AgBr/BiOBr Nano-Heterostructure-Decorated Polyacrylonitrile Nanofibers: A Recyclable High-Performance Photocatalyst for Dye Degradation under Visible-Light Irradiation
Abstract
:1. Introduction
2. Experiment
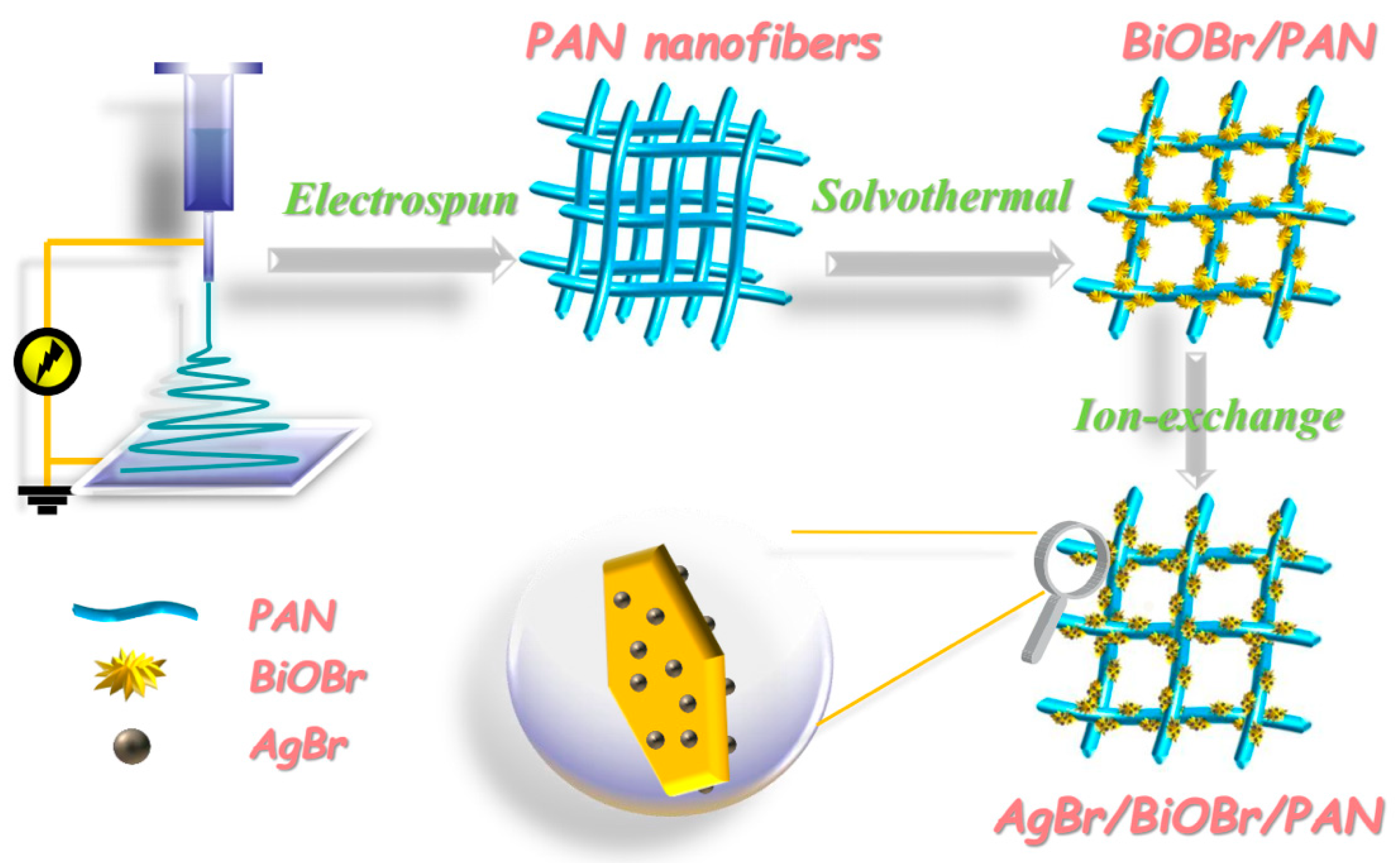
2.1. Fabrication of PAN Nanofiber (NF) Mats
2.2. Fabrication of BiOBr/PAN Composite Mats (CMs)
2.3. Fabrication of AgBr/BiOBr/PAN CMs
2.4. Characterization
2.5. Photocatalytic Tests
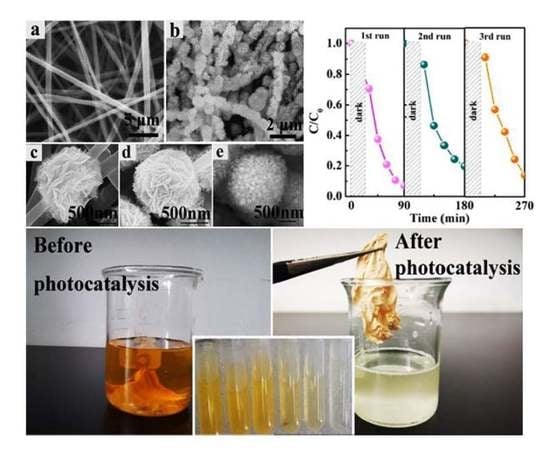
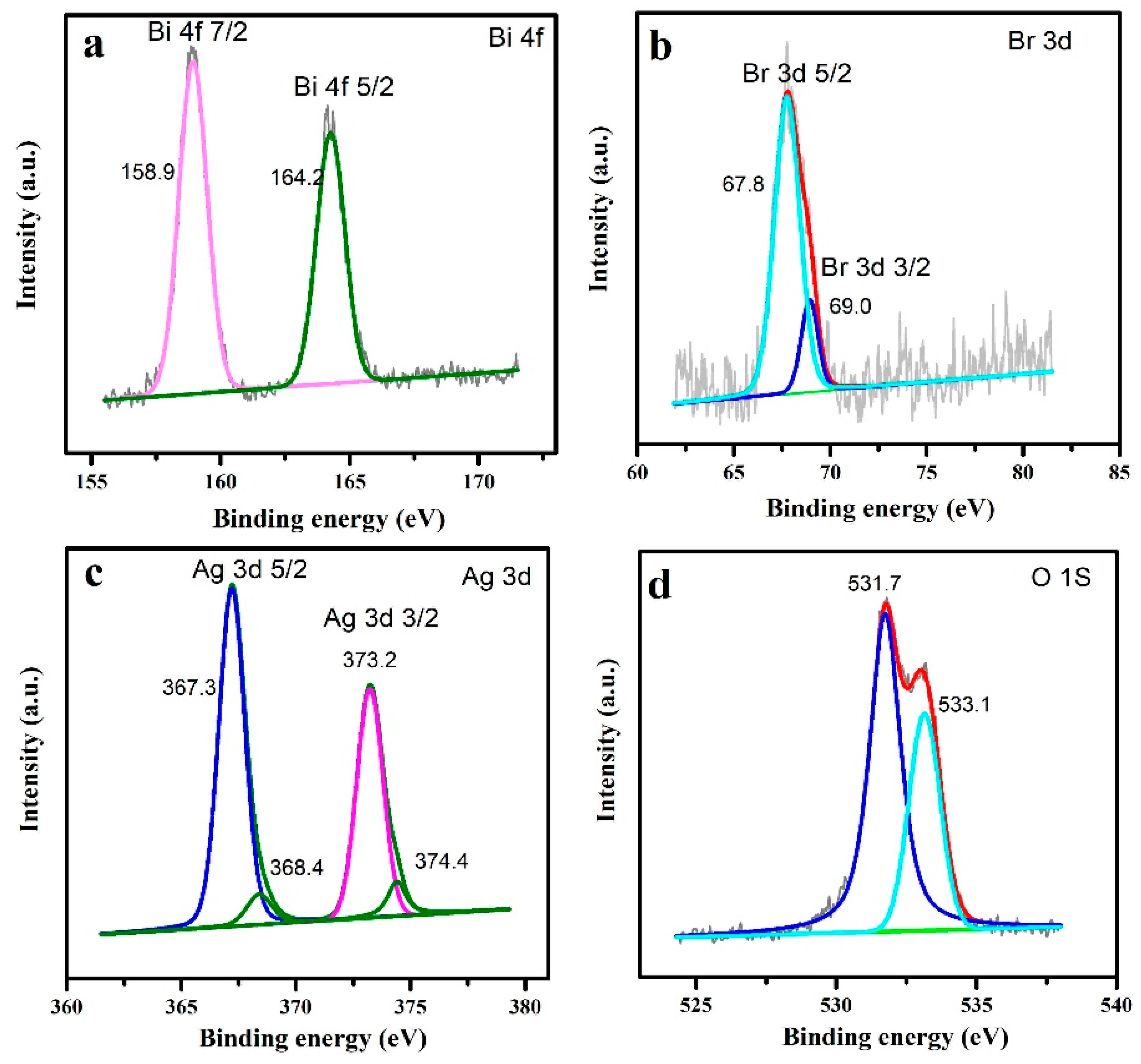
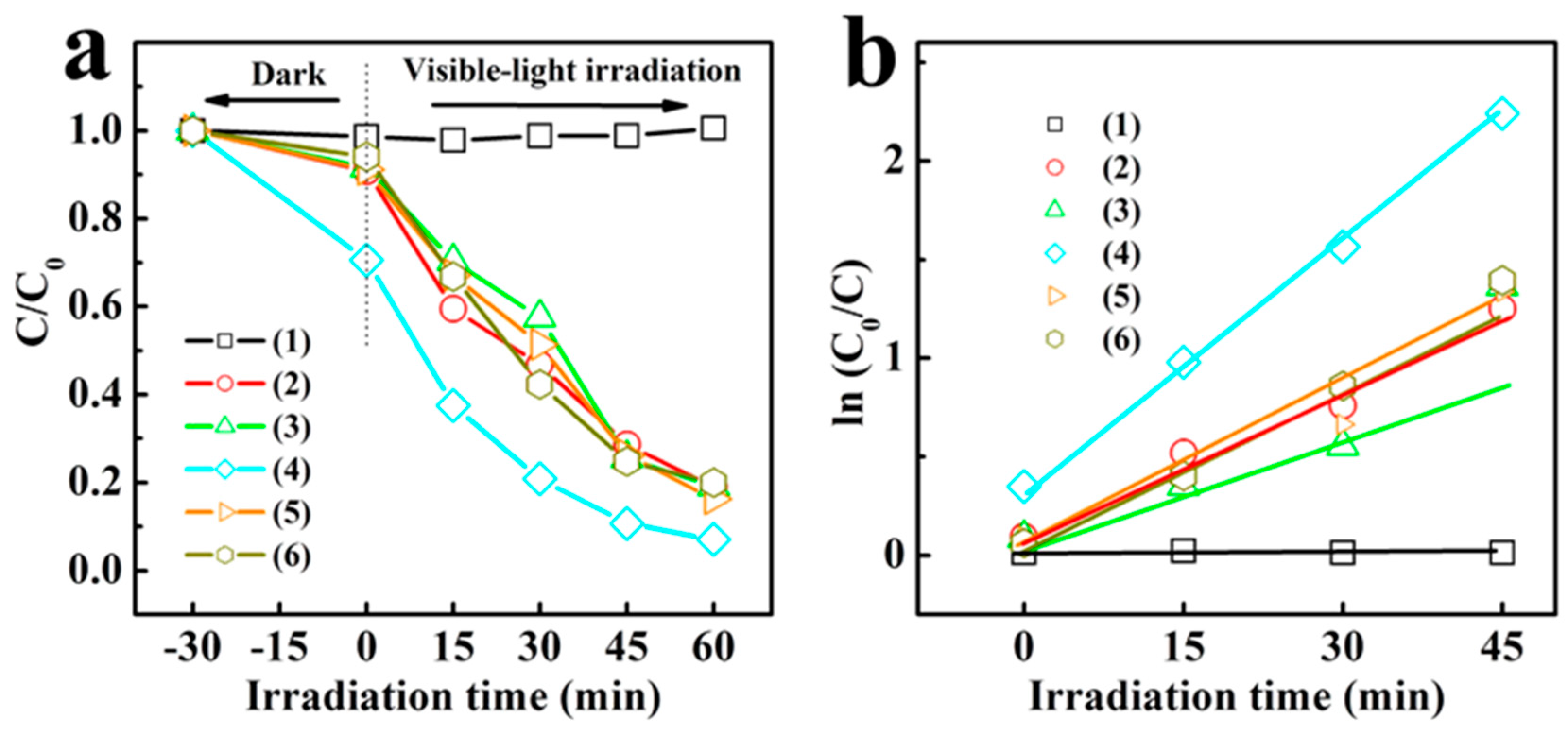
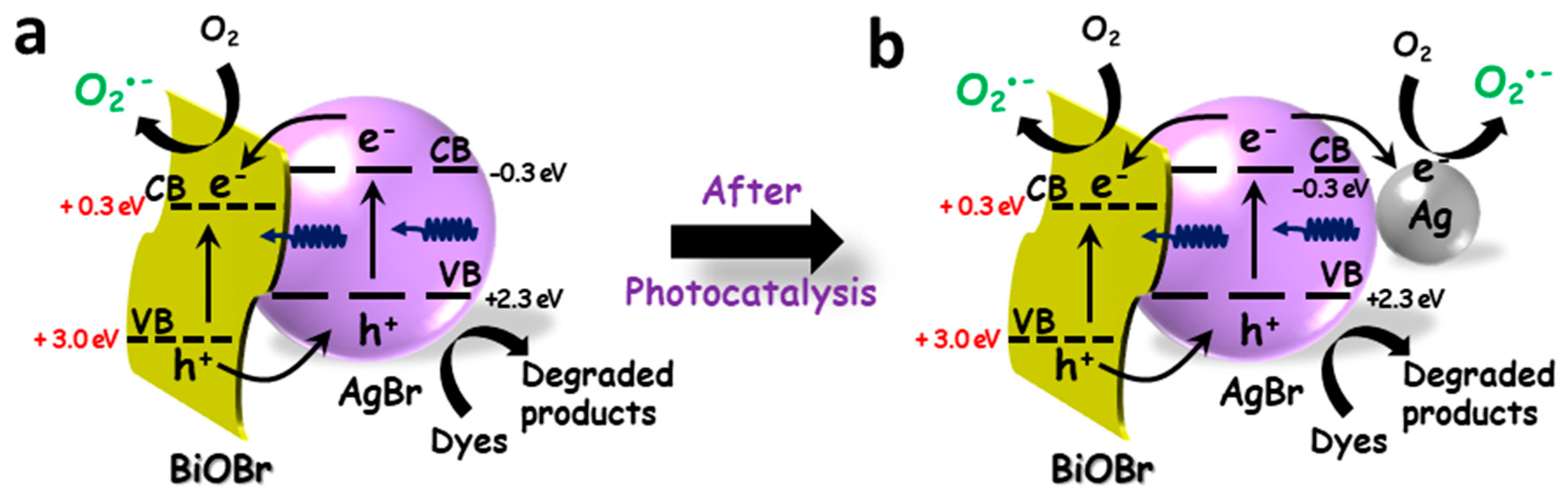
3. Results and Discussion
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Wang, W.; Tadé, M.O.; Shao, Z.P. Research progress of perovskite materials in photocatalysis- and photovoltaics-related energy conversion and environmental treatment. Chem. Soc. Rev. 2015, 44, 5371–5408. [Google Scholar] [CrossRef] [PubMed]
- Miao, F.; Lu, N.; Zhang, P.; Zhang, Z.; Shao, G. Multidimension-Controllable Synthesis of Ant Nest-Structural Electrode Materials with Unique 3D Hierarchical Porous Features toward Electrochemical Applications. Adv. Funct. Mater. 2019, 29, 1808994. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Z.; Fang, Y.; Liu, B.; Huang, J.; Miao, F.; Bao, Y.; Dong, B. IR-Driven strong plasmonic-coupling on Ag nanorices/W18O49 nanowires heterostructures for photo/thermal synergistic enhancement of H2 evolution from ammonia borane. Appl. Catal. B Environ. 2019, 252, 164–173. [Google Scholar] [CrossRef]
- Wei, R.-B.; Huang, Z.-L.; Gu, G.-H.; Wang, Z.; Zeng, L.; Chen, Y.; Liu, Z.-Q. Dual-cocatalysts decorated rimous CdS spheres advancing highly-efficient visible-light photocatalytic hydrogen production. Appl. Catal. B Environ. 2018, 231, 101–107. [Google Scholar] [CrossRef]
- Shandilya, P.; Mittal, D.; Raizada, P.; Hosseini-Bandegharaei, A.; Saini, A.K.; Singh, P. Fabrication of fluorine doped graphene and SmVO4 based dispersed and adsorptive photocatalyst for abatement of phenolic compounds from water and bacterial disinfection. J. Clean. Prod. 2018, 203, 386–399. [Google Scholar] [CrossRef]
- Zhang, P.; Wan, D.; Zhang, Z.; Wang, G.; Hu, J.; Shao, G. RGO-functionalized polymer nanofibrous membrane with exceptional surface activity and ultra-low airflow resistance for PM2.5 filtration. Environ. Sci. Nano 2018, 5, 1813–1820. [Google Scholar] [CrossRef]
- Marschall, R. Semiconductor Composites: Strategies for Enhancing Charge Carrier Separation to Improve Photocatalytic Activity. Adv. Funct. Mater. 2014, 17, 2421–2440. [Google Scholar] [CrossRef]
- Chu, K.W.; Lee, S.L.; Chang, C.J.; Liu, L.Y. Recent Progress of Carbon Dot Precursors and Photocatalysis Applications. Polymers 2019, 11, 689. [Google Scholar] [CrossRef]
- Zheng, N.-C.; Ouyang, T.; Chen, Y.; Wang, Z.; Chen, D.-Y.; Liu, Z.-Q. Ultrathin CdS shell sensitized hollow S-doped CeO2 spheres for efficient visible-light photocatalysis. Catal. Sci. Technol. 2019, 9, 1357–1364. [Google Scholar] [CrossRef]
- Ye, L.Q.; Su, Y.R.; Jin, X.L.; Xie, H.Q.; Zhang, C. Recent advances in BiOX (X = Cl, Br and I) photocatalysts: Synthesis, modification, facet effects and mechanisms. Environ. Sci. Nano 2014, 1, 90–112. [Google Scholar] [CrossRef]
- Sharma, K.; Dutta, V.; Sharma, S.; Raizada, P.; Hosseini-Bandegharaei, A.; Thakur, P.; Singh, P. Recent advances in enhanced photocatalytic activity of bismuth oxyhalides for efficient photocatalysis of organic pollutants in water: A review. J. Ind. Eng. Chem. 2019, 78, 1–20. [Google Scholar] [CrossRef]
- Cheng, H.F.; Huang, B.B.; Dai, Y. Engineering BiOX (X = Cl, Br, I) nanostructures for highly efficient photocatalytic applications. Nanoscale 2014, 6, 2009–2026. [Google Scholar] [CrossRef]
- Wang, Y.J.; Wang, Q.S.; Zhan, X.Y.; Wang, F.M.; Safdar, M.; He, J. Visible light driven type II heterostructures and their enhanced photocatalysis properties: A review. Nanoscale 2013, 5, 8326–8339. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Bai, J.; Liang, H.O.; Li, C.P. Synthesis of the novel nanostructured AgI-BiOI/PAN composite photocatalyst with highly enhanced visible-light catalytic performances. J. Photochem. Photobiol. A Chem. 2018, 357, 132–139. [Google Scholar] [CrossRef]
- Guo, X.H.; Zhou, X.J.; Li, X.H.; Shao, C.L.; Han, C.H.; Li, X.W.; Liu, Y.C. Bismuth oxychloride (BiOCl)/copper phthalocyanine (CuTNPc) heterostructures immobilized on electrospun polyacrylonitrile nanofibers with enhanced activity for floating photocatalysis. J. Colloid Interface Sci. 2018, 525, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Shao, C.L.; Li, X.H.; Xin, J.Y.; Yang, S.; Liu, Y.C. Electrospun CuAl2O4 hollow nanofibers as visible light photocatalyst with enhanced activity and excellent stability under acid and alkali conditions. CrystEngComm 2018, 20, 312–322. [Google Scholar] [CrossRef]
- Shi, H.F.; Yu, Y.C.; Zhang, Y.; Feng, X.J.; Zhao, X.Y.; Tan, H.Q.; Ullah Khan, S.; Li, Y.G.; Wang, E.B. Polyoxometalate/TiO2/Ag composite nanofibers with enhanced photocatalytic performance under visible light. Appl. Catal. B Environ. 2018, 221, 280–289. [Google Scholar] [CrossRef]
- Zhou, X.J.; Shao, C.L.; Yang, S.; Li, X.W.; Guo, X.H.; Wang, X.X.; Li, X.H.; Liu, Y.C. Heterojunction of g-C3N4/BiOI Immobilized on Flexible Electrospun Polyacrylonitrile Nanofibers: Facile Preparation and Enhanced Visible Photocatalytic Activity for Floating Photocatalysis. ACS Sustain. Chem. Eng. 2018, 6, 2316–2323. [Google Scholar] [CrossRef]
- Zhou, X.J.; Shao, C.L.; Li, X.H.; Wang, X.X.; Guo, X.H.; Liu, Y.C. Three dimensional hierarchical heterostructures of g-C3N4 nanosheets/TiO2 nanofibers: Controllable growth via gas-solid reaction and enhanced photocatalytic activity under visible light. J. Hazard. Mater. 2018, 344, 113–122. [Google Scholar] [CrossRef]
- Sun, Y.C.; Shao, C.L.; Li, X.H.; Guo, X.H.; Zhou, X.J.; Li, X.W.; Liu, Y.C. Hierarchical heterostructures of p-type bismuth oxychloride nanosheets on n-type zinc ferrite electrospun nanofibers with enhanced visible-light photocatalytic activities and magnetic separation properties. J. Colloid Interface Sci. 2018, 516, 110–120. [Google Scholar] [CrossRef]
- Cheng, Z.Q.; Zhao, S.Z.; Han, L.H. A novel preparation method for ZnO/γ-Al2O3 nanofibers with enhanced absorbability and improved photocatalytic water-treatment performance by Ag nanoparticles. Nanoscale 2018, 10, 6892–6899. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, C.F.; Ye, H.W.; Jia, Y.S.; Wu, Y.Y.; Jin, A.L.; Wang, Y.B.; Chen, X.S. One-step hydrothermal growth of carbon nanofibers and in-situ assembly of Ag nanowire@carbon nanofiber@Ag nanoparticles ternary composites for efficient photocatalytic removal of organic pollutants. Carbon 2018, 131, 213–222. [Google Scholar] [CrossRef]
- Zeng, Q.X.; Xu, G.C.; Zhang, L.; Lin, H.; Lv, Y.; Jia, D.Z. Porous CuO nanofibers derived from a Cu-based coordination polymer as a photocatalyst for the degradation of rhodamine B. New J. Chem. 2018, 42, 7016–7024. [Google Scholar] [CrossRef]
- Li, S.J.; Hu, S.W.; Jiang, W.; Liu, Y.P.; Zhou, Y.T.; Liu, Y.; Mo, L.Y. Hierarchical architectures of bismuth molybdate nanosheets onto nickel titanate nanofibers: Facile synthesis and efficient photocatalytic removal of tetracycline hydrochloride. J. Colloid Interface Sci. 2018, 521, 42–49. [Google Scholar] [CrossRef]
- Tao, R.; Zhao, C.C.; Shao, C.L.; Li, X.H.; Li, X.W.; Zhang, J.; Yang, S.; Liu, Y.C. Bi2WO6/ZnFe2O4 heterostructures nanofibers: Enhanced visible-light photocatalytic activity and magnetically separable property. Mater. Res. Bull. 2018, 104, 124–133. [Google Scholar] [CrossRef]
- Chen, S.S.; Li, S.Z.; Xiong, L.B.; Wang, G.H. In-situ growth of ZnIn2S4 decorated on electrospun TiO2 nanofibers with enhanced visible-light photocatalytic activity. Chem. Phys. Lett. 2018, 706, 68–75. [Google Scholar] [CrossRef]
- Tao, R.; Shao, C.L.; Li, X.H.; Li, X.W.; Liu, S.; Yang, S.; Zhao, C.C.; Liu, Y.C. Bi2MoO6/BiFeO3 heterojunction nanofibers: Enhanced photocatalytic activity, charge separation mechanism and magnetic separability. J. Colloid Interface Sci. 2018, 529, 404–414. [Google Scholar] [CrossRef]
- Dursun, S.; Cihan Kaya, I.; Kalem, V.; Akyildiz, H. UV/visible light active CuCrO2 nanoparticle–SnO2 nanofiber p–n heterostructured photocatalysts for photocatalytic applications. Dalton Trans. 2018, 47, 14662–14678. [Google Scholar] [CrossRef]
- Liu, G.; Wang, G.H.; Hu, Z.H.; Su, Y.R.; Zhao, L. Ag2O nanoparticles decorated TiO2 nanofibers as a p-n heterojunction for enhanced photocatalytic decomposition of RhB under visible light irradiation. Appl. Surf. Sci. 2019, 465, 902–910. [Google Scholar] [CrossRef]
- Li, Y.A.; Li, J.; Chen, L.; Sun, H.B.; Zhang, H.; Guo, H.; Feng, L. In situ Synthesis of Au-Induced Hierarchical Nanofibers/Nanoflakes Structured BiFeO3 Homojunction Photocatalyst With Enhanced Photocatalytic Activity. Front. Chem. 2019, 6, 649. [Google Scholar] [CrossRef]
- Karim, S.A.; Mohamed, A.; Abdel-Mottaleb, M.M.; Osman, T.A.; Khattab, A. Visible light photocatalytic activity of PAN-CNTs/ZnO-NH2 electrospun nanofibers. J. Alloy. Compd. 2019, 772, 650–655. [Google Scholar] [CrossRef]
- Cai, Y.T.; Song, J.; Liu, X.Y.; Yin, X.; Li, X.R.; Yu, J.Y.; Ding, B. Soft BiOBr@TiO2 nanofibrous membranes with hierarchical heterostructures as efficient and recyclable visible-light photocatalysts. Environ. Sci. Nano 2018, 5, 2631–2640. [Google Scholar] [CrossRef]
- Ma, G.; Lu, J.L.; Meng, Q.G.; Lv, H.Q.; Shui, L.L.; Zhang, Y.G.; Jin, M.L.; Chen, Z.H.; Yuan, M.Z.; Nötzel, R.; et al. Synergistic effect of Cu-ion and WO3 nanofibers on the enhanced photocatalytic degradation of Rhodamine B and aniline solution. Appl. Surf. Sci. 2018, 451, 306–314. [Google Scholar] [CrossRef]




| Photocatalyst | Light | Photocatalytic Results | Year | Ref. |
|---|---|---|---|---|
| AgI–BiOI/PAN composite nanofibers | 300-W Xe lamp (λ > 400 nm) | The photodegradation efficiency of Rhodamine B (RhB) could reach 98.5% within 210 min | 2018 | [14] |
| BiOCl/CuTNPc/PAN nanofibers | 150-W xenon lamp (λ > 420 nm) | The degradation rate of RhB reached 75% within 180 min | 2018 | [15] |
| CuAl2O4 hollow nanofibers | 500-W xenon lamp (λ ≧ 420 nm) | The RhB and MO solutions were degraded by 83.5% and 94% within 360 and 300 min | 2018 | [16] |
| Polyoxometalate/TiO2/Ag composite nanofibers | 300-W Xe lamp (λ ≧ 420 nm) | MO (20 mL, 20 ppm) could be completely degraded within 160 min | 2018 | [17] |
| PAN/g-C3N4/BiOI nanofibers | 500-W Xe lamp (λ ≧ 400 nm) | The degradation efficiency of RhB could reach 98.0% after irradiation for 90 min | 2018 | [18] |
| g-C3N4 nanosheets/TiO2 nanofibers | 500-W xenon lamp (λ ≧ 420 nm) | The photocatalytic degradation ratios of RhB could reach 93.6% after irradiation for 120 min | 2018 | [19] |
| p-BiOCl/nZnFe2O4 nanofibers | 150-W xenon lamp (λ > 420 nm) | The photodegradation efficiency of RhB could reach 97% within 180 min | 2018 | [20] |
| ZnO/γ-Al2O3 nanofibers | 120-W ultraviolet radiation lamp | The MO could be completely degraded in 120 min | 2018 | [21] |
| Ag nanowire@carbon nanofiber@Ag nanoparticles | 300-W Xe lamp (λ > 400 nm) | 91% of methylene blue (MB) could be eliminated within 120 min | 2018 | [22] |
| CuO nanofibers | The degradation rate of RhB reached 96% within 160 min | 2018 | [23] | |
| Bi2MoO6/NiTiO3 nanofibers | 300-W Xe lamp (λ ≧ 420 nm) | The degradation efficiency of MB, MO, and RhB achieved using 1.0 Bi–Ni was 100%, 86.7%, or 100% within 90 min | 2018 | [24] |
| Bi2WO6/ZnFe2O4 nanofibers | 150-W xenon lamp (λ > 420 nm) | The degradation rate of RhB reached 98% within 300 min | 2018 | [25] |
| ZnIn2S4/TiO2 nanofibers | 350-W Xe lamp (λ ≧ 420 nm) | The MO could be completely degraded within 75 min | 2018 | [26] |
| Bi2MoO6/BiFeO3 heterojunction nanofibers | 150-W xenon lamp (λ > 420 nm) | The degradation rate of RhB reached 98% within 5 h | 2018 | [27] |
| CuCrO2/SnO2 nanofibers | 250-W metal halide lamp | The MB could be completely degraded within 120 min | 2018 | [28] |
| Ag2O/TiO2 composite nanofibers | visible light irradiation (>420 nm) | The degradation rate of RhB reached 87.7% within 80 min | 2019 | [29] |
| Au/BiFeO3 composite nanofibers | 500W xenon lamp | The degradation rate of RhB reached 85.76% within 3h | 2019 | [30] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Qi, Y.; Zhang, Z. AgBr/BiOBr Nano-Heterostructure-Decorated Polyacrylonitrile Nanofibers: A Recyclable High-Performance Photocatalyst for Dye Degradation under Visible-Light Irradiation. Polymers 2019, 11, 1718. https://doi.org/10.3390/polym11101718
Zhang M, Qi Y, Zhang Z. AgBr/BiOBr Nano-Heterostructure-Decorated Polyacrylonitrile Nanofibers: A Recyclable High-Performance Photocatalyst for Dye Degradation under Visible-Light Irradiation. Polymers. 2019; 11(10):1718. https://doi.org/10.3390/polym11101718
Chicago/Turabian StyleZhang, Mingyi, Ying Qi, and Zhenyi Zhang. 2019. "AgBr/BiOBr Nano-Heterostructure-Decorated Polyacrylonitrile Nanofibers: A Recyclable High-Performance Photocatalyst for Dye Degradation under Visible-Light Irradiation" Polymers 11, no. 10: 1718. https://doi.org/10.3390/polym11101718
APA StyleZhang, M., Qi, Y., & Zhang, Z. (2019). AgBr/BiOBr Nano-Heterostructure-Decorated Polyacrylonitrile Nanofibers: A Recyclable High-Performance Photocatalyst for Dye Degradation under Visible-Light Irradiation. Polymers, 11(10), 1718. https://doi.org/10.3390/polym11101718