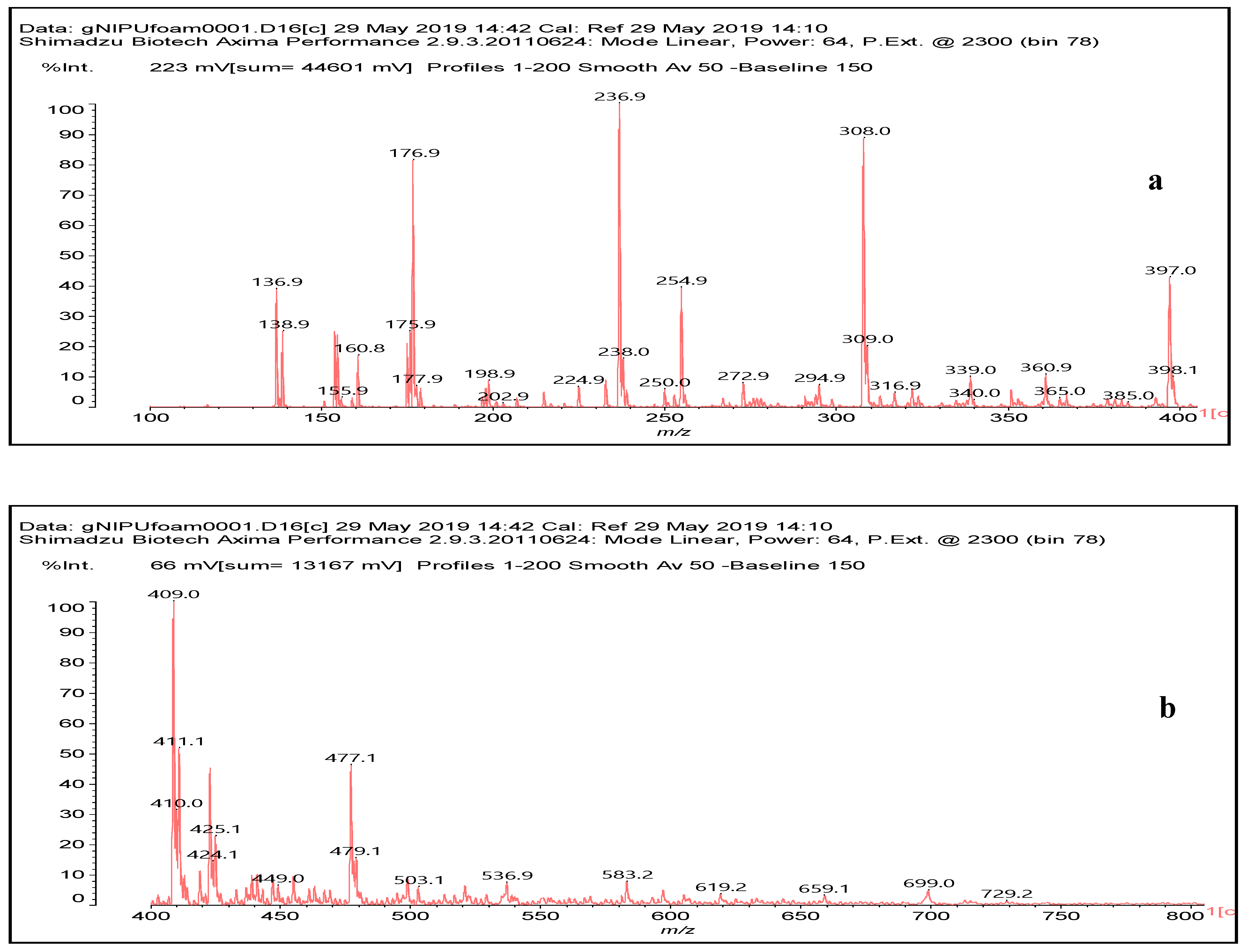
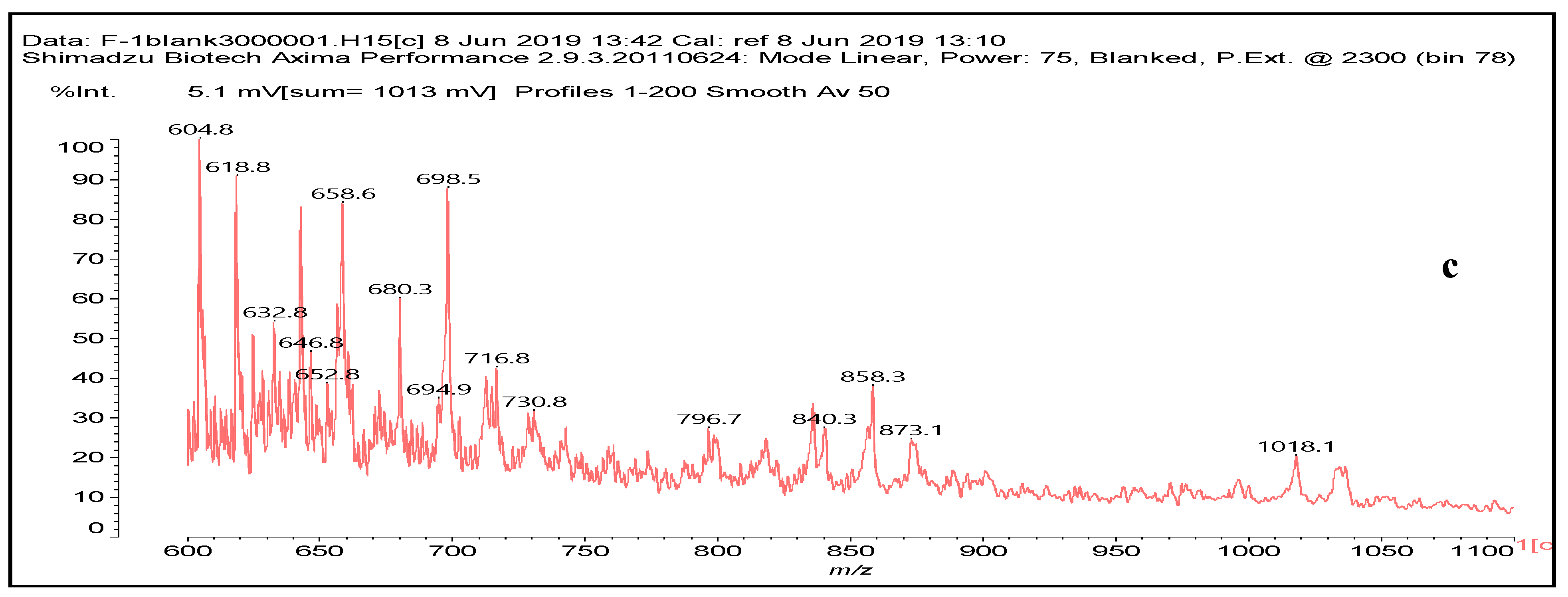
3.6. MALDI ToF Analysis
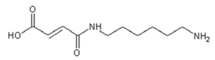
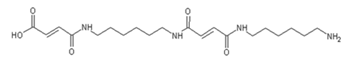
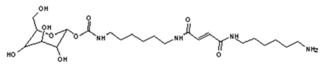
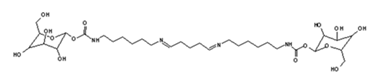
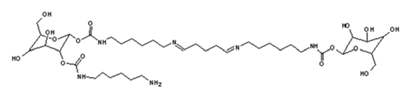
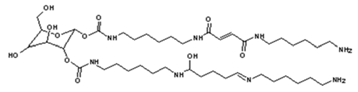
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Kihara, N.; Endo, T. Synthesis and properties of poly (hydroxyurethane)s. J. Polym. Sci. Part A 1993, 31, 2765–2773. [Google Scholar] [CrossRef]
- Rokicki, G.; Piotrowska, A. A new route to polyurethanes from ethylene carbonate, diamines and diols. Polymer 2002, 43, 2927–2935. [Google Scholar] [CrossRef]
- Ochiai, B.; Inoue, S.; Endo, T. One-pot non-isocyanate synthesis of polyurethanes from bisepoxide, carbon dioxide, and diamine. J. Polym. Sci. Part A 2005, 43, 6613–6618. [Google Scholar] [CrossRef]
- Bähr, M.; Bitto, A.; Mülhaupt, R. Cyclic limonene dicarbonate as a new monomer for non-isocyanate oligo-and polyurethanes (NIPU) based upon terpenes. Green Chem. 2012, 14, 1447–1454. [Google Scholar] [CrossRef]
- Blattmann, H.; Fleischer, M.; Bähr, M.; Mülhaupt, R. Isocyanate-and phosgene-free routes to polyfunctional cyclic carbonates and green polyurethanes by fixation of carbon dioxide. Macromol. Rapid Commun. 2014, 35, 1238–1254. [Google Scholar] [CrossRef]
- Büttner, H.; Steinbauer, J.; Wulf, C.; Dindaroglu, M.; Schmalz, H.G.; Werner, T. Organocatalyzed synthesis of oleochemical carbonates from CO2 and renewables. ChemSusChem 2017, 10, 1076–1079. [Google Scholar] [CrossRef] [PubMed]
- Figovsky, O.; Shapovalov, L.; Leykin, A.; Birukova, O.; Potashnikova, R. Recent advances in the development of non-isocyanate polyurethanes based on cyclic carbonates. PU Mag. 2013, 10, 1–9. [Google Scholar]
- Sheng, X.; Ren, G.; Qin, Y.; Chen, X.; Wang, X.; Wang, F. Quantitative synthesis of bis (cyclic carbonate)s by iron catalyst for non-isocyanate polyurethane synthesis. Green Chem. 2015, 17, 373–379. [Google Scholar] [CrossRef]
- Kathalewar, M.S.; Joshi, P.B.; Sabnis, A.S.; Malshe, V.C. Non-isocyanate polyurethanes: From chemistry to applications. RSC Adv. 2013, 3, 4110–4129. [Google Scholar] [CrossRef]
- Rokicki, G.; Parzuchowski, P.G.; Mazurek, M. Non-isocyanate polyurethanes: Synthesis, properties, and applications. Polym. Adv. Technol. 2015, 26, 707–761. [Google Scholar] [CrossRef]
- Wu, Z.; Dai, J.; Tang, L.; Qu, J. Sorbitol-based aqueous cyclic carbonate dispersion for waterborne nonisocyanate polyurethane coatings via an environment-friendly route. J. Coat. Technol. Res. 2019, 16, 721–732. [Google Scholar] [CrossRef]
- Lee, A.; Deng, Y. Green polyurethane from lignin and soybean oil through non-isocyanate reactions. Eur. Polym. J. 2015, 63, 67–73. [Google Scholar] [CrossRef]
- Tamami, B.; Sohn, S.; Wilkes, G.L. Incorporation of carbon dioxide into soybean oil and subsequent preparation and studies of nonisocyanate polyurethane networks. J. Appl. Polym. Sci. 2004, 92, 883–891. [Google Scholar] [CrossRef]
- Zhang, K.; Nelson, A.M.; Talley, S.J.; Chen, M.; Margaretta, E.; Hudson, A.G.; Moore, R.B.; Long, T.E. Non-isocyanate poly (amide-hydroxyurethane) s from sustainable resources. Green Chem. 2016, 18, 4667–4681. [Google Scholar] [CrossRef]
- Poussard, L.; Mariage, J.; Grignard, B.; Detrembleur, C.; Jérôme, C.; Calberg, C.; Heinrichs, B.; De Winter, J.; Gerbaux, P.; Raquez, J.-M.; et al. Non-isocyanate polyurethanes from carbonated soybean oil using monomeric or oligomeric diamines to achieve thermosets or thermoplastics. Macromolecules 2016, 49, 2162–2171. [Google Scholar] [CrossRef]
- Grignard, B.; Thomassin, J.M.; Gennen, S.; Poussard, L.; Bonnaud, L.; Raquez, J.-M.; Dubois, P.; Tran, M.-P.; Park, C.B.; Jerome, C.; et al. CO2-blown microcellular non-isocyanate polyurethane (NIPU) foams: From bio-and CO2-sourced monomers to potentially thermal insulating materials. Green Chem. 2016, 18, 2206–2215. [Google Scholar] [CrossRef]
- Esmaeili, N.; Zohuriaan-Mehr, M.J.; Salimi, A.; Vafayan, M.; Meyer, W. Tannic acid derived non-isocyanate polyurethane networks: Synthesis, curing kinetics, antioxidizing activity and cell viability. Thermochim. Acta 2018, 664, 64–72. [Google Scholar] [CrossRef]
- Tundo, P.; Selva, M. The chemistry of dimethyl carbonate. Acc. Chem. Res. 2002, 35, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Lamarzelle, O.; Durand, P.L.; Wirotius, A.L.; Chollet, G.; Grau, E.; Cramail, H. Activated lipidic cyclic carbonates for non-isocyanate polyurethane synthesis. Polym. Chem. 2016, 7, 1439–1451. [Google Scholar] [CrossRef]
- Unverferth, M.; Kreye, O.; Prohammer, A.; Meier, M.A. Renewable Non-Isocyanate Based Thermoplastic Polyurethanes via Polycondensation of Dimethyl Carbamate Monomers with Diols. Macromol. Rapid Commun. 2013, 34, 1569–1574. [Google Scholar] [CrossRef]
- Tang, D.; Mulder, D.J.; Noordover, B.A.J.; Koning, C.E. Well-defined Biobased Segmented Polyureas Synthesis via a TBD-catalyzed Isocyanate-free Route. Macromol. Rapid Commun. 2011, 32, 1379–1385. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, V.M.; Dhulst, E.A.; Leitsch, E.K.; Wilmot, N.; Heath, W.H.; Gies, A.P.; Miller, M.D.; Torkelson, J.M.; Scheidt, K.A. Cooperative Catalysis of Cyclic Carbonate Ring Opening: Application Towards Non-Isocyanate Polyurethane Materials. Eur. J. Org. Chem. 2015, 13, 2791–2795. [Google Scholar] [CrossRef]
- Duval, C.; Kébir, N.; Charvet, A.; Martin, A.; Burel, F. Synthesis and properties of renewable nonisocyanate polyurethanes (NIPU s) from dimethylcarbonate. J. Polym. Sci. Part A 2015, 53, 1351–1359. [Google Scholar] [CrossRef]
- Thebault, M.; Pizzi, A.; Dumarçay, S.; Gerardin, P.; Fredon, E.; Delmotte, L. Polyurethanes from hydrolysable tannins obtained without using isocyanates. Ind. Crop. Prod. 2014, 59, 329–336. [Google Scholar] [CrossRef]
- Thebault, M.; Pizzi, A.; Essawy, H.A.; Barhoum, A.; Assche, G.V. Isocyanate free condensed tannin-based polyurethanes. Eur. Polym. J. 2015, 67, 513–526. [Google Scholar] [CrossRef]
- Thebault, M.; Pizzi, A.; Santiago-Medina, F.J.; Al-Marzouki, F.M.; Abdalla, S. Isocyanate-free polyurethanes by coreaction of condensed tannins with aminated tannins. J. Renew. Mater. 2017, 5, 21–29. [Google Scholar] [CrossRef]
- Santiago-Medina, F.J.; Basso, M.C.; Pizzi, A.; Delmotte, L. Polyurethanes from kraft lignin without using isocyanates. J. Renew. Mater. 2018, 6, 413–425. [Google Scholar] [CrossRef]
- Xi, X.; Pizzi, A.; Delmotte, L. Isocyanate-free polyurethane coatings and adhesives from mono-and di-saccharides. Polymers 2018, 10, 402. [Google Scholar] [CrossRef]
- Xi, X.; Wu, Z.; Pizzi, A.; Gerardin, C.; Lei, H.; Zhang, B.; Du, G. Non-isocyanate polyurethane adhesive from sucrose used for particleboard. Wood Sci. Technol. 2019, 53, 393–405. [Google Scholar] [CrossRef]
- Xi, X.; Pizzi, A.; Gerardin, C.; Du, J. Glucose-biobased Non-Isocyanate Polyurethane Rigid Foams. J. Renew. Mater. 2019, 7, 301–312. [Google Scholar] [CrossRef]
- Sprung, M.A. A Summary of the Reactions of Aldehydes with Amines. Chem. Rev. 1940, 26, 297–338. [Google Scholar] [CrossRef]
- Tanaka, K.; Shiraishi, R. Clean and efficient condensation reactions of aldehydes and amines in a water suspension medium. Green Chem. 2000, 2, 272–273. [Google Scholar] [CrossRef]
- Tondi, G.; Pizzi, A. Tannin-based rigid foams: Characterization and modification. Ind. Crop. Prod. 2009, 29, 356–363. [Google Scholar] [CrossRef]
- He, X.; Xu, X.; Wan, Q.; Bo, G.; Yan, Y. Solvent-and Catalyst-free Synthesis, Hybridization and Characterization of Biobased Nonisocyanate Polyurethane (NIPU). Polymers 2019, 11, 1026. [Google Scholar] [CrossRef] [PubMed]
- Pathak, R.; Kathalewar, M.; Wazarkar, K.; Sabnis, A. Non-isocyanate polyurethane (NIPU) from tris-2-hydroxy ethyl isocyanurate modified fatty acid for coating applications. Prog. Org. Coat. 2015, 89, 160–169. [Google Scholar] [CrossRef]
- Ke, J.; Li, X.; Jiang, S.; Liamg, C.; Wang, J.; Kang, M.; Li, Q.; Zhao, Y. Promising approaches to improve the performances of hybrid non-isocyanate polyurethane. Polym. Int. 2019, 68, 651–660. [Google Scholar] [CrossRef]
- Marques, M.M.B. Catalytic Enantioselective Cross-Mannich Reaction of Aldehydes. Angew. Chem. Int. Ed. 2006, 45, 348–352. [Google Scholar] [CrossRef]
- Melchiorre, P. Cinchona-based primary amine catalysis in the asymmetric functionalization of carbonyl compounds. Angew. Chem. Int. Ed. 2012, 51, 9748–9770. [Google Scholar] [CrossRef]
- Tolbert, T.L.; Houston, B. The Preparation of Aldimines through the Stephen Reaction1. J. Org. Chem. 1963, 28, 695–697. [Google Scholar] [CrossRef]
- Hasell, T.; Schmidtmann, M.; Stone, C.A.; Smith, M.W.; Cooper, A.I. Reversible water uptake by a stable imine-based porous organic cage. Chem. Commun. 2012, 48, 4689–4691. [Google Scholar] [CrossRef]
- Basso, M.C.; Pizzi, A.; Al-Marzouki, F.; Abdalla, S. Horticultural/hydroponics and floral foams from tannins. Ind. Crop. Prod. 2016, 87, 177–181. [Google Scholar] [CrossRef]

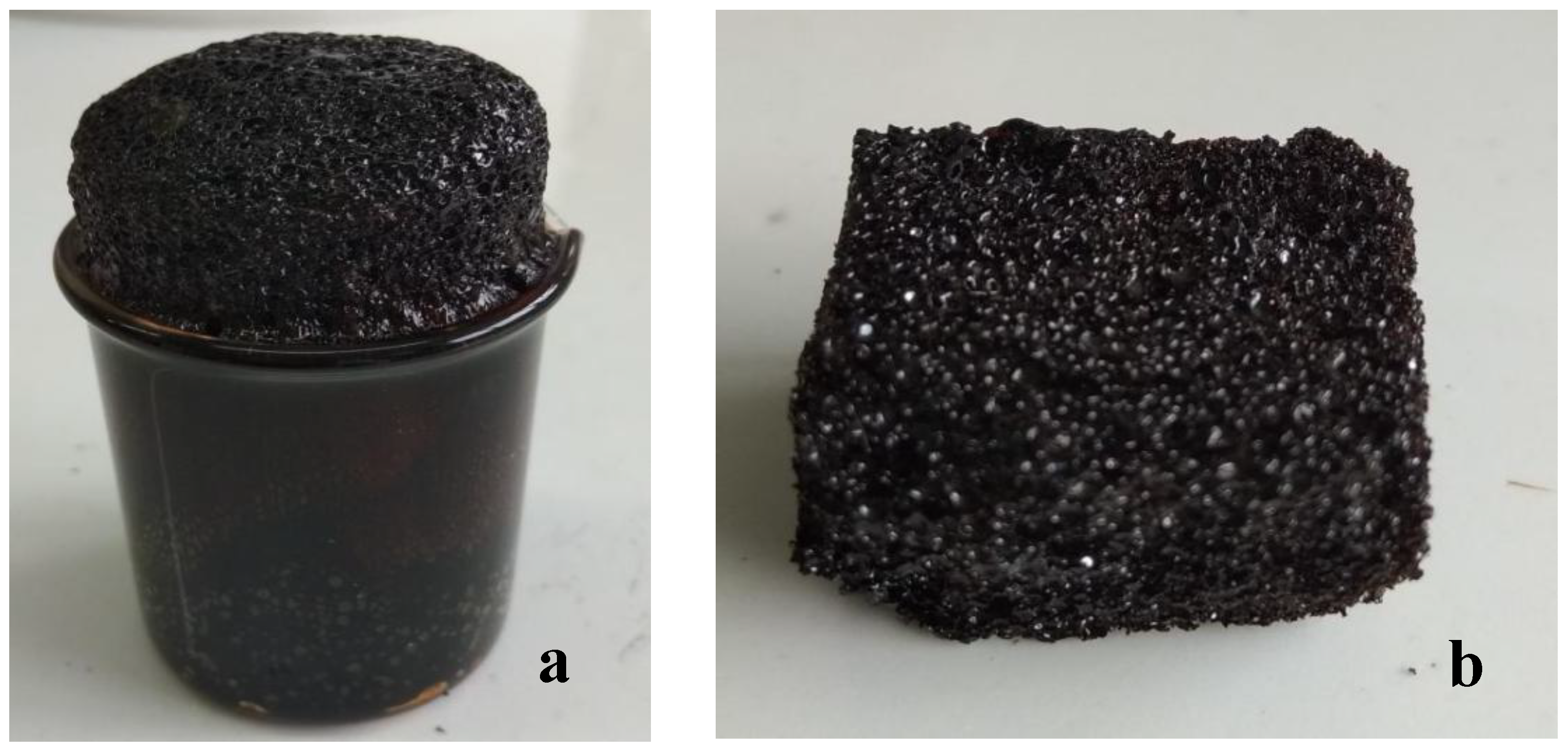



| No. | g-NIPU (g) | Maleic Acid (g) | Glutaraldehyde (g) |
|---|---|---|---|
| F2 | 10 | 1.0 | 2.5 |
| F3 | 10 | 1.2 | 2.5 |
| F4 | 10 | 1.5 | 2.5 |
| F5 | 10 | 1.2 | 1.8 |
| F6 | 10 | 1.2 | 3.6 |
| No. | Formulations | Density (g/cm3) | 2 h Water Absorption (%) | Ignition Time (s) |
|---|---|---|---|---|
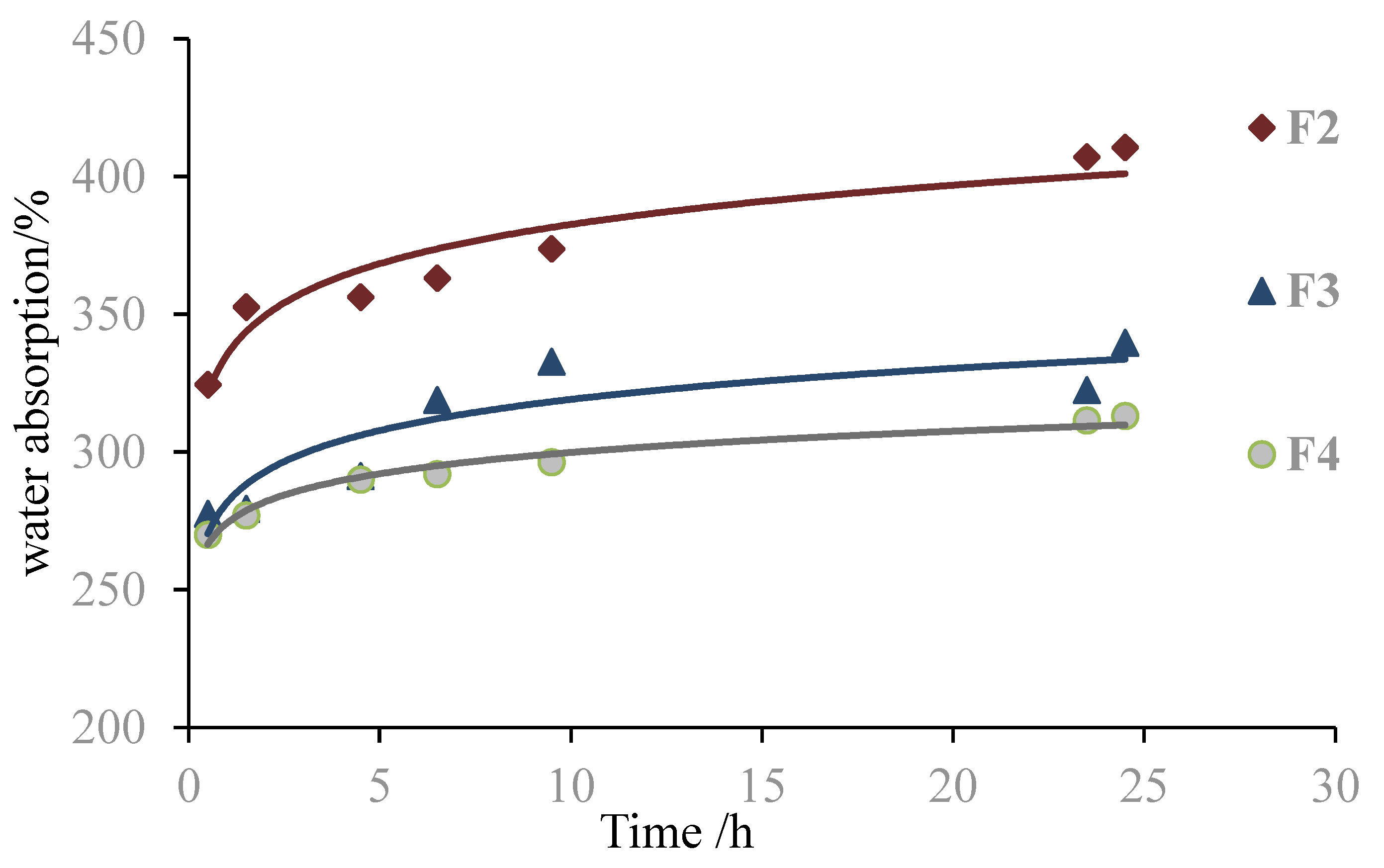
| F2 | 10 g g-NIPU + 1.0 g MA + 2.5 g G | 0.13 | 352.6 | 140 |
| F3 | 10 g g-NIPU + 1.2 g MA + 2.5 g G | 0.09 | 279.3 | 120 |
| F4 | 10 g g-NIPU + 1.5 g MA + 2.5 g G | 0.08 | 276.9 | 88 |
| F5 | 10 g g-NIPU + 1.2 g MA + 1.8 g G | 0.10 | 253.5 | 130 |
| F6 | 10 g g-NIPU + 1.2 g MA + 3.6 g G | 0.13 | 332.4 | 175 |
| 236.9 Da (calc. 237) = Small molecule of maleic acid reacts with hexamethylenediamine with 23 (Na+) |
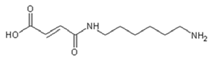 |
| 322 Da (calc. 322) = molecule of urethane structure of “glucose–carbonate–diamine” without Na+ |
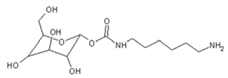 |
| 409–411 Da (calc. 410) = reaction results of two molecules “maleic acid–diamine” without Na+ |
 |
| 422 Da = 322Da react with glutaraldehyde without Na+ |
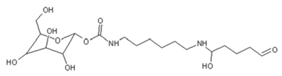 |
| 520 Da = 422 Da react with diamine without Na+ |
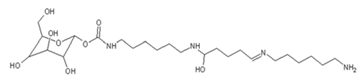 |
| 528 Da = urethane structure of “glucose–carbonate–diamine–carbonate–glucose” without Na+ |
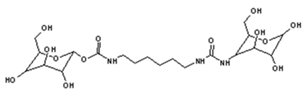 |
| 541 Da = molecule of “glucose–carbonate–diamine–maleic acid–diamine” with Na+ |
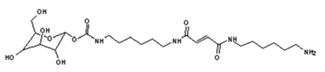 |
| 564 Da = urethane structure of “glucose–carbonate–diamine–carbonate–diamine” with one aldehyde without Na+ |
 |
| 618 Da = molecule of “glucose–carbonate–diamine–aldehyde–diamine–maleic acid” without Na+ |
 |
| 644 Da = molecule of “glucose–2×carbonate–2×diamine–aldehyde–maleic acid” without Na+ |
 |
| 731 Da = molecule of” 2×glucose–carbonate–diamine” connected with aldehyde as a bridge, with Na+ |
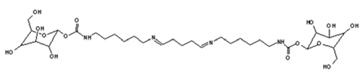 |
| 858 Da = Dendrimer of “glucose–2×carbonate–4×diamine–aldehyde–maleic acid” without Na+ |
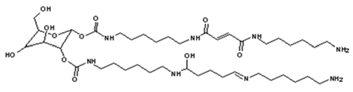 |
| 873 Da = 731 Da react with carbonate and diamine, without Na+ |
 |
| 1019 Da = 873 Da react with one aldehyde, with Na+ |
 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xi, X.; Pizzi, A.; Gerardin, C.; Lei, H.; Chen, X.; Amirou, S. Preparation and Evaluation of Glucose Based Non-Isocyanate Polyurethane Self-Blowing Rigid Foams. Polymers 2019, 11, 1802. https://doi.org/10.3390/polym11111802
Xi X, Pizzi A, Gerardin C, Lei H, Chen X, Amirou S. Preparation and Evaluation of Glucose Based Non-Isocyanate Polyurethane Self-Blowing Rigid Foams. Polymers. 2019; 11(11):1802. https://doi.org/10.3390/polym11111802
Chicago/Turabian StyleXi, Xuedong, Antonio Pizzi, Christine Gerardin, Hong Lei, Xinyi Chen, and Siham Amirou. 2019. "Preparation and Evaluation of Glucose Based Non-Isocyanate Polyurethane Self-Blowing Rigid Foams" Polymers 11, no. 11: 1802. https://doi.org/10.3390/polym11111802
APA StyleXi, X., Pizzi, A., Gerardin, C., Lei, H., Chen, X., & Amirou, S. (2019). Preparation and Evaluation of Glucose Based Non-Isocyanate Polyurethane Self-Blowing Rigid Foams. Polymers, 11(11), 1802. https://doi.org/10.3390/polym11111802







