Development of Self-Healable Organic/Inorganic Hybrid Materials Containing a Biobased Copolymer via Diels–Alder Chemistry and Their Application in Electromagnetic Interference Shielding
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Synthesis of Poly(ethylene brassylate)-Poly(furfuryl glycidyl ether) Copolymer (PEBF)
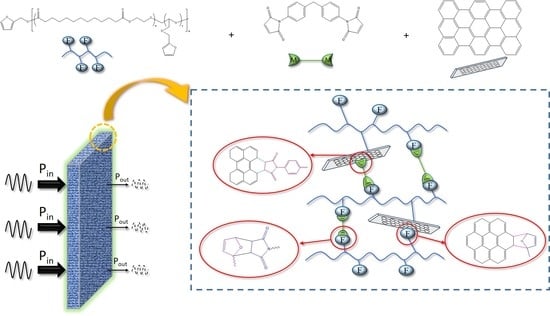
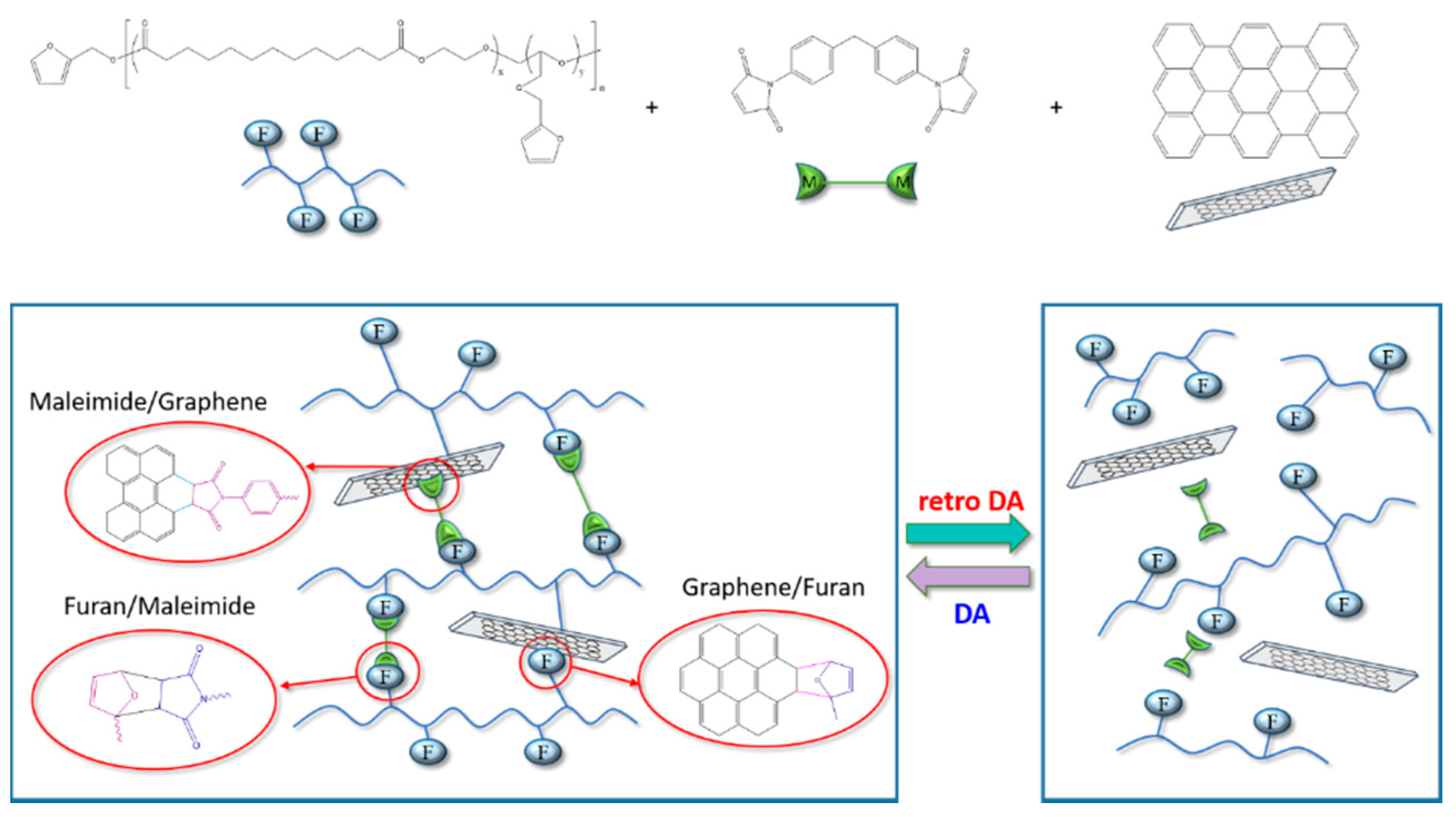
2.2.2. Preparation of Self-Healing PEBF/Graphene/BMI Hybrid Material
2.2.3. Characterization Methods
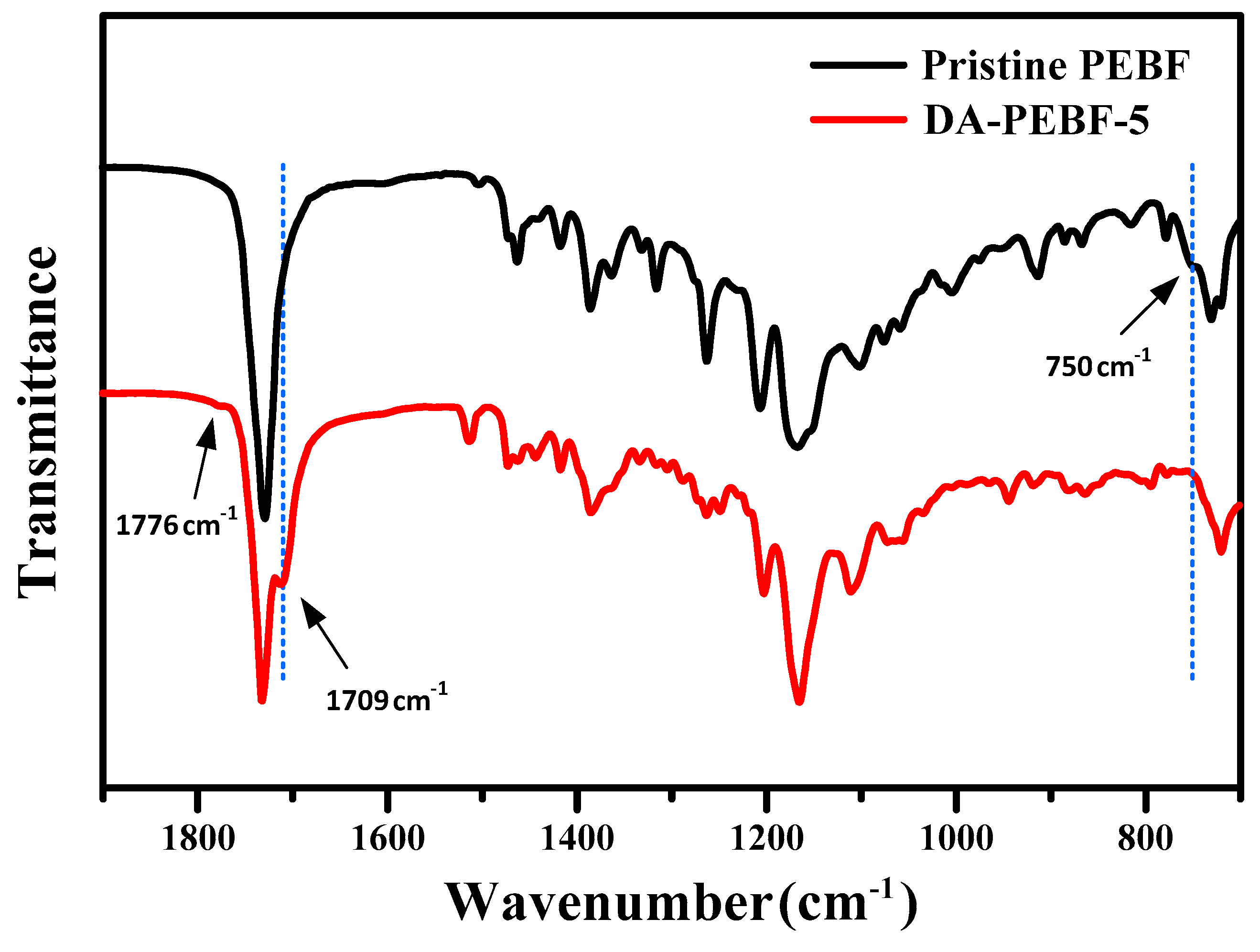
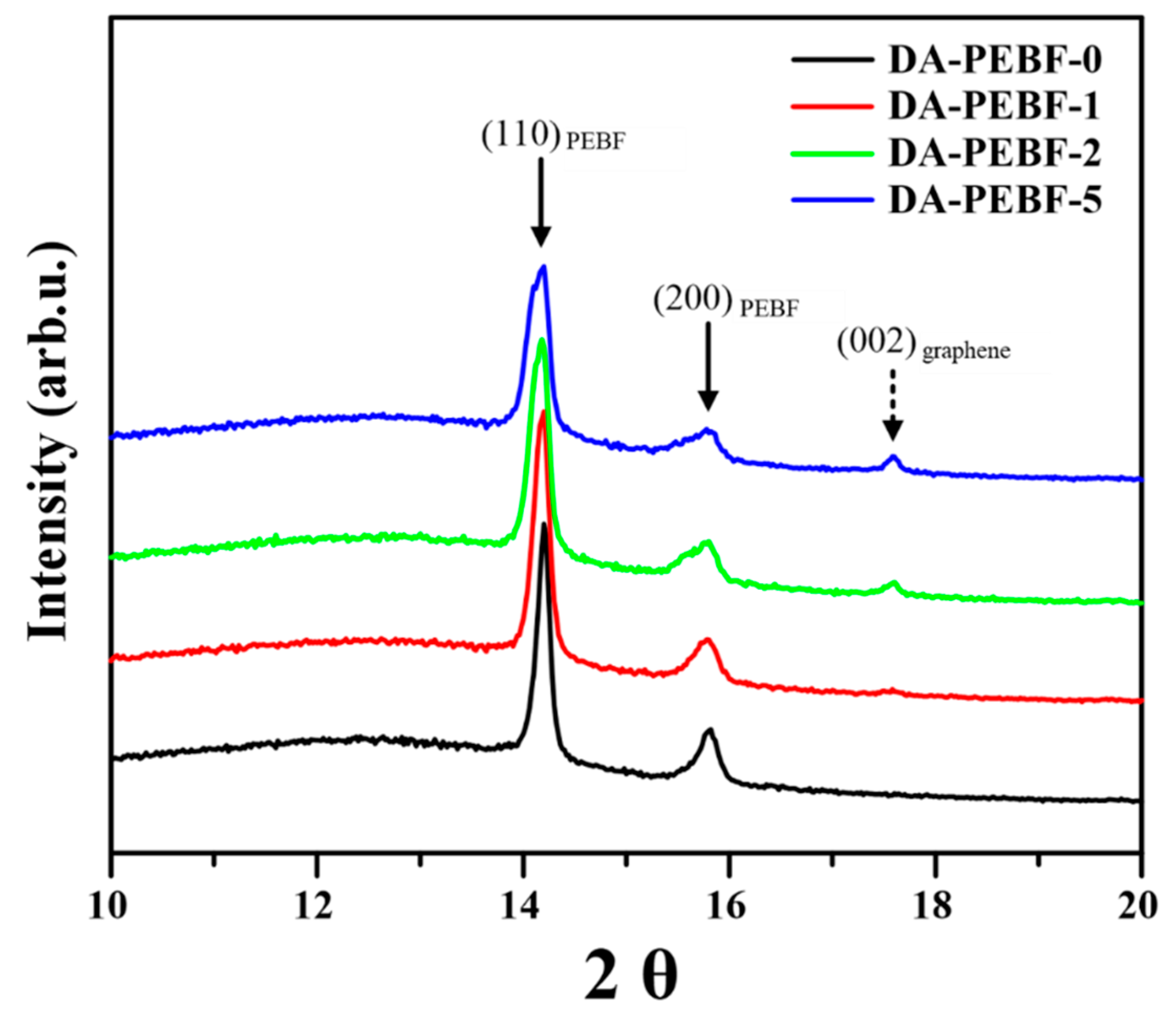
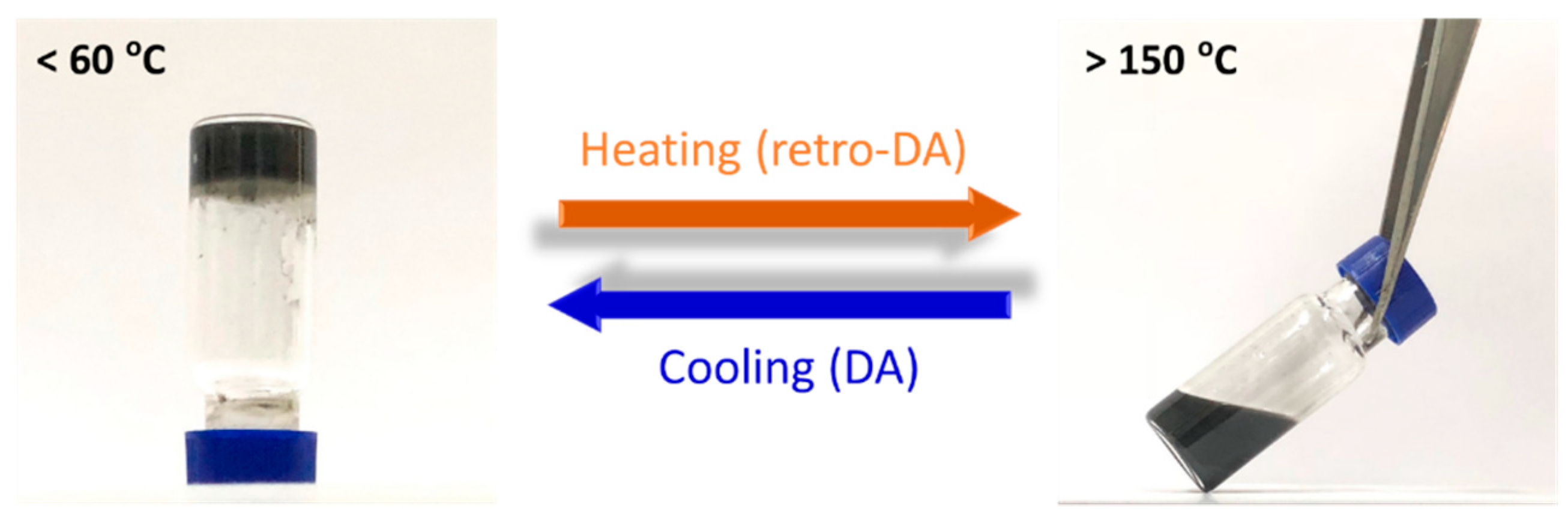
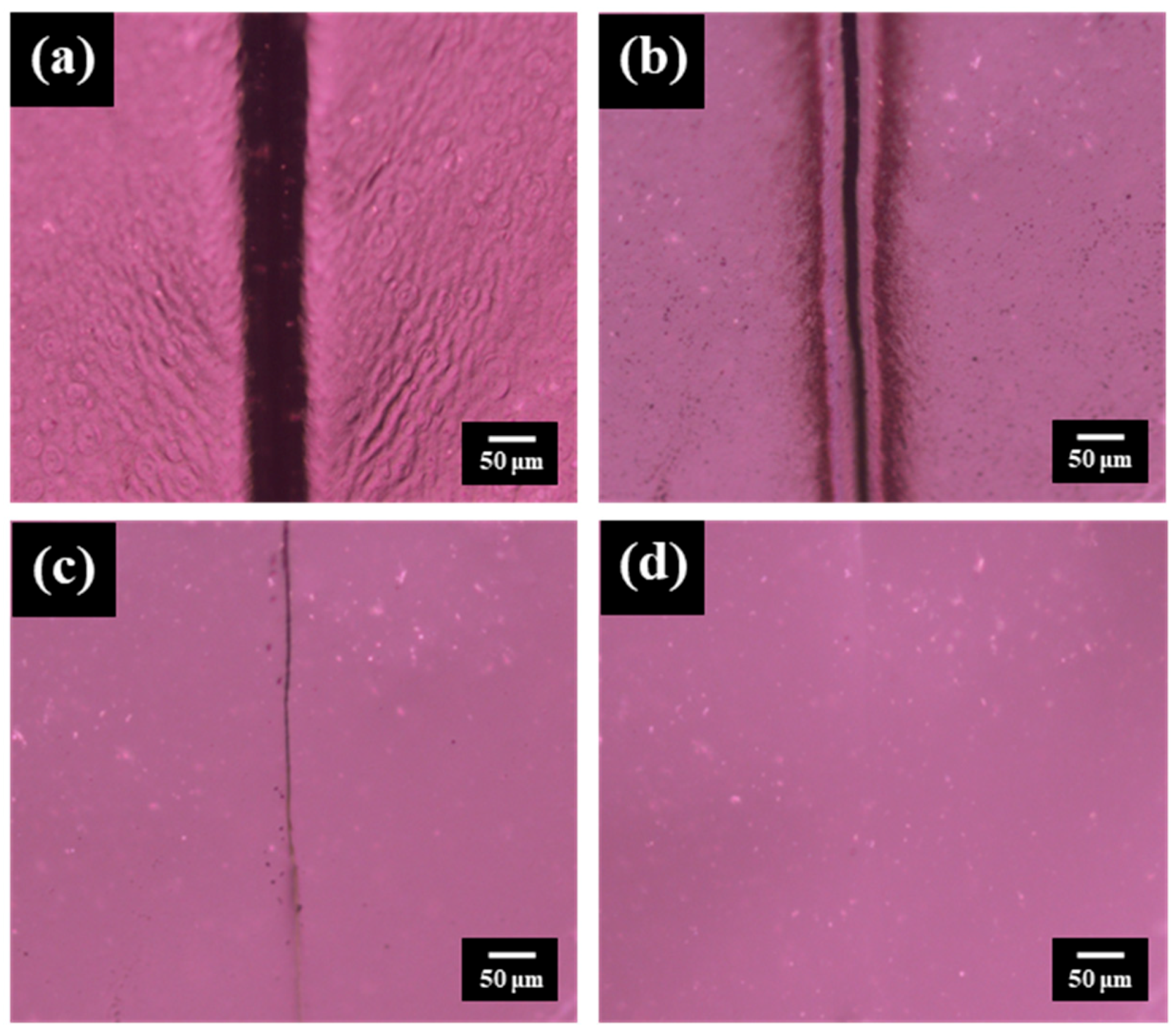
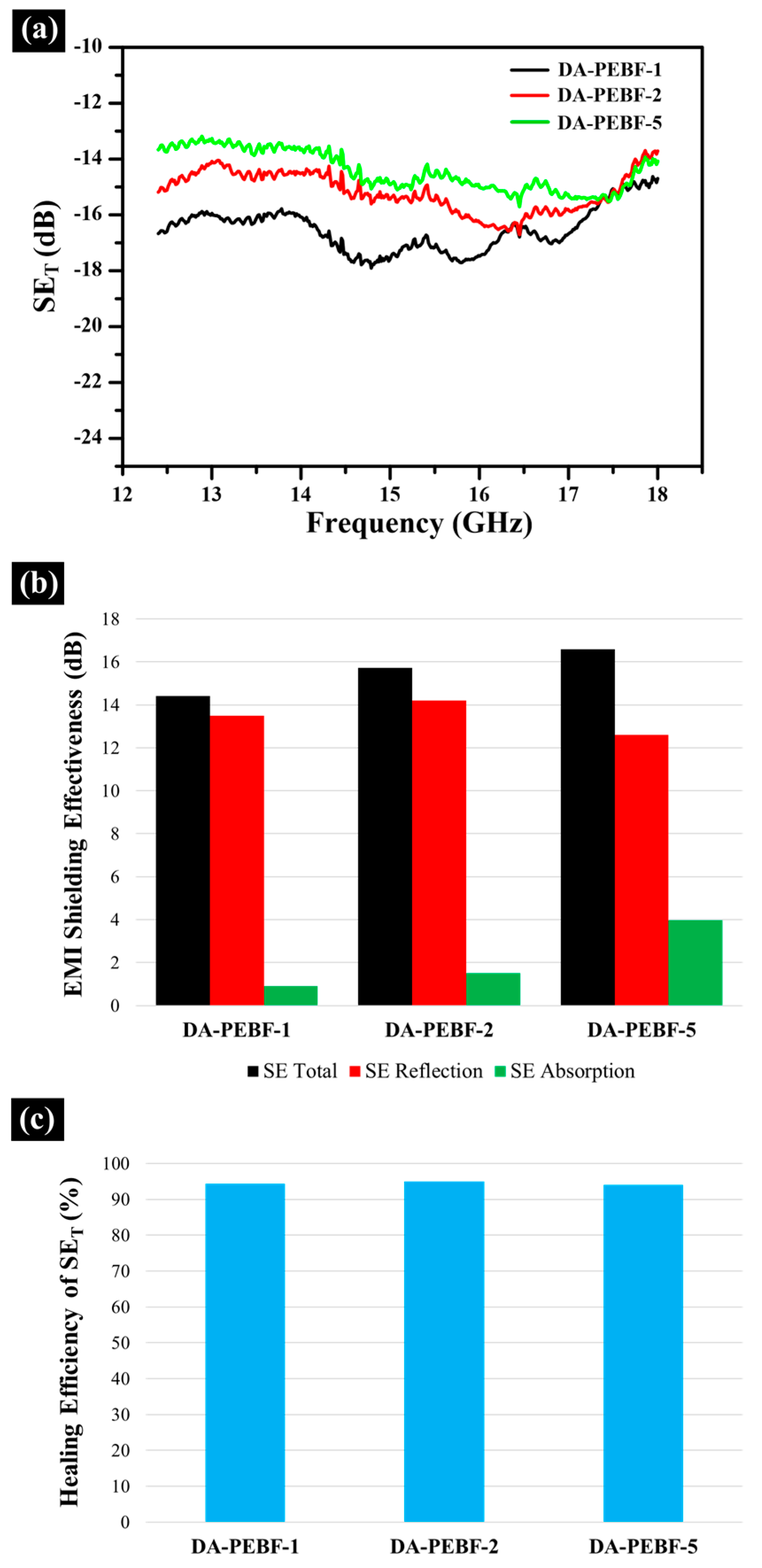
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- White, S.R.; Sottos, N.R.; Geubelle, P.H.; Moore, J.S.; Kessler, M.R.; Sriram, S.R.; Brown, E.N.; Viswanatha, S. Autonomic healing of polymer composites. Nature 2001, 409, 794–797. [Google Scholar] [CrossRef]
- Samadzadeh, M.; Boura, S.H.; Peikari, M.; Kasiriha, S.M.; Ashrafi, A. A review on self-healing coatings based on micro/nanocapsules. Prog. Org. Coat. 2010, 68, 159–164. [Google Scholar] [CrossRef]
- Song, Y.-K.; Jo, Y.-H.; Lim, Y.-J.; Cho, S.-Y.; Yu, H.-C.; Ryu, B.-C.; Lee, S.-I.; Chung, C.-M. Sunlight-induced self-healing of a microcapsule-type protective coating. Appl. Mater. Interfaces 2013, 5, 1378–1384. [Google Scholar] [CrossRef]
- Toohey, K.S.; Sottos, N.R.; Lewis, J.A.; Moore, J.S.; White, S.R. Self-healing materials with microvascular networks. Nat. Mater. 2007, 6, 581–585. [Google Scholar] [CrossRef]
- Hansen, C.J.; Wu, W.; Toohey, K.S.; Sottos, N.R.; White, S.R.; Lewis, J.A. Self-healing materials with interpenetrating microvascular networks. Adv. Mater. 2009, 21, 4143–4147. [Google Scholar] [CrossRef]
- Hansen, C.J.; White, S.R.; Sottos, N.R.; Lewis, J.A. Accelerated self-healing via ternary interpenetrating microvascular networks. Adv. Funct. Mater. 2011, 21, 4320–4326. [Google Scholar] [CrossRef]
- Shchukin, D.G.; Möhwald, H. Self-repairing coatings containing active nanoreservoirs. Small 2007, 3, 926–943. [Google Scholar] [CrossRef]
- Lanzara, G.; Yoon, Y.; Liu, H.; Peng, S.; Lee, W.-I. Carbon nanotube reservoirs for self-healing materials. Nanotechnology 2009, 20, 335704. [Google Scholar] [CrossRef]
- Syrett, J.A.; Becer, C.R.; Haddleton, D.M. Self-healing and self-mendable polymers. Polym. Chem. 2010, 1, 978–987. [Google Scholar] [CrossRef]
- Wu, D.Y.; Meure, S.; Solomon, D. Self-healing polymeric materials: a review of recent developments. Prog. Polym. Sci. 2008, 33, 479–522. [Google Scholar] [CrossRef]
- Yang, Y.; Urban, M.W. Self-healing polymeric materials. Chem. Soc. Rev. 2013, 42, 7446–7467. [Google Scholar] [CrossRef]
- Chung, C.-M.; Roh, Y.-S.; Cho, S.-Y.; Kim, J.-G. Crack healing in polymeric materials via photochemical [2+2] cycloaddition. Chem. Mater. 2004, 16, 3982–3984. [Google Scholar] [CrossRef]
- Ghosh, B.; Urban, M.W. Self-repairing oxetane-substituted chitosan polyurethane networks. Science 2009, 323, 1458–1460. [Google Scholar] [CrossRef]
- Scott, T.F.; Schneider, A.D.; Cook, W.D.; Bowman, C.N. Photoinduced plasticity in cross-linked polymers. Science 2005, 308, 1615–1617. [Google Scholar] [CrossRef]
- Gruendling, T.; Kaupp, M.; Blinco, J.P.; Barner-Kowollik, C. Photoinduced conjugation of dithioester- and trithiocarbonate-functional RAFT polymers with Alkenes. Macromolecules 2011, 44, 166–174. [Google Scholar] [CrossRef]
- Holten-Andersen, N.; Harrington, M.J.; Birkedal, H.; Lee, B.P.; Messersmith, P.B.; Lee, K.Y.C.; Waite, J.H. pH-induced metal-ligand cross-links inspired by mussel yield self-healing polymer networks with near-covalent elastic moduli. PNAS 2011, 108, 2651–2655. [Google Scholar] [CrossRef] [Green Version]
- Mozhdehi, D.; Ayala, S.; Cromwell, O.R.; Guan, Z. Self-healing multiphase polymers via dynamic metal−ligand interactions. J. Am. Chem. Soc. 2014, 136, 16128–16131. [Google Scholar] [CrossRef]
- Rao, Y.-L.; Chortos, A.; Pfattner, R.; Lissel, F.; Chiu, Y.-C.; Feig, V.; Xu, J.; Kurosawa, T.; Gu, X.; Wang, C.; et al. Stretchable Self-healing polymeric dielectrics cross-linked through metal−ligand coordination. J. Am. Chem. Soc. 2016, 138, 6020–6027. [Google Scholar] [CrossRef]
- Nakahata, M.; Takashima, Y.; Yamaguchi, H.; Harada, A. Redox-responsive self-healing materials formed from host-guest polymers. Nat. Commun. 2011, 2, 511. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, D.; Yan, X.; Chen, J.; Dong, S.; Zheng, B.; Huang, F. Self-healing supramolecular gels formed by crown ether based host–guest interactions. Angew. Chem. Int. Ed. 2012, 51, 7011–70015. [Google Scholar] [CrossRef]
- Kakuta, T.; Takashima, Y.; Nakahata, M.; Otsubo, M.; Yamaguchi, H.; Harada, A. Preorganized hydrogel: Self-healing properties of supramolecular hydrogels formed by polymerization of host-guest monomers that contain cyclodextrins and hydrophobic guest groups. Adv. Mater. 2013, 25, 2849–2853. [Google Scholar] [CrossRef]
- Diels, O.; Alder, K. Synthesen in der hydroaromatischen reihe. Justus Liebigs Ann. Chem. 1928, 460, 98–122. [Google Scholar] [CrossRef]
- Ripoll, J.L.; Rouessac, A.; Rouessac, F. Applications recentes de la reaction de retro-Diels-Alder en synthese organique. Tetrahedron 1978, 34, 19–40. [Google Scholar] [CrossRef]
- McElhanon, J.R.; Wheeler, D.R. Thermally responsive dendrons and dendrimers based on reversible furan-maleimide Diels-Alder adducts. Org. Lett. 2001, 3, 2681–2683. [Google Scholar] [CrossRef]
- Szalai, M.L.; McGrath, D.V.; Wheeler, D.R.; Zifer, T.; McElhanon, J.R. Dendrimers based on thermally reversible furan-maleimide Diels-Alder adducts. Macromolecules 2007, 40, 818–823. [Google Scholar] [CrossRef]
- Tasdelen, M.A. Diels–Alder “click” reactions: recent applications in polymer and material science. Polym. Chem. 2011, 2, 2133–2145. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Chuo, T.-W. Self-healing polymers based on thermally reversible Diels–Alder chemistry. Polym. Chem. 2013, 4, 2194–2205. [Google Scholar] [CrossRef]
- Mallek, H.; Jegat, C.; Mignard, N.; Abid, M.; Abid, S.; Taha, M. Reversibly crosslinked self-healing PCL-based networks. J. Appl. Polym. Sci. 2013, 129, 954–964. [Google Scholar] [CrossRef]
- Barthel, M.J.; Rudolph, T.; Teichler, A.; Paulus, R.M.; Vitz, J.; Hoeppener, S.; Hager, M.D.; Schacher, F.H.; Schubert, U.C. Self-healing materials via reversible crosslinking of poly(ethylene oxide)-block-poly(furfuryl glycidyl ether) (PEO-b-PFGE) block copolymer films. Adv. Funct. Mater. 2013, 23, 4921–4932. [Google Scholar] [CrossRef]
- Yang, L.; Lu, X.; Wang, Z.; Xia, H. Diels–Alder dynamic crosslinked polyurethane/ polydopamine composites with NIR triggered self-healing function. Polym. Chem. 2018, 9, 2166–2172. [Google Scholar] [CrossRef]
- Truong, T.T.; Thai, S.H.; Nguyen, H.T.; Phung, D.T.T.; Nguyen, L.T.; Pham, H.Q.; Nguyen, L.-T.T. Tailoring the hard−soft interface with dynamic Diels−Alder linkages in polyurethanes: toward superior mechanical properties and healability at mild temperature. Chem. Mater. 2019, 31, 2347–2357. [Google Scholar] [CrossRef]
- Banerjee, S.; Tawadeb, B.V.; Améduri, B. Functional fluorinated polymer materials and preliminary self-healing behavior. Polym. Chem. 2019, 10, 1993–1997. [Google Scholar] [CrossRef]
- Han, J.T.; Kim, B.K.; Woo, J.S.; Jang, J.I.; Cho, J.Y.; Jeong, H.J.; Jeong, S.Y.; Seo, S.H.; Lee, G.-W. Bioinspired multifunctional superhydrophobic surfaces with carbon-nanotube-based conducting pastes by facile and scalable printing. Appl. Mater. Interfaces 2017, 9, 7780–7786. [Google Scholar] [CrossRef] [PubMed]
- Darabi, M.A.; Khosrozadeh, A.; Mbeleck, R.; Liu, Y.; Chang, Q.; Jiang, J.; Cai, J.; Wang, Q.; Luo, G.; Xing, M. Skin-inspired multifunctional autonomic-intrinsic conductive self-healing hydrogels with pressure sensitivity, stretchability, and 3D printability. Adv. Mater. 2017, 29, 1700533. [Google Scholar] [CrossRef]
- Tee, B.C.-K.; Wang, C.; Allen, R.; Bao, Z. An electrically and mechanically self-healing composite with pressure- and flexion-sensitive properties for electronic skin applications. Nat. Nanotechnol. 2012, 7, 825–832. [Google Scholar] [CrossRef]
- Gnidakouong, J.R.N.; Kim, M.; Park, H.W.; Park, Y.B.; Jeong, H.S.; Jung, Y.B.; Ahn, S.K.; Han, K.; Park, J.-M. Electromagnetic interference shielding of composites consisting of a polyester matrix and carbon nanotube-coated fiber reinforcement. Compos. Part A 2013, 50, 73–80. [Google Scholar] [CrossRef]
- Schäfer, S.; Kickelbick, G. Diels−Alder Reactions on surface-modified magnetite/maghemite nanoparticles: application in self-healing nanocomposites. Appl. Nano Mater. 2018, 1, 2640–2652. [Google Scholar] [CrossRef]
- Willocq, B.; Bose, R.K.; Khelifa, F.; Garcia, S.J.; Dubois, P.; Raquez, J.-M. Healing by the joule effect of electrically conductive poly(ester-urethane)/carbon nanotube nanocomposites. J. Mater. Chem. A. 2016, 4, 4089–4097. [Google Scholar] [CrossRef]
- Li, Q.-T.; Jiang, M.-J.; Wu, G.; Chen, L.; Chen, S.-C.; Cao, Y.-X.; Wang, Y.-Z. Photothermal conversion triggered precisely targeted healing of epoxy resin based on thermoreversible Diels−Alder network and amino-functionalized carbon nanotubes. Appl. Mater. Interfaces 2017, 9, 20797–20807. [Google Scholar] [CrossRef]
- Lee, Y.-H.; Zhuang, Y.N.; Wang, H.-T.; Wei, M.-F.; KO, W.-C.; Chang, W.-J.; Way, T.-F.; Rwei, S.-P. Fabrication of self-healable magnetic nanocomposites via Diels-Alder click chemistry. Appl. Sci. 2019, 9, 506. [Google Scholar] [CrossRef]
- Wu, S.; Li, J.; Zhang, G.; Yao, Y.; Li, G.; Sun, R.; Wong, C. Ultrafast self-healing nanocomposites via infrared laser and their application in flexible electronics. Appl. Mater. Interfaces 2017, 9, 3040–3049. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Xiao, P.; Hou, S.; Huang, Y. Rapid and efficient polymer/graphene based multichannel self-healing material via Diels-Alder reaction. Carbon 2019, 147, 398–407. [Google Scholar] [CrossRef]
- Menon, A.V.; Madras, G.; Bose, S. Ultrafast self-healable interfaces in polyurethane nanocomposites designed using Diels−Alder “click” as an efficient microwave absorber. Omega 2018, 3, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Wang, Y.; Huang, Y.; Ma, Y.; Liu, Z.; Cai, J.; Zhang, C.; Gao, H.; Chen, Y. Electromagnetic interference shielding of graphene/epoxy composites. Carbon 2009, 47, 922–925. [Google Scholar] [CrossRef]
- Chen, Z.; Xu, C.; Ma, C.; Ren, W.; Cheng, H.-M. Lightweight and flexible graphene foam composites for high-performance electromagnetic interference shielding. Adv. Mater. 2013, 25, 1296–1300. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-Q.; Patel, M.K. Plastics derived from biological sources: present and future: a technical and environmental review. Chem. Rev. 2012, 112, 2082–2099. [Google Scholar] [CrossRef]
- Gandini, A.; Lacerda, T.M. From monomers to polymers from renewable resources: recent advances. Prog. Polym. Sci. 2015, 48, 1–39. [Google Scholar] [CrossRef]
- Zhu, Y.; Romain, C.; Williams, C.K. Sustainable polymers from renewable resources. Nature 2016, 540, 354–362. [Google Scholar] [CrossRef]
- Chang, C.-M.; Liu, Y.-L. Functionalization of multi-walled carbon nanotubes with furan and maleimide compounds through Diels-Alder cycloaddition. Carbon 2009, 47, 3041–3049. [Google Scholar] [CrossRef]
- Jin, C.; Wei, Z.; Yu, Y.; Sui, M.; Leng, X.; Li, Y. Copolymerization of ethylene brassylate with δ-valerolactone towards isodimorphic random copolyesters with continuously tunable mechanical properties. Eur. Polym. J. 2018, 102, 90–100. [Google Scholar] [CrossRef]
- Ding, J.; Yan, W.; Xie, W.; Sun, S.; Bao, J.; Gao, C. Highly efficient photocatalytic hrdrogen evolution of graphene/YInO3 nanocomposites under visible light irradiation. Nanoscale 2014, 6, 2299–2306. [Google Scholar] [CrossRef] [PubMed]
- Jana, P.B.; Mallick, A.K.; De, S.K. Effects of sample thickless and fiber aspect ratio on EMI shielding effectiveness of carbon fiber filled polychloroprene composites in the X-band frequency range. IEEE Trans. Electromagn. Compat. 1992, 34, 478–481. [Google Scholar] [CrossRef]
- Hong, Y.; Lee, C.; Jeong, C.; Lee, D.; Kim, K.; Joo, J. Method and apparatus to measure electromagnetic interference shielding efficiency and its shielding characteristics in broadband frequency range. Rev. Sci. Instrum. 2003, 74, 1098–1103. [Google Scholar] [CrossRef]
- Ji, H.; Zhao, R.; Zhang, N.; Jin, C.; Lu, X.; Wang, C. Lightweight and flexible electrospun polymer nanofiber/metal nanoparticle hybrid membrane for high-performance electromagnetic interference shielding. NPG Asia Mater. 2018, 10, 749–760. [Google Scholar] [CrossRef]
- Yan, D.-X.; Pang, H.; Li, B.; Vajtai, R.; Xu, L.; Ren, P.-G.; Wang, J.-H.; Li, Z.-M. Structured reduced graphene oxide/polymer composites for ultra-efficient electromagnetic interference shielding. Adv. Funct. Mater. 2015, 25, 559–566. [Google Scholar] [CrossRef]









| Sample Code 1 | FGE/BMI Ratio 2 | Graphene Amount 3 |
|---|---|---|
| DA-PEBF-0 | 1:1 | 0 wt % |
| DA-PEBF-1 | 1:1 | 1 wt % |
| DA-PEBF-2 | 1:1 | 2 wt % |
| DA-PEBF-5 | 1:1 | 5 wt % |
| Sample 1 | 5wt % Loss Temperature (°C) 2 | Char Residue at 600 °C (%) 3 | Retro-DA Onset Temperature (°C) 4 | Retro-DA Heat Flow (J/g) 5 |
|---|---|---|---|---|
| DA-PEBF-0 | 369 | 10.6 | 95 | 8.0 |
| DA-PEBF-1 | 369 | 15.5 | 94 | 9.8 |
| DA-PEBF-2 | 370 | 16.2 | 95 | 11.4 |
| DA-PEBF-5 | 368 | 16.9 | 96 | 16.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.-H.; Ko, W.-C.; Zhuang, Y.-N.; Wang, L.-Y.; Yu, T.-W.; Lee, S.-Y.; Way, T.-F.; Rwei, S.-P. Development of Self-Healable Organic/Inorganic Hybrid Materials Containing a Biobased Copolymer via Diels–Alder Chemistry and Their Application in Electromagnetic Interference Shielding. Polymers 2019, 11, 1755. https://doi.org/10.3390/polym11111755
Lee Y-H, Ko W-C, Zhuang Y-N, Wang L-Y, Yu T-W, Lee S-Y, Way T-F, Rwei S-P. Development of Self-Healable Organic/Inorganic Hybrid Materials Containing a Biobased Copolymer via Diels–Alder Chemistry and Their Application in Electromagnetic Interference Shielding. Polymers. 2019; 11(11):1755. https://doi.org/10.3390/polym11111755
Chicago/Turabian StyleLee, Yi-Huan, Wen-Chi Ko, Yan-Nian Zhuang, Lu-Ying Wang, Tao-Wei Yu, Shaio-Yen Lee, Tun-Fun Way, and Syang-Peng Rwei. 2019. "Development of Self-Healable Organic/Inorganic Hybrid Materials Containing a Biobased Copolymer via Diels–Alder Chemistry and Their Application in Electromagnetic Interference Shielding" Polymers 11, no. 11: 1755. https://doi.org/10.3390/polym11111755
APA StyleLee, Y.-H., Ko, W.-C., Zhuang, Y.-N., Wang, L.-Y., Yu, T.-W., Lee, S.-Y., Way, T.-F., & Rwei, S.-P. (2019). Development of Self-Healable Organic/Inorganic Hybrid Materials Containing a Biobased Copolymer via Diels–Alder Chemistry and Their Application in Electromagnetic Interference Shielding. Polymers, 11(11), 1755. https://doi.org/10.3390/polym11111755