Preparation of Progressive Antibacterial LDPE Surface via Active Biomolecule Deposition Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Plasma Treatment of LDPE
2.3. Antibacterial Agent Grafting
2.4. Hydroperoxide Determination
2.5. Surface Wettability Measurements
2.6. Graft Yield Analysis
2.7. Film Thickness Investigation
2.8. Peel Test
2.9. Surface Chemistry Characterization
2.10. Surface Morphology Analysis
2.11. Antibacterial Tests
3. Results
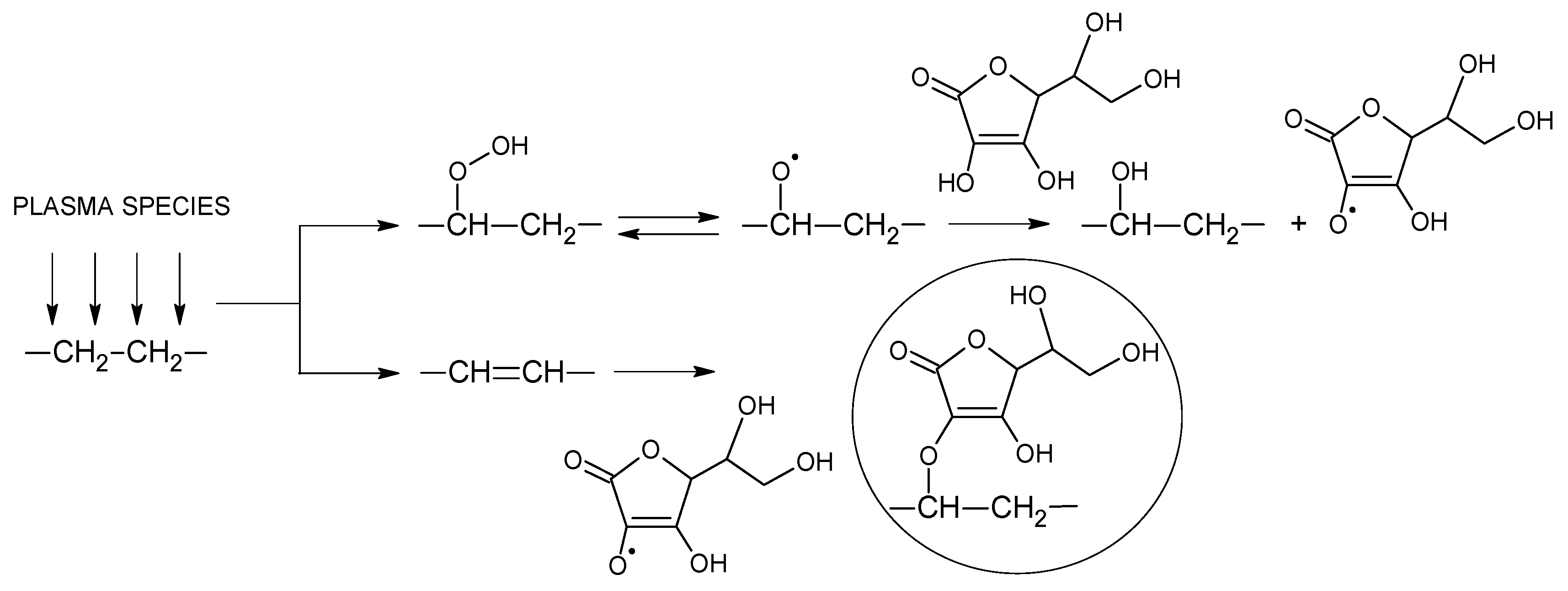
3.1. Hydroperoxide Concentration
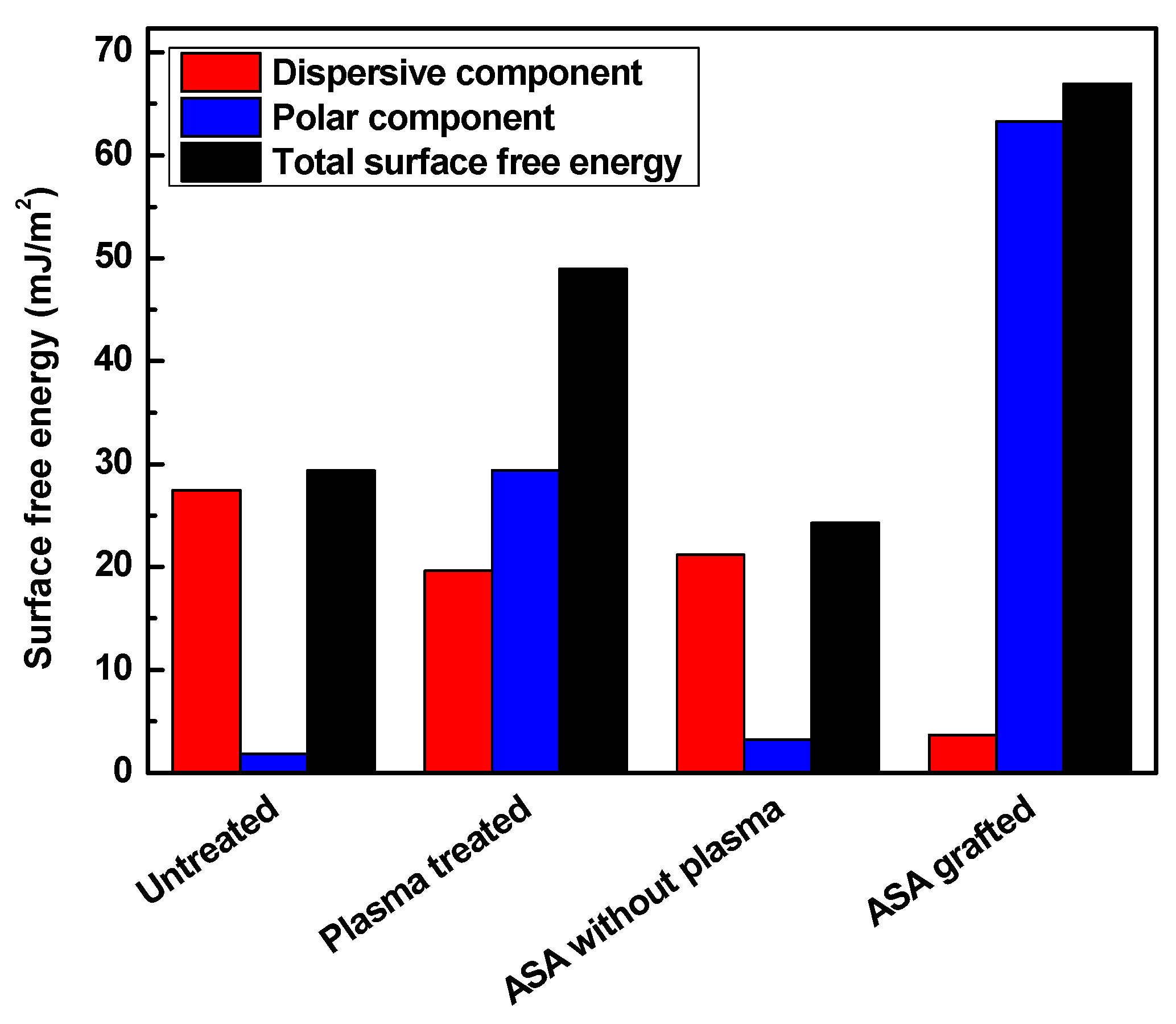
3.2. Surface Wettability Analysis
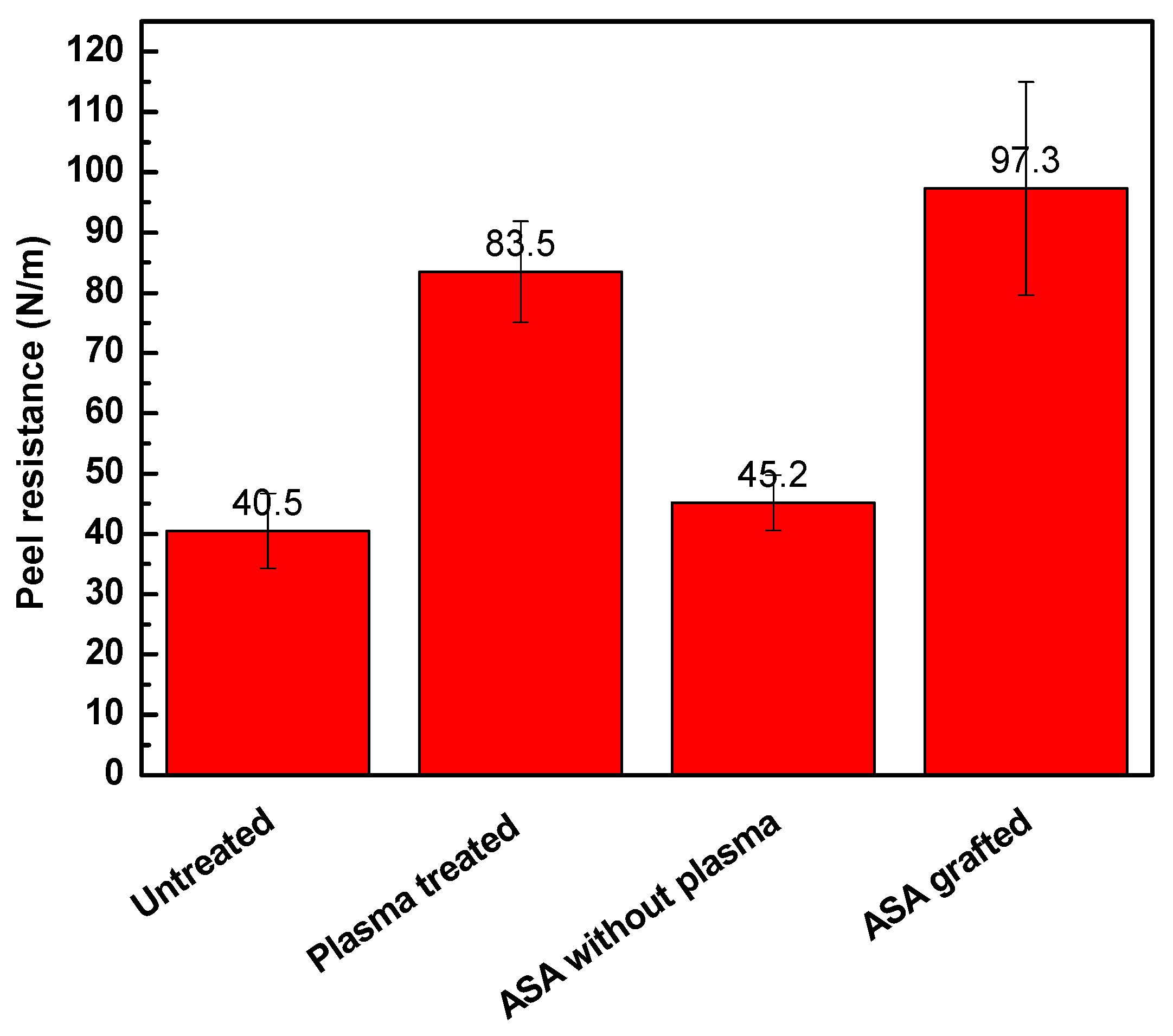
3.3. Adhesion Analysis
3.4. Chemical Composition Investigation
3.5. Surface Morphology Analysis
3.6. Antibacterial Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Han, J.H.; Floros, J.D. Casting Antimicrobial Packaging Films and Measuring Their Physical Properties and Antimicrobial Activity. J. Plast. Film Sheeting 1997, 13, 287–298. [Google Scholar] [CrossRef]
- De Azeredo, H.M.C. Nanocomposites for food packaging applications. Food Res. Int. 2009, 42, 1240–1253. [Google Scholar] [CrossRef]
- Pankaj, S.; Bueno-Ferrer, C.; Misra, N.; Milosavljević, V.; O’Donnell, C.; Bourke, P.; Keener, K.; Cullen, P. Applications of cold plasma technology in food packaging. Trends Food Sci. Technol. 2014, 35, 5–17. [Google Scholar] [CrossRef]
- Dowling, D.; Tynan, J.; Ward, P.; Hynes, A.; Cullen, J.; Byrne, G. Atmospheric pressure plasma treatment of amorphous polyethylene terephthalate for enhanced heatsealing properties. Int. J. Adhes. Adhes. 2012, 35, 1–8. [Google Scholar] [CrossRef]
- Kavc, T.; Kern, W.; Ebel, M.F.; Svagera, R.; Pölt, P. Surface Modification of Polyethylene by Photochemical Introduction of Sulfonic Acid Groups. Chem. Mater. 2000, 12, 1053–1059. [Google Scholar] [CrossRef]
- Pukánszky, B.; Fekete, E. Adhesion and Surface Modification. In Mineral Fillers in Thermoplastics I: Raw Materials and Processing; Springer: Berlin/Heidelberg, Germany, 2007; pp. 109–153. [Google Scholar]
- Chanunpanich, N.; Ulman, A.; Strzhemechny, Y.M.; Schwarz, S.A.; Janke, A.; Braun, H.G.; Kraztmuller, T. Surface Modification of Polyethylene through Bromination. Langmuir 1999, 15, 2089–2094. [Google Scholar] [CrossRef]
- Griesser, H.J.; Da, Y.; Hughes, A.E.; Gengenbach, T.R.; Mau, A.W.H. Shallow reorientation in the surface dynamics of plasma-treated fluorinated ethylene-propylene polymer. Langmuir 1991, 7, 2484–2491. [Google Scholar] [CrossRef]
- Liston, E.; Martinu, L.; Wertheimer, M. Plasma surface modification of polymers for improved adhesion: A critical review. J. Adhes. Sci. Technol. 1993, 7, 1091–1127. [Google Scholar] [CrossRef]
- Albertsson, A.C. (Ed.) Long Term Properties of Polyolefins; Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 2004; Volume 169, ISBN 978-3-540-40769-0. [Google Scholar]
- Desai, S.M.; Singh, R.P. Surface Modification of Polyethylene. In Long Term Properties of Polyolefins; Springer: Berlin/Heidelberg, Germany, 2004; pp. 231–294. [Google Scholar]
- Allen, K.W. Polymer surface modification: Relevance to adhesion. Polym. Int. 2002, 42, 235. [Google Scholar]
- Biederman, H. Plasma Polymer Films; Imperial College Press and Distributed by World Scientific Publishing Co.: Singapore, 2012; ISBN 978-1-86094-467-3. [Google Scholar]
- Pascual, M.; Balart, R.; Sánchez, L.; Fenollar, O.; Calvo, O. Study of the aging process of corona discharge plasma effects on low density polyethylene film surface. J. Mater. Sci. 2008, 43, 4901–4909. [Google Scholar] [CrossRef]
- Popelka, A.; Novák, I.; Lehocký, M.; Junkar, I.; Mozetič, M.; Kleinová, A.; Janigová, I.; Slouf, M.; Bílek, F.; Chodák, I. A new route for chitosan immobilization onto polyethylene surface. Carbohydr. Polym. 2012, 90, 1501–1508. [Google Scholar] [CrossRef]
- Graves, D.B. Low temperature plasma biomedicine: A tutorial review. Phys. Plasmas 2014, 21, 80901. [Google Scholar] [CrossRef]
- Popelka, A.; Novák, I.; Al-maadeed, M.A.S.A.; Ouederni, M.; Krupa, I. Effect of corona treatment on adhesion enhancement of LLDPE. Surf. Coat. Technol. 2018, 335, 118–125. [Google Scholar] [CrossRef]
- Abusrafa, A.E.; Habib, S.; Krupa, I.; Ouederni, M.; Popelka, A. Modification of Polyethylene by RF Plasma in Different/Mixture Gases. Coatings 2019, 9, 145. [Google Scholar] [CrossRef]
- Kong, M.G.; Kroesen, G.; Morfill, G.; Nosenko, T.; Shimizu, T.; Van Dijk, J.; Zimmermann, J.L. Plasma medicine: An introductory review. New J. Phys. 2009, 11, 115012. [Google Scholar] [CrossRef]
- Bardos, L.; Barankova, H. Cold atmospheric plasma: Sources, processes, and applications. Thin Solid Films 2010, 518, 6705–6713. [Google Scholar] [CrossRef]
- Helgadóttir, S.; Pandit, S.; Mokkapati, V.R.S.S.; Westerlund, F.; Apell, P.; Mijakovic, I. Vitamin C Pretreatment Enhances the Antibacterial Effect of Cold Atmospheric Plasma. Front. Cell. Infect. Microbiol. 2017, 7, 43. [Google Scholar] [CrossRef] [PubMed]
- De Geyter, N.; Morent, R. Nonthermal Plasma Sterilization of Living and Nonliving Surfaces. Annu. Rev. Biomed. Eng. 2012, 14, 255–274. [Google Scholar] [CrossRef]
- Joshi, S.G.; Paff, M.; Friedman, G.; Fridman, G.; Fridman, A.; Brooks, A.D. Control of methicillin-resistant Staphylococcus aureus in planktonic form and biofilms: A biocidal efficacy study of nonthermal dielectric-barrier discharge plasma. Am. J. Infect. Control 2010, 38, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Bazaka, K.; Jacob, M.V.; Chrzanowski, W.; Ostrikov, K. Anti-bacterial surfaces: Natural agents, mechanisms of action, and plasma surface modification. RSC Adv. 2015, 5, 48739–48759. [Google Scholar] [CrossRef]
- Weng, Y.M.; Chen, M.J.; Chen, W. Antimicrobial Food Packaging Materials from Poly (ethylene-co-methacrylic acid). LWT 1999, 32, 191–195. [Google Scholar] [CrossRef]
- Ahmed, I.; Ready, D.; Wilson, M.; Knowles, J.C. Antimicrobial effect of silver-doped phosphate-based glasses. J. Biomed. Mater. Res. Part A 2006, 79, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Valappil, S.P.; Pickup, D.M.; Carroll, D.L.; Hope, C.K.; Pratten, J.; Newport, R.J.; Smith, M.E.; Wilson, M.; Knowles, J.C. Effect of Silver Content on the Structure and Antibacterial Activity of Silver-Doped Phosphate-Based Glasses. Antimicrob. Agents Chemother. 2007, 51, 4453–4461. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Haag, R. Universal polymer coatings and their representative biomedical applications. Mater. Horiz. 2015, 2, 567–577. [Google Scholar] [CrossRef]
- Muñoz-Bonilla, A.; Fernández-García, M. Polymeric materials with antimicrobial activity. Prog. Polym. Sci. 2012, 37, 281–339. [Google Scholar] [CrossRef]
- Aider, M. Chitosan application for active bio-based films production and potential in the food industry: Review. LWT 2010, 43, 837–842. [Google Scholar] [CrossRef]
- Leceta, I.; Guerrero, P.; De La Caba, K. Functional properties of chitosan-based films. Carbohydr. Polym. 2013, 93, 339–346. [Google Scholar] [CrossRef]
- Theapsak, S.; Watthanaphanit, A.; Rujiravanit, R. Preparation of Chitosan-Coated Polyethylene Packaging Films by DBD Plasma Treatment. ACS Appl. Mater. Interfaces 2012, 4, 2474–2482. [Google Scholar] [CrossRef]
- Correia, V.G.; Ferraria, A.M.; Pinho, M.G.; Aguiar-Ricardo, A. Antimicrobial Contact-Active Oligo (2-oxazoline) s-Grafted Surfaces for Fast Water Disinfection at the Point-of-Use. Biomacromolecules 2015, 16, 3904–3915. [Google Scholar] [CrossRef]
- Tiller, J.C.; Lee, S.B.; Lewis, K.; Klibanov, A.M. Polymer surfaces derivatized with poly (vinyl-N-hexylpyridinium) kill airborne and waterborne bacteria. Biotechnol. Bioeng. 2002, 79, 465–471. [Google Scholar] [CrossRef]
- Ardjani, T.E.A.; Alvarez-Idaboy, J.R. Radical scavenging activity of ascorbic acid analogs: Kinetics and mechanisms. Theor. Chem. Acc. 2018, 137, 69. [Google Scholar] [CrossRef]
- Niki, E. Action of ascorbic acid as a scavenger of active and stable oxygen radicals. Am. J. Clin. Nutr. 1991, 54, 1119–1124. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Yuan, C.; Chen, Y.; Li, H.; Liu, J. Combined effects of ascorbic acid and chitosan on the quality maintenance and shelf life of plums. Sci. Hortic. 2014, 176, 45–53. [Google Scholar] [CrossRef]
- Verghese, R.J.; Ramya, S.; Kanungo, R. In vitro Antibacterial Activity of Vitamin C and in Combination with Ciprofloxacin against Uropathogenic Escherichia coli. J. Clin. Diagn. Res. 2017, 11, 1–5. [Google Scholar] [CrossRef]
- Tajkarimi, M.; Ibrahim, S.A. Antimicrobial activity of ascorbic acid alone or in combination with lactic acid on Escherichia coli O157:H7 in laboratory medium and carrot juice. Food Control 2011, 22, 801–804. [Google Scholar] [CrossRef]
- Hemilä, H. Vitamin C and infections. Nutrients 2017, 9, 339. [Google Scholar] [CrossRef]
- Vrijsen, R.; Everaert, L.; Boeyé, A. Antiviral Activity of Flavones and Potentiation by Ascorbate. J. Gen. Virol. 1988, 69, 1749–1751. [Google Scholar] [CrossRef]
- Verghese, R.; Mathew, S.; David, A. Antimicrobial activity of Vitamin C demonstrated on uropathogenic Escherichia coli and Klebsiella pneumoniae. J. Curr. Res. Sci. Med. 2018, 3, 88–93. [Google Scholar]
- Kallio, J.; Jaakkola, M.; Mäki, M.; Kilpeläinen, P.; Virtanen, V. Vitamin C Inhibits Staphylococcus aureus Growth and Enhances the Inhibitory Effect of Quercetin on Growth of Escherichia coli In Vitro. Planta Med. 2012, 78, 1824–1830. [Google Scholar] [CrossRef]
- Li, S.; Taylor, K.B.; Kelly, S.J.; Jedrzejas, M.J. Vitamin C Inhibits the Enzymatic Activity of Streptococcus pneumoniae Hyaluronate Lyase. J. Biol. Chem. 2001, 276, 15125–15130. [Google Scholar] [CrossRef]
- Isela, S.R.; Sergio, N.; José, M.J. Ascorbic Acid On Oral Microbial Growth and Biofilm. Pharma Innov. J. 2013, 2, 103–109. [Google Scholar]
- Eddy, B.P.; Ingram, M. Interactions between ascorbic acid and bacteria. Bacteriol. Rev. 1953, 17, 93–107. [Google Scholar] [PubMed]
- Du, J.; Cullen, J.J.; Buettner, G.R. Ascorbic acid: Chemistry, biology and the treatment of cancer. Biochim. Biophys. Acta BBA Bioenerg. 2012, 1826, 443–457. [Google Scholar] [CrossRef] [PubMed]
- Pal, D.; Nimse, S.B. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar]
- JIS Z 2801/ISO 22196—Microbe Investigations (MIS). Available online: https://www.microbe-investigations.com/testing-methods/jis-z-2801-iso-22196/ (accessed on 6 August 2019).
- Suzuki, M.; Kishida, A.; Iwata, H.; Hata, Y.; Ikada, Y. Graft copolymerization of Acrylamide onto a polythylene surface pretreated with a glow discharge. Macromolecules 1986, 19, 1804–1808. [Google Scholar] [CrossRef]
- Wagner, C.D.; Smith, R.H.; Peters, E.D. Determination of Organic Peroxides—Evaluation of a Modified lodometric Method. Anal. Chem. 1947, 19, 976–979. [Google Scholar] [CrossRef]
- Thelen, H.; Kaufmann, R.; Klee, D. Development and characterization of a wettable surface modified aromatic polyethersulphone using glow discharge induced HEMA-graft polymerisation. Anal. Bioanal. Chem. 1995, 353, 290–296. [Google Scholar] [CrossRef]
- Busscher, H.; Van Pelt, A.; De Boer, P.; De Jong, H.; Arends, J. The effect of surface roughening of polymers on measured contact angles of liquids. Colloids Surf. 1984, 9, 319–331. [Google Scholar] [CrossRef]
- Erbil, H.Y. Surface tension of polymers. In CRC Handbook of Surface and Colloid Chemistry; Birdi, K.S., Ed.; CRC Press: Boca Raton, FL, USA, 2015; pp. 265–312. [Google Scholar]
- Jay, J.M. Modern Food Microbiology; Springer: Berlin/Heidelberg, Germany, 2012; ISBN 978-0-387-23180-8. [Google Scholar]

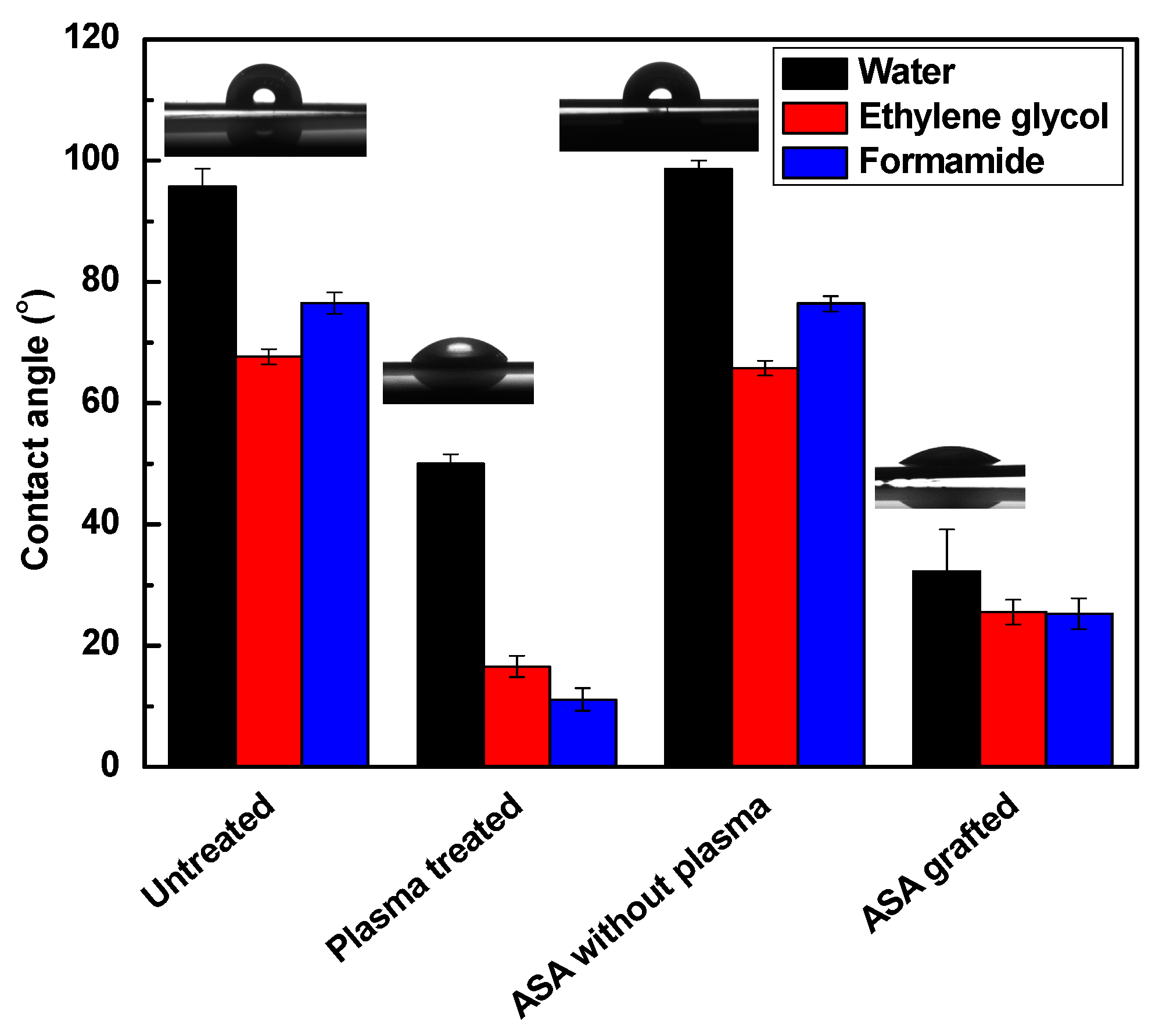

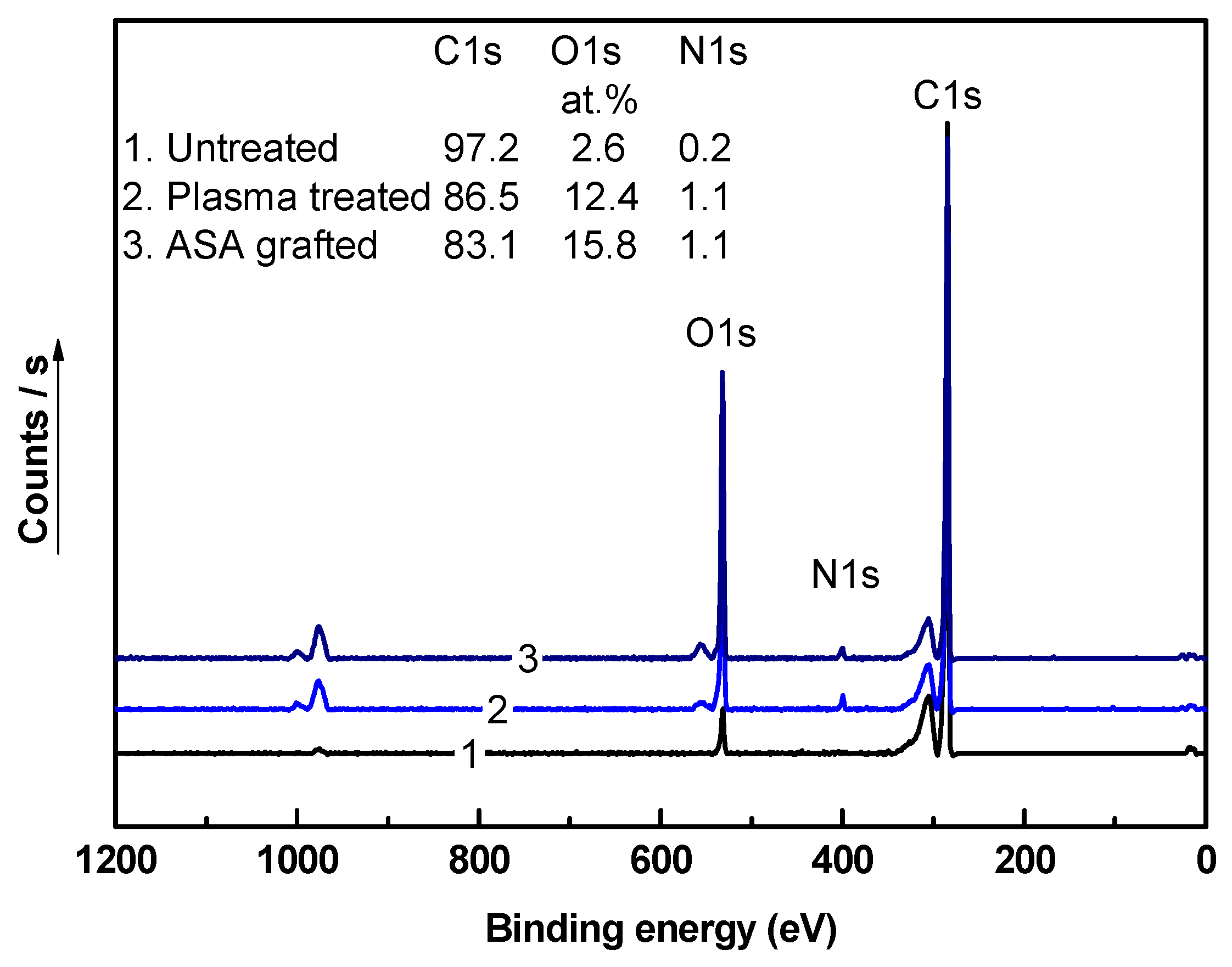
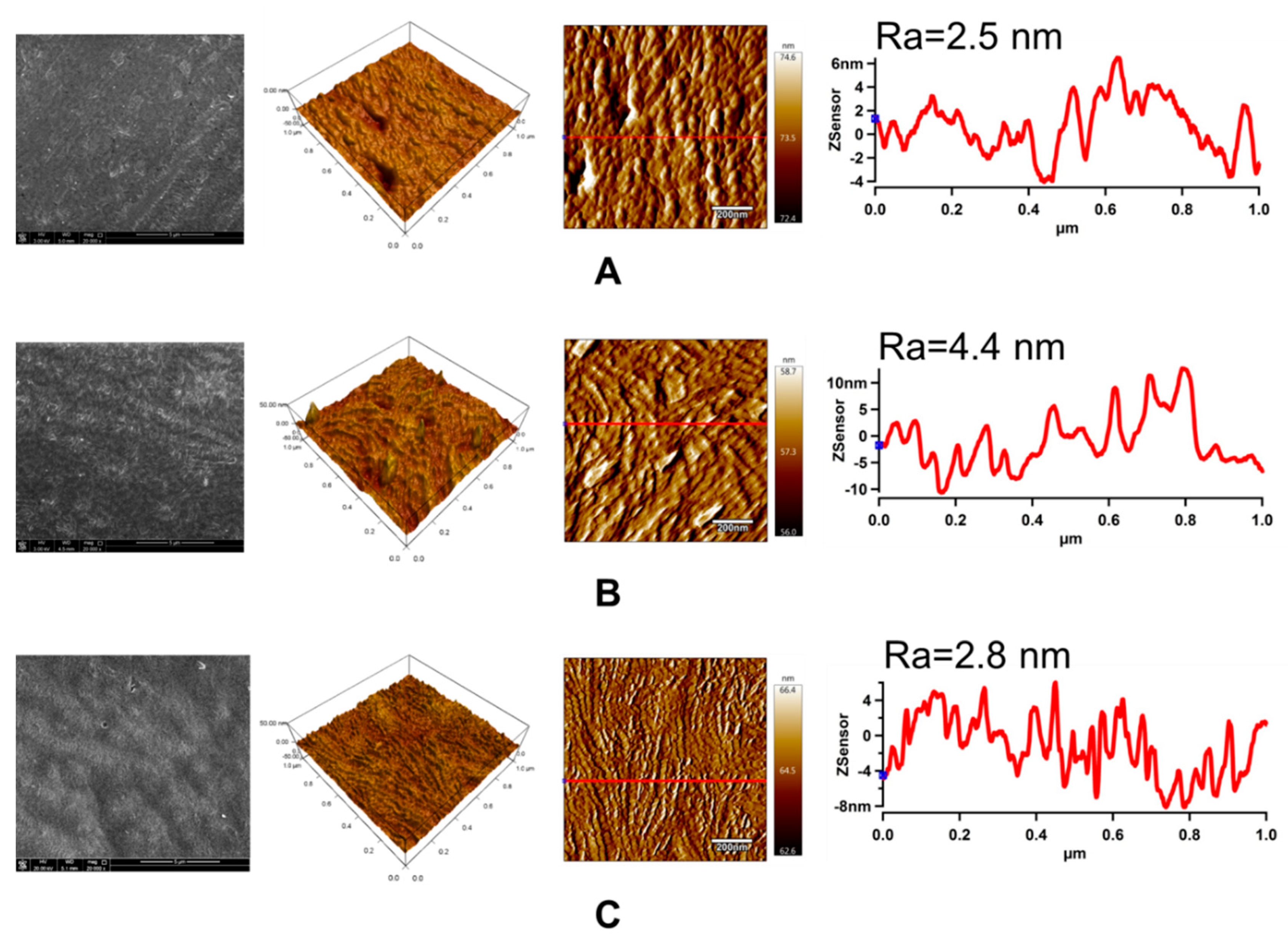

| LDPE | Water (°) | Ethylene Glycol (°) | Formamide (°) | GY (%) | Film Thickness (nm) |
|---|---|---|---|---|---|
| Untreated (A) | 95.7 (±3.0) | 67.7 (±1.2) | 76.5 (±1.8) | - | - |
| Plasma-treated (B) | 50.0 (±1.6) | 16.6 (±1.7) | 11.1 (±1.8) | - | 28.2 (±4.0) |
| A + ASA | 98.6 (±1.4) | 65.8 (±1.2) | 76.4 (±1.2) | 0.0 | - |
| B + ASA | 32.3 (±6.9) | 25.5 (±2.0) | 25.3 (±2.5) | 0.4 | 10.1 (1.0) |
| LDPE | Dispersive (mJ/m2) | Polar (mJ/m2) | Total Surface Free Energy (mJ/m2) |
|---|---|---|---|
| Untreated (A) | 27.5 | 1.9 | 29.3 |
| Plasma-treated (B) | 19.6 | 29.4 | 49.0 |
| A + ASA | 21.2 | 3.2 | 24.3 |
| B + ASA | 3.7 | 63.3 | 67.0 |
| Increase in Bacterial Colonies 1 | ||
|---|---|---|
| LDPE | S. aureus | E. coli |
| Untreated (A) | 4, 4–5, 4–5 | 4, 4, 4–5 |
| Plasma-treated (B) | 5, 5, 5 | 5, 5, 5 |
| B + ASA | 0, 1, 1 | 4, 4, 4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Habib, S.; Lehocky, M.; Vesela, D.; Humpolíček, P.; Krupa, I.; Popelka, A. Preparation of Progressive Antibacterial LDPE Surface via Active Biomolecule Deposition Approach. Polymers 2019, 11, 1704. https://doi.org/10.3390/polym11101704
Habib S, Lehocky M, Vesela D, Humpolíček P, Krupa I, Popelka A. Preparation of Progressive Antibacterial LDPE Surface via Active Biomolecule Deposition Approach. Polymers. 2019; 11(10):1704. https://doi.org/10.3390/polym11101704
Chicago/Turabian StyleHabib, Salma, Marian Lehocky, Daniela Vesela, Petr Humpolíček, Igor Krupa, and Anton Popelka. 2019. "Preparation of Progressive Antibacterial LDPE Surface via Active Biomolecule Deposition Approach" Polymers 11, no. 10: 1704. https://doi.org/10.3390/polym11101704
APA StyleHabib, S., Lehocky, M., Vesela, D., Humpolíček, P., Krupa, I., & Popelka, A. (2019). Preparation of Progressive Antibacterial LDPE Surface via Active Biomolecule Deposition Approach. Polymers, 11(10), 1704. https://doi.org/10.3390/polym11101704








