Preparation and Characterization of Flame-Retarded Poly(butylene terephthalate)/Poly(ethylene terephthalate) Blends: Effect of Content and Type of Flame Retardant
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of TAD
2.3. Preparation of Flame-Retardant PBT/PET Blend
2.4. Characterization
3. Results and Discussion
3.1. Flame-Retardant Performance
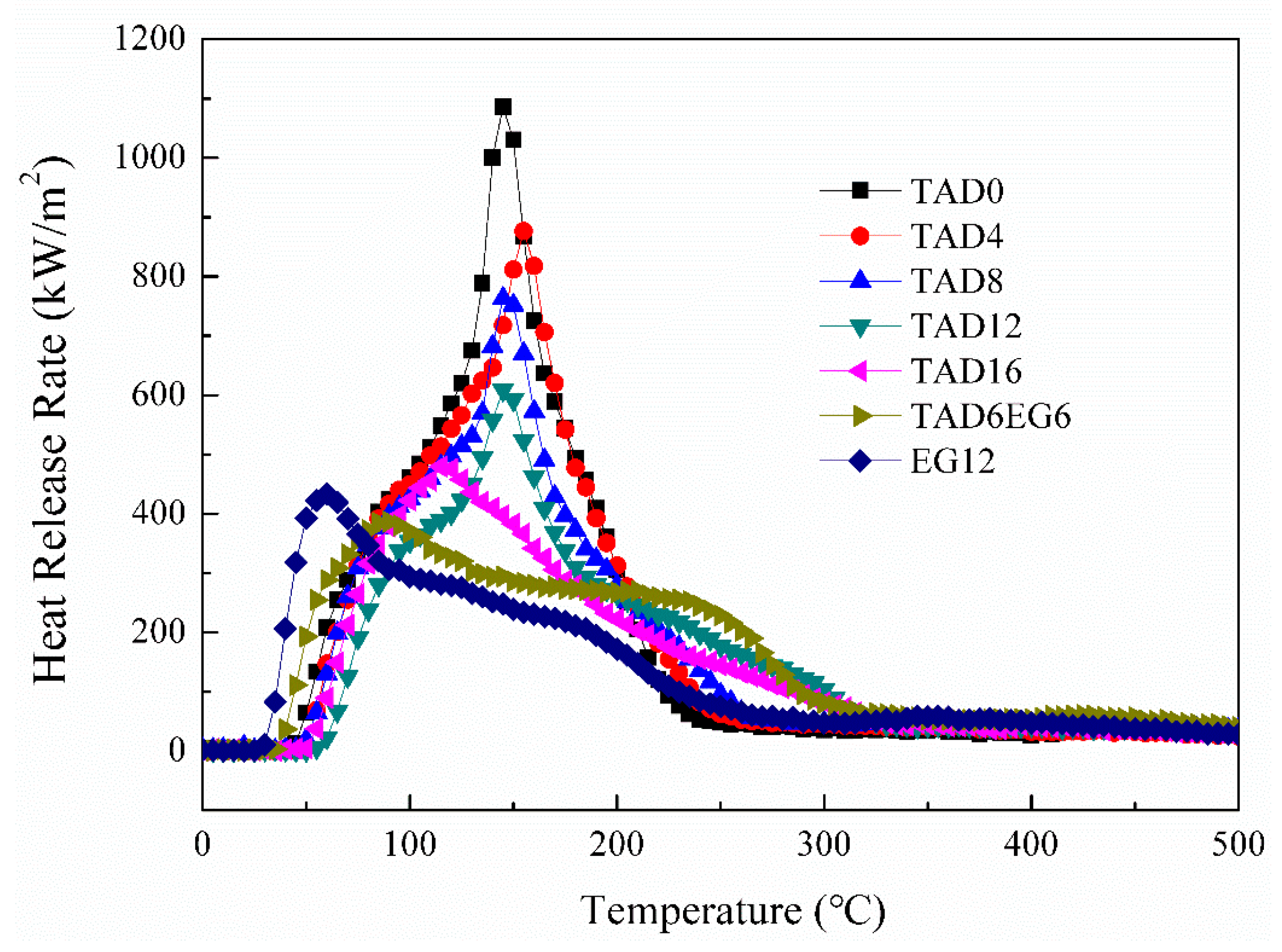
3.2. Cone Calorimeter Test
3.3. Thermal Stability
3.4. Morphology
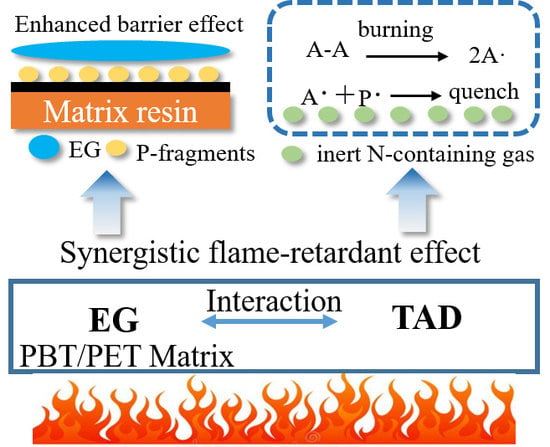
3.5. Flame-Retardant Mechanism
3.6. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hao, Y.; Li, W.; Sun, S.; Zhang, H.; Gao, G.; Dong, L. Poly(butylene terephthalate) Toughening with Butadiene-Epoxy-Functionalized Methyl Methacrylate Core–Shell Copolymer. J. Macromol. Sci. B 2015, 54, 1267–1281. [Google Scholar] [CrossRef]
- Pesetskii, S.S.; Jurkowski, B.; Filimonov, O.V.; Koval, V.N.; Golubovich, V.V. PET/PC Blends: Effect of Chain Extender and Impact Strength Modifier on Their Structure and Properties. J. Appl. Polym. Sci. 2011, 119, 225–234. [Google Scholar] [CrossRef]
- Xiao, J.; Hu, Y.; Wang, Z.; Tang, Y.; Chen, Z.; Fan, W. Preparation and characterization of poly(butylene terephthalate) nanocomposites from thermally stable organic-modified montmorillonite. Eur. Polym. J. 2005, 41, 1030–1035. [Google Scholar] [CrossRef]
- Liu, P.; Liu, M.; Gao, C.; Wang, F.; Ding, Y.; Wen, B.; Zhang, S.; Yang, M. Preparation, characterization and properties of a halogen-free phosphorous flame-retarded poly(butylene terephthalate) composite based on a DOPO derivative. J. Appl. Polym. Sci. 2013, 130, 1301–1307. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, S.H. Poly (ethylene terephthalate) recycling for high value added textiles. Fash. Text. 2014, 1, 1–7. [Google Scholar] [CrossRef]
- Laoutid, F.; Ferry, L.; Lopez-Cuesta, J.M.; Crespy, A. Red phosphorus/aluminium oxide compositions as flame retardants in recycled poly(ethylene terephthalate). Polym. Degrad. Stab. 2003, 82, 357–363. [Google Scholar] [CrossRef]
- Giraldi, A.L.F.D.; Bartoli, J.R.; Velasco, J.I.; Mei, L.H.I. Glass fibre recycled poly(ethylene terephthalate) composites: Mechanical and thermal properties. Polym. Test. 2005, 24, 507–512. [Google Scholar] [CrossRef]
- Wang, F.; Meng, X.F.; Xu, X.F.; Wen, B.; Qian, Z.Z.; Gao, X.W.; Ding, Y.F.; Zhang, S.M.; Yang, M.S. Inhibited transesterification of PET/PBT blends filled with silica nanoparticles during melt processing. Polym. Degrad. Stab. 2008, 93, 1397–1404. [Google Scholar] [CrossRef]
- Zhang, W.Z.; Wang, K.; Yan, W.; Guo, W.H. Toughening modification of poly(butylene terephthalate)/poly(ethylene terephthalate) blends by an epoxy-functionalized elastomer. Mater. Res. Express 2017, 4, 105303. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.S.; Zhang, L.; Li, C.Z. Inhibited Transesterification of Poly(Butylene Terephthalate)/Poly(Ethylene Terephthalate)/SiO(2) Nanocomposites by Two Processing Methods. J. Macromol. Sci. B 2011, 50, 453–462. [Google Scholar]
- Higgins, J.S.; Tambasco, M.; Lipson, J.E.G. Polymer blends; stretching what we can learn through the combination of experiment and theory. Prog. Polym. Sci. 2005, 30, 832–843. [Google Scholar] [CrossRef]
- Ryan, A.J. Designer polymer blends. Nat. Mater. 2002, 1, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Andresen, E.; Zachmann, H.G. Studies of miscibility, transesterification and crystallization in blends of poly(ethylene terephthalate) and Poly(ethylene-2,6-naphthalene dicarboxylate). Colloid Polym. Sci. 1994, 272, 1352–1362. [Google Scholar] [CrossRef]
- Aravinthan, G.; Kale, D.D. Blends of poly(ethylene terephthalate) and poly(butylene terephthalate). J. Appl. Polym. Sci. 2005, 98, 75–82. [Google Scholar] [CrossRef]
- Supaphol, P.; Dangseeyun, N.; Thanomkiat, P.; Nithitanakul, M. Thermal, crystallization, mechanical, and rheological characteristics of poly(trimethylene terephthalate)/poly(ethylene terephthalate) blends. J. Polym. Sci. Polym. Phys. 2004, 42, 676–686. [Google Scholar] [CrossRef]
- Kuo, Y.H.; Woo, E.M. Miscibility in Two Blend Systems of Homologous Semicrystalline Aryl Polyesters Involving Poly(trimethylene terephthalate). Polym. J. 2003, 35, 236–244. [Google Scholar] [CrossRef] [Green Version]
- Shin, H.; Park, E.S. Mechanical and Dielectric Breakdown Properties of PBT/TPE, PBT/PBT/PET, and PBT/Antioxidant Blends. J. Appl. Polym. Sci. 2009, 114, 3008–3015. [Google Scholar] [CrossRef]
- Song, K.; White, J.L. Double bubble tubular film extrusion of polybutylene terephthalate–polyethylene terephthalate blends. Polym. Eng. Sci. 2000, 40, 902–916. [Google Scholar] [CrossRef]
- Zhang, W.Z.; Ren, J.W.; Wei, T.; Guo, W.H. Synergistic effect between ammonium polyphosphate and expandable graphite on flame-retarded poly(butylene terephthalate). Mater. Res. Express 2018, 5, 025310. [Google Scholar] [CrossRef]
- Brehme, S.; Schartel, B.; Goebbels, J.; Fischer, O.; Pospiech, D.; Bykov, Y.; Doring, M. Phosphorus polyester versus aluminium phosphinate in poly(butylene terephthalate) (PBT): Flame retardancy performance and mechanisms. Polym. Degrad. Stab. 2011, 96, 875–884. [Google Scholar] [CrossRef]
- Weil, E.D.; Levchik, S.V. Commercial flame retardancy of thermoplastic polyesters—A review. J. Fire Sci. 2004, 22, 339–350. [Google Scholar] [CrossRef]
- Hoque, M.A.; Cho, Y.H.; Kawakami, Y. High performance holographic gratings formed with novel photopolymer films containing hyper-branched silsesquioxane. React. Funct. Polym. 2007, 67, 1192–1199. [Google Scholar] [CrossRef]
- Wang, Z.; Yonggang, L.; Huijuan, M.; Wenpeng, S.; Tao, L.; Cuifen, L.; Junqi, N.; Guichun, Y.; Zuxing, C. Novel phosphorus-nitrogen-silicon copolymers with double-decker silsesquioxane in the main chain and their flame retardancy application in PC/ABS. Fire Mater. 2018, 42, 946–957. [Google Scholar] [CrossRef]
- Xie, M.C.; Zhang, S.M.; Ding, Y.F.; Wang, F.; Liu, P.; Tang, H.Y.; Wang, Y.T.; Yang, M.S. Synthesis of a heat-resistant DOPO derivative and its application as flame-retardant in engineering plastics. J. Appl. Polym. Sci. 2017, 134, 44892. [Google Scholar] [CrossRef]
- Buczko, A.; Stelzig, T.; Bommer, L.; Rentsch, D.; Heneczkowski, M.; Gaan, S. Bridged DOPO derivatives as flame retardants for PA6. Polym. Degrad. Stab. 2014, 107, 158–165. [Google Scholar] [CrossRef]
- Yang, S.; Wang, J.; Huo, S.; Cheng, L.; Wang, M. Preparation and flame retardancy of an intumescent flame-retardant epoxy resin system constructed by multiple flame-retardant compositions containing phosphorus and nitrogen heterocycle. Polym. Degrad. Stab. 2015, 119, 251–259. [Google Scholar] [CrossRef]
- Patrick Lim, W.K.; Mariatti, M.; Chow, W.S.; Mar, K.T. Effect of intumescent ammonium polyphosphate (APP) and melamine cyanurate (MC) on the properties of epoxy/glass fiber composites. Compos. Part B Eng. 2012, 43, 124–128. [Google Scholar] [CrossRef]
- Wen, P.; Feng, X.; Kan, Y.; Hu, Y.; Yuen, R.K.K. Synthesis of a novel triazine-based polymeric flame retardant and its application in polypropylene. Polym. Degrad. Stab. 2016, 134, 202–210. [Google Scholar] [CrossRef]
- Tang, S.; Qian, L.J.; Liu, X.X.; Dong, Y.P. Gas-phase flame-retardant effects of a bi-group compound based on phosphaphenanthrene and triazine-trione groups in epoxy resin. Polym. Degrad. Stab. 2016, 133, 350–357. [Google Scholar] [CrossRef]
- Tang, S.; Wachtendorf, V.; Klack, P.; Qian, L.; Dong, Y.; Schartel, B. Enhanced flame-retardant effect of a montmorillonite/phosphaphenanthrene compound in an epoxy thermoset. RSC Adv. 2017, 7, 720–728. [Google Scholar] [CrossRef] [Green Version]
- Alongi, J.; Frache, A.; Gioffredi, E. Fire-retardant poly(ethylene terephthalate) by combination of expandable graphite and layered clays for plastics and textiles. Fire Mater. 2011, 35, 383–396. [Google Scholar] [CrossRef]
- Awad, W.H.; Wilkie, C.A. Investigation of the thermal degradation of polyurea: The effect of ammonium polyphosphate and expandable graphite. Polymer 2010, 51, 2277–2285. [Google Scholar] [CrossRef] [Green Version]
- Duquesne, S.; Delobel, R.; Le Bras, M.; Camino, G. A comparative study of the mechanism of action of ammonium polyphosphate and expandable graphite in polyurethane. Polym. Degrad. Stab. 2002, 77, 333–344. [Google Scholar] [CrossRef]
- Han, J.; Liang, G.; Gu, A.; Ye, J.; Zhang, Z.; Yuan, L. A novel inorganic-organic hybridized intumescent flame retardant and its super flame retarding cyanate ester resins. J. Mater. Chem. A 2013, 1, 2169–2182. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Y.Z. A review on flame retardant technology in China. Part I: Development of flame retardants. Polym. Adv. Technol. 2010, 21, 1–26. [Google Scholar] [CrossRef]
- Tang, M.Q.; Qi, F.; Chen, M.; Sun, Z.D.; Xu, Y.; Chen, X.L.; Zhang, Z.B.; Shen, R. Synergistic effects of ammonium polyphosphate and red phosphorus with expandable graphite on flammability and thermal properties of HDPE/EVA blends. Polym. Adv. Technol. 2016, 27, 52–60. [Google Scholar] [CrossRef]
- Fang, Y.; Qian, L.; Huang, Z.; Tang, S.; Qiu, Y. Synergistic charring effect of triazinetrione-alkyl-phosphinate and phosphaphenanthrene derivatives in epoxy thermosets. RSC Adv. 2017, 7, 46505–46513. [Google Scholar] [CrossRef] [Green Version]
- He, M.; Zhang, D.; Zhao, W.; Qin, S.; Yu, J. Flame retardant and thermal decomposition mechanism of poly(butylene terephthalate)/DOPO-HQ composites. Polym. Compos. 2019, 40, 974–985. [Google Scholar] [CrossRef]




| Samples | Resin Matrix | TAD (wt%) | EG (wt%) | TAD/EG | LOI | UL-94 |
|---|---|---|---|---|---|---|
| TAD0 | 100 | 0 | 0 | / | 22.0 | NR |
| TAD4 | 96 | 4 | 0 | / | 25.2 | NR |
| TAD8 | 92 | 8 | 0 | / | 26.9 | V-2 |
| TAD12 | 88 | 12 | 0 | / | 28.4 | V-1 |
| TAD16 | 84 | 16 | 0 | / | 28.1 | V-1 |
| TAD6EG6 | 88 | 6 | 6 | 1/1 | 29.2 | V-0 |
| EG12 | 88 | 0 | 12 | / | 25.8 | NR |
| Sample | PHRR (kW/m2) | Mean-EHC (MJ/kg) | THR (MJ/m2) | Mean-CO2Y(kg/kg) | TSR (m2/m2) |
|---|---|---|---|---|---|
| TAD0 | 1087.7 | 17.1 | 92.0 | 1.48 | 1530.9 |
| TAD4 | 885.1 | 8.6 | 94.2 | 0.91 | 1853.5 |
| TAD8 | 777.9 | 8.9 | 91.0 | 0.92 | 1944.3 |
| TAD12 | 616.7 | 10.4 | 84.3 | 0.99 | 2105.0 |
| TAD16 | 486.7 | 10.6 | 72.1 | 1.05 | 2236.5 |
| TAD6EG6 | 390.4 | 7.8 | 84.7 | 0.67 | 2622.9 |
| EG12 | 435.2 | 10.2 | 78.3 | 0.83 | 2412.5 |
| Sample | T5% (°C) | Char Residue (%) |
|---|---|---|
| TAD0 | 390 | 7.8 |
| TAD12 | 389 | 9.3 |
| TAD16 | 391 | 13.9 |
| TAD6EG6 | 390 | 16.9 |
| EG12 | 390 | 14.4 |
| TAD | 218 | 7.2 |
| EG | 211 | 60.2 |
| Sample | C (%) | N (%) | O (%) | P (%) |
|---|---|---|---|---|
| TAD12 | 81.2 | 1.9 | 14.9 | 2.1 |
| TAD6EG6 | 90.5 | 0.8 | 7.2 | 1.5 |
| Samples | Notched Impact Strength (kJ/m2) | Bending Modulus (MPa) | Bending Strength (MPa) | Tensile Strength (MPa) |
|---|---|---|---|---|
| TAD0 | 53.1± 4.7 | 1454 ± 12 | 47.5 ± 1.8 | 36.4 ± 0.5 |
| TAD4 | 33.4 ± 2.4 | 1530 ± 25 | 50.6 ± 1.2 | 37.9 ± 0.6 |
| TAD12 | 18.5 ± 1.6 | 1376 ± 16 | 44.0 ± 1.4 | 33.5 ± 1.6 |
| TAD6EG6 | 15.1 ± 3.5 | 1312 ± 19 | 39.9 ± 1.7 | 30.7 ± 2.5 |
| EG12 | 11.6 ± 4.9 | 1220 ± 36 | 36.7 ± 2.2 | 28.2 ± 2.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Zheng, C.; Zhang, Y.; Guo, W. Preparation and Characterization of Flame-Retarded Poly(butylene terephthalate)/Poly(ethylene terephthalate) Blends: Effect of Content and Type of Flame Retardant. Polymers 2019, 11, 1784. https://doi.org/10.3390/polym11111784
Zhang W, Zheng C, Zhang Y, Guo W. Preparation and Characterization of Flame-Retarded Poly(butylene terephthalate)/Poly(ethylene terephthalate) Blends: Effect of Content and Type of Flame Retardant. Polymers. 2019; 11(11):1784. https://doi.org/10.3390/polym11111784
Chicago/Turabian StyleZhang, Weizhou, Cheng Zheng, Yuhui Zhang, and Weihong Guo. 2019. "Preparation and Characterization of Flame-Retarded Poly(butylene terephthalate)/Poly(ethylene terephthalate) Blends: Effect of Content and Type of Flame Retardant" Polymers 11, no. 11: 1784. https://doi.org/10.3390/polym11111784
APA StyleZhang, W., Zheng, C., Zhang, Y., & Guo, W. (2019). Preparation and Characterization of Flame-Retarded Poly(butylene terephthalate)/Poly(ethylene terephthalate) Blends: Effect of Content and Type of Flame Retardant. Polymers, 11(11), 1784. https://doi.org/10.3390/polym11111784